Effect of Biostimulants on Drought Tolerance of Greenhouse-Grown Tomato
Abstract
1. Introduction
2. Materials and Methods
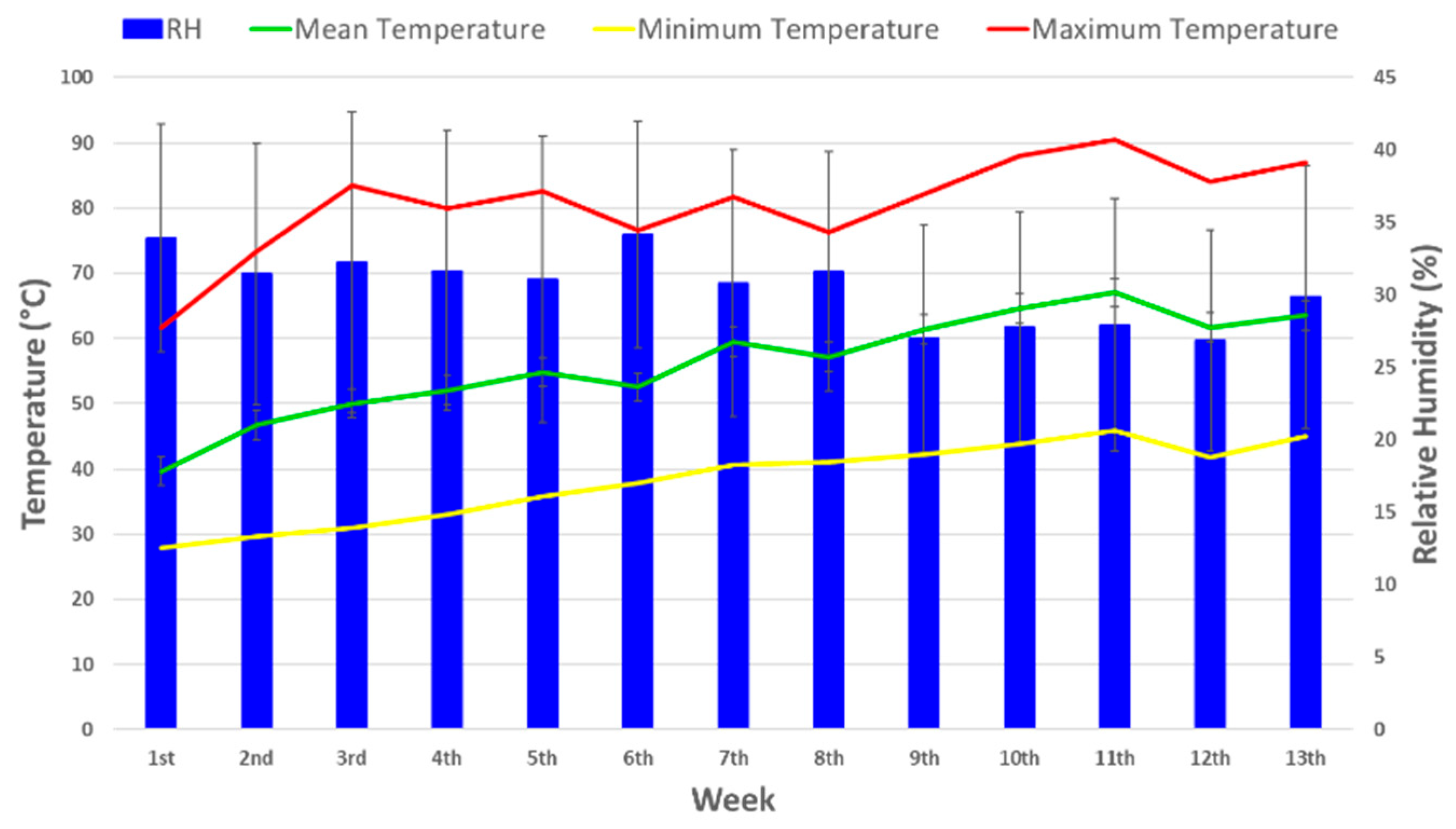
2.1. Plant Material, Greenhouse Experiment, and Drought Stress Treatment
2.2. Evaluated Features
2.2.1. Plant Growth and Physiology Assessments
