Abstract
Lobelia chinensis (Lour.) is a medicinal plant that contains phytochemicals, such as phenolics and polyacetylene compounds, with beneficial biological activities. In vitro cultures are typically employed for biomass generation and plant multiplication. However, the current biotechnological approaches for producing these chemicals are ineffective, which is why bioelicitors are being used to boost synthesis of these molecules. Plantlet cultures were established in vitro using Murashige and Skoog medium supplemented with 3% sucrose (w/v). Following 4 weeks of culture initiation, the plantlet cultures were treated with 0, 25, 50, 100, or 200 mg L−1 of yeast extract (YE) or with 0, 25, 50, 100, or 200 µM of salicylic acid (SA) for 1 week to boost the synthesis of bioactive compounds. The amounts of total phenolics, total flavonoids, specific phenolics including catechin, phloretic acid, linarin, and polyacetylenes, including lobetyolinin and lobetylin, were considerably elevated in the plantlet cultures treated with 50 mg L−1 YE and/or 25 µM SA. The 2,2 Diphenyl 1 picrylhydrazyl (DPPH) radical scavenging assay, 2,2′-azino-bis (3-ethybenzothiazoline-6-sulphonic acid) (ABTS) assay, and ferric reducing antioxidant power (FRAP) assay were performed to assess the antioxidant properties of the plantlets. The elicitor-treated plantlets were found to have higher antioxidant activity. Thus, plantlet biomass produced in vitro can be used as a raw material to produce medicinal and nutraceutical products.
1. Introduction
Lobelia chinensis Lour., a member of the Campanulaceae family and commonly known as “Chinese lobelia”, is a medicinal perennial herb found in China, Korea, and Japan. In Chinese medicine, this plant is frequently used to treat edema, malarial fever, jaundice, diarrhea, and snakebites [1]. L. chinensis is reportedly abundant with numerous phytochemicals, including phenolics, alkaloids, coumarins, terpenoids, and polyacetylenes [2]. Several biological activities of L. chinensis, including anti-inflammatory [3], antioxidant [4], antiviral [5], anti-obesity [6], anti-tuberculosis [7], and anticancer [8] properties, have been identified. This well-known herb is in high demand as a raw material and is utilized in Chinese, Japanese, and Oriental medicine [2]. Multiple attempts have been made to grow this plant using tissue culture techniques, because overexploitation has reduced the number of natural stands of L. chinensis [9]. Additionally, employing cell and organ cultures to produce important phytochemicals like glucosinolates, phenolics, terpenoids, and alkaloids on a large scale is feasible with liquid cultures [10,11].
Elicitation techniques are frequently used to generate secondary metabolites (SMs) in tissue cultures to increase the output [11]. Additionally, cells and organs of plants are first grown in the best biomass growth medium before being transferred to the best production medium to promote the synthesis of the desired bioactive compounds [11]. Numerous biotic elicitors, including chitin, pectin, chitosan, cellulose, and yeast extract, as well as abiotic elicitors, such as physical stressors (e.g., light, heavy metal salts) and intracellular signaling molecules (e.g., salicylic and jasmonic acids), have been effectively employed to improve the accumulation of bioactive compounds in plant tissue cultures [12,13].
Yeast extract (YE) is a common elicitor used in vitro and in vivo to boost plant growth, productivity, and secondary metabolite accumulation [13]. YE significantly increased the production of lupeol in shoot cultures of Hemidesmus indicus and Tylophora indica [14], flavonoid accumulation in adventitious roots of Oplapanax elatus [15], and accumulation of phenolics in apple cell cultures [16]. In plant tissue cultures, salicylic acid (SA) is an effective elicitor that promotes growth and metabolite accumulation. For example, SA is responsible for enhanced phenolic accumulation in Moringa oleifera callus cultures [17] and increased stevioside production in Stevia rebaudiana plantlet cultures [18]. Although attempts have been made to study L. chinensis in tissue culture, there have been no reports on the use of elicitors to increase the levels of target bioactive molecules. In the present study, we established plantlet cultures of L. chinensis and used YE and SA as elicitors to produce phenolics and polyacetylenes.
2. Materials and Methods
2.1. Plant Material and Culture Conditions
Lobelia chinensis Lour. axenic cultures, which are maintained at the Department of Horticultural Science at Chungbuk National University in the Republic of Korea, were utilized to initiate the experiment. To maintain the cultures, the nodal explants were sub cultured every four weeks and regenerated via organogenesis [9]. The plantlets of L. chinensis were maintained on Murashige and Skoog (MS) [19] medium supplemented with 3% sucrose (w/v) and 7 g L−1 agar. The hydrogen ion concentration (pH) was adjusted to 5.8 before autoclaving. The cultures were maintained at 25 ± 2 °C with 70% relative humidity, a 16/8 h photoperiod, and 40 µmol m−2 s−1 light intensity (fluorescent lamps). After four weeks of culture, 1.5 cm stem segments containing two nodes with alternating leaves and no roots (explants) were used as the experimental material.
2.2. Elicitation
Cultures were established in 500 mL conical flasks containing 200 mL of MS medium with 3% sucrose (w/v). Explants (3 g L−1) were cultured and maintained on a rotary shaker (OS 4000 orbital shaker, Jeio Tech, Daejon, Republic of Korea) at 100 rpm. The cultures were maintained at 25 ± 2 °C with 70% relative humidity, a 16/8 h photoperiod, and 60 µmol m−2 s−1 light intensity provided by red + blue + green light-emitting diodes (LEDs) equipped with a red 20 stick/400 tip, blue 20 stick/400 tip, and green 20 stick/400 tp to arrange the panel (Itswell Co., Incheon, Republic of Korea). The light intensity and spectral parameters were adjusted using an LI-250A light meter with Q50604 (LI-COR, Lincoln, NE, USA). After 4 weeks of culture establishment, elicitation effects of YE (0, 25, 50, 100, and 200 mg L−1) or SA (0, 25, 50, 100, and 200 µM) were evaluated, and cultures were maintained under the same conditions for another week. The stock solution of YE (Duchefa, Haarlem, The Netherlands) was prepared in distilled water, whereas stock 10 mM of SA (Sigma, St. Louis, CA, USA) was prepared in ethanol. Solutions were filter sterilized through a 0.22 µm Millipore syringe filter (Sartorius, Gottingen, Germany) and then added to cultures. At the end of 5 weeks, the fresh and dry biomasses of plantlets were collected, and secondary metabolites accumulated in the regenerated plants were estimated.
