Sweet Chestnut Wood Distillate’s Role in Reducing Helicoverpa armigera Damage and Enhancing Chickpea Performance: Evidence from Field Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Wood Distillate Characteristics
2.2. Study Site and Experimental Setup
2.3. An Evaluation of H. armigera Damage Across the Reproductive Stage
2.4. Seed Nutritional Parameters
2.4.1. Polyphenol Concentration
2.4.2. Flavonoid Concentration
2.4.3. Starch Concentration
2.4.4. Element Concentration
2.5. Statistical Analyses
3. Results
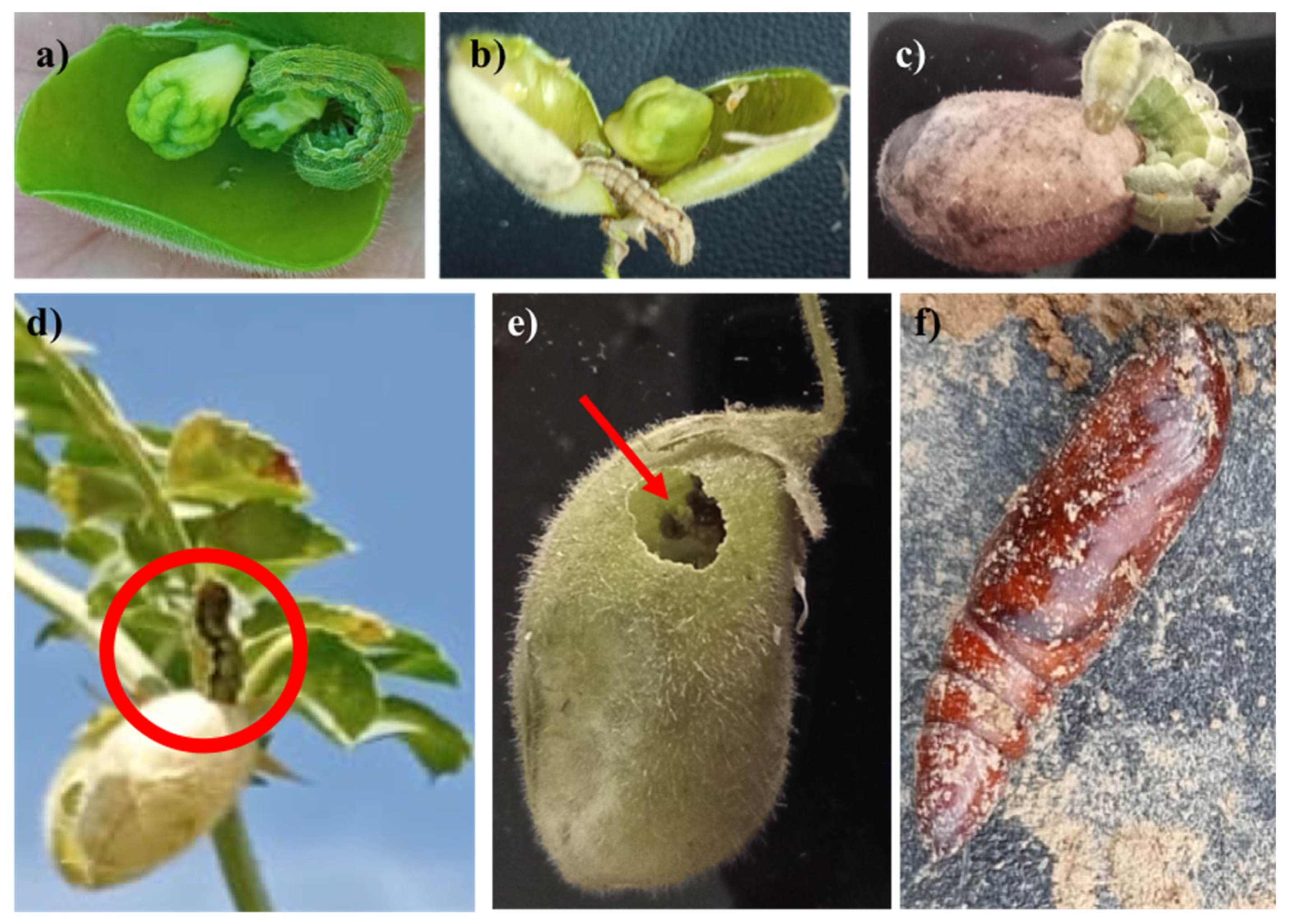
3.1. Observations of H. armigera Lifecycle in Chickpea Crops
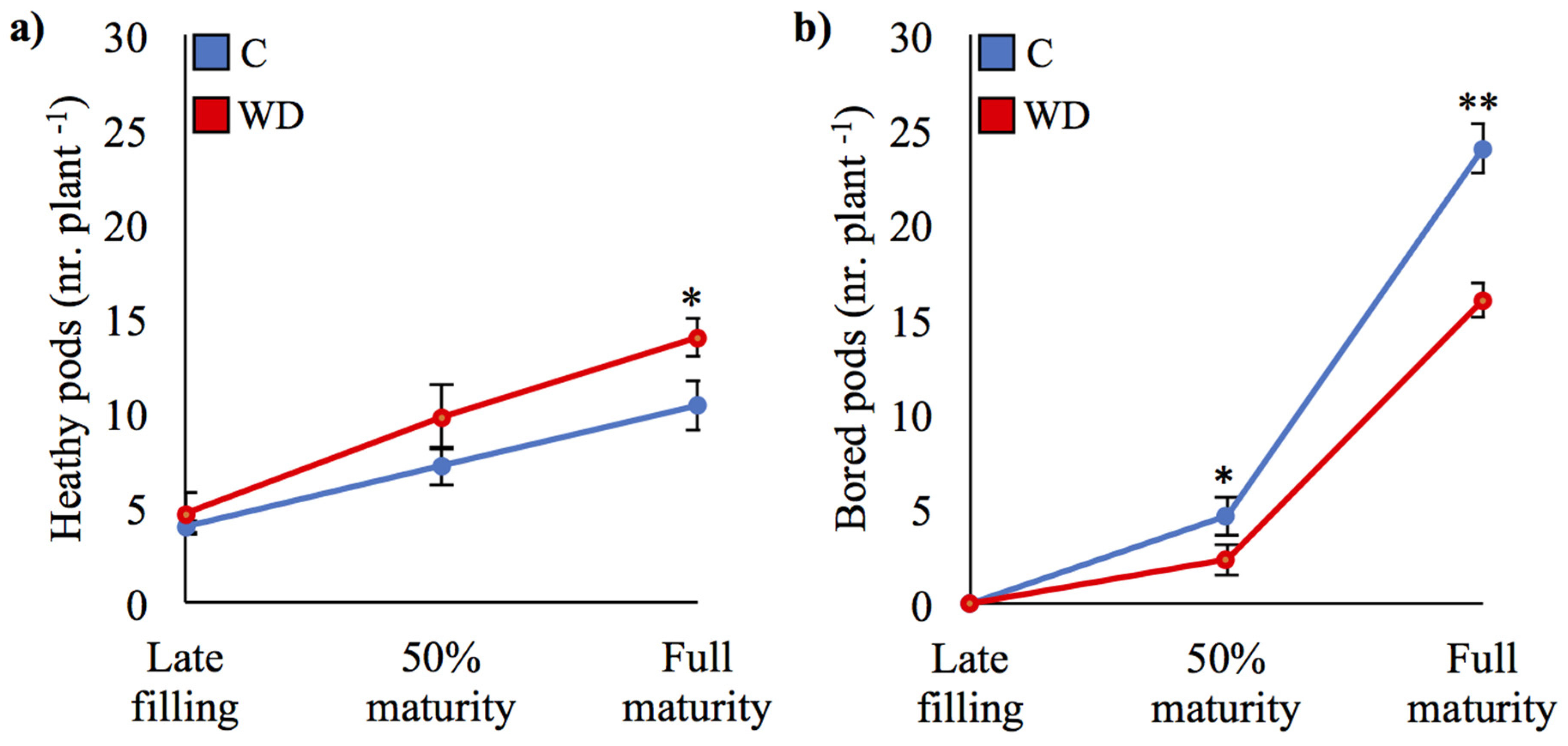
3.2. Reduced Damage in WD-Treated Plants
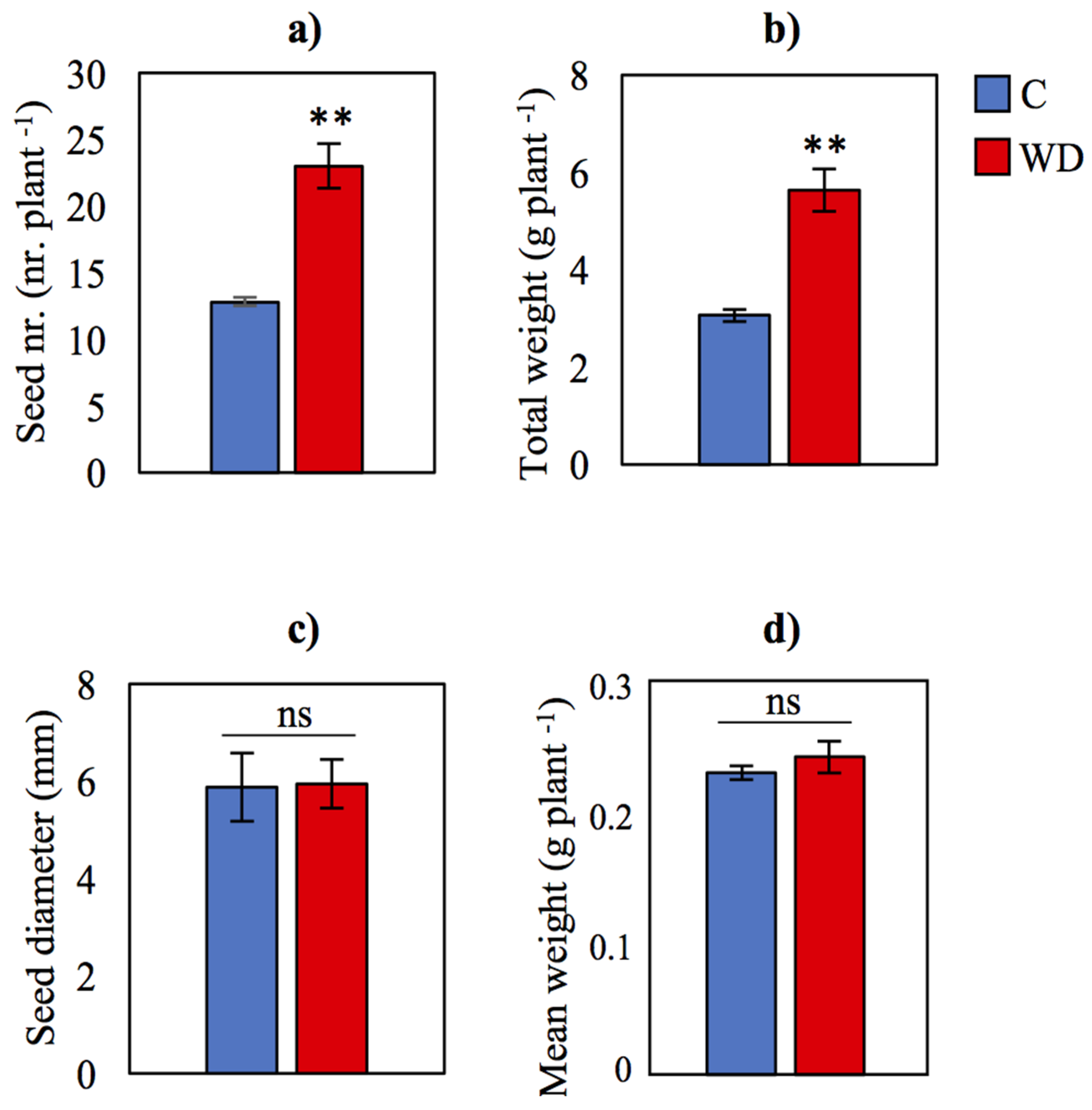
3.3. Seed Yield and Nutritional Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WD | Wood distillate |
| IPM | Integrated pest management |
References
- Begum, N.; Khan, Q.U.; Liu, L.G.; Li, W.; Liu, D.; Haq, I.U. Nutritional composition, health benefits and bio-active compounds of chickpea (Cicer arietinum L.). Front. Nutr. 2023, 10, 1218468. [Google Scholar] [CrossRef] [PubMed]
- Togola, A.; Ongom, P.O.; Mohammed, S.B.; Fatokun, C.; Tamò, M.; Boukar, O. Host plant resistance to insects in pulse crops. In Plant Resistance to Insects in Major Field Crops; Springer Nature: Singapore, 2024; pp. 169–182. [Google Scholar]
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Rogers, D.J.; Brier, H.B. Pest-damage relationships for Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on soybean (Glycine max) and dry bean (Phaseolus vulgaris) during pod-fill. Crop Prot. 2010, 29, 47–57. [Google Scholar] [CrossRef]
- Hakeem, S.A.; Wani, R.A.; Baba, J.A.; Allie, B.A.; Dar, N.A.; Bashir, S.; Nissa, S.U.; Zaffer, G.; Dar, S.A.; Sofi, M.A. Evaluation of different insecticides against pod borer (Helicoverpa armigera) in Lentil. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 681–685. [Google Scholar] [CrossRef]
