Expression Analysis of Citrate Metabolism-Related Genes Reveals New Insights into High Citrate Accumulation in a Bingtang Orange Bud Mutant (Citrus sinensis cv. Jinyan)
Abstract
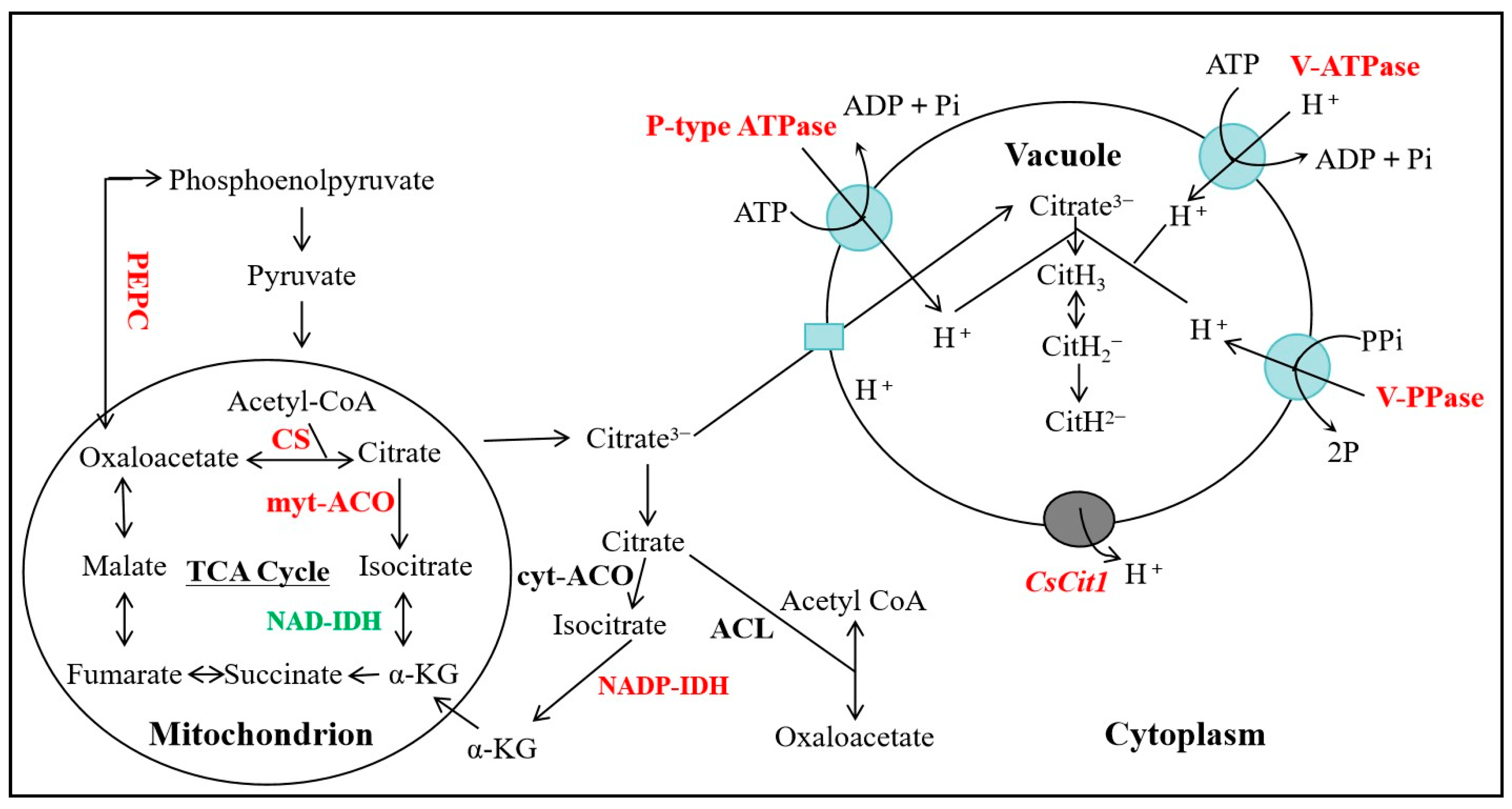
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Physical and Inclusion Indicators
2.3. Measurement of Soluble Sugars and Organic Acids
2.4. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.5. Statistical Analysis
3. Results
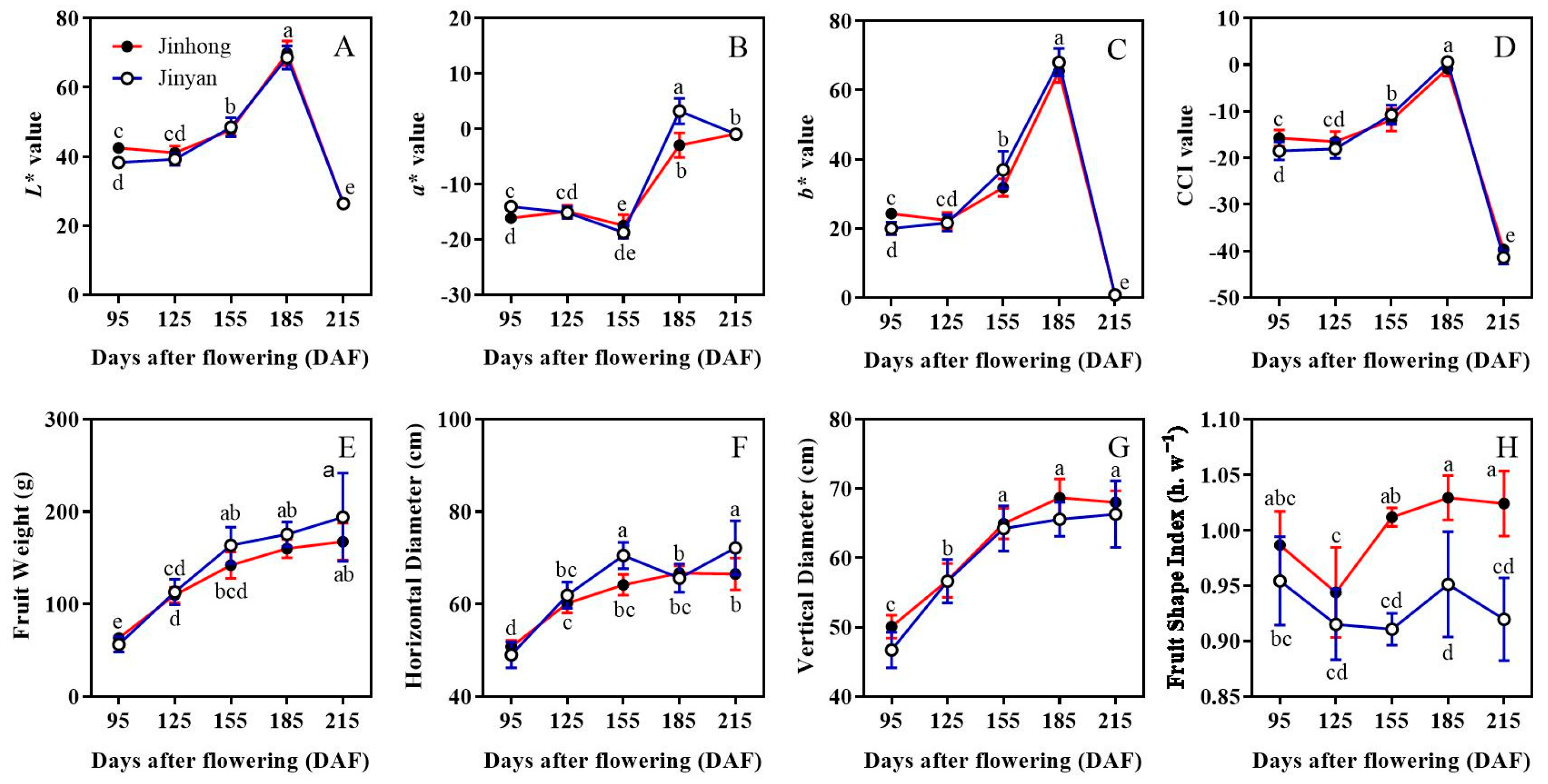
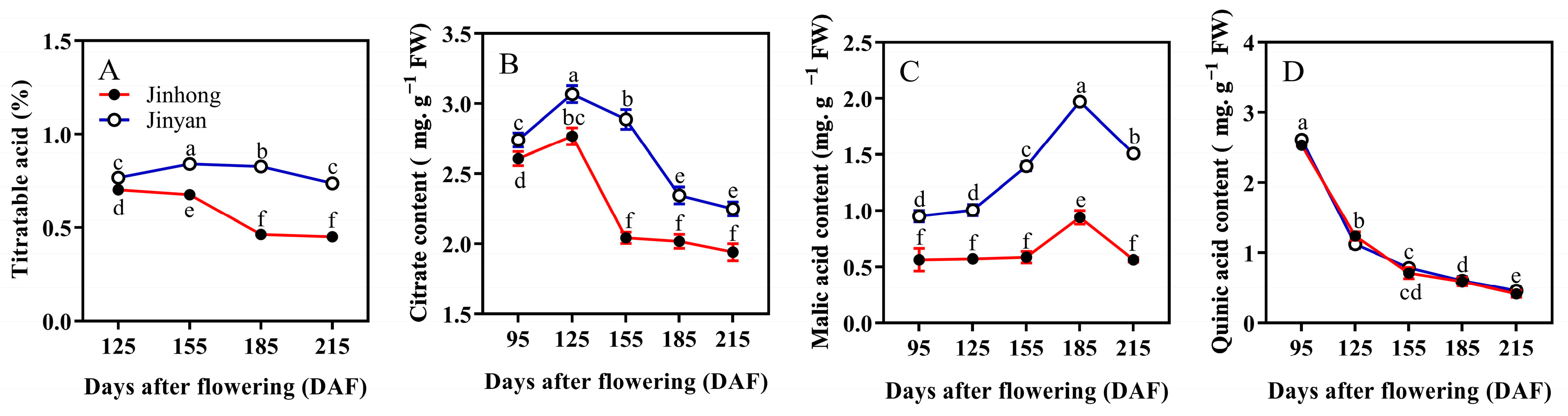
3.1. Fruit Quality Characteristics of ‘Jinhong’ and ‘Jinyan’ Bingtang Orange
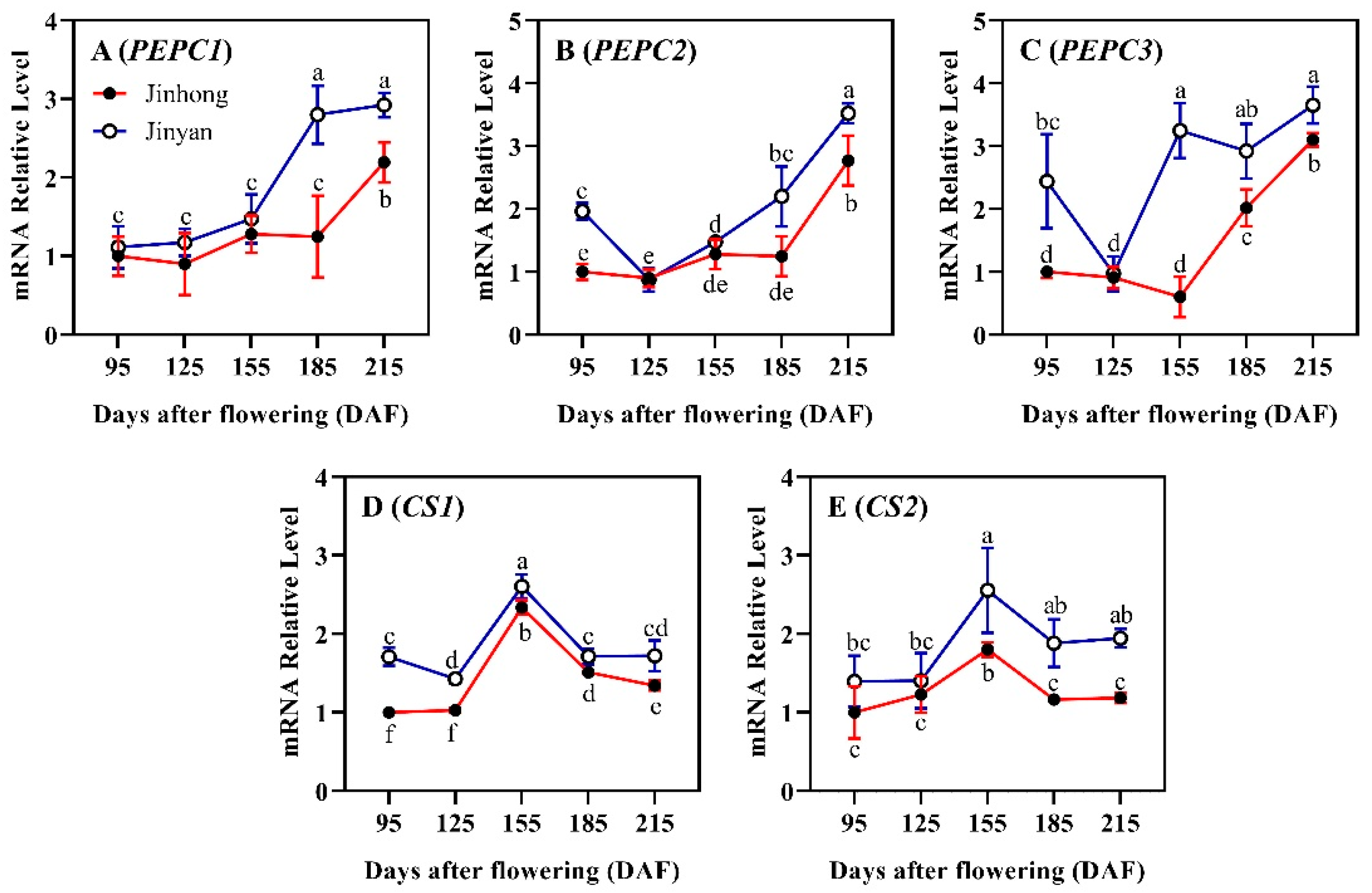
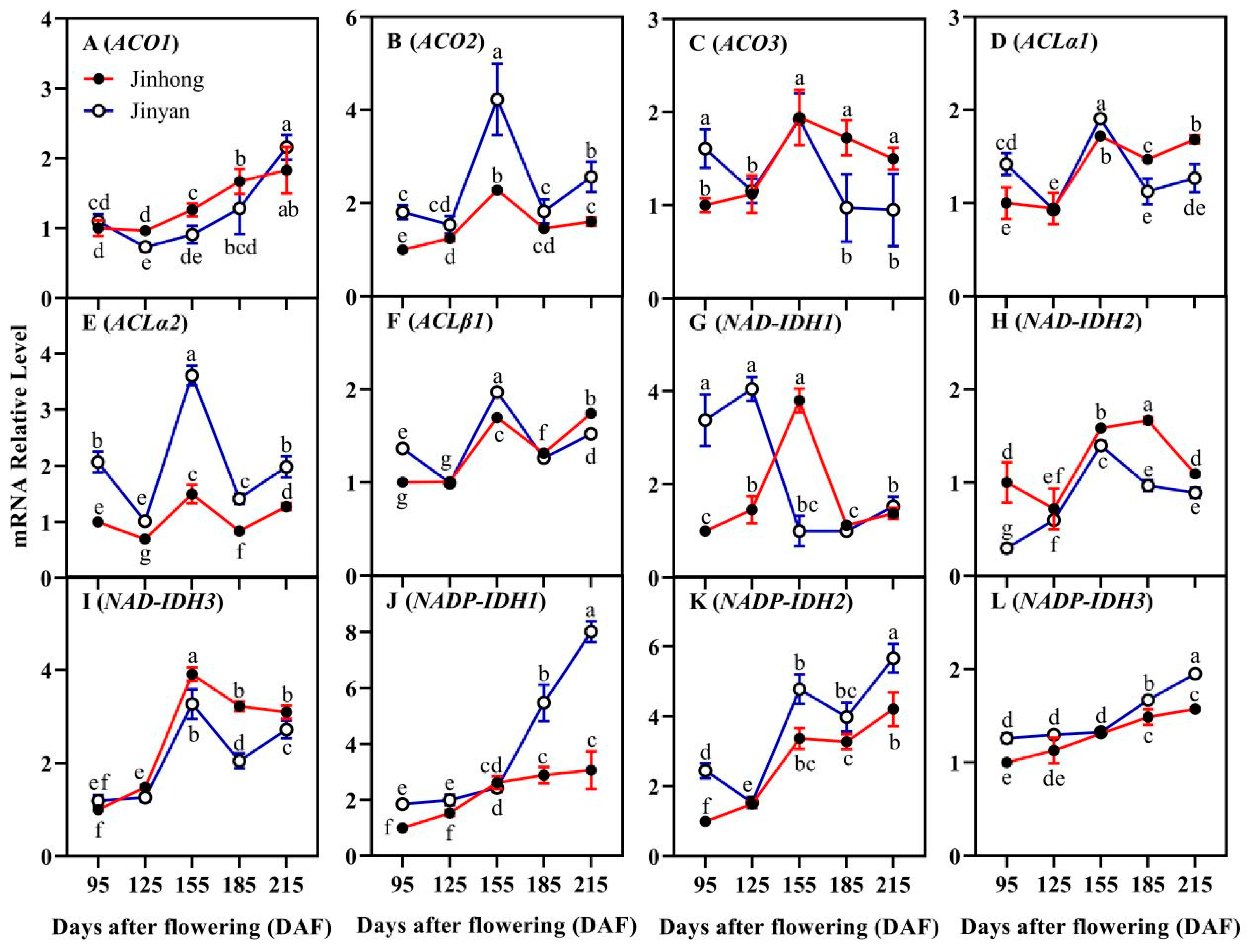
3.2. Comparative Analysis of Citrate Biosynthesis-Related Genes in Developing Juice Sacs
3.3. Comparative Analysis of Citrate Transport-Related Genes in Developing Juice Sacs
3.4. Comparative Analysis of Citrate Degradation-Related Genes in Developing Juice Sacs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Wang, P.; Chen, J.; Li, L.; Tang, L.; Huang, W.; Wei, X.; Xu, J. The accumulation pattern of sugars and organic acids in diverse fruit pulps coordinates sweet-sour taste of passion fruit. LWT 2025, 225, 117948. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in Organic Acid Profiles During Fruit Development and Ripening: Correlation or Causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.B.; Shi, C.Y.; Guo, L.X.; Kamran, H.M.; Sadka, A.; Liu, Y.Z. Recent