2.3. Biomass Estimation
The plantlet biomass harvested after 5 weeks of culture was washed twice with tap water to remove the medium, and the surface water was blotted away with tissue paper. The fresh weight (FW) was estimated. The dry weight (DW) was estimated after desiccating the biomass at 50 °C for 72 h in an oven (Sanyo, MoV-112V, Aichi, Japan). The growth ratio was calculated as follows: [harvested DW (g) − inoculated DW (g)/inoculated DW].
2.4. Preparation of Plant Extract
The dried samples were pulverized using a pestle and mortar, and 1 g of powdered sample was used for extraction using an extraction apparatus (LS-2050-S10; LS-TECH, Seoul, Republic of Korea) with 30 mL 80% ethanol at 80 °C for 1 h and passed through filter paper (Advantec 110 mm; Toyo Roshi Kaisha Ltd., Tokyo, Japan). The final volume of the solution was adjusted to 30 mL using 80% ethanol.
2.5. Estimation of Total Phenolic Content
Total phenolic content was estimated using the Folin–Ciocalteu method [20]. Briefly, a known amount of sample was taken and made up to 3 mL with distilled water, and 0.1 mL of 2 N Folin–Ciocalteu solution was added, followed by incubation for 6 min, and then 0.5 mL of 20% Na2CO3 was added to each tube. The tubes were kept in warm water for 30 min, and the absorbance was measured at 760 nm using a UV–visible spectrophotometer (Libra S22, Biochrome Ltd., Cambridge, UK). Gallic acid was used as the standard. The results were expressed as milligrams gallic acid equivalents (GAE) per gram of dry weight (DW). The equation of the calibration curve was y = 0.0023x + 0.0022 R2 = 0.9985.
2.6. Estimation of Total Flavonoid Content
The flavonoid content of the extracts was analyzed as described by Harborne [20]. The extract (0.1 mL) was added to 3 mL of distilled water, followed by the addition of 0.15 mL of 10% AlCl3 and 2 mL of 1 M NaOH after 5 min of incubation at room temperature. The solutions were vortexed, and the absorbance was measured at 510 nm using a UV–visible spectrophotometer (Libra S22, Biochrome Ltd., Cambridge, UK). Catechin was used as the standard. The results were expressed as milligrams catechin equivalents (CE) per gram of dry weight (DW). The equation of the calibration curve was y = 0.0024x + 0.0562 R2 = 0.995.
2.7. Quantification of Phenolic Compounds
Phenolic compounds were extracted and analyzed as described by Burin et al. [21]. Powdered plant samples (0.1 g) were extracted in 2.0 mL of 70% methanol containing 2% formic acid and centrifuged at 10,000× g for 10 min at 4 °C. The supernatant was filtered through 0.22 µm syringe filters prior to analysis. High-performance liquid chromatography (HPLC) analysis was performed using a Shimadzu (Kyoto, Japan) liquid chromatography system equipped with a vacuum degasser (DGU-14A), quaternary pump LC-10AT, UV–vis detector (SPD-10AV), and an injector (Rheodyne) with a 20 µL loop. CLASS-V software (version 6.1) was used to control the gradient settings, UV–vis, and data acquisition. A C18 reversed-phased column (4.6 mm × 250 mm, 5 µm particle size, Waters XBridge) was used. Gradient elution was conducted with water (0.1% formic acid) (A) and acetonitrile (0.1% formic acid) (B): 0 min, 95% A; 25 min, 72%, and 40 min, 72% A, at a flow rate of 1.2 mL/min. The detection wavelength was 300 nm. Phenolic compounds were identified by comparing retention times and UV spectra with authentic standards (Sigma-Aldrich, St. Louis, MO, USA).
2.8. Quantification of Polyacetylenes
Polyacetylenes (lobetyolin and lobetyolinin) were extracted according to the protocol described by Qiao et al. [22]. HPLC analysis was performed using a Shimadzu (Kyoto, Japan) liquid chromatography system using C18 column (4.65 × 250 mm, 5 µM, Waters XBridge). The column temperature was set at 20 °C. The mobile phase comprised acetonitrile and water. Linear gradient elution was performed from 10% to 40%, and then to 100% acetonitrile for 25 and 35 min, respectively. The detection wavelengths were 267 and 295 nm. The standard compounds were procured from Sigma-Aldrich (St. Louis, MO, USA).
2.9. Analysis of Antioxidant Activities
2.9.1. 2,2 Diphenyl 1 Picrylhydrazyl (DPPH) Radical Scavenging Assay
The extract (0.1 mL) was added to 1.9 mL of 0.1 mM DPPH solution prepared in ethanol. The tubes were then vortexed and incubated in the dark for 15 min. The discoloration of the DPPH solution was measured at 517 nm against ethanol as a blank, using a UV–visible spectrophotometer (Libra S22, Biochrome Ltd., Cambridge, UK). Gallic acid was used as a standard, and the activity of the extracts was expressed as milligrams GAE per gram of extract [23]. The equation for the DPPH assay was as follows (I%): I% = [(Ablank − Asample)/Ablank] × 100, where I% is the percent inhibition of the DPPH radical and Ablank and Asample are the absorbance of the control reaction (containing all reagents except the extract) and the extract, respectively.