- Mahmood, M.T.; Akhtar, M.; Ahmad, M.; Saleem, M.; Aziz, A.; Rasool, I.; Amin, Z.A.M. An update on biology, extent of damage and effective management strategies of chickpea pod borer (Helicoverpa armigera). Pak. J. Agric. Res. 2021, 34, 91–101. [Google Scholar] [CrossRef]
- Stacke, R.F.; Arnemann, J.A.; Rogers, J.; Stacke, R.S.; Strahl, T.T.; Perini, C.R.; Dossin, M.F.; Pozeban, H.; Cavallin, L.A.; Guedes, J.V. Damage assessment of Helicoverpa armigera (Lepidoptera: Noctuidae) in soybean reproductive stages. Crop Prot. 2018, 112, 10–17. [Google Scholar] [CrossRef]
- Feng, H.; Wu, X.; Wu, B.; Wu, K. Seasonal migration of Helicoverpa armigera (Lepidoptera: Noctuidae) over the Bohai sea. J. Econ. Entomol. 2009, 102, 95–104. [Google Scholar] [CrossRef]
- Balzan, M.V.; Moonen, A.C. Management strategies for the control of Tuta absoluta (Lepidoptera: Gelechiidae) damage in open-field cultivations of processing tomato in Tuscany (Italy). EPPO Bull. 2012, 42, 217–225. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.C. Landscape complexity and field margin vegetation diversity enhance natural enemies and reduce herbivory by Lepidoptera pests on tomato crop. BioControl 2016, 61, 141–154. [Google Scholar] [CrossRef]
- Burgio, G.; Ravaglia, F.; Maini, S.; Bazzocchi, G.G.; Masetti, A.; Lanzoni, A. Mating disruption of Helicoverpa armigera (Lepidoptera: Noctuidae) on processing tomato: First applications in Northern Italy. Insects 2020, 11, 206. [Google Scholar] [CrossRef]
- Ruiu, L.; Lentini, A. Sustainable Silage Maize Integrated Protection against the European Corn Borer Ostrinia nubilalis and the Corn Earworm Helicoverpa armigera Employing the Farm Irrigation System. Agronomy 2022, 12, 362. [Google Scholar] [CrossRef]
- Sannino, L.; Espinosa, B.; Caponero, A. Helicoverpa armigera (Hübner) harmful to pepper crops in Italy. Inf. Fitopatol. 2004, 54, 23–25. [Google Scholar]
- Efsa PLH 2014 Panel (EFSA Panel on Plant Health). Scientific Opinion on the risk of Phyllosticta citricarpa (Guignardia citricarpa) for the EU territory with identification and evaluation of risk reduction options. EFSA J. 2014, 12, 3557. [Google Scholar]
