Functional Characterization of CpPIP1;1 and Genome-Wide Analysis of PIPs in Wintersweet (Chimonanthus praecox (L.) Link)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Cloning of Genes and Promoters
2.3. Bioinformatic Analysis
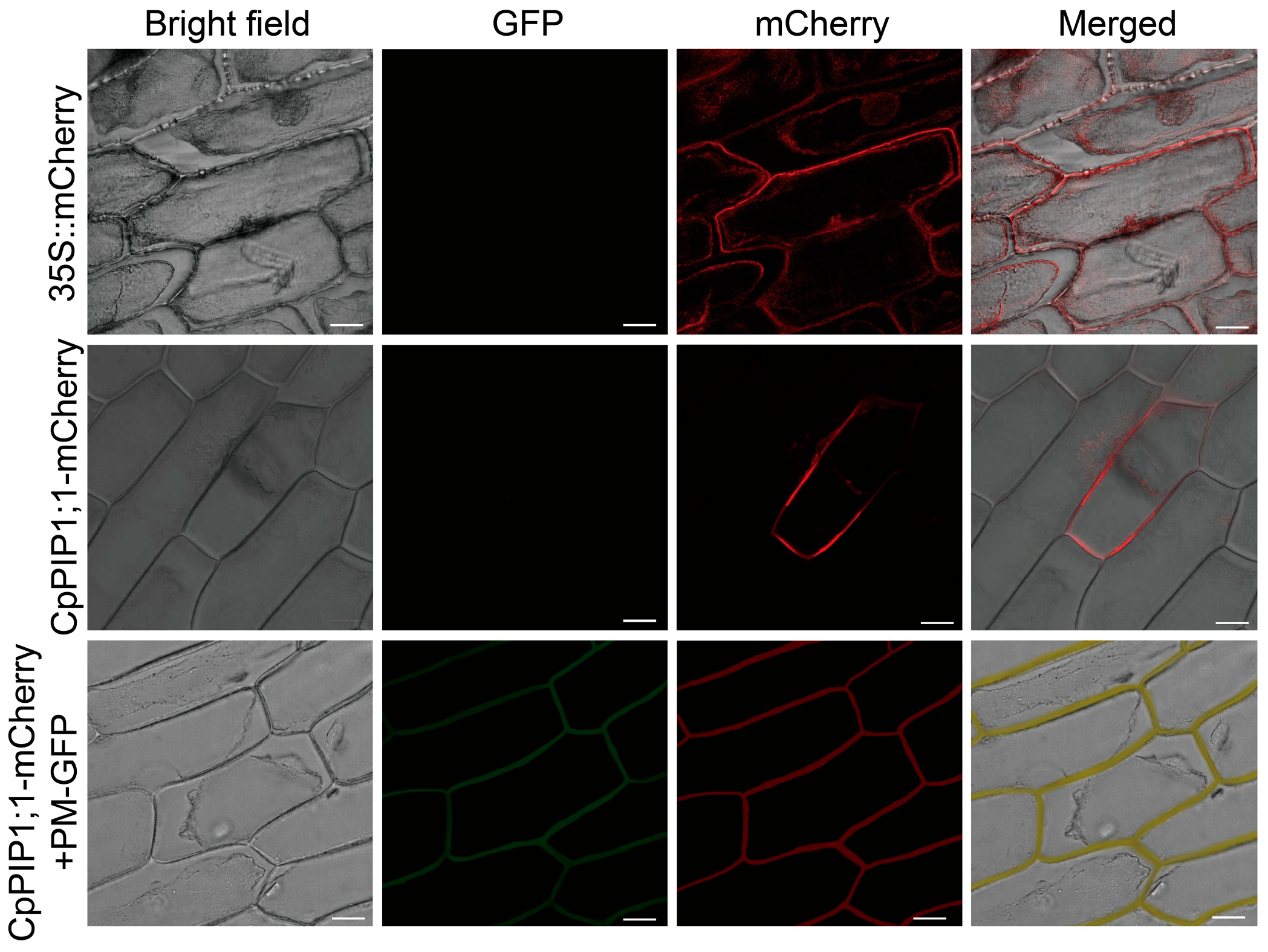
2.4. Subcellular Localization of CpPIP1;1
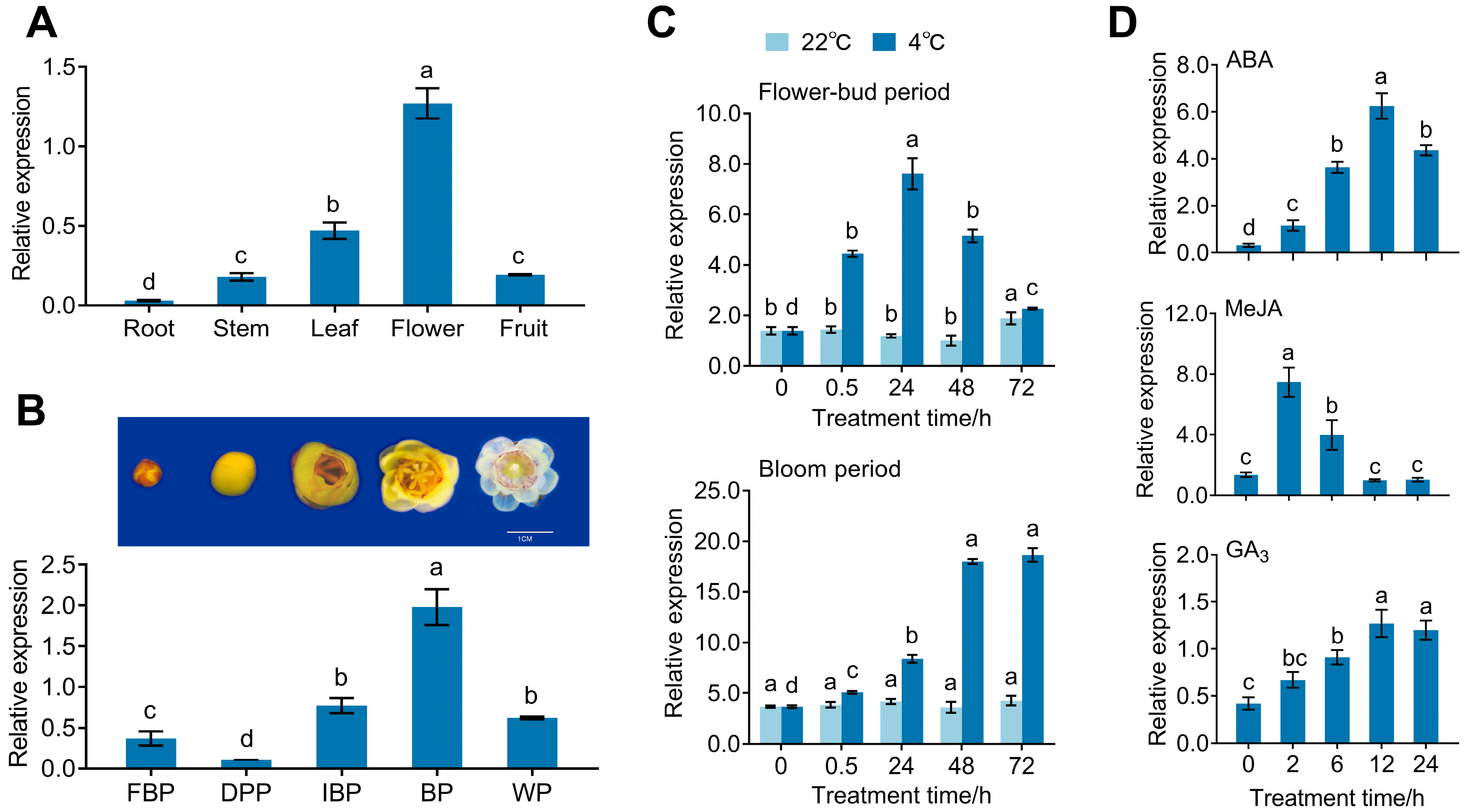
2.5. Expression Patterns of CpPIP1;1 in Wintersweet
2.6. Extraction of RNA and Analysis of Gene Expression
2.7. Vector Construction and Genetic Transformation
2.8. Evaluation of Transgenic A. thaliana Plants
2.9. GUS Histochemical and Activity Assays
3. Results
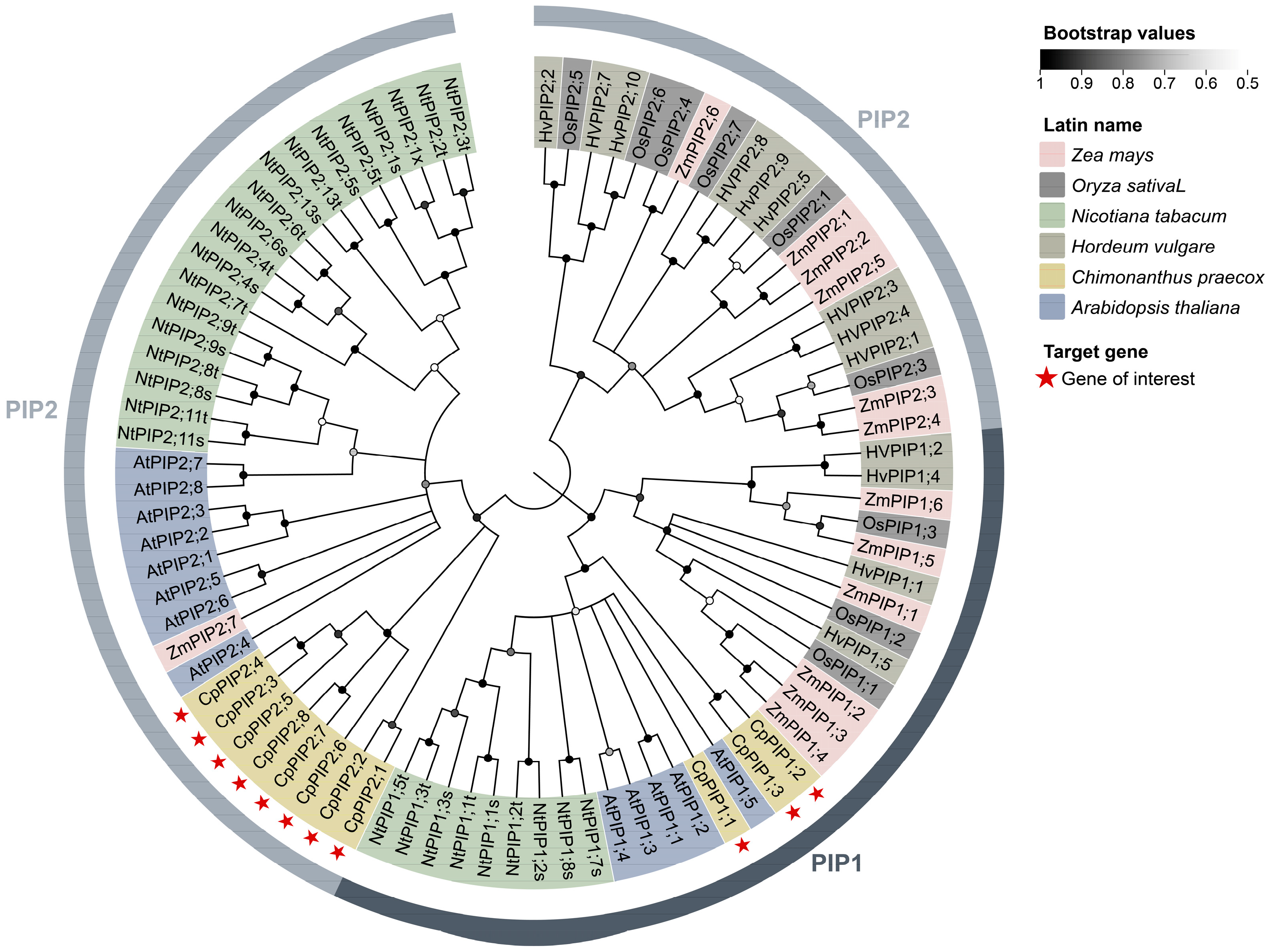
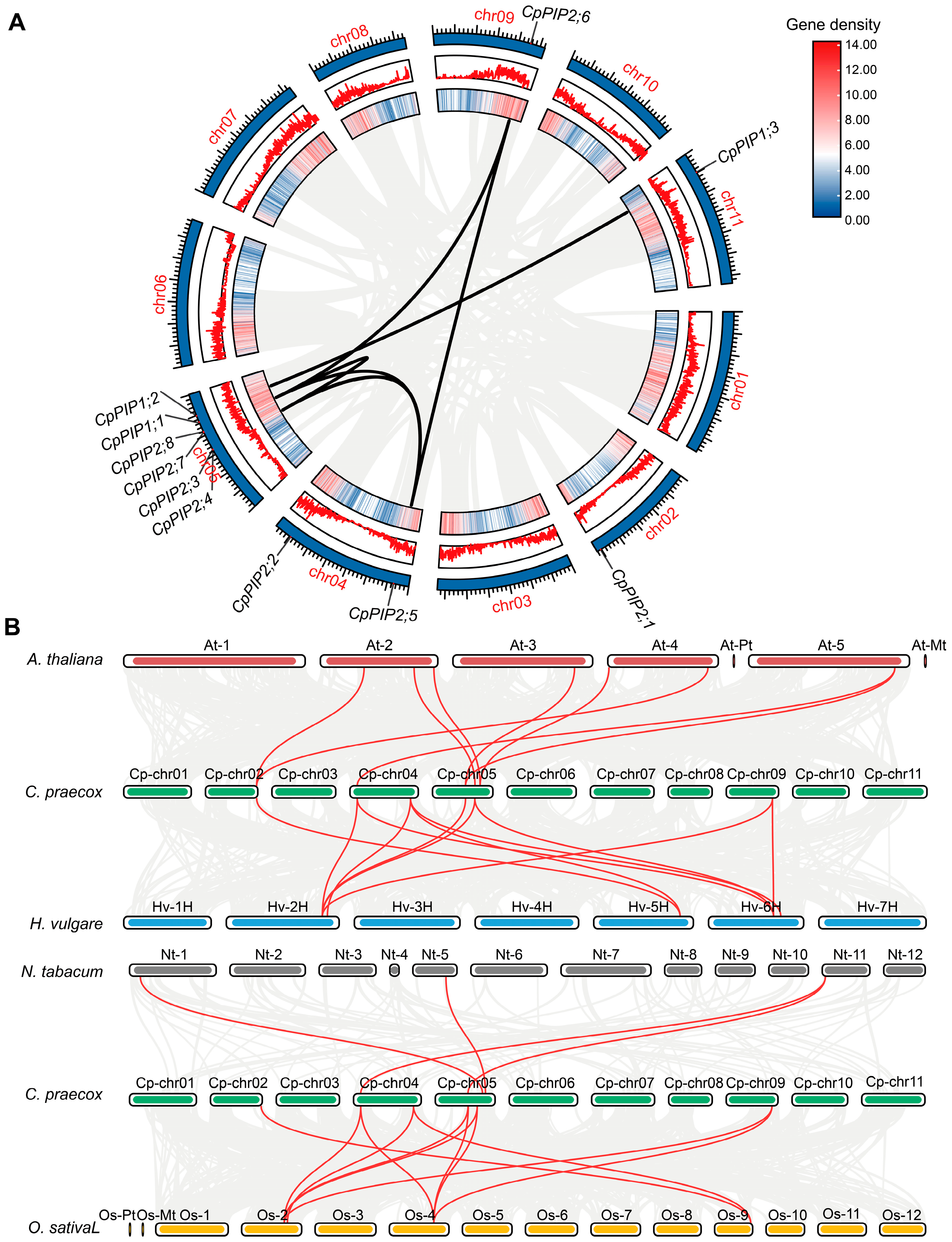
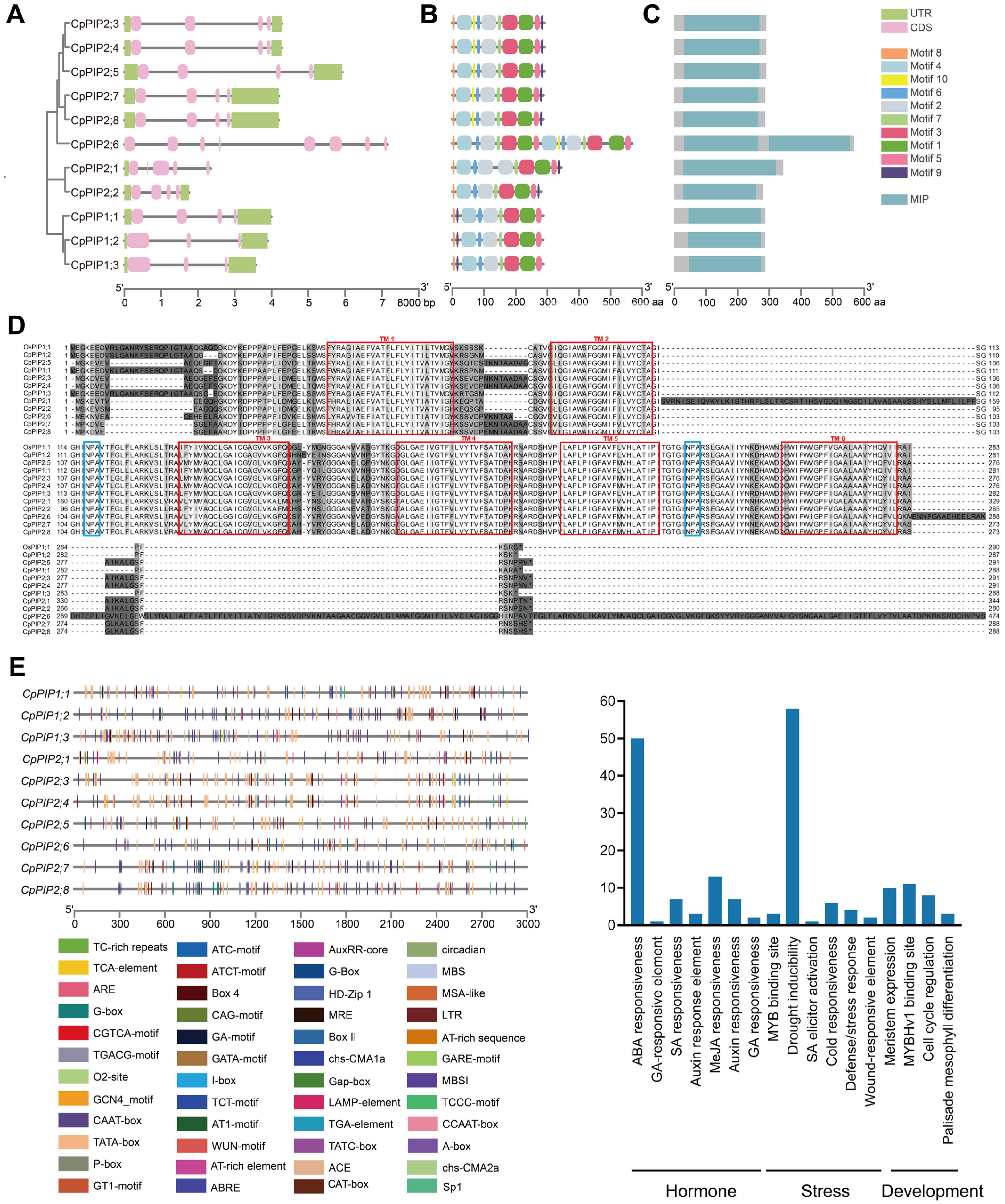
3.1. Identification, Sequence, and Phylogenetic Analysis of PIP Genes in C. praecox
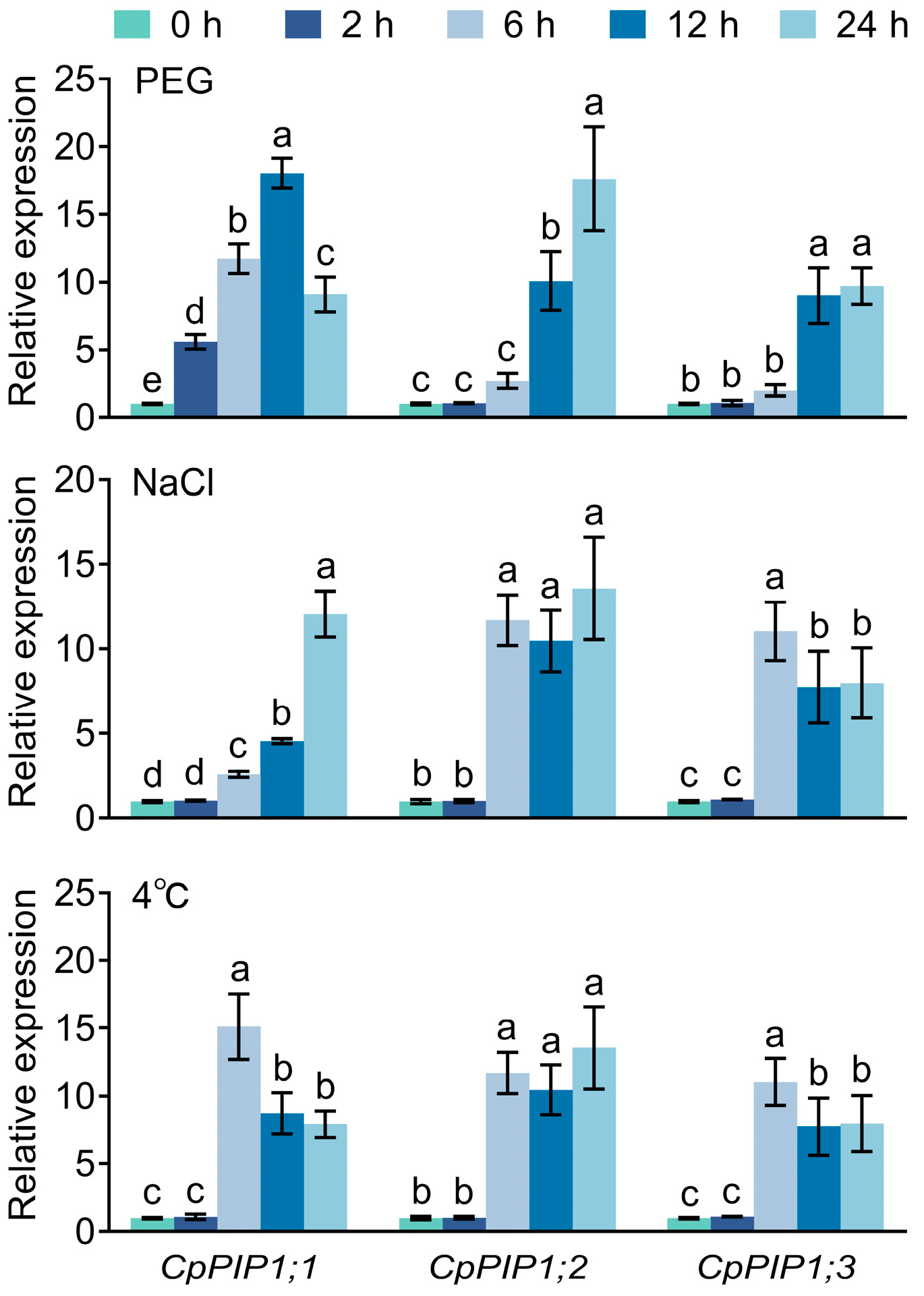
3.2. Expression Pattern of CpPIP1 Gene Family Members in Stressed Wintersweet
3.3. Subcellular Localization and Expression of CpPIP1;1
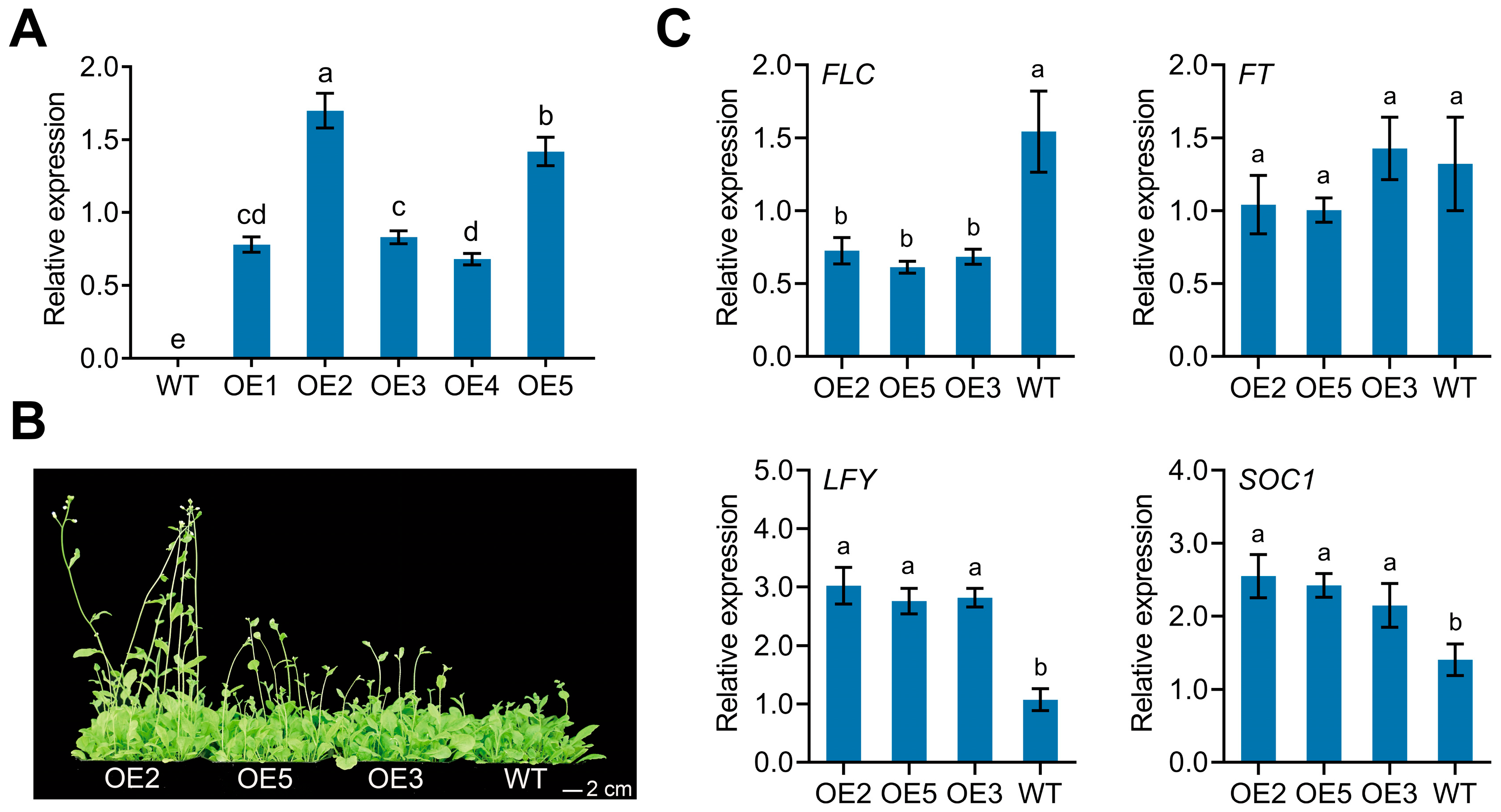
3.4. Overexpression of CpPIP1;1 Improves Flowering and Stress Tolerance in A. thaliana
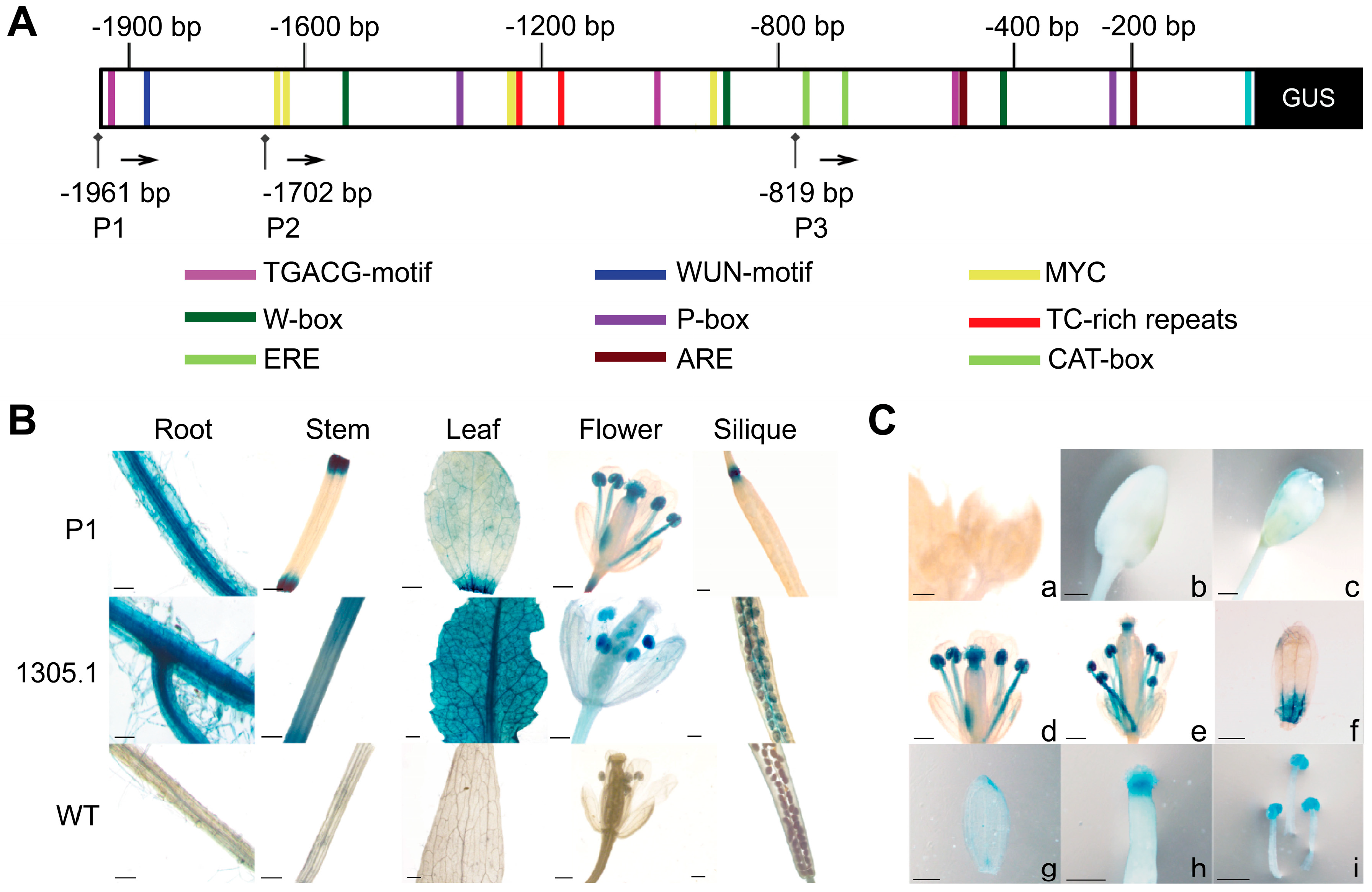
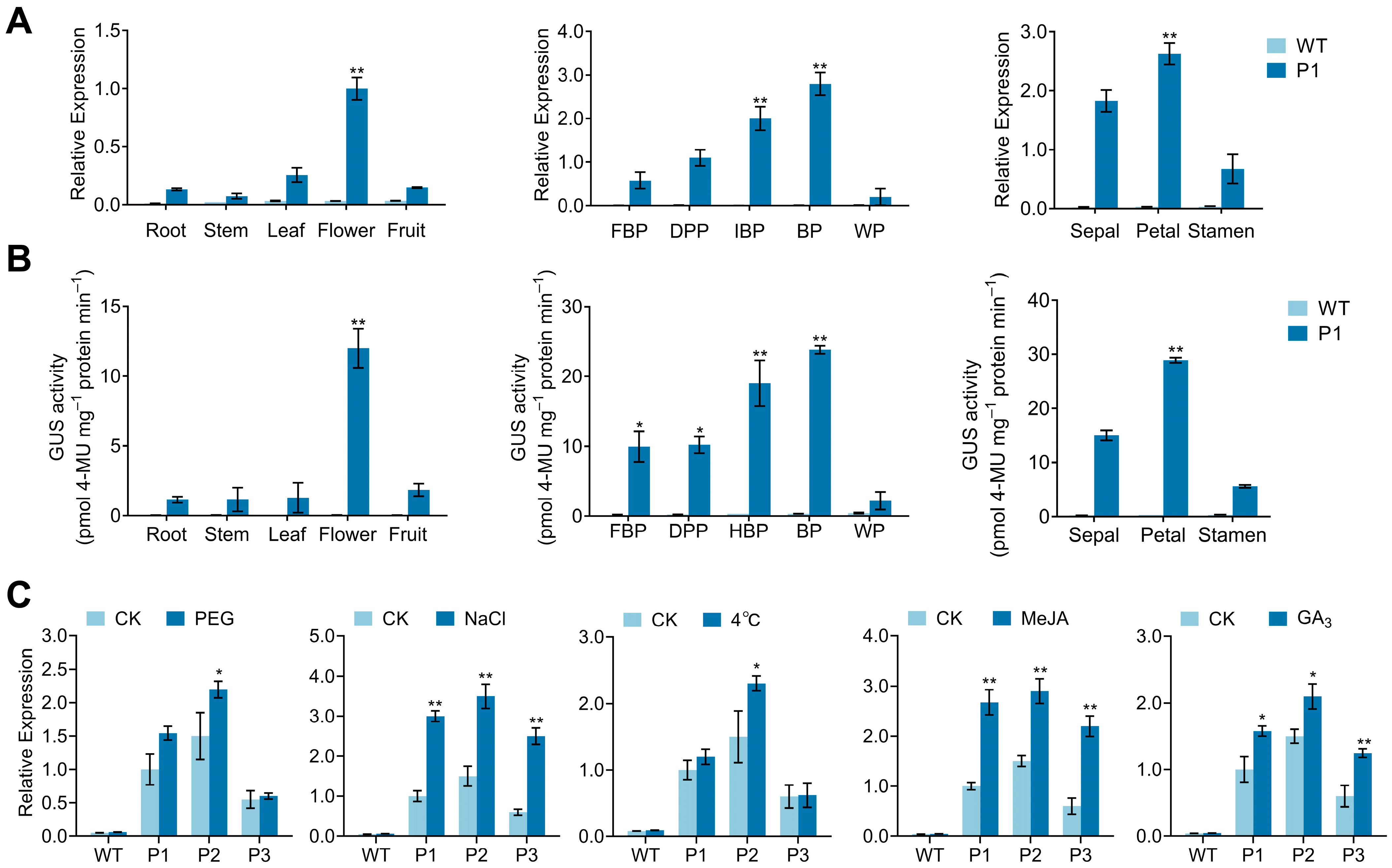
3.5. Analysis of CpPIP1;1 Promoter Cis-Acting Elements and GUS Histochemical Staining in Transgenic A. thaliana
3.6. Analysis of CpPIP1;1 Promoter Expression and Activity in Tobacco
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paluch-Lubawa, E.; Polcyn, W. Tissue-specific accumulation of PIP aquaporins of a particular heteromeric composition is part of the maize response to mycorrhiza and drought. Sci. Rep. 2024, 14, 21712. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, L.; Xin, M.; Ma, F.; Liu, J. Gene-Wide Analysis of Aquaporin Gene Family in Malus domestica and Heterologous Expression of the Gene MpPIP2;1 Confers Drought and Salinity Tolerance in Arabidposis thaliana. Int. J. Mol. Sci. 2019, 20, 3710. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zhang, Y.; Zhu, G.; Zhu, X.; Xia, Y.; Li, J.; Gao, X.; Wang, S.; Zhang, J.; et al. Genome-Wide Identification and Function of Aquaporin Genes During Dormancy and Sprouting Periods of Kernel-Using Apricot (Prunus armeniaca L.). Front. Plant Sci. 2021, 12, 690040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, W.; Li, H.; Lu, Q.; Xu, B.; Huang, B. Overexpression of an aquaporin gene PvPIP2;9 improved biomass yield, protein content, drought tolerance and water use efficiency in switchgrass (Panicum virgatum L.). Glob. Change Biol. Bioenergy 2020, 12, 979–991. [Google Scholar] [CrossRef]
- Ayadi, M.; Brini, F.; Masmoudi, K. Overexpression of a Wheat Aquaporin Gene, TdPIP2;1, Enhances Salt and Drought Tolerance in Transgenic Durum Wheat cv. Maali. Int. J. Mol. Sci. 2019, 20, 2389. [Google Scholar] [CrossRef]
- Fan, S.; Han, N.; Wu, H.; Jia, J.; Guo, J. Plasma membrane intrinsic protein SlPIP1;7 promotes root growth and enhances drought stress tolerance in transgenic tomato (Solanum lycopersicum) plants. Plant Breed. 2021, 140, 1102–1114. [Google Scholar] [CrossRef]
- Rabeh, K.; Sallami, A.; Gaboun, F.; Filali-Maltouf, A.; Sbabou, L.; Belkadi, B. Genome-wide analysis of aquaporin and their responses to abiotic stresses in plants: A systematic review and meta-analysis. Plant Stress 2024, 11, 100362. [Google Scholar] [CrossRef]
- Chen, W.; Yin, X.; Wang, L.; Tian, J.; Yang, R.; Liu, D.; Yu, Z.; Ma, N.; Gao, J. Involvement of rose aquaporin RhPIP1;1 in ethylene-regulated petal expansion through interaction with RhPIP2;1. Plant Mol. Biol. 2013, 83, 219–233. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, M.; Chen, W.; Zhou, X.; Lu, J.; Wang, Y.; Li, Y.; Jiang, C.-Z.; Gan, S.-S.; Ma, N.; et al. In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat. Plants 2019, 5, 290–299. [Google Scholar] [CrossRef]