2.9.2. 2,2′-Azino-bis (3-ethybenzothiazoline-6-sulphonic acid) (ABTS) Assay
The ABTS solution was prepared by mixing 7 mM ABTS and 2.45 mM potassium persulfate in a 1:1 ratio and storing the mixture in the dark for 24 h. At the time of analysis, the ABTS solution was diluted using phosphate buffer (pH 7.3) to obtain a value of 0.70 at 732 nm. Fifty microliters of the extract was added to 950.0 μL of diluted ABTS solution, which was then placed in the dark for 10 min. Subsequently, the absorbance of the mixture was measured at 732 nm using UV–visible spectrophotometry (Libra S22, Biochrome Ltd., Cambridge, UK). The equation for the ABTS assay was as follows (I%): I% = [(Ablank − Asample)/Ablank] × 100, where I% is the percent inhibition of the ABTS radical and Ablank and Asample are the absorbance of the control reaction (containing all reagents except the extract) and the extract, respectively [23].
2.9.3. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP reagent was prepared by mixing three solutions: 300 mM acetate buffer (pH 3.6), a 10 mM solution of 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl, and 20 mM FeCl2·6H2O, which were mixed in a ratio of 10:1:1. The extract (0.2 mL) was added to 3.0 mL of FRAP reagent, the tubes were vortexed and incubated for 6 min at room temperature, and the absorbance was measured at 593 nm using a UV–visible spectrophotometer (Libra S22, Biochrome Ltd., Cambridge, UK). Ascorbic acid was used as a standard, and the activity was expressed as milligrams ascorbic acid equivalents (AAE) per gram of extract [23]. The equation of the calibration curve was y = 0.0028x + 0.5795 R2 = 0.7853.
2.10. Statistical Analysis
All experiments were set up in a completely randomized design, and data were collected from three replicates. A one-way analysis of variance (ANOVA) was performed to determine significant differences between treatments. The statistical significance of the differences between mean values was assessed using Duncan’s multiple range test (DMRT) at p < 0.05. All statistical analyses were performed using the SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Elicitation of Cultures with Yeast Extract
Table 1 and Figure 1 show biomass accumulation in L. chinensis plantlet cultures treated with YE (0, 25, 50, 100, and 200 mg L−1). A stimulatory effect on biomass accumulation was observed following the administration of 50 mg L−1 YE, leading to an optimal accumulation of 97.3 g L−1 fresh and 9.8 g L−1 dry biomass. In contrast, the fresh biomass in the control cultures was 84 g L−1, and the dried biomass was 8.5 g L−1. However, biomass accumulation decreased with a further increase in YE concentration (Table 1).

Table 1.
Effect of different concentrations of yeast extract on the growth of L. chinensis plantlets.

Figure 1.
Lobelia chinensis biomass obtained after 5 weeks of culture in MS medium. Cultures were elicited after 4 weeks with 0, 25, 50, 100, and 200 mg L−1 YE.
Addition of YE to the L. chinensis shoot cultures also enhanced the accumulation of total phenolics (1.62 mg GAE g−1 DW) and total flavonoids (8.46 mg CE g−1 DW) at 50 mg L−1, whereas the corresponding values for control cultures were 1.12 mg GAE g−1 DW and 5.39 mg CE g−1 DW, respectively (Figure 2A, B). Additionally, we assessed the effects of YE treatment on the accumulation of distinct phenolic compounds and polyacetylenes using HPLC. When 50 mg L−1 YE was added to the cultures, the amounts of catechin (26.73 µg g−1 DW), phloretic acid (88.58 µg g−1 DW), naringin (96.66 µg g−1 DW), and linarin (52.75 µg g−1 DW) increased by 10%, 17%, 124%, and 138%, respectively (Figure 2C). However, this treatment reduced the levels of chlorogenic acid, coumaric acid, SA, and myricetin by 27%, 18%, 36%, and 3%, respectively. Supplementing the cultures with 50 mg L−1 YE significantly enhanced the concentration of the two estimated polyacetylenes, lobetyolinin (200.88 µg g−1 DW) and lobetyolin (576.71 µg g−1 DW), by 26% and 30%, respectively (Figure 2D).
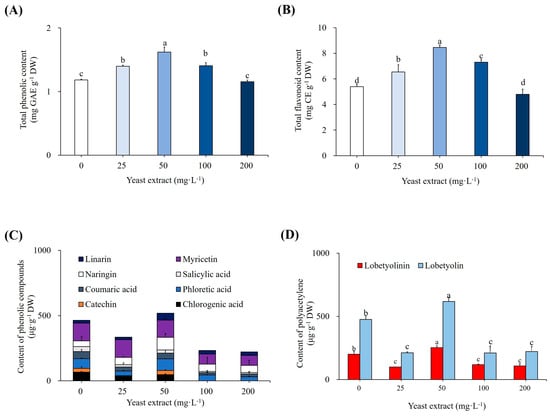
Figure 2.
Effects of YE elicitation on accumulation of bioactive compounds. (A) Total phenolic content, (B) Total flavonoid content, (C) Content of several phenolics as per HPLC analysis, and (D) Content of polyacetylenes as per HPLC analysis. Cultures were elicited after 4 weeks with 0, 25, 50, 100, and 200 mg L−1 YE. Values followed by the same letter within the columns are not significantly different at p ≤ 0.05 using DMRT.
Finally, using the DPPH, ABTS, and FRAP assay techniques, the antioxidant properties of plantlet extract that were elicited and un-elicited by YE were assessed. The results are shown in Figure 3. The ABTS scavenging activity (Figure 3A), DPPH scavenging activity (Figure 3B), and reducing power (Figure 3C) of YE-elicited plants were higher than those of control plants.