- Kora, D.; Teshome, E.; Biftu, A. On-farm demonstration and evaluation of synthetic insecticides for the control of pod borer (Helicoverpa armigera Hubner) on chickpea in Bale zone. Am. J. Plant Biol. 2018, 3, 29–32. [Google Scholar] [CrossRef]
- El Fakhouri, K.; Boulamtat, R.; Sabraoui, A.; El Bouhssini, M. The chickpea pod borer, Helicoverpa armigera (Hübner): Yield loss estimation and biorational insecticide assessment in Morocco. Agronomy 2022, 12, 3017. [Google Scholar] [CrossRef]
- Saroop, S.; Tamchos, S. Impact of pesticide application: Positive and negative side. In Pesticides in a Changing Environment; Elsevier: Amsterdam, The Netherlands, 2024; pp. 155–178. [Google Scholar]
- Urrutia, R.I.; Gutierrez, V.S.; Stefanazzi, N.; Volpe, M.A.; González, J.O.W. Pyrolysis liquids from lignocellulosic biomass as a potential tool for insect pest management: A comprehensive review. Ind. Crop Prod. 2022, 177, 114533. [Google Scholar] [CrossRef]
- Fedeli, R.; Fiaschi, T.; Angiolini, C.; Maccherini, S.; Loppi, S.; Fanfarillo, E. Dose-Dependent and Species-Specific Effects of Wood Distillate Addition on the Germination Performance of Threatened Arable Plants. Plants 2023, 12, 3028. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Uptake of trace elements in the water fern Azolla filiculoides after short-term application of chestnut wood distillate (Pyroligneous Acid). Plants 2020, 9, 1179. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Becagli, M.; Santin, M.; Cardelli, R. Co-application of wood distillate and biochar improves soil quality and plant growth in basil (Ocimum basilicum). J. Plant Nutr. Soil Sci. 2022, 185, 120–131. [Google Scholar] [CrossRef]
- Ghorbani, M.; Azarnejad, N.; Carril, P.; Celletti, S.; Loppi, S. Boosting the resilience to drought of crop plants using wood distillate: A pilot study with lettuce (Lactuca sativa L.). Plant Stress 2024, 12, 100450. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.A.F.; Ferreira, M.B.; Favero, S.; Carollo, C.A. Biological activity of sugarcane pyroligneous acid against Spodoptera frugiperda (JE Smith, 1797) (Lepidoptera: Noctuidae) larvae. Afr. J. Biotechnol. 2013, 12, 6241–6244. [Google Scholar]
