Abstract
Citric acid serves as the principal organic acid in citrus fruits, with its concentration critically determining fruit flavor and market acceptability. Isocitrate dehydrogenase (IDH), a key enzyme in citric acid metabolism, mediates the conversion of citrate to α-ketoglutarate. This study cloned six candidate genes encoding IDH from grapefruit (Citrus paradisi). Bioinformatics analysis showed that all six genes contained the typical characteristic structure of IDH. Gene expression analysis found that CpNADP-IDH1 is highly expressed in mature and low-acid varieties. Overexpression of CpNADP-IDH1 significantly increased IDH enzyme activity and decreased citric acid content in transgenic grapefruit callus. These results showed that at least six genes encoding IDH exist in grapefruit, among which CpNADP-IDH1 catalyzes the decomposition of citric acid and regulates the organic acid content in fruits at maturity. CpNADP-IDH1 can be used as a candidate gene for molecular breeding of low-acid citrus varieties and as an essential target gene for developing citrus cultivation technology for reducing acid content.
1. Introduction
Citrus is one of the most important fruit crops in the world, with 169 million tons of fruit produced in 2023 (https://www.fao.org/faostat/en/#home, accessed on 14 February 2025). Citrus fruits are rich in dietary fibers, sugars, organic acids, mineral nutrients, vitamins, carotenoids, and flavonoids, which benefit human health [1]. Citrus fruits accumulate high concentrations of organic acids, predominantly citric acid (66–99% of total acids), followed by malic, tartaric, and quinic acids [2]. The citric acid content serves as a key determinant of both fruit flavor and commercial value due to its critical impact on organoleptic quality and consumer preference. Citric acid is involved in energy metabolism, providing energy and intermediates for citrus fruit development, maintaining cell osmotic pressure, and promoting cell expansion during growth and development [3]. The citric acid content effectively improves fruit sink strength and promotes sugar accumulation [4]. Meanwhile, citric acid content affects fruit storage ability, and higher citric acid content significantly extends the storage period [5].
During the expansion phase of citrus fruit development, citric acid is biosynthesized in mitochondria via the tricarboxylic acid (TCA) cycle and subsequently transported into vacuoles mediated by citrate/H+ symporter [6,7,8]. In maturation, vacuolar-stored citric acid is gradually translocated into the cytosol for enzymatic catabolism [9]. Multiple studies have shown that γ-aminobutyric acid (GABA) and the ATP-citrate lyase pathway play important roles in the degradation of citric acid [10,11]. Cis-aconitase (ACO) and isocitrate dehydrogenase (IDH) sequentially mediate citrate catabolism through isomerization of citrate to isocitrate and subsequent oxidative decarboxylation to α-ketoglutarate, respectively [12,13]. Based on subcellular localization and biological functions, IDH can be divided into NAD-IDH and NADP-IDH [14]. The organic acid content of citrus fruits is closely related to IDH enzyme activity and IDH gene expression. For example, Sadka found that the IDH enzyme activity and gene expression of NADP-IDH are low in the early stage of lemon development and increase with fruit maturation [12]. Lu et al. identified three genes encoding IDH in sweet oranges, and expression analysis found that the expression levels of CsIDH1, CsIDH2, and CsIDH3 in the low-acid variety were significantly higher than those in the high-acid variety [11].
So far, the IDH gene family has been identified in Arabidopsis, rice, tomato, maize, and other species. IDH plays a key regulatory role in plant growth [15], fruit development [16,17], and responses to biotic [18] and abiotic stresses [19,20]. In maize, eleven IDH genes were identified, and ZmIDH1.4 responded to drought stress [21]. In pepper, three CaNADP-IDHs regulated fruit ripening through modulating nitric oxide [16]. However, the regulatory mechanism of IDH in citric acid metabolism during citrus fruit ripening remains unclear. Grapefruit is an important part of citrus fruits and is favored by consumers. To investigate the citric acid metabolism mechanism, two grapefruit varieties including ‘Cock tail’ (low acid) and ‘Flame’ (high acid) were chosen as experimental materials. In this study, six genes encoding IDH were identified from grapefruit fruits. The phylogeny, conserved motifs, structures, chromosome distribution, and promoter cis-acting elements were analyzed. The gene expression characteristics were determined in two grapefruit varieties with significant differences in acid content using qRT-PCR. In addition, CpNADP-IDH1 was functionally characterized by genetically transforming citrus callus.