advances in the regulation of citric acid metabolism in citrus fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Huang, Y.; Wang, L.; Liu, S.; Jiang, Z.; Wang, X.; Xu, Q. Transcriptome analysis reveals the common and specific pathways of citric acid accumulation in different citrus species. Hortic. Plant J. 2025, 11, 520–534. [Google Scholar] [CrossRef]
- He, J.X.; Xu, Y.T.; Huang, D.; Fu, J.L.; Liu, Z.A.; Wang, L.; Zhang, Y.; Xu, R.W.; Li, L.; Deng, X.X.; et al. TRIPTYCHON-LIKE regulates aspects of both fruit flavor and color in citrus. J. Exp. Bot. 2022, 73, 3610–3624. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric Acid-Mediated Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- Guo, L.X.; Hussain, S.B.; Fernie, A.R.; Liu, Y.Z.; Yan, M.; Chen, H.; Alam, S.M. Multiomic analysis elucidates the reasons underlying the differential metabolite accumulation in citrus mature leaves and fruit juice sacs. J. Agric. Food Chem. 2020, 68, 11863–11874. [Google Scholar] [CrossRef]
- Han, H.; Li, Y.J.; Alam, S.M.; Zhou, T.; Khan, M.A.; Thu, A.M.; Liu, Y.Z. AP2 transcription factor CsAIL6 negatively regulates citric acid accumulation in citrus fruits by interacting with a WD40 protein CsAN11. Hortic. Res. 2025, 12, uhaf002. [Google Scholar] [CrossRef]
- Sadka, A.; Dahan, E.; Or, E.; Cohen, L. NADP+-isocitrate dehydrogenase gene expression and isozyme activity during citrus fruit development. Plant Sci. 2000, 158, 173–181. [Google Scholar] [CrossRef]