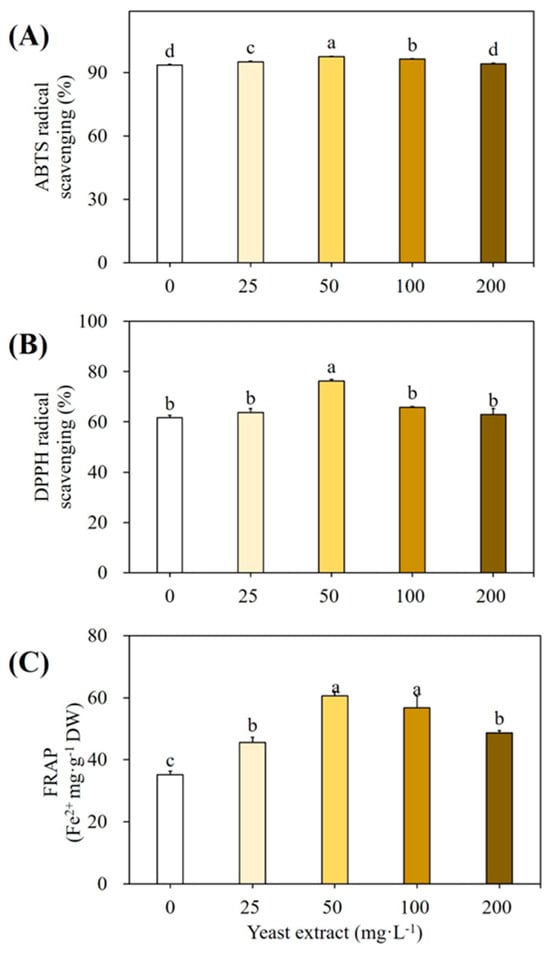
Figure 3.
Antioxidant activity of plantlet extracts elicited with various YE concentrations. (A) ABTS radical scavenging, (B) DPPH radical scavenging, and (C) FRAP activities. Cultures were elicited after 4 weeks with 0, 25, 50, 100, and 200 mg L−1 YE. Values followed by the same letter within the columns are not significantly different at p ≤ 0.05 using DMRT.
3.2. Elicitation of Cultures with Salicylic Acid
SA is a well-known elicitor and signaling molecule that synthesizes bioactive chemicals in plants both in vitro and in vivo. The cultures were treated with 0, 25, 50, 100, and 200 µM SA in this study. The cultures treated with 25 µM SA accumulated higher amounts of fresh (93.0 g L−1) and dry (9.3 g L−1) biomass than the control cultures (84 g L−1 fresh and 8.5 g L−1 dry biomass) (Table 2, Figure 4). In contrast to 25-µM SA treatment, treatments with greater doses of SA (50, 100, and 200 µM) resulted in lower biomass levels.

Table 2.
Effect of different concentrations of salicylic acid on the growth of Lobelia chinensis.

Figure 4.
Lobelia chinensis biomass obtained after 5 weeks of culture in MS medium. Cultures were elicited after 4 weeks with 0, 25, 50, 100, and 200 µM SA.
SA treatment had a substantial impact on the total phenolic and flavonoid contents of L. chinensis plantlets (Figure 5A,B). Under 25-µM SA treatment, the maximum levels of total phenolic content (1.61 mg GAE g−1 DW) and flavonoids (7.67 mg CE g−1 DW) were observed. The total phenolic and flavonoid contents in plantlets decreased with higher SA concentrations (50, 100, and 200 µM). According to the HPLC data, plantlets treated with SA displayed unusual fluctuations in specific phenolic compounds (Figure 5C). Specifically, when compared to control, the levels of linarin (178.09 µg g−1 DW), SA (96.36 µg g−1 DW), coumaric acid (102.72 µg g−1 DW), phloretic acid (312.90 µg g−1 DW), chlorogenic acid (136.20 µg g−1 DW), and catechin (18.41 µg g−1 DW) increased by 122%, 112%, 86%, 496%, 62%, and 12%, respectively, in plantlets treated with 25 µM SA. However, following 25 µM SA treatment, the quantities of myricetin (112.59 µg g−1 DW) and narigin (92.66 µg g−1 DW) were reduced by 17% and 14%, respectively (Figure 5C). The concentration of lobetyolinin (87.09 µg g−1 DW) dropped by 40%, whereas that of lobetyolin (1019 µg g−1 DW) increased by 171% (Figure 5D).
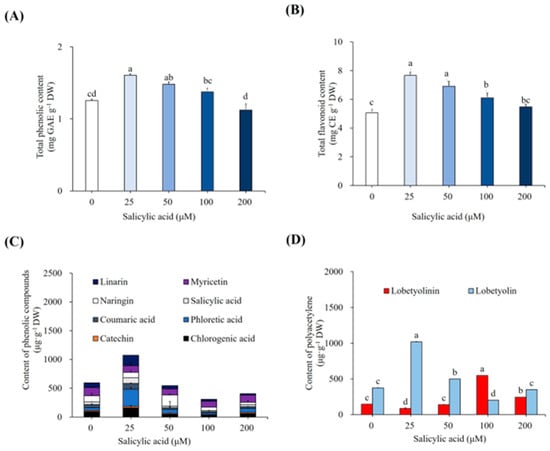
Figure 5.
Effect of SA elicitation on accumulation of bioactive compounds. (A) Total phenolic content, (B) Total flavonoid content, (C) Content of several phenolics as per HPLC analysis, and (D) Content of polyacetylenes as per HPLC analysis. Cultures were elicited after 4 weeks with 0, 25, 50, 100, and 200 µM SA. Values followed by the same letter within the columns are not significantly different at p ≤ 0.05 using DMRT.