2. Materials and Methods
2.1. Plant Material
The experimental materials including ‘Cock tail’ and ‘Flame’ grapefruit (Citrus paradisi) were collected from the germplasm resource nursery of Zhejiang Citrus Research Institute (28.64 N, 121.16 E). Both are 5-year-old varieties grafted onto the trifoliate orange (Poncirus trifoliata) rootstock. The grapefruit samples were collected from three separate trees for each variety, with four fruits from each tree. The fruits were collected 90, 120, 150, 180, 210, and 240 days after flowering (DAF). Pulp tissues were cut into small pieces, flash-frozen in liquid nitrogen, and stored at −80 °C for later use. Tissue culture was performed using the ovary walls of grapefruits as explants. The callus was induced and proliferated in MT basal medium supplemented with 4 mg/L. The specific method was slightly modified according to previous research [22].
2.2. Bioinformatics Analysis
The amino acid sequences of IDH of Arabidopsis thaliana were used as a reference and downloaded from the TAIR database (https://www.arabidopsis.org/, accessed on 14 February 2022). Candidate genes coding citrus IDH were obtained by comparison and analysis using BlastP in the citrus genome database (http://citrus.hzau.edu.cn/download.php, accessed on 14 February 2022). The BlastP parameter was scored a value ≥ 100 and e value ≤ 10−10. The conserved domain analysis of IDH was performed using the Batch Web CD-search Tool [23]. The physicochemical parameters, including protein molecular weight (MW), isoelectric point (IP), and total mean hydrophilicity of the citrus IDH gene, were analyzed using ExPASy online tools (http://www.expasy.org, accessed on 14 February 2022). The chromosome localization and gene structure of citrus IHD were analyzed using TB tool [24]. The amino acid sequences of multiple comparisons of citrus, Arabidopsis, rice, corn, tobacco, soybeans, and potatoes were performed using ClustalW [25], and the phylogenetic tree was constructed using the neighbor-joining method of MEGA X [26]. Heat maps were generated using the FPKM values of different tissues in the citrus genome database (http://citrus.hzau.edu.cn/geneExpression/query.php, accessed on 14 February 2022). The TB tool was used in the visual analysis of gene-conserved motifs and functional domains [24].
2.3. RNA Extraction and Gene Expression Analysis
A plant total RNA extraction kit (Takara, Dalian, China) was used for RNA extraction, and a HiScript II 1st Strand cDNA Synthesis kit (Shenggong, Shanghai, China) was used for DNA first strand synthesis. Real-time fluorescent quantitative PCR analysis was performed using a SYBR® Select Master Mix (Takara, Dalian, China) in the CFX96 PCR Detection System (Bio-Rad, Hercules, CA, USA). β-Actin was used as the internal reference gene. The reaction system and operation procedure were carried out according to the operation instructions of the kit. Primer-blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/, accessed on 14 February 2022) was used to design primers (Supplementary Table S1), and the 2−ΔΔCT method was used to calculate the relative expression of target genes [27].
2.4. Expression Vector Construction and Genetic Transformation
The CpNADP-IDH1 coding region was amplified using the grapefruit cDNA as the template with gene-specific primers (Supplementary Table S1) with the 2 × Phanta® Max Master Mix (Vazyme, Nanjing, China). The CpNADP-IDH1-pMD18 plasmid (Takara, Dalian, China) was obtained and sequenced using TA cloning. The plasmid was linearized using restriction endonucleases (Bam HI and MluI), and the CpNADP-IDH1 was inserted into the pCAMBIA1300-35S-E9 expression vector using the ClonExpress® II One Step cloning kit (Vazyme, Nanjing, China). After sequencing confirmation, CpNADP-IDH1-pCAMBIA1300-35S-E9 was transformed into Agrobacterium GV3101. The genetic transformation of citrus callus using the Agrobacterium-mediated method is referred to in previous research [28]. The independent transformed callus lines were screened from tiny pieces of antibiotic-resistant callus. The wild-type and transgenic calluses were sub-cultured on MT medium every three weeks.