- Tong, Z.; Li, Q.; Yang, Y.; Dai, F.; Gao, J.; Hong, B. Isolation and expression analysis of LoPIP2, a lily (Lilium Oriental Hybrids) aquaporin gene involved in desiccation-induced anther dehiscence. Sci. Hortic. 2013, 164, 316–322. [Google Scholar] [CrossRef]
- Nemoto, K.; Niinae, T.; Goto, F.; Sugiyama, N.; Watanabe, A.; Shimizu, M.; Shiratake, K.; Nishihara, M. Calcium-dependent protein kinase 16 phosphorylates and activates the aquaporin PIP2;2 to regulate reversible flower opening in Gentiana scabra. Plant Cell 2022, 34, 2652–2670. [Google Scholar] [CrossRef]
- Kong, W.; Bendahmane, M.; Fu, X. Genome-Wide Identification and Characterization of Aquaporins and Their Role in the Flower Opening Processes in Carnation (Dianthus caryophyllus). Molecules 2018, 23, 1895. [Google Scholar] [CrossRef]
- Lv, Y.; Xie, M.; Zhou, S.; Wen, B.; Sui, S.; Li, M.; Ma, J. CpCAF1 from Chimonanthus praecox Promotes Flowering and Low-Temperature Tolerance When Expressed in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 12945. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yang, F.; Gao, J. Isolation of Rh-TIP1;1, an aquaporin gene and its expression in rose flowers in response to ethylene and water deficit. Postharvest Biol. Technol. 2009, 51, 407–413. [Google Scholar] [CrossRef]
- Wu, C.L.; Hu, N.Z. Studies on the flower form and blooming characteristics of the wintersweet. Acta Hortic. Sin. 1995, 22, 277–282. [Google Scholar]