2.2.2. Fruit Yield and Quality Assessments
2.2.3. Sample Extraction, Fruit Bioactive Compound Assessments, and Antioxidant Activity
- Sample extraction
- Total Phenolic Content (TPC)
- Total Flavonoid Content (TFC)
- Antioxidant activity
2.3. Statistical Analysis
3. Results and Discussion
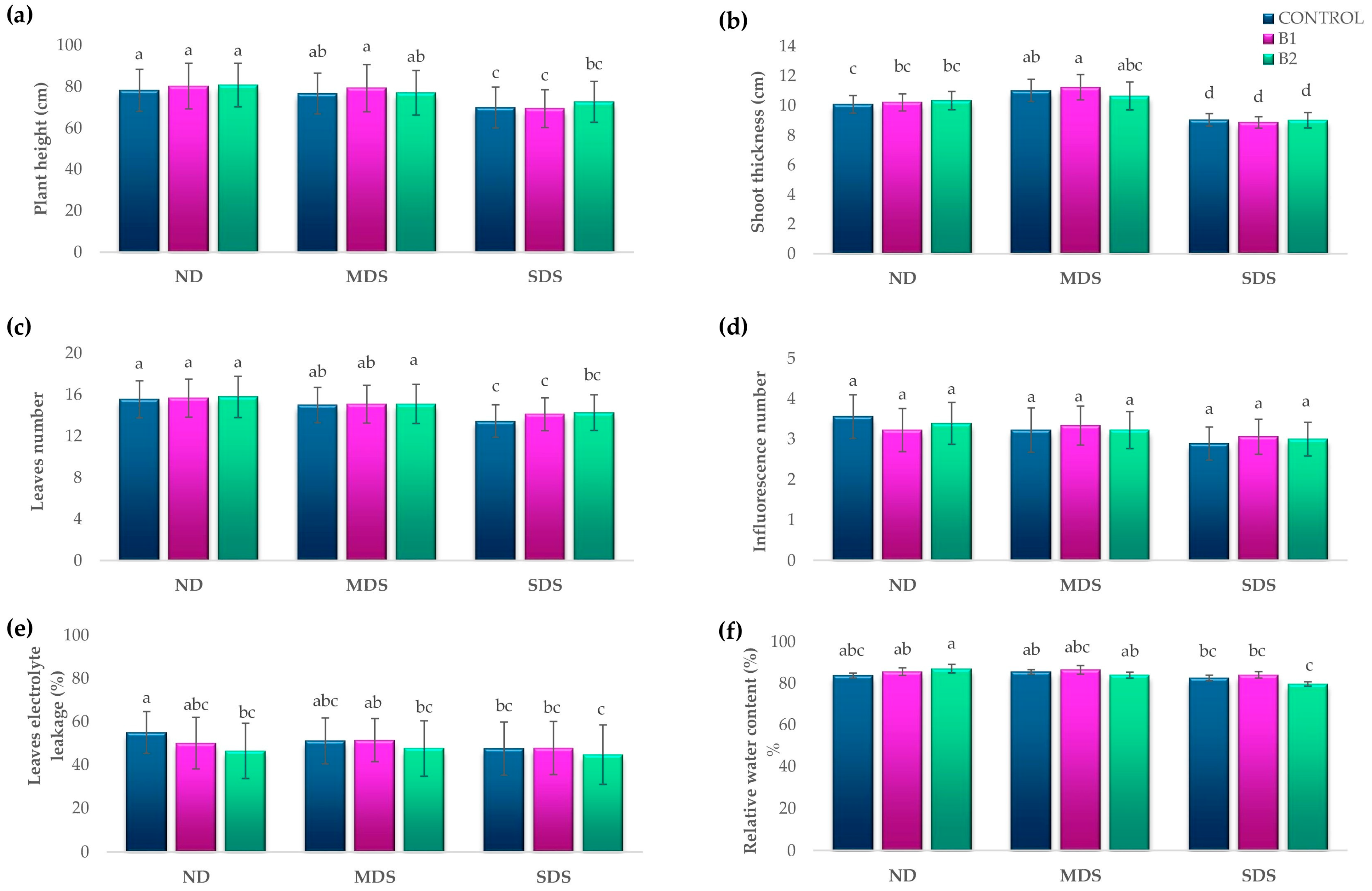