2.5. Determination of IDH Enzyme Activity, Soluble Sugar, and Citric Acid Content
The extraction and activity assays of IDH enzymes in callus were determined using the method according to previous research [29]. About 0.5 g callus was homogenized using 5 mL extraction buffer (50 mM MOPS, pH = 7.5, 0.1% (v/v) Triton X-100, 10 mM MgCl2, 1 mM EDTA, 20 mM KCl, 10 mM DTT, 10 mM β-mercaptoethanol, 2.5% PVPP, 2 mM PMSF and a protease inhibition cocktail tablet) pre-cooled at 4 °C and centrifuged at 12,000× g and 4 °C for 20 min. Then, 2 mL of the supernatant was transferred to a new centrifuge tube for the determination of IDH activity. The reaction mixture was composed of 40 mM Hepes (pH = 8.2), 800 mM NAD, 200 mM MnSO4, and 2 mM isocitrate. The absorption was recorded at 340 nm after reaction at 30 °C for 15 min using a UV–Vis spectrophotometer (2800 UV/VIS, Unico, Franksville, WI, USA). The soluble sugar and citric acid content in the fruit and callus of grapefruit was determined using liquid chromatography with ultraviolet spectrophotometry (Agilent 1200, Agilent, Santa Clara, CA, USA). The method was slightly modified according to previous research [19]. The citrus pulp and callus were extracted with gauze. Then, 1 g of the juice was mixed with distilled water to yield a total volume of 50 mL and then filtered through a 0.22 μm filter. Citric acid was analyzed using a Titank C18 chromatographic column (250 mm × 4.6 mm, 5 μm, Firomon, Guangzhou, China). The injection conditions for the chromatography were as follows: the injection volume is 10 μL; the mobile phase was methanol and potassium dihydrogen phosphate (40 mmol/L, pH = 2.4) (volume ratio 2:98); the flow rate was 0.8 mL/min; the column temperature is 35 °C; the detection wavelength was 210 nm. Soluble sugars were analyzed using a Rezex RCM-Monosaccharide Ca (300 mm × 7.8 mm, 5 μm, Phenomenex, Tianjing, China) chromatographic column. The chromatography injection conditions were as follows: the injection volume was 20 μL; the mobile phase was pure water; the flow rate was 0.5 mL/min; the column temperature was 63 °C. A refractive index detector detected sucrose, glucose, and fructose at 40 °C. Three biological replicates were measured for each sample.
2.6. Data Analysis
All data are shown as mean ± standard error (SE). SPSS 13.0 was used for data analysis, and the Duncan test was used to analyze the significance of differences. GraphPad Prism 8 software was used for plotting.
3. Results
3.1. Determination of Sugar and Acid Content in Two Varieties of Grapefruit
During fruit maturation, ‘Flame’ grapefruit mesocarp transitioned from pink to ruby-red, while ‘Cock tail’ mesocarp transitioned from off-white to orange (Figure 1). The citric acid content of the two grapefruit varieties was higher in the early stage. The citric acid contents in the ‘Cock tail’ grapefruit and the ‘Flame’ grapefruit were 11.27 mg/g and 15.22 mg/g at 150 DAF, respectively. With the ripening process, the citric acid content in ‘Cock tail’ grapefruit gradually decreased, and the citric acid content was 4.89 mg/g at 210 DAF. The citric acid content in ‘Flame’ grapefruit always remained high, and its content was 13.18 mg/g at 210 DAF, which was significantly higher than that in ‘Cock tail’ grapefruit. Sucrose, fructose, and glucose are the three main sugars in grapefruit. The sucrose content in ‘Cock tail’ grapefruit fruit accumulated rapidly from 150 DAF to 210 DAF. The sucrose in the ‘Flame’ grapefruit fruit gradually rose, peaked (42.45 mg/g) at 180 DAF, and then gradually decreased. During the ripening period, the accumulation of fructose and glucose in the two grapefruit fruits tended to stabilize (Figure 2).

Figure 1.
The fruit of ‘Flame’ and ‘Cock tail’ grapefruit varieties at five stages. The white scale is 4 cm.
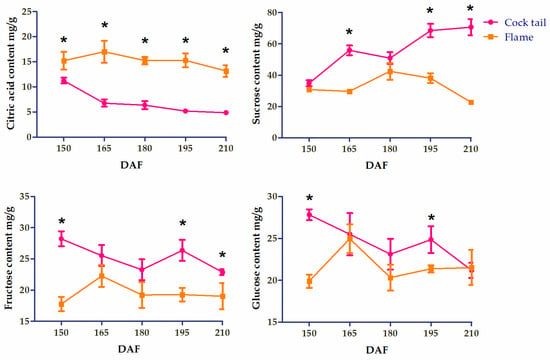
Figure 2.
Sugar and acid content of two varieties of grapefruit in different developmental stages. The asterisk (*) indicates significant differences (p < 0.05).