- Prabowo, H.; Martono, E.; Witjaksono, W. Activity of liquid smoke of tobacco stem waste as an insecticide on Spodoptera litura Fabricius larvae. J. Perlindungan Tanam. Indones 2016, 20, 22–27. [Google Scholar] [CrossRef]
- Tavares, W.S.; Costa, M.A.; Cruz, I.; Silveira, R.D.; Serrao, J.E.; Zanuncio, J.C. Selective effects of natural and synthetic insecticides on mortality of Spodoptera frugiperda (Lepidoptera: Noctuidae) and its predator Eriopis connexa (Coleoptera: Coccinellidae). J. Environ. Sci. Health 2010, 45, 557–561. [Google Scholar] [CrossRef]
- Sayed, A.M.; Behle, R.W.; Tiilikkala, K.; Vaughn, S.F. Insecticidal activity of bio-oils and biochar as pyrolysis products and their combination with microbial agents against Agrotis ipsilon (Lepidoptera: Noctuidae). Pestic. Fitomedicina 2018, 33, 39–52. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Grattacaso, M.; Loppi, S. Wood distillate (pyroligneous acid) boosts nutritional traits of potato tubers. Ann. App. Biol. 2023, 183, 135–140. [Google Scholar] [CrossRef]
- Vannini, A.; Moratelli, F.; Monaci, F.; Loppi, S. Effects of wood distillate and soy lecithin on the photosynthetic performance and growth of lettuce (Lactuca sativa L.). SN Appl. Sci. 2021, 3, 113. [Google Scholar] [CrossRef]
- Carril, P.; Bianchi, E.; Cicchi, C.; Coppi, A.; Dainelli, M.; Gonnelli, C.; Loppi, S.; Pazzagli, L.; Colzi, I. Effects of Wood Distillate (Pyroligneous Acid) on the Yield Parameters and Mineral Composition of Three Leguminous Crops. Environments 2023, 10, 126. [Google Scholar] [CrossRef]
- Carril, P.; Colzi, I.; Salvini, R.; Beltramone, L.; Rindinella, A.; Ermini, A.; Gonnelli, C.; Garzelli, A.; Loppi, S. Multispectral, Thermographic and Spectroradiometric Analyses Unravel Bio-Stimulatory Effects of Wood Distillate in Field-Grown Chickpea (Cicer arietinum L.). Remote Sens. 2024, 16, 2524. [Google Scholar] [CrossRef]