- Perotti, V.E.; Figueroa, C.M.; Andreo, C.S.; Iglesias, A.A.; Podestá, F.E. Cloning, expression, purification and physical and kinetic characterization of the phosphoenolpyruvate carboxylase from orange (Citrus sinensis osbeck var. Valencia) fruit juice sacs. Plant Sci. 2010, 179, 527–535. [Google Scholar]
- Sweetlove, L.J.; Beard, K.F.M.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, D.; Martinoia, E. Citrate transport into barley mesophyll vacuoles: Comparison with malate-uptake activity. Planta 1991, 184, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, R.; Luttge, U.; Gonzalez, P.; Etxeberria, E. Malate and malate-channel antibodies inhibit electrogenic and ATP-dependent citrate transport across the tonoplast of citrus juice cells. J. Plant Physiol. 2003, 160, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Palmgren, M.G.; Schumacher, K. Plant proton pumps. FEBS Lett. 2007, 581, 2204–2214. [Google Scholar] [CrossRef]
- Muller, M.L.; Irkens, K.U.; Rubinstein, B.; Taiz, L. On the mechanism of hyperacidification in lemon-comparison of the vacuolar H+-ATPase activities of fruits and epicotyls. J. Biol. Chem. 1996, 271, 1916–1924. [Google Scholar]
- Shimada, T.; Nakano, R.; Shulaev, V.; Sadka, A.; Blumwald, E. Vacuolar citrate/H+ symporter of citrus juice cells. Planta 2006, 224, 472–480. [Google Scholar] [CrossRef]
- Sadka, A.; Dahan, E.; Cohen, L.; Marsh, K.B. Aconitase activity and expression during the development of lemon fruit. Physiol. Plant. 2003, 108, 255–262. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef]