3.2. Identification and Bioinformatics Analysis of the IDH Gene Family in Citrus
Six highly homologous sequences were obtained using BLASTP comparison of AtIDH amino acid sequences in the sweet orange genome. Using grapefruit fruit cDNA as template, six IDH genes were cloned and named CpNADP-IDH1, CpNADP-IDH2, CpNADP-IDH3, CpNAD-IDH1, CpNAD-IDH2, and CpNAD-IDH3 (the sequences are listed in Table S2). The number of amino acids in the six IDH genes ranged from 359 to 533, the molecular weights ranged from 39.20 to 60.053 kDa, and the isoelectric points ranged from 7.52 to 8.75. The genetic structures of the IDH gene family vary significantly. The exon numbers of the six IDH genes were 15, 16, 15, 4, 7, and 4, respectively. Chromosome localization analysis showed that the IDH genes are located on chromosomes 3, 2, 9, 8, 6, and 1, respectively. The total average hydrophilicity values of the six IDHs were negative, ranging from −3.17 to −0.100 (Supplementary Table S3). CpNADP-IDH1, CpNADP-IDH2, and CpNADP-IDH3 contained six conserved motifs, and CpNAD-IDH1, CpNAD-IDH2, and CpNAD-IDH3 contained three conserved motifs (Figure 3). Evolutionary analysis showed that 32 genes encoding IDH in different plants were divided into two categories: NAP-dependent and NADP-independent. CpNADP-IDH1 clustered with OsICDHa and OsICDHa. CpNADP-IDH2 clustered with AtICDHa. CpNADP-IDH3 clustered with AtICDHb. CpNAD-IDH1 and CpNAD-IDH3 clustered with AtIDH1. CpNAD-IDH2 clustered with NtIDHa of tobacco (Figure 4).
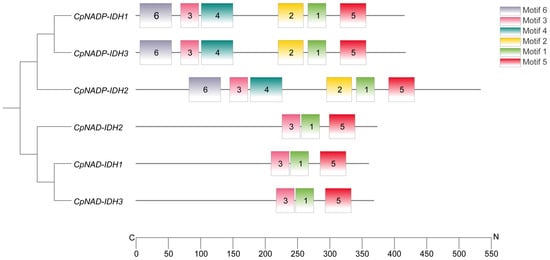
Figure 3.
The conservative motif analysis of IDH. Six motifs were identified in the CpIDH protein and marked by different colors. The sequences of the conserved motifs are listed in Supplementary Table S4.
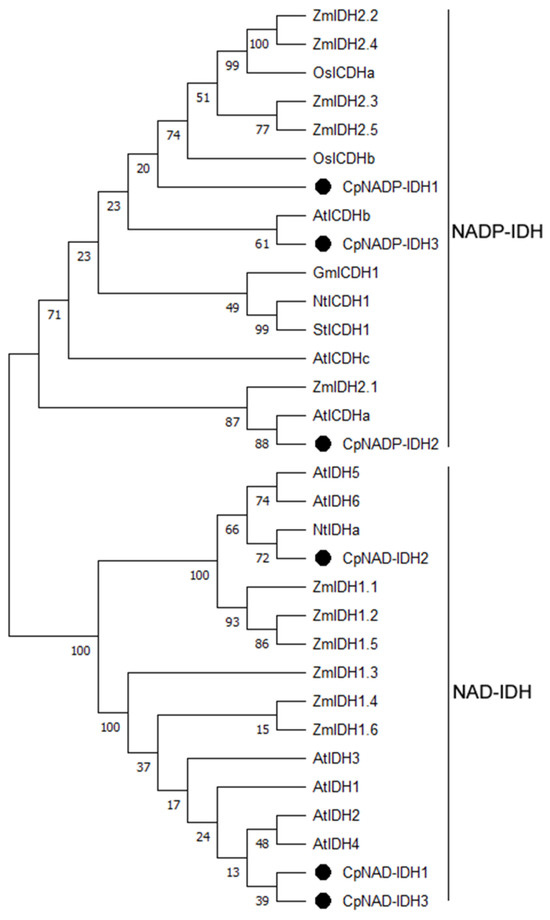
Figure 4.
Phylogenetic analysis of IDH in different plants. At indicates Arabidopsis, Os indicates rice, Gm indicates soybean, Nt indicates tobacco, and St indicates potato. The different numbers refer to bootstrap values. The amino acid sequences of IDH in different plants are listed in Supplementary Table S5.
3.3. Expression Analysis of IDH Genes
To investigate the biological function of IDH in citrus, the expression characteristics of IDH in leaf, root, and fruit were analyzed using RPKM values from the public database through a heatmap (Figure 5). CpNADP-IDH1 was expressed highly in the root and mature fruit. The expression of CpNADP-IDH1 and CpNADP-IDH1 was higher in mature fruit and leaves. CpNAD-IDH1, CpNAD-IDH2, and CpNAD-IDH3 were expressed highly in the root. These results indicated that IDHs played important roles in citrus leaf, root, and fruit growth. To further investigate the gene function of IDH in citrus ripening, IDH expression in ‘Cock tail’ and ‘Flame’ grapefruit at five stages were analyzed using qRT-PCR (Figure 6). The results showed that the expression of CpNADP-IDH1 increased gradually during the ripening process and peaked at 210 DAF. In addition, the expression level of CpNADP-IDH1 in ‘Cock tail’ grapefruit was significantly higher than that in ‘Flame’ grapefruit. The other five genes encoding IDH were not significantly different between the two grapefruit varieties. These results suggested that CpNADP-IDH1 might be a key candidate gene for regulating citric acid content during citrus fruit ripening.