- Fedeli, R.; Marotta, L.; Frattaruolo, L.; Panti, A.; Carullo, G.; Fusi, F.; Loppi, S. Nutritionally enriched tomatoes (Solanum lycopersicum L.) grown with wood distillate: Chemical and biological characterization for quality assessment. J. Food Sci. 2023, 88, 5324–5338. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Cruz, C.; Loppi, S.; Munzi, S. Hormetic Effect of Wood Distillate on Hydroponically Grown Lettuce. Plants 2024, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Alexandrov, D. Guarnieri. M.; Loppi, S. Foliar application of wood distillate boosts plant yield and nutritional parameters of chickpea. Ann. App. Biol. 2023, 182, 57–64. [Google Scholar] [CrossRef]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Essential mineral elements and potentially toxic elements in orange-fleshed sweet potato cultivated in northern Ethiopia. Biology 2023, 12, 266. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahmad, R. Bioefficacy of Steinernema masoodi (Ali et al., 2005) against pre pupa/pupa of Helicoverpa armigera. Biosciences 2009, 2, 18–19. [Google Scholar]
- Hagner, M.; Kuoppala, E.; Fagernäs, L.; Tiilikkala, K.; Setälä, H. Using the Copse snail Arianta arbustorum (Linnaeus) to detect repellent compounds and the quality of wood Vinegarv. Int. J. Environ. Res. 2015, 9, 53–60. [Google Scholar]
- Cândido, N.R.; Pasa, V.M.D.; de Oliveira Vilela, A.; Campos, Â.D.; de Fátima, Â.; Modolo, L.V. Understanding the multifunctionality of pyroligneous acid from waste biomass and the potential applications in agriculture. Sci. Total Environ. 2023, 881, 163519. [Google Scholar] [CrossRef]
- Yatagai, M.; Nishimoto, M.; Hori, K.; Ohira, T.; Shibata, A. Termiticidal activity of wood vinegar, its components and their homologues. J. Wood Sci. 2002, 48, 338–342. [Google Scholar] [CrossRef]
- Oramahi, H.A.; Yoshimura, T.; Diba, F.; Setyawati, D.; Nurhaida. Antifungal and antitermitic activities of wood vinegar from oil palm trunk. J. Wood Sci. 2018, 64, 311–317. [Google Scholar] [CrossRef]