- Huang, R.; Sui, S.; Liu, H.; Li, M.; Liu, D. Overexpression of CpWRKY75 from Chimonanthus praecox Promotes Flowering Time in Transgenic Arabidopsis. Genes 2022, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.S.; Stevenson, F.O.; Zimmer, E.A. Evaluation and comparison of FTA card and CTAB DNA extraction methods for non-agricultural taxa. Appl. Plant Sci. 2017, 5, 1600109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, F.; Song, A.; Zhao, Y.; Chen, X.; Gao, Y.; Wei, G.; Zhang, W.; Guan, Y.; Fu, J. The genome assembly of Chimonanthus praecox var. concolor and comparative genomic analysis highlight the genetic basis underlying conserved and variable floral traits of wintersweet. Ind. Crops Prod. 2023, 206, 117603. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wang, S.; Liu, Y.; Yang, M.; Huang, Y. Genome-wide identification of the Pyrus R2R3-MYB gene family and PhMYB62 regulation analysis in Pyrus hopeiensis flowers at low temperature. Int. J. Biol. Macromol. 2024, 257, 128611. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Lin, Y.; Wei, G.; Hu, X.Q.; Zheng, Y.H.; Ma, J. Overexpression of the CpCOR413PM1 Gene from Wintersweet (Chimonanthus praecox) Enhances Cold and Drought Tolerance in Arabidopsis. Horticulturae 2024, 10, 599. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, D.; Wang, X.; Ahmed, S.; Li, M.; Kovinich, N.; Sui, S. Transgene CpNAC68 from Wintersweet (Chimonanthus praecox) Improves Arabidopsis Survival of Multiple Abiotic Stresses. Plants 2021, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, Z.Y.; Huang, Y.; Xu, J.J.; Liu, Z.; Xiang, Z.M.; Zhao, F.L.; Xue, J.P.; Xue, T.; Duan, Y.B. Functional characterization of the Pinellia ternata cytoplasmic class II small heat shock protein gene PtsHSP17.2 via promoter analysis and overexpression in tobacco. Plant Physiol. Biochem. 2022, 177, 1–9. [Google Scholar] [CrossRef]