- Mafrica, R.; De Bruno, A.; Mafrica, D.L.; Merlo, C.; Gattuso, A.; Poiana, M. Ripening Dynamics and Optimal Harvest Timing of ‘Fantastico’ and ‘Femminello’ Bergamot Fruit. Agriculture 2025, 15, 737. [Google Scholar] [CrossRef]
- Bartolozzi, F.; Bertazza, G.; Bassi, D.; Cristoferi, G. Simultaneous determination of soluble sugars and organic acids as their trimethylsilyl derivatives in apricot fruits by gas-liquid chromatography. J. Chromatogr. A. 1997, 758, 99–107. [Google Scholar] [CrossRef]
- Guo, L.X.; Shi, C.Y.; Liu, X.; Ning, D.Y.; Jin, L.F.; Yang, H.; Liu, Y.Z. Citrate accumulation-related gene expression and/or enzyme activity analysis combined with metabolomics provide a novel insight for an orange mutant. Sci. Rep. 2016, 6, 29343. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 [−Delta Delta C (T)] Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landazuri, P.; Loango, N.; Aguillon, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Chen, M.; Xie, X.L.; Lin, Q.; Chen, J.Y.; Grierson, D.; Yin, X.R.; Sun, C.D.; Chen, K.S. Differential expression of organic acid degradation-related genes during fruit development of Navel oranges (Citrus sinensis) in two habitats. Plant Mol. Biol. Rep. 2013, 31, 1131–1140. [Google Scholar] [CrossRef]