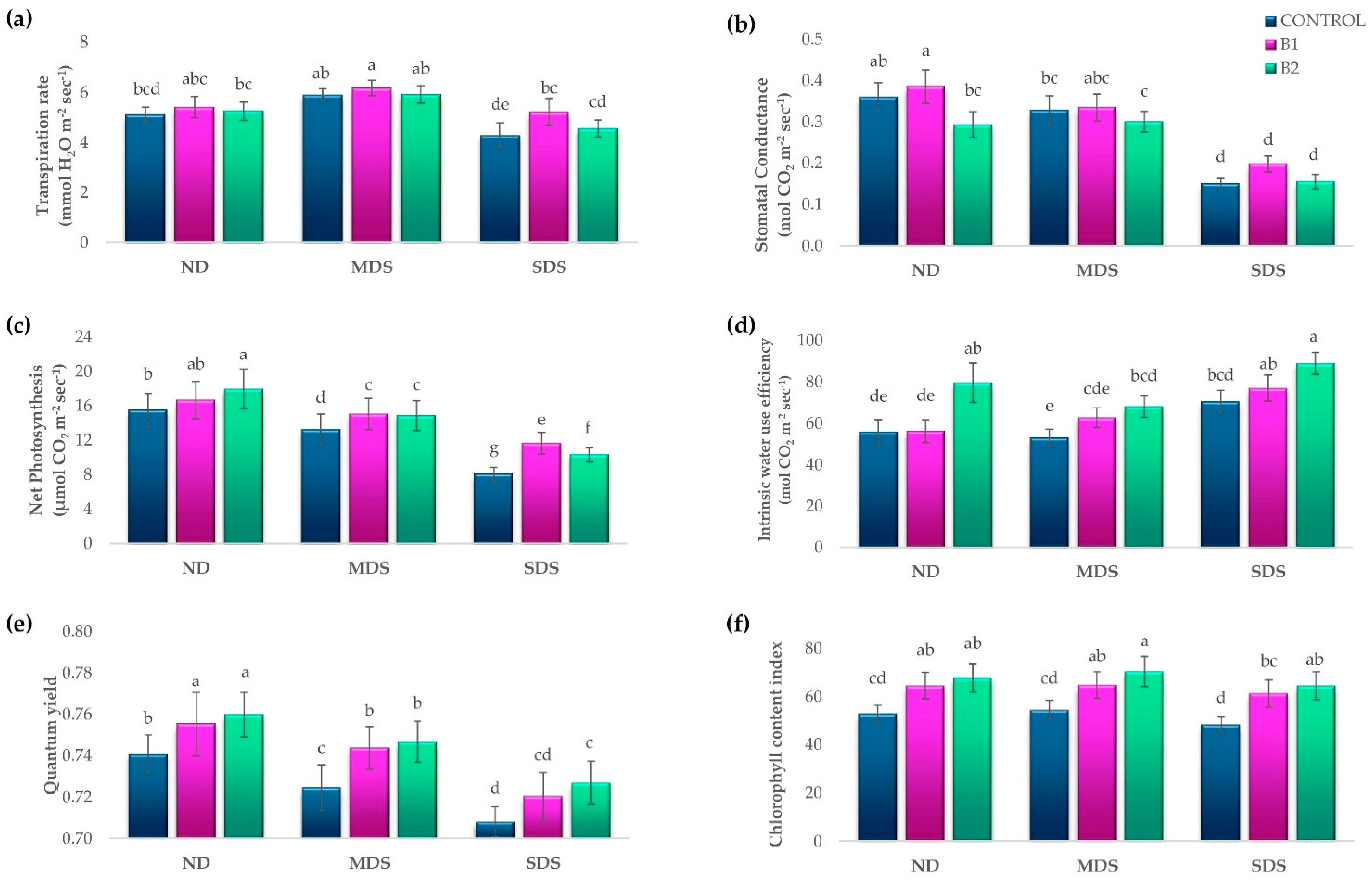
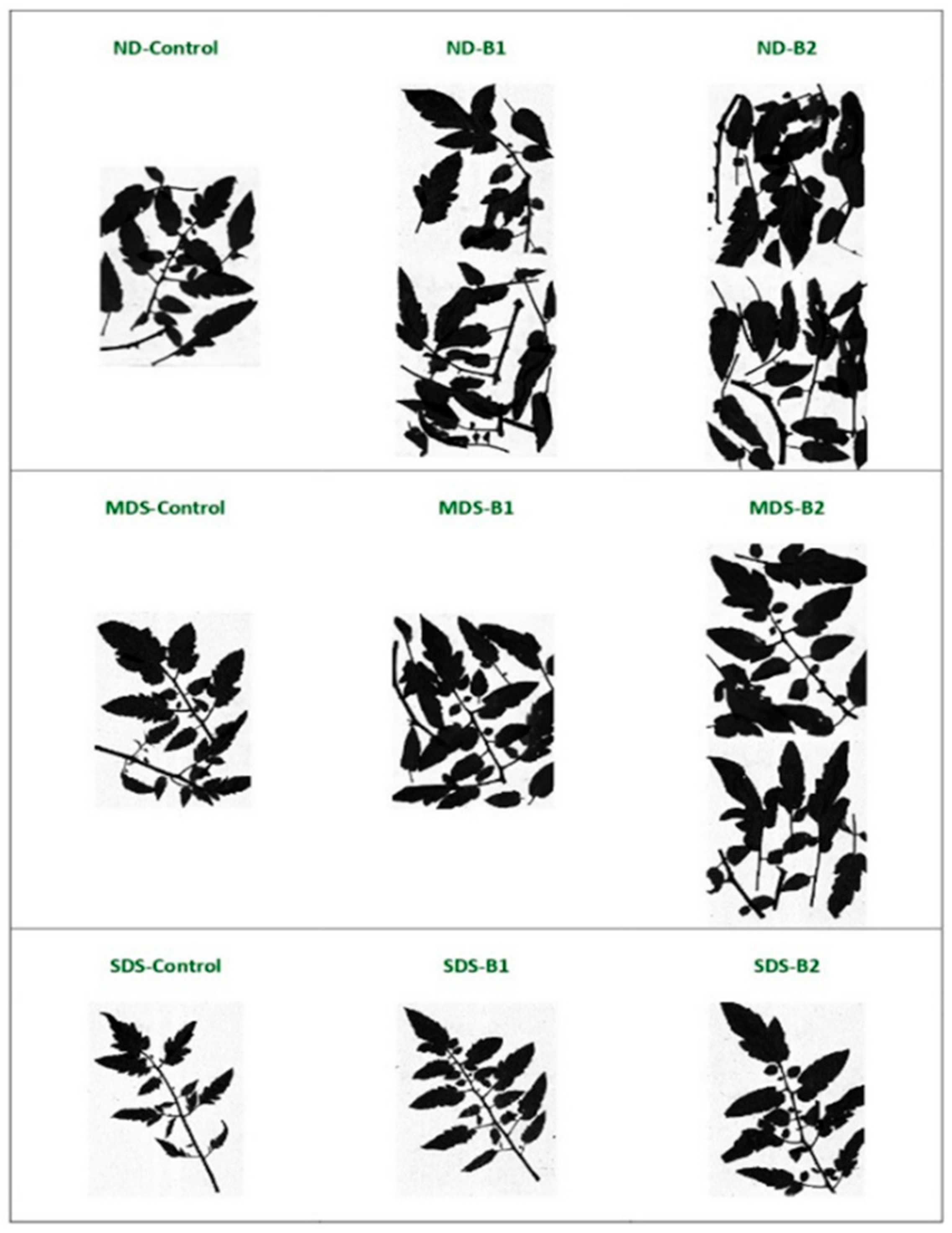
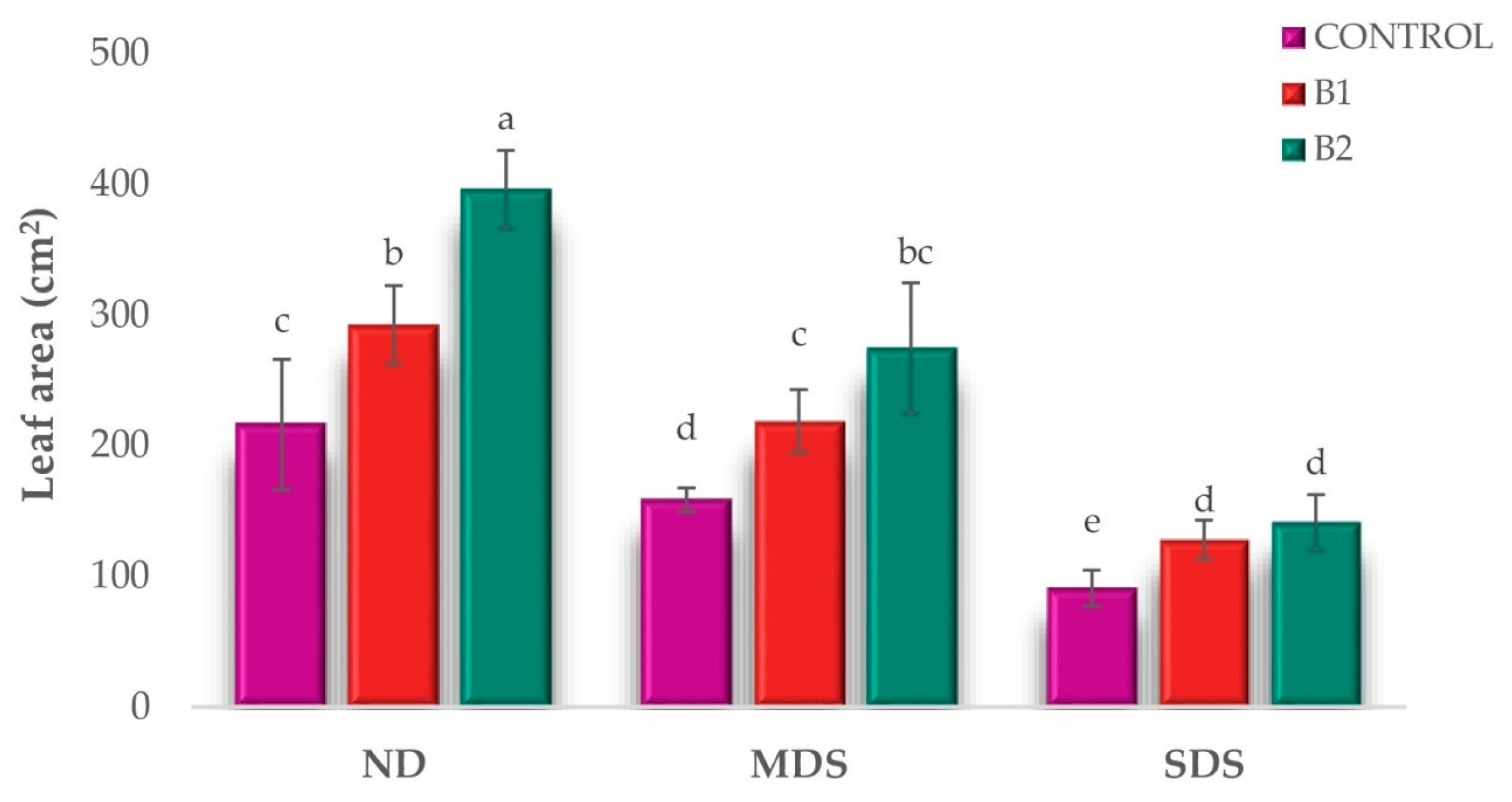
3.1. Effects of Biostimulant Treatments on Tomato Growth and Physiology Under Drought Stress
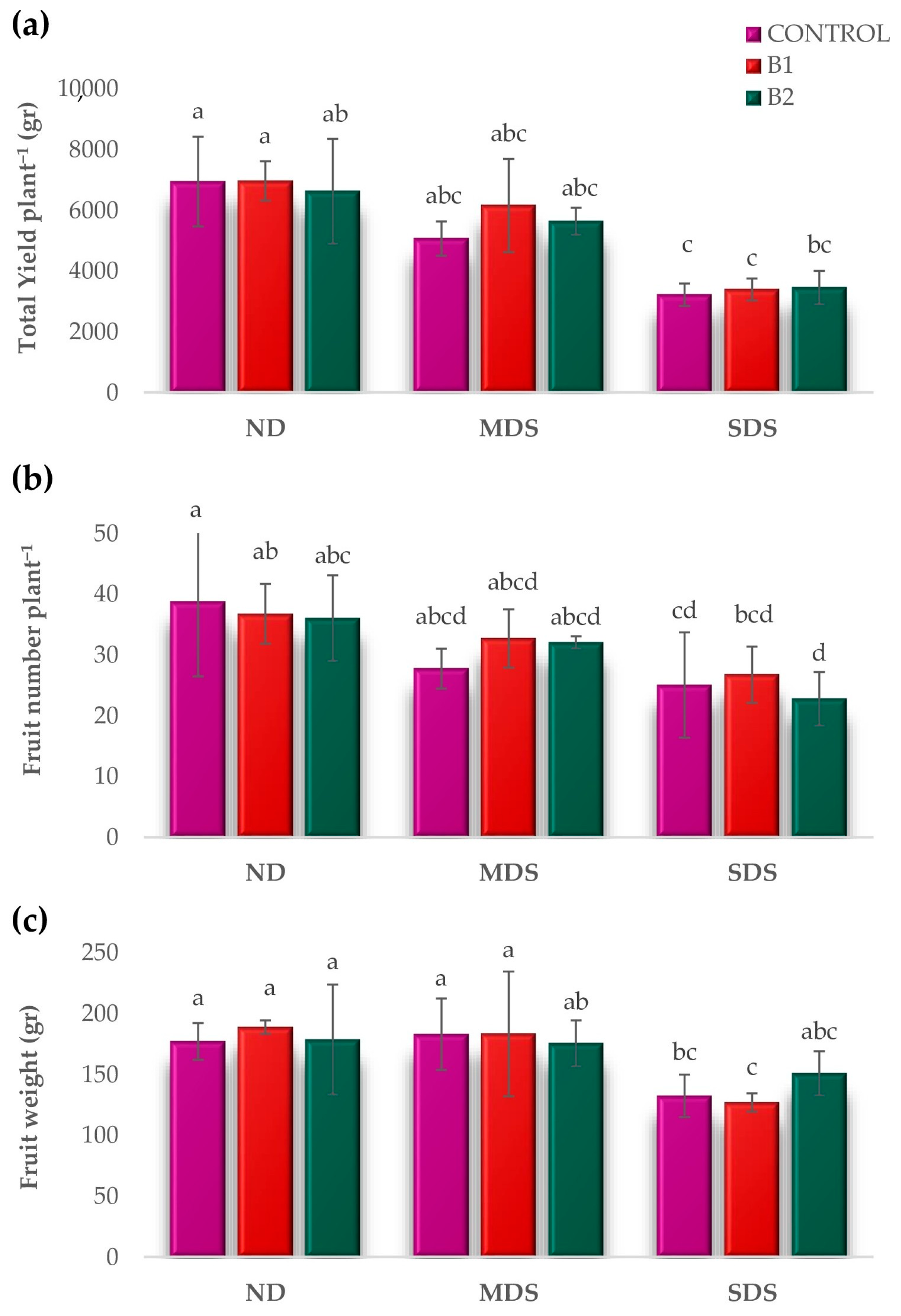
3.2. Effects of Biostimulant Treatments on Fruit Yield and Quality Indices Under Drought Stress
3.3. Effect of Biostimulant Treatments on Bioactive Compounds and on Antioxidant Activity of Tomato Fruits Under Drought Stress
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- FAOSTAT. The Food and Agriculture Organization (FAO). 2022. Available online: http://www.fao.org/faostat/ (accessed on 18 February 2025).