Figure 6 shows the antioxidant activity of extracts derived from plants treated with SA. As compared to the extracts of control plants, the extract of 25 µM SA-treated plants showed higher levels of ABTS (97.98%; Figure 6A), DPPH (72.43%; Figure 6B), scavenging activities, and antioxidant power as per FRAP assay (62.90 Fe2+ mg g−1 DW; Figure 6C).
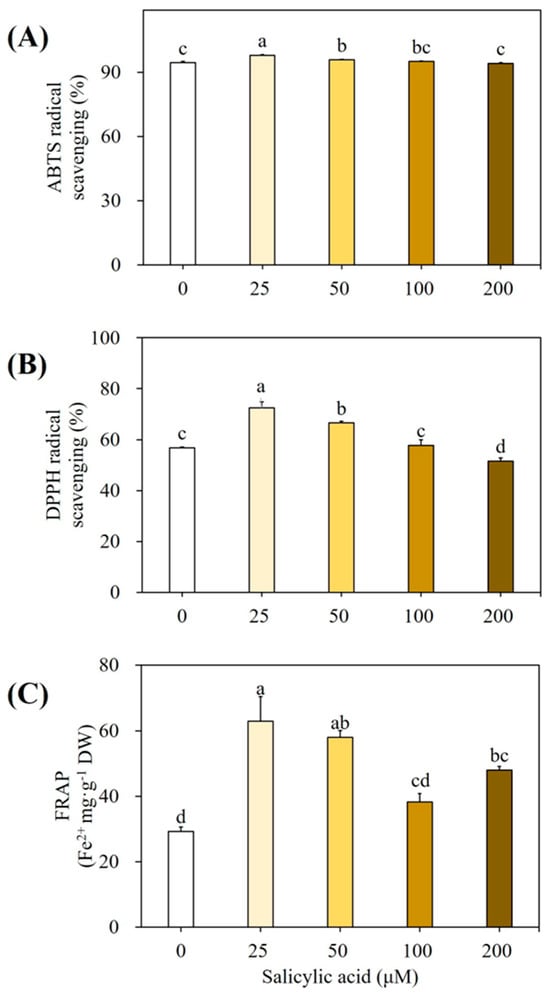
Figure 6.
Antioxidant activity of plantlet extracts elicited with various SA concentrations. (A) ABTS radical scavenging, (B) DPPH radical scavenging, and (C) FRAP activities. Cultures were elicited after 4 weeks with 0, 25, 50, 100, and 200 mg µM SA. Values followed by the same letter within the columns are not significantly different at p ≤ 0.05 using DMRT.
4. Discussion
Plants synthesize and acquire a great number of SMs in order to inhabit extreme environments and interact with other species. These SMs are extremely beneficial to humans as bioactive molecules [12,13]. The accumulation of SMs in wild or cultivated plants is less than 1%, and production is mostly regulated by the plant’s developmental and physiological phases. Furthermore, genetic and environmental factors influence the accumulation of SMs in natural plants [24]. As a result, plant cell, organ, and tissue cultures have been shown to be effective methods for the reliable and widespread synthesis of SMs. A variety of strategies have been used to increase the accumulation of SMs in plant tissue cultures, and elicitation is one of the most effective techniques for SM hyperaccumulation [12,13]. A variety of biotic and abiotic elicitors, such as chitin, pectin, chitosan, cellulose, and yeast extract [12,13], as well as physical stressors like light [25], γ-rays [26], nanoparticles [27], and intracellular signaling molecules like salicylic acid and jasmonic acid [12,13], have been used to enhance bioactive compound accumulation in plant tissue cultures.
4.1. Effect of YE on Growth of Plantlets
YE is a widely used biotic elicitor that has wide applications for large-scale plant and tissue culture systems due to its affordability and accessibility. Since YE is rich in amino acids, nutrients, vitamins, and minerals, it is utilized as a supplement to increase plant growth [28,29]. The amount of YE present in the nutritional medium, however, has varying effects on different plant species. A lower concentration of YE has been shown to be advantageous when added to the medium, while a larger concentration has been shown to hinder growth [30]. YE was added to the cultures of L. chinensis at several doses (25–200 mg L–1); however, the highest biomass accumulation was caused by 50 mg L−1. YE functions as an elicitor, promoting cellular growth, division, and biomass accumulation in in vitro cultures through its abundance in proteins, carbohydrates, amino acids, nucleotides, and several trace elements [28]. In vitro cultures triggered with YE showed increased shoot multiplication and biomass accumulation in several medicinal plants, including Arnica motana [31] and Plumbago indica [32]. A few researchers documented the detrimental impact on biomass production in Curcuma mangga, Glehnia littralis, Thymus lotocephalus, and Knautia sarjevensis [33,34,35,36].
4.2. Effect of YE on Accumulation of SMs and Antioxidant Enzymes
Total phenolics and flavonoids, together with catechin, phloretic acid, naringin, linarin (phenolics), and lobetyolinin and lobetyolin (polyacetylenes), increased in accumulation when L. chinensis plantlet cultures were elicited with 50 mg L−1 YE. These results are similar to those of Laezza et al. [16], who showed that adding 300 and 500 mg L−1 YE to Malus pumila cell suspension cultures increased the concentration of specific phenolics like catechin, phloridzin, chlorogenic acid, and total polyphenols at 100 mg L−1 YE treatment. Likewise, the administration of 100 mg L−1 YE raised the phenolic content in the callus cultures of Ocimum basilicum and the hairy root culture of Aster scaber [37,38]. In tissue cultures of various plant species, the use of YE as an elicitor has also increased a number of bioactive compounds, such as flavonoids in Oplapanax elaus [15], caffeoylquinic acids in Inula crithmoides [39], tanshinones in Perovkia abrotanoides [40], and phenolics in Polygonum multiflorum [41].