- Guo, L.X.; Liu, Y.Z.; Luo, L.J.; Hussain, S.B.; Bai, Y.X.; Alam, S.M. Comparative Metabolites and Citrate-Degrading Enzymes Activities in Citrus Fruits Reveal the Role of Balance between ACL and Cyt-ACO in Metabolite Conversions. Plants 2020, 9, 350. [Google Scholar] [CrossRef]
- Wei, Q.J.; Ma, Q.L.; Zhou, G.F.; Liu, X.; Ma, Z.Z.; Gu, Q.Q. Identification of genes associated with soluble sugar and organic acid accumulation in ‘Huapi’ kumquat (Fortunella crassifolia Swingle) via transcriptome analysis. J. Sci. Food Agric. 2021, 101, 4321–4331. [Google Scholar] [CrossRef]
- Yamaki, Y.T. Effect of lead arsenate on citrate synthase activity in fruit pulp of Satsuma mandarin. J. Japan. Soc. Hort. Sci. 1990, 58, 899–905. [Google Scholar] [CrossRef][Green Version]
- Wen, T.; Xiong, Q.E.; Zeng, W.G.; Liu, Y.P. Changes of organic acid synthetase activity during fruit development of navel orange (Citrus sinesis Osbeck). Acta. Hortic. Sinica 2001, 28, 161–163. [Google Scholar]
- Sadka, A.; Dahan, E.; Or, E.; Roose, M.L.; Marsh, K.B.; Cohen, L. Comparative analysis of mitochondrial citrate synthase gene structure, transcript level and enzymatic activity in acidless and acid-containing Citrus varieties. Funct. Plant Biol. 2001, 28, 383–390. [Google Scholar] [CrossRef]
- Etienne, C.; Moing, A.; Dirlewanger, E.; Raymond, P.; Monet, R.; Rothan, C. Isolation and characterization of six peach cDNAs encoding key proteins in organic acid metabolism and solute accumulation: Involvement in regulating peach fruit acidity. Physiol. Plant. 2002, 114, 259–270. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R.; Hocking, P.J.; Richardson, A.E. Effects of altered citrate synthase and isocitrate dehydrogenase expression on internal citrate contents and citrate efflux from tobacco (Nicotiana tabacum L.) roots. Plant Soil. 2003, 248, 137–144. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, C.Y.; Dong, W.C.; Jiang, Q.; Wang, D.L.; Li, S.J.; Chen, M.; Liu, C.R.; Sun, C.D.; Chen, K.S. Transcriptome and metabolome analyses of sugar and organic acid metabolism in Ponkan (Citrus reticulata) fruit during fruit maturation. Gene 2015, 554, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.P.; Cao, X.J.; Li, F.F.; Li, J.; Xiong, J.; Long, G.Y.; Cao, S.Y.; Xie, S.X. Comparative transcriptome analysis reveals a global insight into molecular processes regulating citrate accumulation in sweet orange (Citrus sinensis). Physiol. Plantarum. 2016, 158, 463–482. [Google Scholar] [CrossRef]
- Bogin, E.; Wallace, A. Organic acid synthesis and accumulation in sweet and sour lemon fruit. J. Am. Soc. Horti. Sci. 1966, 89, 182–194. [Google Scholar]
- Degu, A.; Hatew, B.; Nunes-Nesi, A.; Shlizerman, L.; Zur, N.; Katz, E.; Fernie, A.R.; Blumwald, E.; Sadka, A. Inhibition of aconitase in citrus fruit callus results in a metabolic shift towards amino acid biosynthesis. Planta 2011, 234, 501–513. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Q.; Yin, X.R.; Lin, Q.; Chen, J.Y.; Allan, A.C.; Xu, C.J.; Chen, K.S. Effect of hot air treatment on organic acid- and sugar-metabolism in Ponkan (Citrus reticulata) fruit. Sci. Hortic. 2012, 147, 118–125. [Google Scholar] [CrossRef]