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of Sugars and Organic Acids in Tomato Fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Kurina, A.B.; Solovieva, A.E.; Khrapalova, I.A.; Artemyeva, A.M. Biochemical Composition of Tomato Fruits of Various Colors. Vavilovskii Zhurnal Genet. Sel. 2021, 25, 514–527. [Google Scholar] [CrossRef]
- Francaviglia, R.; Di Bene, C. Deficit Drip Irrigation in Processing Tomato Production in the Mediterranean Basin. A Data Analysis for Italy. Agriculture 2019, 9, 79. [Google Scholar] [CrossRef]
- Liava, V.; Chaski, C.; Añibarro-Ortega, M.; Pereira, A.; Pinela, J.; Barros, L.; Petropoulos, S.A. The Effect of Biostimulants on Fruit Quality of Processing Tomato Grown under Deficit Irrigation. Horticulturae 2023, 9, 1184. [Google Scholar] [CrossRef]
- Fernandes, Â.; Chaski, C.; Pereira, C.; Kostić, M.; Rouphael, Y.; Soković, M.; Barros, L.; Petropoulos, S.A. Water Stress Alleviation Effects of Biostimulants on Greenhouse-Grown Tomato Fruit. Horticulturae 2022, 8, 645. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Argin, S.; Brinza, M.; Yildirim, E. Drought Stress Amelioration in Tomato (Solanum lycopersicum L.) Seedlings by Biostimulant as Regenerative Agent. Front. Plant Sci. 2023, 14, 1211210. [Google Scholar] [CrossRef]
- Kumar, R.; Berwal, M.K. Morphological, Physiological, Biochemical and Molecular Facet of Drought Stress in Horticultural Crops. IJBSM 2019, 10, 545–560. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum Extract Biostimulants and Their Role in Enhancing Tolerance to Drought Stress in Tomato Plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Conti, V.; Romi, M.; Guarnieri, M.; Cantini, C.; Cai, G. Italian Tomato Cultivars under Drought Stress Show Different Content of Bioactives in Pulp and Peel of Fruits. Foods 2022, 11, 270. [Google Scholar] [CrossRef]
- Damalas, C.A. Improving Drought Tolerance in Sweet Basil (Ocimum basilicum) with Salicylic Acid. Sci. Hortic. 2019, 246, 360–365. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Hanaka, A.; Ozimek, E.; Reszczyńska, E.; Jaroszuk-Ściseł, J.; Stolarz, M. Plant Tolerance to Drought Stress in the Presence of Supporting Bacteria and Fungi: An Efficient Strategy in Horticulture. Horticulturae 2021, 7, 390. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009. Available online: https://data.europa.eu/eli/reg/2019/1009/oj (accessed on 20 November 2024).
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Gil-Ortiz, R.; Naranjo, M.Á.; Atares, S.; Vicente, O. Antioxidant Responses of Water-Stressed Cherry Tomato Plants to Natural Biostimulants. Agronomy 2023, 13, 2314. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Sánchez-Hernández, C.V.; Palmeros-Suárez, P.A.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Meza-Canales, I.D.; Becerril-Espinosa, A. Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress. Agronomy 2022, 12, 2495. [Google Scholar] [CrossRef]
- Sariñana-Aldaco, O.; Benavides-Mendoza, A.; Robledo-Olivo, A.; González-Morales, S. The Biostimulant Effect of Hydroalcoholic Extracts of Sargassum spp. in Tomato Seedlings under Salt Stress. Plants 2022, 11, 3180. [Google Scholar] [CrossRef]
- Kocira, A.; Lamorska, J.; Kornas, R.; Nowosad, N.; Tomaszewska, M.; Leszczyńska, D.; Kozłowicz, K.; Tabor, S. Changes in Biochemistry and Yield in Response to Biostimulants Applied in Bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 189. [Google Scholar] [CrossRef]
- Consentino, B.B.; Vultaggio, L.; Sabatino, L.; Ntatsi, G.; Rouphael, Y.; Bondì, C.; De Pasquale, C.; Guarino, V.; Iacuzzi, N.; Capodici, G.; et al. Combined Effects of Biostimulants, N Level and Drought Stress on Yield, Quality and Physiology of Greenhouse-Grown Basil. Plant Stress 2023, 10, 100268. [Google Scholar] [CrossRef]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing Chlorophyll Fluorescence for Phenotyping Analysis of Wild and Cultivated Tomato for High Photochemical Efficiency under Water Deficit for Climate Change Resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of Water-Deficit Stress and Paclobutrazol on Growth, Relative Water Content, Electrolyte Leakage, Proline Content and Some Antioxidant Changes in Curcuma alismatifolia Gagnep. Cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Kadoglidou, K.; Xanthopoulou, A.; Kalyvas, A.; Mellidou, I. Utilization of Tomato Landraces to Improve Seedling Performance under Salt Stress. Stresses 2021, 1, 238–252. [Google Scholar] [CrossRef]
- Liang, J.; Krauss, K.W.; Finnigan, J.; Stuart-Williams, H.; Farquhar, G.D.; Ball, M.C. Linking Water Use Efficiency with Water Use Strategy from Leaves to Communities. New Phytol. 2023, 240, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Kadoglidou, K.; Kalaitzidis, A.; Stavrakoudis, D.; Mygdalia, A.; Katsantonis, D. A Novel Compost for Rice Cultivation Developed by Rice Industrial By-Products to Serve Circular Economy. Agronomy 2019, 9, 553. [Google Scholar] [CrossRef]