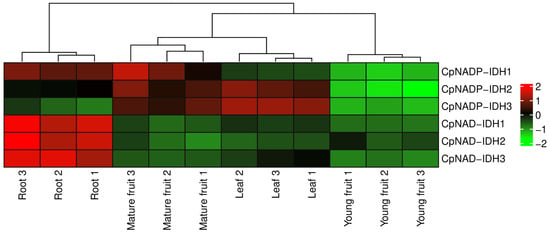
Figure 5.
The expression characteristics of IDHs in different tissues (root, leaf, young fruit, and mature fruit) of citrus. Different numbers represent three replicates.
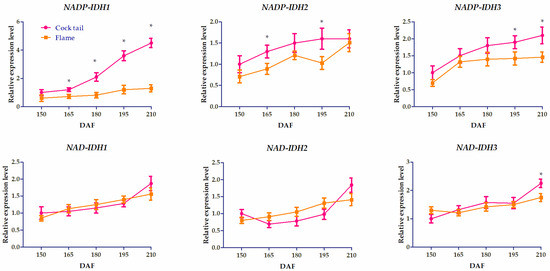
Figure 6.
Expression characteristics of IDH in two varieties of grapefruit at different maturity stages. The asterisk (*) indicates significant differences (p < 0.05).
3.4. Promoter Component Analysis of IDHs
To clarify the function of IDH in citrus growth and fruit development, the cis-acting elements on the 2 kb promoter upstream of each IDH gene were analyzed. A total of 75 cis-acting elements were identified in six IDH genes, including 22 light-responsive elements, 4 low-temperature-responsive elements, 3 drought-responsive elements, 4 defense and stress elements, 10 abscisic acid-responsive elements, 2 auxin-responsive elements, 3 gibberellin-responsive elements, 22 MeJA-responsive elements, and 5 salicylic acid-responsive elements (Figure 7). These results suggested that the expression of IDHs in citrus might be regulated by environmental factors such as light, temperature, water, and plant hormones.
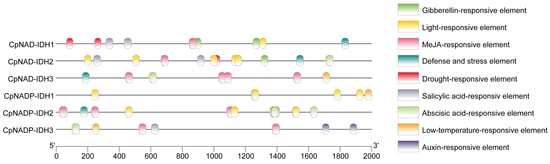
Figure 7.
Analysis of the cis-acting elements present in the promoter regions of IDHs.
3.5. Functional Analysis of CpNADP-IDH1
To further study the function of CpNADP-IDH1 in citric acid metabolism of citrus fruits, an overexpression vector pCAMBIA1300-35S-E9 was constructed and transformed into grapefruit callus using Agrobacterium GV3101. Three overexpression (OE) callus lines with high expression of CpNADP-IDH1 were screened using kanamycin selection and qRT-PCR (Figure 8A,B). The IDH enzyme activities in OE callus were significantly higher than those of the wild-type callus (Figure 8C). The citric acid content in transgenic and wild-type (WT) callus was determined. The results showed that the citric acid contents in the callus of the three transgenic lines were 0.08 mg/g, 0.13 mg/g, and 0.09 mg/g, respectively. These values were significantly lower than that in the WT callus (0.34 mg/g) (Figure 8D). These results indicated that overexpression of CpNADP-IDH1 could significantly reduce citric acid content in citrus.
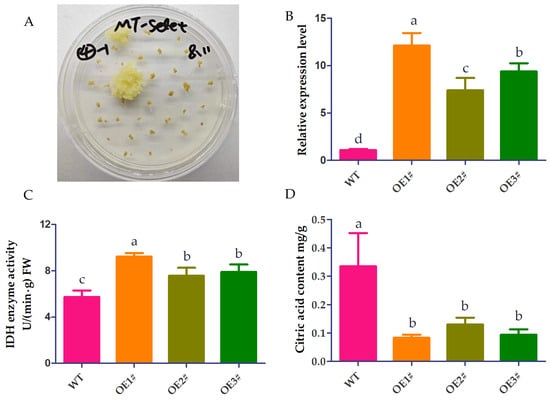
Figure 8.
Functional analysis of CpNADP-IDH1 in callus. (A) Screening of transgenic callus, (B) Analysis of CpNADP-IDH1 expression in transgenic and wild type (WT) callus, (C) Enzyme activity analysis of IDH in transgenic and wild type callus, (D) Citric acid content in transgenic and WT callus. Different lowercase letters indicate significant differences (p < 0.05).