- Iacomino, G.; Idbella, M.; Staropoli, A.; Nanni, B.; Bertoli, T.; Vinale, F.; Bonanomi, G. Exploring the potential of wood vinegar: Chemical composition and biological effects on crops and pests. Agronomy 2024, 14, 114. [Google Scholar] [CrossRef]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Repellents effectively disrupt the olfactory orientation of Sitophilus granarius to wheat kernels. J. Pest Sci. 2015, 88, 675–684. [Google Scholar] [CrossRef]
- Saxena, H.O.; Tripathi, Y.C.; Pawar, G.; Kakkar, A.; Mohammad, N. Botanicals as biopesticides: Active chemical constituents and biocidal action. In Familiarizing with Local Biodiversity; Tropical Forest Research Institute (ICFRE): Jabalpur, India, 2014; pp. 222–240. [Google Scholar]
- Cheng, S.Y.; Li, Q. Effects of pyroligneous acid amendment on avoidance, survival and biomass change of earthworms in an agricultural soil. In Advances in Food Safety and Environmental Engineering; CRC Press: Boca Raton, FL, USA, 2022; pp. 14–18. [Google Scholar]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.; Pogačnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef]
- Salgado, P.; Lallès, J.P.; Toullec, R.; Mourato, M.; Cabral, F.; Freire, J.P. Nutrient digestibility of chickpea (Cicer arietinum L.) seeds and effects on the small intestine of weaned piglets. Anim. Feed. Sci. Technol. 2001, 91, 197–212. [Google Scholar] [CrossRef]
- Segev, A.; Badani, H.; Kapulnik, Y.; Shomer, I.; Oren-Shamir, M.; Galili, S. Determination of polyphenols, flavonoids, and antioxidant capacity in colored chickpea (Cicer arietinum L.). J. Food Sci. 2010, 75, S115–S119. [Google Scholar] [CrossRef]
- Sahu, V.K.; Tiwari, S.; Gupta, N.; Tripathi, M.K.; Yasin, M. Evaluation of physiological and biochemical contents in desi and Kabuli chickpea. Legume Res.-Int. J. 2022, 45, 1197–1208. [Google Scholar]
- Macar, T.K.; Macar, O.; Mart, D.İ. Variability in some biochemical and nutritional characteristics in desi and Turkish kabuli chickpea (Cicer arietinum L.) types. Celal Bayar Univ. J. Sci. 2017, 13, 677–680. [Google Scholar]