- Wang, L.; He, F.; Huang, Y.; He, J.X.; Yang, S.Z.; Zeng, J.W.; Deng, C.L.; Jiang, X.L.; Fang, Y.W.; Wen, S.H.; et al. Genome of wild mandarin and domestication history of mandarin. Mol. Plant. 2018, 11, 1024–1037. [Google Scholar] [CrossRef]
- Katz, E.; Fon, M.; Lee, Y.J.; Phinney, B.S.; Sadka, A.; Blumwald, E. The citrus fruit proteome: Insights into citrus fruit metabolism. Planta 2007, 226, 989–1005. [Google Scholar] [CrossRef]
- Eisenach, C.; Baetz, U.; Martinoia, E. Vacuolar proton pumping: More than the sum of its parts? Trends Plant Sci. 2014, 19, 344–346. [Google Scholar] [CrossRef][Green Version]
- Tan, F.Q.; Zhang, M.; Xie, K.D.; Fan, Y.J.; Song, X.; Wang, R.; Wu, X.M.; Zhang, H.Y.; Guo, W.W. Polyploidy remodels fruit metabolism by modifying carbon source utilization and metabolic flux in Ponkan mandarin (Citrus reticulata Blanco). Plant Sci. 2019, 289, 110276. [Google Scholar] [CrossRef]
- Shi, C.Y.; Hussain, S.B.; Yang, H.; Bai, Y.X.; Khan, M.A.; Liu, Y.Z. CsPH8, a P-type proton pump gene, plays a key role in the diversity of citrate accumulation in citrus fruits. Plant Sci. 2019, 289, 110288. [Google Scholar] [CrossRef] [PubMed]