4. Discussion
The citric acid content in citrus fruits is comprehensively regulated by three biological processes: synthesis, transport, and decomposition [6,7,8]. The GABA pathway involving IDH plays a significant role in the degradation of citric acid [5]. IDH is a class of enzymes that widely exist in animals, plants, and microorganisms and is a core enzyme that links carbon and nitrogen metabolism by supplying carbon skeletons for primary nitrogen assimilation [30]. Under the catalysis of IDH, isocitrate is decomposed into α-ketoglutaric acid and carbon dioxide, while NAD+ and NADP+ are reduced to NADH and NADPH [14]. As α-ketoglutaric acid is an important precursor for amino acid synthesis, IDH is also involved in growth and development [16,17]. In addition, the reduced coenzyme NAD(P)H supplies the reducing power needed for cellular anabolism and plays a crucial role in maintaining the balance of reactive oxygen species (ROS) within cells. IDH is also a key candidate gene in plant stress response [20,31,32]. Most fruits ripen with the gradual degradation of organic acids and the rapid accumulation of sugar. As citric acid in the cytoplasm is mostly decomposed by ACO and IDH in the GABA degradation pathway [10], IDH may play a role in regulating fruit quality. To understand the function of the IDH genes in citrus fruit development, six IDH family members were identified and characterized in grapefruit.
In this study, a total of six genes encoding IDH in grapefruit were identified. The numbers of IDH family members of Arabidopsis, rice, sorghum, and maize were 10, 7, 9, and 11, respectively. This interspecific variation likely resulted from divergent evolutionary selection pressures and specific gene duplication events. Physical and chemical property analyses showed that the amino acid number, molecular weight, isoelectric point, and hydrophilicity of the six candidate IDHs were similar to those of the plants, indicating that IDH was conservative among different species. According to the conservative motif prediction, gene structure, and phylogenetic tree analysis, the six candidate genes encoding citrus IDH can be divided into two categories: CpNAD-IDH and CpNADP-IDH. The study of maize suggests that all ZmNADP-IDHs are predicted to be located in the cytoplasmic fraction, while all ZmNAD-IDHs are predicted to be located in the mitochondrial fraction [21]. In total, 29 IDHs from different species were divided into NAD-dependent IDH and NADP-dependent IDH, which suggested that IDH was relatively conserved during plant evolution. However, in phylogenetic tree analysis, 13 out of 29 bootstrap values were below 70, which indicated that the amino acid sequences of IDH varied greatly among different species. A similar result was also found in the study of the IDH gene family in maize [21]. The evolution difference might be caused by the diversity of citric acid content among different species. Tissue expression analysis showed that CpNAD-IDHs were mainly expressed in citrus roots, suggesting that NAD-IDH may be involved in citrus root growth and development. Meanwhile, CpNADP-IDHs were highly expressed in leaves, fruits, and roots, and the expression level in mature fruits was higher than that in young fruits, suggesting that CpNADP-IDH might play important regulatory roles in citrus fruit ripening. Studies on other crops found that IDH regulated fruit development and ripening. For example, NADP-ICDH presented incremental expression levels during peach fruit softening along with a concomitant decrease in organic acid content [17]. In tomato, SlICDH1 is involved in fruit ripening via ethylene regulation [33].
IDH enzymatic activity and transcriptional levels exhibited inverse correlations with citric acid accumulation. In sweet oranges, the expression levels of CsIDH1 and CsIDH2 were significantly higher in low-acid varieties than in high-acid varieties [11]. In kumquat, high expression of NAD-IDH resulted in citric acid degradation during fruit development [3]. In this study, transcriptional upregulation of CpNADP-IDH1 in ‘Cock tail’ grapefruit (low acid) indicates that CpNADP-IDH1 might serve as a rate-limiting enzyme in citrate degradation at maturity. In addition, functional validation of CpNADP-IDH1 in transgenic grapefruit callus confirmed the modulation. Meanwhile, citric acid accumulation in citrus fruits is regulated through IDH-mediated metabolic modulation, responding to environmental factors and hormonal signaling networks. For example, drought treatment significantly decreased the enzyme activity of IDH and increased the citric acid content in the Ponkan fruits [34]. The citric acid content in ABA-treated Ponkan fruits was significantly lower than control, and the expression of IDH in ABA-treated fruits was significantly higher during the late ripening stages [35]. In this study, three drought-responsive elements, four defense and stress elements, and ten abscisic acid-responsive elements were identified in the promoter of IDHs. Particularly, four light response elements, one ABA response element, and one low-temperature response element were identified in the CpNADP-IDH1 promoter, which suggests that CpNADP-IDH1 may be involved in regulating citric acid metabolism during citrus fruit ripening through environmental factors and phytohormones homeostasis.