Strong antioxidant activity was demonstrated by YE-induced L. chinensis plantlets (50 mg L−1) in the DPPH, ABTS, and FRAP tests. Total phenolics (1.62 mg GAE g−1 DW) and flavonoids (8.46 mg CE g−1 DW) were greater in YE-elicited (50 mg L−1) plantlets, along with catechin (26.73 µg g−1 DW), phloretic acid (88.58 µg g−1 DW), naringin (96.66 µg g−1 DW), and linarin (52.75 µg g−1 DW). The antioxidant capacity of L. chinensis elicited plants is due to these bioactive substances. When elicitors were applied to in vitro plant cultures, oxidative stress was caused. Therefore, they activate both non-enzymatic antioxidants such as phenolic compounds, flavonoid compounds, ascorbic acid, α-tocopherol, and others, as well as enzymatic antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX) [42]. Treatment with 100 mg L−1 YE raised total phenolic levels and enhanced antioxidant activity in Arnica motana shoot cultures grown in vitro [31]. In vitro-grown Glehnia littoralis shoots and roots have shown increased phenyl alanine lyase (PAL) activity and secondary metabolite synthesis as a result of YE treatment [36].
4.3. Effect of SA on Growth of Plantlets
SA, a phenolic molecule found in plants, is known to be a crucial signal in their defense system against both biotic and abiotic stressors [43]. SA is also a well-known plant hormone that affects both in vitro and in vivo plant growth and development [44]. The type of plant and concentration of SA determine how it affects plant growth when administered exogenously [45]. For example, 50 µM SA significantly enhanced the growth of Matricaria chamomilla leaves and roots by 32% and 65%, respectively, whereas 250 µM SA significantly decreased the growth of leaves and roots by 40% and 43%, respectively, according to Kavacik et al. [46]. Fresh and dry biomass levels were increased in L. chinensis plantlet cultures treated with 25 µM SA, whereas biomass levels were lower at higher doses (50, 100, and 200 µM). Applying 100 µM SA to Arnica motana shoot cultures in vitro demonstrated strong growth of plantlets, which is comparable to the current results [31]. In contrast to these findings, Bayraktar et al. [47] found that SA treatment resulted in thinner and shorter shoots and inhibited the growth of Stevia rebaudiana plants grown in vitro.
4.4. Effect of SA on Accumulation of SMs and Antioxidant Enzymes
Larger amounts of total phenolics, total flavonoids, and specific phenolics like linarin, coumaric acid, phloretic acid, chlorogenic acid, catechin, and lobetyolin (polyacetylene) were accumulated in L. chinensis plantlet cultures elicited with 25 µM SA as compared to control cultures. In comparison to non-elicited cultures, plantlet cultures treated with 25 µM SA exhibited significant antioxidant activity as determined by DPPH, ABTS, and FRAP tests. SA is a plant hormone that affects the growth and development of plants, enhances SMs in in vitro cultures of different medicinal plants, and engages in certain signal transduction pathways to stimulate specific enzymes [44]. In vitro cultures of Salvia vulgaris and Salvia officinalis accumulated phenolic compounds as a result of exogenous SA treatment, which increased the expression of phenylalanine ammonia-lyase (PAL), a crucial enzyme in the phenylpropanoid pathway [48]. Following SA treatment, Dong et al. [49] observed increased accumulation of caffeic acid and salvianolic acid B in Salvia miltiorrhiza cell cultures. However, Petrova et al. [31] found that adding SA to Arnica motana plantlet cultures in vitro resulted in a decrease in the accumulation of total flavonoids and total phenolics. Similar findings were noted by Gadzovska et al. [50], who investigated how SA elicitation affected the buildup of phenylpropanoids in suspension cultures of Hypericum perforatum shoots, calluses, and cells.
5. Conclusions
The current study documented elicitor-induced SM production in L. chinensis, and YE at 50 mg L−1 was found to be more advantageous than SA since it led to increased plantlet biomass production and the accumulation of larger amounts of total flavonoids and total phenolics. Increased accumulation of catechin, phloretic acid, naringin, linarin, and lobetyolinin and lobetyolin was caused by YE elicitation (50 mg L−1). As determined by the DPPH, ABTS, and FRAP assays, plantlets induced by YE also showed increased levels of antioxidant activity. Although SA at 25 µM was responsible for the reduction of some phenolic and polyacetylene compounds, it was also responsible for the accumulation of greater levels of total phenolics and total flavonoids. The results of this study could pave the way for further investigation into the increase in potent antioxidant phenolic and polyacetylene chemicals in this valued medicinal plant.
Author Contributions
X.B. conducted the experiments; H.-S.L. and J.-E.H. helped in the analysis; H.N.M. was involved in the analysis of data and writing; and S.-Y.P. was involved in conceptualization, procuring funding, and guiding the students. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Industrial Strategic Technology Development Program (Grant No. P0018148) funded by the Ministry of Trade, Industry and Energy, Republic of Korea.
Data Availability Statement
Raw data are available from So Young Park.