- Seliem, M.; El-Mahrouk, M.; El-Banna, A.; Hafez, Y.; Dewir, Y. Micropropagation of Philodendron selloum: Influence of copper sulfate on endophytic bacterial contamination, antioxidant enzyme activity, electrolyte leakage, and plant survival. S. Afr. J. Bot. 2021, 139, 230–240. [Google Scholar] [CrossRef]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Report. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Quigley, F.; Rosenberg, J.M.; Shachar-Hill, Y.; Bohnert, H.J. From genome to function: The Arabidopsis aquaporins. Genome Biol. 2001, 3, 1–17. [Google Scholar] [CrossRef]
- Groszmann, M.; De Rosa, A.; Chen, W.; Qiu, J.; McGaughey, S.A.; Byrt, C.S.; Evans, J.R. A high-throughput yeast approach to characterize aquaporin permeabilities: Profiling the Arabidopsis PIP aquaporin sub-family. Front. Plant Sci. 2023, 14, 1078220. [Google Scholar] [CrossRef]
- Hachez, C.; Moshelion, M.; Zelazny, E.; Cavez, D.; Chaumont, F. Localization and quantification of plasma membrane aquaporin expression in maize primary root: A clue to understanding their role as cellular plumbers. Plant Mol. Biol. 2006, 62, 305–323. [Google Scholar] [CrossRef]
- Maistriaux, L.C.; Laurent, M.J.; Jeanguenin, L.; Prado, S.A.; Nader, J.; Welcker, C.; Charcosset, A.; Tardieu, F.; Nicolas, S.D.; Chaumont, F. Genetic variability of aquaporin expression in maize: From eQTLs to a MITE insertion regulating PIP2;5 expression. Plant Physiol. 2024, 196, 368–384. [Google Scholar] [CrossRef]
- Yi, X.F.; Sun, X.C.; Tian, R.; Li, K.X.; Ni, M.; Ying, J.L.; Xu, L.; Liu, L.W.; Wang, Y. Genome-Wide Characterization of the Aquaporin Gene Family in Radish and Functional Analysis of RsPIP2-6 Involved in Salt Stress. Front. Plant Sci. 2022, 13, 860742. [Google Scholar] [CrossRef] [PubMed]