- Kadoglidou, K.I.; Krommydas, K.; Ralli, P.; Mellidou, I.; Kalyvas, A.; Irakli, M. Assessing Physicochemical Parameters, Bioactive Profile and Antioxidant Status of Different Fruit Parts of Greek Eggplant Germplasm. Horticulturae 2022, 8, 1113. [Google Scholar] [CrossRef]
- Singleton, V. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, Flavonols, and Free Radical Scavenging Activity of Chinese Bayberry (Myrica rubra) Extracts and Their Color Properties and Stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ntinas, G.K.; Kadoglidou, K.; Tsivelika, N.; Krommydas, K.; Kalivas, A.; Ralli, P.; Irakli, M. Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material. Horticulturae 2019, 5, 42. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Kadoglidou, K.I.; Maliachovas, C.; Ralli, P.; Irakli, M.; Tsivelika, N.; Mavromatis, A.G.; Chatzopoulou, P. Integrated Characterization of Greek Fennel Genotypes through Morpho-Agronomical Characteristics, Yield Components and Phytochemical Compounds. JSFA Rep. 2025, 5, 91–103. [Google Scholar] [CrossRef]
- Sudiro, C.; Guglielmi, F.; Hochart, M.; Senizza, B.; Zhang, L.; Lucini, L.; Altissimo, A. A Phenomics and Metabolomics Investigation on the Modulation of Drought Stress by a Biostimulant Plant Extract in Tomato (Solanum lycopersicum). Agronomy 2022, 12, 764. [Google Scholar] [CrossRef]
- Showemimo, F.A.; Olarewaju, J.D. Drought tolerance indices in sweet pepper (Capsicum annuum L.). Int. J. Plant Breed. Genet. 2007, 1, 29–33. [Google Scholar] [CrossRef]
- Earl, H.J.; Davis, R.F. Effect of Drought Stress on Leaf and Whole Canopy Radiation Use Efficiency and Yield of Maize. Agron. J. 2003, 95, 688–696. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought Stress Had a Predominant Effect over Heat Stress on Three Tomato Cultivars Subjected to Combined Stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A Biostimulant Based on Seaweed (Ascophyllum nodosum and Laminaria digitata) and Yeast Extracts Mitigates Water Stress Effects on Tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Samarah, N.; Mullen, R.; Cianzio, S. Size Distribution and Mineral Nutrients of Soybean Seeds in Response to Drought Stress. J. Plant Nutr. 2004, 27, 815–835. [Google Scholar] [CrossRef]
- Top, S.; Vandoorne, B.; Pauwels, E.; Perneel, M.; Van Labeke, M.-C.; Steppe, K. Plant Sensors Untangle the Water-Use and Growth Effects of Selected Seaweed-Derived Biostimulants on Drought-Stressed Tomato Plants (Solanum lycopersicum). J. Plant Growth Regul. 2023, 42, 5615–5627. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Effects of NAA and Ecklonia Maxima Extracts on Lettuce and Tomato Transplant Production. Agronomy 2022, 12, 329. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Khan, M.N.; Mukherjee, S.; Alamri, S.; Basahi, R.A.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.M.A.; Ali, H.M.; Almohisen, I.A.A. Hydrogen Sulfide (H2S) and Potassium (K+) Synergistically Induce Drought Stress Tolerance through Regulation of H+-ATPase Activity, Sugar Metabolism, and Antioxidative Defense in Tomato Seedlings. Plant Cell Rep. 2021, 40, 1543–1564. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Agrawal, V.; Gupta, S.C. Comparison of Drought-Induced Polypeptides and Ion Leakage in Three Tomato Cultivars. Biol. Plant. 2009, 53, 685–690. [Google Scholar] [CrossRef]
- Sousaraei, N.; Mashayekhi, K.; Mousavizadeh, S.J.; Akbarpour, V.; Medina, J.; Aliniaeifard, S. Screening of Tomato Landraces for Drought Tolerance Based on Growth and Chlorophyll Fluorescence Analyses. Hortic. Environ. Biotechnol. 2021, 62, 521–535. [Google Scholar] [CrossRef]
- Hamedeh, H.; Antoni, S.; Cocciaglia, L.; Ciccolini, V. Molecular and Physiological Effects of Magnesium–Polyphenolic Compound as Biostimulant in Drought Stress Mitigation in Tomato. Plants 2022, 11, 586. [Google Scholar] [CrossRef]
- Senousy, H.H.; Hamoud, Y.A.; Abu-Elsaoud, A.M.; Mahmoud Al zoubi, O.; Abdelbaky, N.F.; Zia-ur-Rehman, M.; Usman, M.; Soliman, M.H. Algal Bio-Stimulants Enhance Salt Tolerance in Common Bean: Dissecting Morphological, Physiological, and Genetic Mechanisms for Stress Adaptation. Plants 2023, 12, 3714. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, H.; Himanshu, S.K.; García-Caparrós, P.; Tisarum, R.; Cha-um, S.; Datta, A. Ascophyllum nodosum Seaweed Extract and Potassium Alleviate Drought Damage in Tomato by Improving Plant Water Relations, Photosynthetic Performance, and Stomatal Function. J. Appl. Phycol. 2024, 36, 2255–2268. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A. Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Drought. Horticulturae 2022, 8, 1177. [Google Scholar] [CrossRef]
- Zivcak, M.; Kalaji, H.M.; Shao, H.-B.; Olsovska, K.; Brestic, M. Photosynthetic Proton and Electron Transport in Wheat Leaves under Prolonged Moderate Drought Stress. J. Photochem. Photobiol. B Biol. 2014, 137, 107–115. [Google Scholar] [CrossRef]