Acknowledgments
Hosakatte Niranjana Murthy is thankful for the “Brain Pool” (Grant No. 2022H1D3A2A02056665) of the National Research Foundation, Republic Korea.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, S. The Ben Cao Gang Mu (Chinese Edition), 1st ed.; University of California Press: Oakland, CA, USA, 2016; p. 265. [Google Scholar]
- Falquitto, D.G.; Swiech, J.N.D.; Pereira, C.B.; Bobek, V.B.; Possagno, G.C.H.; Farago, P.V.; Miguel, M.D.; Durate, J.L.; Miguel, O.G. Biological activity, phytochemistry and traditional uses of genus Lobelia (Campanulaceae): A systematic review. Fitoterapia 2019, 134, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Ho, Y.L.; Huang, G.J.; Chang, Y.S. Anti-oxidative and anti-inflammatory effects of Lobelia chinensis in vitro and in vivo. Am. J. Chin. Med. 2015, 43, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Reddy, N.; Khoo, C.; Koyyalamudi, S.R.; Jone, C.E. Antioxidant and immunomodulatory activities and structural characterization of polysaccharides isolated from Lobelia chinensis Lour. Pharmacologia 2018, 9, 157–168. [Google Scholar] [CrossRef]
- Kuo, P.C.; Hwang, T.L.; Lin, Y.T.; Kuo, Y.C.; Leu, Y.L. Chemical constituents from Lobelia chinensis and their anti-virus and anti-inflammatory bioactivates. Arch. Pham. Res. 2011, 34, 715–722. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, P.; Zhang, X.; Li, X. Chemical structure elucidation of an inulin-type fructan isolated from Lobelia chinensis Lour. with anti-obesity on diet-induced mice. Carbohyr. Polym. 2020, 240, 116357. [Google Scholar] [CrossRef]
- Choi, W.H.; Lee, I.A. The anti-tubercular activity of Melia azaedarach L. and Lobelia chinensis Lour. and their potential as effect anti-Mycobacterium tuberculosis candidate agents. Asian Pac. J. Trop. Biomed. 2016, 6, 830–835. [Google Scholar] [CrossRef]
- Chen, M.S.; Chen, W.R.; Zhang, J.M.; Long, X.Y.; Wang, Y.T. Lobelia chinensis: Chemical constituents and anticancer activity perspective. Chin. J. Nat. Med. 2014, 12, 103–107. [Google Scholar] [CrossRef]
- Bai, X.; Lee, H.S.; Han, E.J.; Murthy, H.N.; Kwon, H.J.; Yeon, S.H.; Park, S.Y. Stimulating the synthesis of phenolics and polyacetylenes in Lobelia chinensis Lour. Plantlets using various bioreactor culture systems: A comparison of parameter effects. Plant Cell Tissue Organ Cult. 2025, 16, 36. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Hahn, J.E.; Lee, H.S.; Paek, K.Y.; Park, S.Y. Suspension culture of somatic embryos for the production of high-value secondary metabolites. Physiol. Mol. Biol. Plants 2023, 29, 1153–1177. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Ramirez-Estrda, K.; Limon, H.V.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cudio, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.R.; Patel, S.R.; Joshi, A.G.; Shrivastava, N.; Sindhav, G.; Sharma, S.; Ansari, H. Elicitor mediated enhancement of shoot biomass and lupeol production in Hemidesmus indicus (L.) R.Br. ex. Schult and Tylophora indica (Burm. F.) Merrill using yeast extract and salicylic acid. Nat. Prod. Res. 2023, 37, 1167–1773. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.Y.; Wang, M.; Wu, X.H.; Fan, M.Z.; Li, H.Z.; Guo, Y.Q.; Jiang, J.; Yin, C.R.; Lian, M.L. Improving flavonoid accumulation of bioreactor-cultured adventitious roots in Oplapanx elatus using yeast extract. Plants 2023, 12, 2174. [Google Scholar] [CrossRef]
- Laezza, C.; Imbimbo, P.; D’Amelia, V.; Marzocchi, A.; Monti, D.M.; di Loria, A.; Monti, S.M.; Novellino, E.; Tenore, G.C.; Rigano, M.M. Use of yeast extract to elicit a pulp-derived callus cultures from Annurca apple and potentiate its biological activity. J. Funct. Foods 2024, 112, 105988. [Google Scholar] [CrossRef]
- Zanella, L.; Gismondi, A.; Di Marco, G.; Barglia, R.; Scuderi, F.; Redi, E.L.; Galgani, A.; Canini, A. Induction of antioxidant metabolites in Moringa oleifera callus by abiotic stresses. J. Nat. Prod. 2019, 82, 23792386. [Google Scholar] [CrossRef]
- Moharramnejad, S.; Azam, A.T.; Panahandeh, J.; Dehghnian, Z.; Ashraf, M. Effect of methyl jasmonate and salicylic acid in vitro growth, sativoside production, and oxidative defense system in Stevia rebaudiana. Sugar Tech. 2019, 21, 1031–1038. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 373–497. [Google Scholar] [CrossRef]
- Harborne, A.J. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 1st ed.; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Burin, V.M.; Arari, S.G.; Costa, L.L.F.; Bordingon-Luiz, A.M.T. Determination of some phenolic compounds in red wind by RP-HPLC: Method development and validation. J. Chromatogr. Sci. 2011, 49, 647–651. [Google Scholar] [CrossRef]
- Qiao, C.F.; He, Z.D.; Han, Q.B.; Hu, H.X.; Jiang, R.W.; Li, S.L.; Zhang, Y.B.; But, P.P.H.; Shaw, P.C. The use of lobetyolin and HPLC-UV fingerprints for quality assessment of radix codonopsis. J. Food Drug Anal. 2007, 15, 258–264. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Jalota, K.; Sharma, V.; Agarwal, C.; Jindal, S. Eco-friendly approaches to phytochemical production: Elicitation and beyond. Nat. Prod. Bioproct. 2024, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Light as an elicitor for enhanced production of secondary metabolites in plant cell, tissue, and organ cultures. Plant Growth Regul. 2024, 104, 31–49. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Production of specialized metabolites in plant cell and organo-cultures: The role of gamma radiation in eliciting secondary metabolites. Int. J. Rad. Biol. 2024, 100, 678–688. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites in cell and organ cultures: Current status and future outlooks. Plant Growth Regul. 2024, 104, 5–30. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast extract: Characteristics, production, applications and future perspectives. J. Micbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Klotz, S.; Kuenz, A.; Prüße, U. Nutritional requirements and the impact of yeast extract on the D-lactic acid production by Sporolactobacillus inulinus. Green Chem. 2017, 19, 4633–4641. [Google Scholar] [CrossRef]