5. Conclusions
In this study, six genes encoding IDH were identified from grapefruit. Bioinformatics and gene expression analysis indicated that CpNADP-IDH1 may be a key candidate gene for regulating citric acid catabolism in citrus maturation. Callus transgenic experiments showed that CpNADP-IDH1 was involved in citric acid decomposition in citrus. While CpNADP-IDH1 potentially represents a good candidate gene for molecular breeding of citrus and a target gene for developing acid-reducing cultivation technology in the citrus industry, further research is required to address the relative importance of other IDH family members in regulating citric acid turnover under particular environmental and/or stress conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11060598/s1, Table S1: List of primers used in this study. Table S2: The gDNA, cDNA and protein sequence of the CpIDH gene family. Table S3: Physicochemical properties of the IDH gene family. Table S4: Conserved motif in CpIDH gene family. Table S5: The amino acid sequences of IDH in different plants.
Author Contributions
Conceptualization, P.W. and L.J.; methodology, L.J. and Y.Y.; investigation, F.L.; data curation, M.W., B.H. and F.L.; writing—original draft preparation, L.J.; writing—review and editing, P.W. and L.J.; supervision, P.W.; project administration, L.J.; funding acquisition, L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32202407).
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aslan, M.N.; Sukan-Karaçağıl, B.; Acar-Tek, N. Roles of citrus fruits on energy expenditure, body weight management, and metabolic biomarkers: A comprehensive review. Nutr. Rev. 2024, 82, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, F.; Huang, Y.; He, J.; Yang, S.; Zeng, J.; Deng, C.; Jiang, X.; Fang, Y.; Wen, S.; et al. Genome of Wild Mandarin and Domestication History of Mandarin. Mol. Plant. 2018, 11, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Ma, Q.; Zhou, G.; Liu, X.; Ma, Z.; Gu, Q. Identification of genes associated with soluble sugar and organic acid accumulation in ‘Huapi’ kumquat (Fortunella crassifolia Swingle) via transcriptome analysis. J. Sci. Food Agric. 2021, 101, 4321–4331. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, C.; Dong, W.; Jiang, Q.; Wang, D.; Li, S.; Chen, M.; Liu, C.; Sun, C.; Chen, K. Transcriptome and metabolome analyses of sugar and organic acid metabolism in Ponkan (Citrus reticulata) fruit during fruit maturation. Gene 2015, 554, 64–74. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Luo, Y.; Sun, X.; Wang, J.; Luo, T.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef]
- Shi, C.; Hussain, S.B.; Han, H.; Alam, S.M.; Liu, D.; Liu, Y. Reduced expression of CsPH8, a P-type ATPase gene, is the major factor leading to the low citrate accumulation in citrus leaves. Plant Physiol. Biochem. 2021, 160, 211–217. [Google Scholar] [CrossRef]
- Shi, C.; Song, R.; Hu, X.; Liu, X.; Jin, L.; Liu, Y. Citrus PH5-like H+-ATPase genes: Identification and transcript analysis to investigate their possible relationship with citrate accumulation in fruits. Front. Plant Sci. 2015, 6, 135. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Xie, X.; Sun, C.; Grierson, D.; Yin, X.; Chen, K. CrMYB73, a PH-like gene, contributes to citric acid accumulation in citrus fruit. Sci. Hortic. 2015, 197, 212–217. [Google Scholar] [CrossRef]
- Hussain, S.B.; Shi, C.; Guo, L.; Kamran, H.M.; Sadka, A.; Liu, Y. Recent Advances in the Regulation of Citric Acid Metabolism in Citrus Fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Yang, W.; Zhang, M.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. GABA Pathway Rate-Limit Citrate Degradation in Postharvest Citrus Fruit Evidence from HB Pumelo (Citrus grandis) × Fairchild (Citrus reticulata) Hybrid Population. J. Agric. Food Chem. 2017, 65, 1669–1676. [Google Scholar] [CrossRef]
- Lu, X.; Cao, X.; Li, F.; Li, J.; Xiong, J.; Long, G.; Cao, S.; Xie, S. Comparative transcriptome analysis reveals a global insight into molecular processes regulating citrate accumulation in sweet orange (Citrus sinensis). Physiol. Plant. 2016, 158, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Sadka, A.; Dahan, E.; Or, E.; Cohen, L. NADP+-isocitrate dehydrogenase gene expression and isozyme activity during citrus fruit development. Plant Sci. 2000, 158, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yin, X.; Wang, W.; Liu, X.; Zhang, B.; Chen, K. Citrus CitNAC62 cooperates with CitWRKY1 to participate in citric acid degradation via up-regulation of CitAco3. J. Exp. Bot. 2017, 68, 3419–3426. [Google Scholar] [CrossRef]