- Macar, T.K.; Macar, O. A comparison of nutritional values and antioxidant levels of desi and Turkish kabuli chickpea (Cicer arietinum L.) seeds. Cumhur. Sci. J. 2020, 41, 764–774. [Google Scholar] [CrossRef]
- Junejo, S.A.; Flanagan, B.M.; Zhang, B.; Dhital, S. Starch structure and nutritional functionality–Past revelations and future prospects. Carbohydr. Polym. 2022, 277, 118837. [Google Scholar] [CrossRef]
- Ghoshal, G.; Kaushal, K. Extraction, characterization, physicochemical and rheological properties of two different varieties of chickpea starch. Legume Sci. 2020, 2, e17. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Starch characteristics of black bean, chickpea, lentil, navy bean and pinto bean cultivars grown in Canada. Food Chem. 2002, 78, 489–498. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Venn, B.J.; Mann, J.I. Cereal grains, legumes and diabetes. Eur. J. Clin. Nutr. 2004, 58, 1443–1461. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef]
- Kumar, R.; Chijina, K.; Mohit, R.; Kumar, B.P. Fortification of micronutrients in chickpea (Cicer arietinum L.): Innovative approaches to combat malnutrition. Pharma Innov. 2022, 11, 886–894. [Google Scholar]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, S.; Krebs-Smith, S.M. Update of the healthy eating index: HEI-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef]
- Wallace, T.C.; Murray, R.; Zelman, K.M. The nutritional value and health benefits of chickpeas and hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A sufficiently effective tool for sustainable agriculture in the era of climate change? Plant Physiol. Biochem. 2024, 211, 108699. [Google Scholar] [CrossRef]
- Ruzzi, M.; Colla, G.; Rouphael, Y. Biostimulants in agriculture II: Towards a sustainable future. Front. Plant Sci. 2024, 15, 1427283. [Google Scholar] [CrossRef]
- Russo, R.O.; Berlyn, G.P. The use of organic biostimulants to help low input sustainable agriculture. J. Sustain. Agric. 1991, 1, 19–42. [Google Scholar] [CrossRef]