- Die, J.V.; Castro, P.; Millán, T.; Gil, J. Segmental and Tandem Duplications Driving the Recent NBS-LRR Gene Expansion in the Asparagus Genome. Genes 2018, 9, 568. [Google Scholar] [CrossRef]
- He, W.; Liu, M.; Qin, X.; Liang, A.; Chen, Y.; Yin, Y.; Qin, K.; Mu, Z. Genome-Wide Identification and Expression Analysis of the Aquaporin Gene Family in Lycium barbarum during Fruit Ripening and Seedling Response to Heat Stress. Curr. Issues Mol. Biol. 2022, 44, 5933–5948. [Google Scholar] [CrossRef]
- Shibasaka, M.; Horie, T.; Katsuhara, M. Mechanisms Activating Latent Functions of PIP Aquaporin Water Channels via the Interaction between PIP1 and PIP2 Proteins. Plant Cell Physiol. 2021, 62, 92–99. [Google Scholar] [CrossRef]
- Guterman, I.; Shalit, M.; Menda, N.; Piestun, D.; Dafny-Yelin, M.; Shalev, G.; Bar, E.; Davydov, O.; Ovadis, M.; Emanuel, M. Rose scent: Genomics approach to discovering novel floral fragrance–related genes. Plant Cell 2002, 14, 2325–2338. [Google Scholar] [CrossRef]
- Ding, X.; Iwasaki, I.; Kitagawa, Y. Overexpression of a lily PIP1 gene in tobacco increased the osmotic water permeability of leaf cells. Plant Cell Environ. 2004, 27, 177–186. [Google Scholar] [CrossRef]
- Kobayashi, H.; Oikawa, Y.; Koiwa, H.; Yamamura, S. Flower-specific gene expression directed by the promoter of a chalcone synthase gene from Gentiana triflora in Petunia hybrida. Plant Sci. 1998, 131, 173–180. [Google Scholar] [CrossRef]
- Li, Y.; Dong, C.; Hu, M.; Bai, Z.; Tong, C.; Zuo, R.; Liu, Y.; Cheng, X.; Cheng, M.; Huang, J.; et al. Identification of Flower-Specific Promoters through Comparative Transcriptome Analysis in Brassica napus. Int. J. Mol. Sci. 2019, 20, 5949. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.F.; Le, C.; Wang, Y.Z.; Li, Z.C.; Jiang, D.H.; Wang, Y.Q.; He, Y.H. Arabidopsis FLC clade members form flowering-repressor complexes coordinating responses to endogenous and environmental cues. Nat. Commun. 2013, 4, 1947. [Google Scholar] [CrossRef]