- Strazzer, P.; Spelt, C.E.; Li, S.J.; Bliek, M.; Federici, C.T.; Roose, M.L.; Koes, R.; Quattrocchio, F.M. Hyperacidification of Citrus fruits by a vacuolar proton-pumping P-ATPase complex. Nat. Commun. 2019, 10, 744. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Hussain, S.B.; Tang, L.; Han, J.; Liao, W.; Zhou, T.; Liu, F.; Wang, C.; Xu, Y.; Chen, P. Expression Analysis of Citrate Metabolism-Related Genes Reveals New Insights into High Citrate Accumulation in a Bingtang Orange Bud Mutant (Citrus sinensis cv. Jinyan). Horticulturae 2025, 11, 616. https://doi.org/10.3390/horticulturae11060616
Guo L, Hussain SB, Tang L, Han J, Liao W, Zhou T, Liu F, Wang C, Xu Y, Chen P. Expression Analysis of Citrate Metabolism-Related Genes Reveals New Insights into High Citrate Accumulation in a Bingtang Orange Bud Mutant (Citrus sinensis cv. Jinyan). Horticulturae. 2025; 11(6):616. https://doi.org/10.3390/horticulturae11060616
Chicago/Turabian StyleGuo, Lingxia, Syed Bilal Hussain, Lei Tang, Jian Han, Wei Liao, Tie Zhou, Fei Liu, Congtian Wang, Yuanyuan Xu, and Peng Chen. 2025. "Expression Analysis of Citrate Metabolism-Related Genes Reveals New Insights into High Citrate Accumulation in a Bingtang Orange Bud Mutant (Citrus sinensis cv. Jinyan)" Horticulturae 11, no. 6: 616. https://doi.org/10.3390/horticulturae11060616
APA StyleGuo, L., Hussain, S. B., Tang, L., Han, J., Liao, W., Zhou, T., Liu, F., Wang, C., Xu, Y., & Chen, P. (2025). Expression Analysis of Citrate Metabolism-Related Genes Reveals New Insights into High Citrate Accumulation in a Bingtang Orange Bud Mutant (Citrus sinensis cv. Jinyan). Horticulturae, 11(6), 616. https://doi.org/10.3390/horticulturae11060616