- Shabbir, A.; Mao, H.; Ullah, I.; Buttar, N.A.; Ajmal, M.; Lakhiar, I.A. Effects of Drip Irrigation Emitter Density with Various Irrigation Levels on Physiological Parameters, Root, Yield, and Quality of Cherry Tomato. Agronomy 2020, 10, 1685. [Google Scholar] [CrossRef]
- Ben Sedrine, I.; Werghi, S.; Hachef, A.; Maalaoui, A.; Zarkouna, R.; Akriche, S.; Hannachi, H.; Zehdi, S.; Fakhfakh, H.; Gorsane, F. Alleviation of Drought Stress in Tomato by Foliar Application of Seafood Waste Extract. Sci. Rep. 2024, 14, 30572. [Google Scholar] [CrossRef] [PubMed]
- Covașă, M.; Slabu, C.; Marta, A.E.; Ostaci, Ș.; Jităreanu, C.D. Impact of the Biostimulants Algevit and Razormin on the Salinity Tolerance of Two Tomato Cultivars. Agronomy 2025, 15, 352. [Google Scholar] [CrossRef]
- Francesca, S.; Raimondi, G.; Cirillo, V.; Maggio, A.; Barone, A.; Rigano, M.M. A Novel Plant-Based Biostimulant Improves Plant Performances under Drought Stress in Tomato. Biol. Life Sci. Forum 2021, 4, 52. [Google Scholar] [CrossRef]
- Della Lucia, M.C.; Baghdadi, A.; Mangione, F.; Borella, M.; Zegada-Lizarazu, W.; Ravi, S.; Deb, S.; Broccanello, C.; Concheri, G.; Monti, A.; et al. Transcriptional and Physiological Analyses to Assess the Effects of a Novel Biostimulant in Tomato. Front. Plant Sci. 2022, 12, 781993. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.-J. Effects of Plant-Derived Protein Hydrolysates on Yield, Quality, and Nitrogen Use Efficiency of Greenhouse Grown Lettuce and Tomato. Agronomy 2022, 12, 1018. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.-K. Methods and Concepts in Quantifying Resistance to Drought, Salt and Freezing, Abiotic Stresses That Affect Plant Water Status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Mishra, K.B.; Iannacone, R.; Petrozza, A.; Mishra, A.; Armentano, N.; La Vecchia, G.; Trtílek, M.; Cellini, F.; Nedbal, L. Engineered Drought Tolerance in Tomato Plants Is Reflected in Chlorophyll Fluorescence Emission. Plant Sci. 2012, 182, 79–86. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhöh, O.; et al. Evaluating Potential of Green Alga Chlorella vulgaris to Accumulate Phosphorus and to Fertilize Nutrient-Poor Soil Substrates for Crop Plants. J. Appl. Phycol. 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of Biostimulants in Tomato Plants (Solanum lycopersicum) to Enhance Plant Growth and Salt Stress Tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Pellegrino, A.; Saita, A.; Calcagno, S.; Cosentino, S.L.; Scandurra, A.; Cafaro, V. A Study on the Effect of Biostimulant Application on Yield and Quality of Tomato under Long-Lasting Water Stress Conditions. Heliyon 2025, 11, e41187. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; El-Nakhel, C.; Fiorentino, N.; Pelosi, M.E.; Rouphael, Y.; Mori, M. Impact of Salinity and Biostimulants on Cherry Tomato Yield and Quality. Horticulturae 2024, 10, 1239. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Ntanasi, T.; Karavidas, I.; Spyrou, G.P.; Giannothanasis, E.; Aliferis, K.A.; Saitanis, C.; Fotopoulos, V.; Sabatino, L.; Savvas, D.; Ntatsi, G. Plant Biostimulants Enhance Tomato Resilience to Salinity Stress: Insights from Two Greek Landraces. Plants 2024, 13, 1404. [Google Scholar] [CrossRef]
- Bai, C.; Zuo, J.; Watkins, C.B.; Wang, Q.; Liang, H.; Zheng, Y.; Liu, M.; Ji, Y. Sugar Accumulation and Fruit Quality of Tomatoes under Water Deficit Irrigation. Postharvest Biol. Technol. 2023, 195, 112112. [Google Scholar] [CrossRef]
- Distefano, M.; Steingass, C.B.; Leonardi, C.; Giuffrida, F.; Schweiggert, R.; Mauro, R.P. Effects of a Plant-Derived Biostimulant Application on Quality and Functional Traits of Greenhouse Cherry Tomato Cultivars. Food Res. Int. 2022, 157, 111218. [Google Scholar] [CrossRef]
- Iacuzzi, N.; Tuttolomondo, T.; Farruggia, D.; Tortorici, N.; Alaimo, F.; De Santis, D.; Rossini, F.; Di Miceli, G. A Two-Year Evaluation of Biostimulant Effects on Yield and Quality Parameters of Tomato Landrace ‘Pizzutello Delle Valli Ericine’ Cultivated Without Irrigation. J. Sustain. Agric. Environ. 2024, 3, 1–11. [Google Scholar] [CrossRef]
| Mean Squares | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variance | df 2 | PH | ST | LN | RWC | E | Gs | Anet | iWUE | QY | CCI |
| Drought Stress (A) | 2 | 616.213 *** | 27.522 *** | 20.783 *** | 107.111 ** | 11.720 *** | 0.252 *** | 308.267 *** | 2426.111 *** | 0.008 *** | 184.922 ns |
| Biostimulant (B) | 2 | 25.383 ns | 0.079 ns | 1.062 ns | 0.929 ns | 1.792 ** | 0.021 ** | 42.175 *** | 2623.597 *** | 0.003 *** | 1795.123 *** |
| A × B | 4 | 18.114 ns | 0.478 ns | 0.386 ns | 29.138 ns | 0.334 ns | 0.004 ns | 5.585 ns | 187.580 ns | 0.001 ns | 6.491 ns |
| Error (a) | 16 | 30.310 | 0.649 | 0.707 | 16.153 | 0.269 | 0.003 | 1.977 | 182.461 | 0.001 | 80.522 |
| Repeated Measures (R) | 2 | 33,498.402 *** | 96.499 *** | 998.799 *** | 67.438 ns | 26.196 *** | 0.164 | 776.342 *** | 4644.685 *** | 0.025 *** | 7051.345 *** |
| A × R | 4 | 30.967 ns | 13.550 *** | 2.127 ** | 18.668 ns | 6.080 *** | 0.009 *** | 39.457 *** | 610.989 * | 0.001 ns | 70.280 ns |