- Monniaux, M.; McKim, S.M.; Cartolano, M.; Thévenon, E.; Parcy, F.; Tsiantis, M.; Hay, A. Conservation vs divergence in LEAFY and APETALA1 functions between Arabidopsis thaliana and Cardamine hirsuta. New Phytol. 2017, 216, 549–561. [Google Scholar] [CrossRef]
- Lian, H.-L.; Yu, X.; Lane, D.; Sun, W.-N.; Tang, Z.-C.; Su, W.-A. Upland rice and lowland rice exhibited different PIP expression under water deficit and ABA treatment. Cell Res. 2006, 16, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Deng, L.; Meng, J.; Wang, Y.; Pan, L.; Niu, L.; Lu, Z.; Cui, G.; Zeng, W.; Wang, Z. Characterization and expression analysis of basic leucine zipper (bZIP) transcription factors responsive to chilling injury in peach fruit. Mol. Biol. Rep. 2023, 50, 361–376. [Google Scholar] [CrossRef]
- Bae, E.-K.; Lee, H.; Lee, J.-S.; Noh, E.-W. Drought, salt and wounding stress induce the expression of the plasma membrane intrinsic protein 1 gene in poplar (Populus alba× P. tremula var. glandulosa). Gene 2011, 483, 43–48. [Google Scholar] [CrossRef]
- Lee, S.H.; Zwiazek, J.J. Regulation of water transport in Arabidopsis by methyl jasmonate. Plant Physiol. Biochem. 2019, 139, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Z.; Ma, N.; Gao, J. Regulation of the rose Rh-PIP2;1 promoter by hormones and abiotic stresses in Arabidopsis. Plant Cell Rep. 2009, 28, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Song, X.Q.; He, H.; Chu, L.W.; Zhou, H.J.; Zhao, Y.Q.; Xu, Y.J.; Zeng, W.; Lin, X.C.; Lu, M.Z. Genome-wide identification of plasma membrane aquaporin gene family in Populus and functional identification of PIP1;1 involved in osmotic stress. Environ. Exp. Bot. 2020, 179, 104200. [Google Scholar] [CrossRef]