- Lancien, M.; Gadal, P.; Hodges, M. Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation. Plant Physiol. 2000, 123, 817–824. [Google Scholar] [CrossRef]
- Zhan, S.; Zhang, Q.; Yao, Y.; Cui, Y.; Huang, T. Cytosolic isocitrate dehydrogenase regulates plant stem cell maintenance in response to nutrient deficiency. Plant Physiol. 2023, 192, 3069–3087. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Taboada, J.; Palma, J.M.; Corpas, F.J. Activity and gene expression analysis of the NADP-dependent isocitrate dehydrogenase (NADP-ICDH) through pepper fruit ripening and its modulation by nitric oxide (NO). Molecular characterization of the peroxisomal isozyme. Plant Sci. 2024, 349, 112269. [Google Scholar] [CrossRef]
- Nilo, R.; Saffie, C.; Lilley, K.; Baeza-Yates, R.; Cambiazo, V.; Campos-Vargas, R.; González, M.; Meisel, L.A.; Retamales, J.; Silva, H.; et al. Proteomic analysis of peach fruit mesocarp softening and chilling injury using difference gel electrophoresis (DIGE). BMC Genom. 2010, 11, 43. [Google Scholar] [CrossRef]
- Margaria, P.; Abba, S.; Palmano, S. Novel aspects of grapevine response to phytoplasma infection investigated by a proteomic and phospho-proteomic approach with data integration into functional networks. BMC Genom. 2013, 14, 38. [Google Scholar] [CrossRef]
- Castañeda, V.; González, E.M. Strategies to Apply Water-Deficit Stress: Similarities and Disparities at the Whole Plant Metabolism Level in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 2813. [Google Scholar] [CrossRef]
- Leterrier, M.; Del Río, L.A.; Corpas, F.J. Cytosolic NADP-isocitrate dehydrogenase of pea plants: Genomic clone characterization and functional analysis under abiotic stress conditions. Free Radic. Res. 2007, 41, 191–199. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, Z.; Yang, H.; Hu, D.; Wu, Y.; Xue, J.; Guo, D.; Xu, S. Characterization of the Isocitrate Dehydrogenase Gene Family and Their Response to Drought Stress in Maize. Plants 2023, 12, 3466. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.M.; Gupta, P. Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Edgar, R.C.; Batzoglou, S. Multiple sequence alignment. Curr. Opin. Struc. Biol. 2006, 16, 368–373. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Long, J.; Liu, C.; Feng, M.; Liu, Y.; Wu, X.; Guo, W. miR156-SPL modules regulate induction of somatic embryogenesis in citrus callus. J. Exp. Bot. 2018, 69, 2979–2993. [Google Scholar] [CrossRef]
- Tang, M.; Bie, Z.; Wu, M.; Yi, H.; Feng, J. Changes in organic acids and acid metabolism enzymes in melon fruit during development. Sci. Hortic. 2010, 123, 360–365. [Google Scholar] [CrossRef]
- Popova, O.V.; Ismailov, S.F.; Popova, T.N.; Dietz, K.; Golldack, D. Salt-induced expression of NADP-dependent isocitrate dehydrogenase and ferredoxin-dependent glutamate synthase in Mesembryanthemum crystallinum. Planta 2002, 215, 906–913. [Google Scholar] [CrossRef]
- Wu, B.; Qi, F.; Liang, Y. Fuels for ROS signaling in plant immunity. Trends Plant Sci. 2023, 28, 1124–1131. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, Z.; Wu, M.; Chan, H.; Yuan, Y.; Tang, N.; Zhang, Q.; Miao, M.; Chang, W.; Li, Z.; et al. Integrative comparative analyses of metabolite and transcript profiles uncovers complex regulatory network in tomato (Solanum lycopersicum L.) fruit undergoing chilling injury. Sci. Rep. 2019, 9, 4470. [Google Scholar] [CrossRef] [PubMed]
- Gamrasni, D.; Erov, M.; Saar, L.; Raz, A.; Glikman, M.; Sonawane, P.D.; Aharoni, A.; Goldway, M. The isocitrate dehydrogenase 1 gene is associated with the climacteric response in tomato fruit ripening. Postharvest Biol. Technol. 2020, 166, 111219. [Google Scholar] [CrossRef]
- Jiang, N.; Jin, L.; Da Silva, J.A.T.; Islam, M.Z.; Gao, H.; Liu, Y.; Peng, S. Activities of enzymes directly related with sucrose and citric acid metabolism in citrus fruit in response to soil plastic film mulch. Sci. Hortic. 2014, 168, 73–80. [Google Scholar] [CrossRef]
- Wang, X.; Yin, W.; Wu, J.; Chai, L.; Yi, H. Effects of exogenous abscisic acid on the expression of citrus fruit ripening-related genes and fruit ripening. Sci. Hortic. 2016, 201, 175–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).