- Rasouli, D.; Werbrouck, S.; Maleki, B.; Jafary, H.; Schurdi-Levraud, V. Elicitor-induced in vitro shoot multiplication and steviol glycosides production in Stevia rebaudiana. S. Afr. J. Bot. 2021, 137, 265–271. [Google Scholar] [CrossRef]
- Petrova, M.; Geneva, M.; Trendafilova, A.; Miladinova-Georgieva, K.; Dimitrova, L.; Sichanowa, M.; Nikolova, M.; Ivanova, V.; Dimitrova, M.; Sozoniuk, M. Antioxidant capacity and accumulation of caffeoylquinic acids in Arnica montana L. in vitro shoots after elicitation with yeast extract or salicylic acid. Plants 2025, 14, 967. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Ruangnoo, S.; Sangmukdee, K.; Chamchusri, K.; Rithichai, P. Enhancement of plumbagin production through elicitation in in vitro-regenerated shoots of Plumbago indica L. Plants 2024, 13, 1450. [Google Scholar] [CrossRef]
- Abraham, F.; Bhatt, A.; Keng, C.L.; Indrayanto, G.; Sulaiman, S.F. Effect of yeast extract and chitosan on shoot proliferation, morphology and antioxidant activity of Curcuma mangga in vitro plantlets. Afr. J. Biotechnol. 2011, 10, 7787–7795. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; SantínE, P.; Coelho, N.; Romano, A. Elicitation improves rosmarinic acid content and antioxidant activity in Thymus lotocephalus shoot cultures. Ind. Crops Prod. 2019, 137, 214–220. [Google Scholar] [CrossRef]
- Karalija, E.; Zeljkovic’, S.C.; Paric’, A. Harvest time-related changes in biomass, phenolics and antioxidant potential in Knautia sarajevensis shoot cultures after elicitation with salicylic acid and yeast. In Vitro Cell Dev. Biol. Plant 2019, 56, 177–183. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kitamura, Y.; Ozeki, Y.; Watanabe, M. Different responses of shoot and root cultures of Glehnia littoralis to yeast extract. J. Nat. Med. 2006, 61, 30–37. [Google Scholar] [CrossRef]
- Zaman, G.; Farooq, U.; Bajwa, M.N.; Jan, H.; Shah, M.; Ahmad, R.; Andleeb, A.; Drouet, S.; Hano, C.; Abbasi, B.H. Effects of yeast extract on the production of phenylpropanoid metabolites in callus culture of purple basil (Ocimum basilicum L. var purpurascens) and their in-vitro evaluation for antioxidant potential. Plant Cell Tissue Organ Cult. 2022, 150, 543–553. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Thiruvengadam, M.; Chung, I.M. Identification of elicitors enhances the polyphenolic compounds and pharmacological potential in hairy root cultures of Aster scaber. S. Afr. J. Bot. 2019, 125, 92–101. [Google Scholar] [CrossRef]
- Custodio, L.; Cziaky, Z.; Castaneda-Loaiza, V.; Rodrigues, M.J. Establishment and elicitation of liquid adventitious root cultures of Inula crithmoides L. for increased caffeoylquinic acid production and hepatoprotective properties. Plant Cell Tissue Organ Cult. 2024, 156, 59. [Google Scholar] [CrossRef]
- Zaker, A.; Sykora, C.; Gossnitzer, F.; Abrishamchi, P.; Aili, J.; Mousavi, S.H.; Wawrosch, C. Effects of some elicitors on tanshinone production in adventitious root cultures of Perovskia arbrotanoides Karel. Ind. Crops Prod. 2015, 67, 97–102. [Google Scholar] [CrossRef]
- Ho, T.T.; Lee, J.D.; Jeong, C.S.; Paek, K.Y.; Park, K.Y. Improvement of biosynthesis and accumulation of bioactive compounds by elicitation in adventitious root cultures of Polygonum multiflorum. Appl. Microbiol. Biotechnol. 2018, 102, 199–209. [Google Scholar] [CrossRef]
- Moharramnejad, S.; Sofalian, O.; Valizadeh, M.; Asgari, A.; Shiri, M. Response of antioxidant defense system to osmotic stress in maize seedlings. Fresenius Environ. Bull. 2016, 25, 805–811. [Google Scholar]
- Zhao, P.; Lu, G.H.; Yang, Y.H. Salicylic acid signaling and its role in responses to stresses in plants. Mech. Plant Horm. Signal Under Stress 2017, 39, 413–441. [Google Scholar]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agric. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]
- Li, A.; Sun, X.; Liu, L. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef]
- Kováčik, J.; Grúz, J.; Bačkor, M.; Strnad, M.; Repčák, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef]
- Bayraktar, M.; Naziri, E.; Akgun, I.H.; Karabey, F.; Ilhan, E.; Akyol, B.; Gurel, A. Elicitor induced stevioside production, in vitro shoot growth, and biomass accumulation in micropropagated Stevia rebaudiana. Plant Cell Tissue Organ Cult. 2016, 127, 289–300. [Google Scholar] [CrossRef]
- Ejtahed, R.; Radjabian, T.; Hoseini Tafreshi, S.A. Expression analysis of phenylalanine ammonia lyase gene and rosmarinic acid production in Salvia officinalis and Salvia virgata shoots under salicylic acid elicitation. Appl. Biochem. Biotechnol. 2015, 176, 1846–1858. [Google Scholar] [CrossRef]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotech. 2010, 148, 99–104. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Hagège, D.; Courtois, D.; Joseph, C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2012, 113, 25–39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).