- He, W.-D.; Gao, J.; Dou, T.-X.; Shao, X.-H.; Bi, F.-C.; Sheng, O.; Deng, G.-M.; Li, C.-Y.; Hu, C.-H.; Liu, J.-H. Early cold-induced peroxidases and aquaporins are associated with high cold tolerance in Dajiao (Musa spp.‘Dajiao’). Front. Plant Sci. 2018, 9, 282. [Google Scholar] [CrossRef]
- Peng, Y.; Ming, Y.H.; Jiang, B.C.; Zhang, X.Y.; Fu, D.Y.; Lin, Q.H.; Zhang, X.Y.; Wang, Y.; Shi, Y.T.; Gong, Z.Z.; et al. Differential phosphorylation of Ca2+-permeable channel CYCLIC NUCLEOTIDE-GATED CHANNEL20 modulates calcium-mediated freezing tolerance in Arabidopsis. Plant Cell 2024, 36, 4356–4371. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, F.; Feng, Z.; Wei, G.; Lv, Y.; Zhao, J.; Deng, Y.; Sui, S.; Ma, J. Functional Characterization of CpPIP1;1 and Genome-Wide Analysis of PIPs in Wintersweet (Chimonanthus praecox (L.) Link). Horticulturae 2025, 11, 581. https://doi.org/10.3390/horticulturae11060581
Ren F, Feng Z, Wei G, Lv Y, Zhao J, Deng Y, Sui S, Ma J. Functional Characterization of CpPIP1;1 and Genome-Wide Analysis of PIPs in Wintersweet (Chimonanthus praecox (L.) Link). Horticulturae. 2025; 11(6):581. https://doi.org/10.3390/horticulturae11060581
Chicago/Turabian StyleRen, Fei, Zhu Feng, Guo Wei, Yimeng Lv, Jia Zhao, Yeyuan Deng, Shunzhao Sui, and Jing Ma. 2025. "Functional Characterization of CpPIP1;1 and Genome-Wide Analysis of PIPs in Wintersweet (Chimonanthus praecox (L.) Link)" Horticulturae 11, no. 6: 581. https://doi.org/10.3390/horticulturae11060581
APA StyleRen, F., Feng, Z., Wei, G., Lv, Y., Zhao, J., Deng, Y., Sui, S., & Ma, J. (2025). Functional Characterization of CpPIP1;1 and Genome-Wide Analysis of PIPs in Wintersweet (Chimonanthus praecox (L.) Link). Horticulturae, 11(6), 581. https://doi.org/10.3390/horticulturae11060581