| B × R | 4 | 10.671 ns | 0.347 ns | 1.254 * | 38.340 ns | 0.125 ns | 0.005 ** | 1.839 ns | 92.010 ns | 0.001 ns | 137.311 ** |
| A × B × R | 8 | 24.553 ns | 0.274 ns | 0.069 ns | 16.192 ns | 0.394 ns | 0.001 ns | 2.280 ns | 65.457 ns | 0.001 ns | 15.001 |
| Error (b) | 36 | 14.265 | 0.324 | 0.367 | 21.429 | 0.232 | 0.002 | 2.558 | 210.243 | 0.001 | 27.280 |
| CV 1 (%) | 4.98 | 5.66 | 4.07 | 5.53 | 9.07 | 17.48 | 11.68 | 21.35 | 2.76 | 8.58 | |
| Mean Squares | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source of Variance | df 2 | IN | LEL | pH | °Brix | df | Yield Plant−1 | Fruit Number Plant−1 | Mean Fruit Weight |
| Drought Stress (A) | 2 | 0.778 * | 77.278 ** | 0.009 ns | 1.292 * | 2 | 4,712,219.614 *** | 57.265 ** | 21,686.056 ** |
| Biostimulant (B) | 2 | 0.002 ns | 115.343 *** | 0.032 ns | 0.510 ns | 2 | 69,535.247 ns | 1.396 ns | 531.429 ns |
| A × B | 4 | 0.116 ns | 19.175 ns | 0.004 ns | 0.068 ns | 4 | 51,786.231 ns | 2.610 ns | 2581.156 ns |
| Error (a) | 16 | 0.192 | 10.188 | 0.022 | 0.345 | 16 | 428,976.273 | 5.410 | 2841.926 |
| Rep. Measures (R) | 1 | 89.063 *** | 56,194.985 *** | 0.001 ns | 0.980 ns | 5 | 8,197,620.232 *** | 263.670 *** | 69,112.120 *** |
| A × R | 2 | 0.403 * | 100.509 ** | 0.023 ns | 0.518 ns | 10 | 879528.970 * | 16.668 * | 6838.995 * |
| B × R | 2 | 0.040 ns | 185.702 *** | 0.001 ns | 0.221 ns | 10 | 191,615.620 ns | 11.365 ns | 1131.876 ns |
| A × B × R | 4 | 0.036 ns | 30.305 ns | 0.013 ns | 1.532 ns | 20 | 200,952.422 ns | 7.299 ns | 1229.603 ns |
| Error (b) | 18 | 0.102 | 11.582 | 0.034 | 4.932 | 90 | 368,658.576 | 8.256 | 3263.665 |
| CV 1 (%) | 9.97 | 6.96 | 4.81 | 11.56 | 13.06 | 15.69 | 14.09 | ||
| Drought Level | Biostimulant Treatment | pH ± SE | °Brix ± SE | TPC (mg GAE 100g−1) ± SE | TFC (mg CE 100g−1) ± SE | ABTS (mg TE g−1) ± SE | DPPH (mg TE g−1) ± SE | FRAP (mg TE g−1) ± SE |
|---|---|---|---|---|---|---|---|---|
| ND | C | 3.79 ± 0.03 a | 4.36 ± 0.17 ab | 435.32 ± 13 a | 41.47 ± 6.05 a | 7.32 ± 2.18 a | 4.35 ± 0.69 a | 6.23 ± 0.60 ab |
| Β1 | 3.88 ± 0.06 a | 4.33 ± 0.15 b | 445.38 ± 42 a | 38.04± 7.97 a | 6.44 ± 0.55 a | 4.36 ± 0.27 a | 6.88 ± 0.64 ab | |
| Β2 | 3.84 ± 0.04 a | 4.17 ± 0.08 b | 444.33 ± 23 a | 31.70 ± 7.90 a | 6.94 ± 0.30 a | 4.90 ± 0.29 a | 6.79 ± 1.45 ab | |
| MDS | C | 3.86 ± 0.05 a | 4.48 ± 0.20 ab | 429.95 ± 42 a | 39.74 ± 3.98 a | 6.82 ± 0.29 a | 4.56 ± 0.54 a | 6.03 ± 0.65 ab |
| Β1 | 3.89 ± 0.06 a | 4.66 ± 0.08 ab | 458.79 ± 29 a | 45.49 ± 5.18 a | 6.52 ± 0.57 a | 4.38 ± 0.43 a | 6.15 ± 0.50 ab | |
| Β2 | 3.89 ± 0.07 a | 4.30 ± 0.20 b | 478.49 ± 49 a | 42.04 ± 6.23 a | 6.98 ± 1.14 a | 4.86 ± 0.69 a | 7.41 ± 0.41 a | |
| SDS | C | 3.79 ± 0.03 a | 4.74 ± 0.29 ab | 483.76 ± 34 a | 47.21 ± 1.73 a | 6.95 ± 0.96 a | 4.43 ± 0.67 a | 4.44 ± 0.63b |
| Β1 | 3.90 ± 0.04 a | 5.10 ± 0.25 a | 427.75 ± 30 a | 36.87 ± 4.57 a | 6.58 ± 0.35 a | 4.44 ± 0.26 a | 5.91 ± 1.58 ab | |
| Β2 | 3.91 ± 0.11 a | 4.60 ± 0.29 ab | 459.78 ± 19 a | 43.21 ± 2.62 a | 7.04 ± 0.33 a | 4.80 ± 0.13 a | 8.35 ± 0.67 a | |
| Tukey’s HSD | 0.0548 | 0.2497 | 20.220 | 3.691 | 0.5562 | 0.2961 | 0.4950 |
| Mean Squares | ||||||
|---|---|---|---|---|---|---|
| Source of Variance | df 2 | TPC | TFC | ABTS | DPPH | FRAP |
| Drought Stress (A) | 2 | 656.261 ns | 86.117 ns | 0.037 ns | 0.008 * | 0.388 ns |
| Error (a) | 4 | 1197.809 | 5.544 | 0.679 | 0.225 | 1.116 |
| Biostimulant (B) | 2 | 664.675 ns | 34.636 ns | 0.736 ns | 0.562 ns | 8.685 ns |
| A × B | 4 | 1792.176 ns | 72.845 ns | 0.097 ns | 0.019 ns | 2.564 ns |
| Error (b) | 12 | 1227.002 | 40.865 | 0.928 | 0.263 | 0.735 |
| CV 1 (%) | 7.76 | 15.73 | 14.07 | 11.25 | 13.27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadoglidou, K.I.; Anthimidou, E.; Krommydas, K.; Papa, E.; Karapatzak, E.; Tsivelika, N.; Irakli, M.; Mellidou, I.; Xanthopoulou, A.; Kalivas, A. Effect of Biostimulants on Drought Tolerance of Greenhouse-Grown Tomato. Horticulturae 2025, 11, 601. https://doi.org/10.3390/horticulturae11060601
Kadoglidou KI, Anthimidou E, Krommydas K, Papa E, Karapatzak E, Tsivelika N, Irakli M, Mellidou I, Xanthopoulou A, Kalivas A. Effect of Biostimulants on Drought Tolerance of Greenhouse-Grown Tomato. Horticulturae. 2025; 11(6):601. https://doi.org/10.3390/horticulturae11060601
Chicago/Turabian StyleKadoglidou, Kalliopi I., Eleni Anthimidou, Konstantinos Krommydas, Eleni Papa, Eleftherios Karapatzak, Nektaria Tsivelika, Maria Irakli, Ifigeneia Mellidou, Aliki Xanthopoulou, and Apostolos Kalivas. 2025. "Effect of Biostimulants on Drought Tolerance of Greenhouse-Grown Tomato" Horticulturae 11, no. 6: 601. https://doi.org/10.3390/horticulturae11060601
APA StyleKadoglidou, K. I., Anthimidou, E., Krommydas, K., Papa, E., Karapatzak, E., Tsivelika, N., Irakli, M., Mellidou, I., Xanthopoulou, A., & Kalivas, A. (2025). Effect of Biostimulants on Drought Tolerance of Greenhouse-Grown Tomato. Horticulturae, 11(6), 601. https://doi.org/10.3390/horticulturae11060601