- Pierre, J.F. Legumes: Cornerstones of Global Food Security and Sustainable Agriculture; IntechOpen: London, UK, 2024. [Google Scholar]
- Grosso, G.; Mateo, A.; Rangelov, N.; Buzeti, T.; Birt, C. Nutrition in the context of the Sustainable Development Goals. Eur. J. Public Health 2020, 30 (Suppl. S1), i19–i23. [Google Scholar] [CrossRef] [PubMed]
| Total Polyphenols (mg g−1) | Flavonoids (mg g−1) | Starch (mg g−1) | |
|---|---|---|---|
| C | 0.507 ± 0.005 | 0.191 ± 0.007 | 161 ± 7 |
| WD | 0.594 ± 0.007 * | 0.298 ± 0.004 ** | 231 ± 4 ** |
| Ca | Mg | Fe | Na | K | P | S | Zn | Cu | |
|---|---|---|---|---|---|---|---|---|---|
| C | 1241 ± 26 | 1589 ± 11 | 76 ± 3 | 153 ± 16 | 10,226 ± 15 | 3660 ± 134 | 2064 ± 113 | 33 ± 1 | 9 ± 1 |
| WD | 1527 ± 34 * | 1741 ± 38 * | 79 ± 7 | 164 ± 33 | 10,791 ± 208 | 3834 ± 179 | 2107 ± 197 | 35 ± 3 | 10 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carril, P.; Colzi, I.; Fedeli, R.; Gonnelli, C.; Loppi, S. Sweet Chestnut Wood Distillate’s Role in Reducing Helicoverpa armigera Damage and Enhancing Chickpea Performance: Evidence from Field Trial. Horticulturae 2025, 11, 613. https://doi.org/10.3390/horticulturae11060613
Carril P, Colzi I, Fedeli R, Gonnelli C, Loppi S. Sweet Chestnut Wood Distillate’s Role in Reducing Helicoverpa armigera Damage and Enhancing Chickpea Performance: Evidence from Field Trial. Horticulturae. 2025; 11(6):613. https://doi.org/10.3390/horticulturae11060613
Chicago/Turabian StyleCarril, Pablo, Ilaria Colzi, Riccardo Fedeli, Cristina Gonnelli, and Stefano Loppi. 2025. "Sweet Chestnut Wood Distillate’s Role in Reducing Helicoverpa armigera Damage and Enhancing Chickpea Performance: Evidence from Field Trial" Horticulturae 11, no. 6: 613. https://doi.org/10.3390/horticulturae11060613
APA StyleCarril, P., Colzi, I., Fedeli, R., Gonnelli, C., & Loppi, S. (2025). Sweet Chestnut Wood Distillate’s Role in Reducing Helicoverpa armigera Damage and Enhancing Chickpea Performance: Evidence from Field Trial. Horticulturae, 11(6), 613. https://doi.org/10.3390/horticulturae11060613











