Abstract
A mixture of amino acids, including aspartic acid, arginine, glycine, and tryptophan, can effectively promote tomato seedling growth. This research aimed to evaluate how the foliar spraying of an amino acid mixture, applied at various doses and intervals, influences the growth of tomato seedlings in a traditional seedbed. An experiment was conducted in the field, with the treatments distributed in a factorial arrangement (3 × 2) in a completely randomized block design with five replications. The growth parameters of tomato seedlings were improved by combining three doses of the amino acid mixture (0.25, 0.50, and 0.75 L ha−1 as VIUSID® agro) with two application intervals (weekly and biweekly). Results indicated that the foliar application of an amino acid mixture at 0.25 L ha−1 weekly enhances stem thickness, stem length, root length, leaf area index, root, shoot, and total dry biomass accumulation. Therefore, investing in the production of robust and high-quality tomato seedlings can lead to improved establishment, faster growth and development, reduced losses, and earlier and higher yields. This discovery indicates that using weekly low doses of the mixture of amino acids could be an effective and environmentally friendly option for improving tomato seedling production in traditional seedbed conditions.
1. Introduction
Global agricultural output faces a serious and complex challenge from climate change [1]. Rising temperatures, altered precipitation patterns, and increased frequency of extreme weather events such as droughts, floods, soil salinization, and heatwaves are disrupting traditional farming practices and affecting crop yields and livestock productivity worldwide [2]. The complex linkages between climate change and other stresses, such as soil degradation, insect and disease spread, and water shortages, may exacerbate the negative consequences on agricultural productivity [3]. Understanding the diverse and developing effects of climate change on agricultural production worldwide is crucial for developing effective adaptation and mitigation strategies to ensure future food security and livelihoods [4].
Tomato (Solanum lycopersicum L.) is one of the world’s most important horticultural crops in terms of economic value and consumption [5]. Global tomato production is a massive and widespread agricultural activity, with China consistently leading as the top producer, accounting for over a third of the world’s output. Other major players include the United States, India, Turkey, Egypt, Italy, and Spain. Production methods vary greatly, from open-field farming to advanced greenhouse cultivation, particularly in regions like Europe, which achieve very high yields [6]. Factors like climate change and technological advancements continue to shape the trends in global tomato production [7]. In Cuba, tomato production typically occurs during the dry season and is among the crops that most benefit producers. It is also the vegetable and fruit most sought after by the population [8]. Tomato production occurs through various systems, including greenhouses, garden conditions, and extensive crop areas; the latter yields the highest volumes of this crop [9].
To achieve high volumes in tomato production, a crucial factor is the cultivation of high-quality seedlings. Tray nurseries or traditional soil beds mainly produce these seedlings [8]. These latter methods are the most widespread in Cuba and present problems with pests, fertilization, and management that decrease the quality and quantity of these seedlings [9]. During this phase, the seedling shows limited growth of foliage and roots. Therefore, agricultural practices during the seedling phase must promote vegetative development and improve the efficiency of the shallow root system [8,9].
Biostimulants play an important role in plant growth because they reduce the effects of biotic and abiotic stresses. Moreover, they can enhance the quality of both production and yield, and therefore provide economic benefits, especially for farmers [10,11]. Moreover, biostimulant formulations may contain microorganisms, minerals, vitamins, amino acids, oligosaccharides, natural plant hormones, and other components [12]. Furthermore, biostimulants can be classified as (a) humic and fulvic acids, (b) protein hydrolysates and other N-containing compounds, (c) seaweed extracts and botanical products, (d) chitosan and other biopolymers, (e) inorganic compounds, (f) beneficial fungi (i.e., mycorrhizal fungi), or (g) beneficial bacteria based on the raw material source [13,14].
Amino acid-based biostimulants can play an important role in the growth and development of plants. Likewise, VIUSID® agro (a commercial amino acid growth promoter) contains a mixture of aspartic acid, arginine, glycine, and tryptophan, leading to stimulated plant growth and productivity due to the activation of diverse physiological and biochemical mechanisms inside plant cells [15,16,17,18]. The direct provision of these crucial components offers the essential building blocks necessary for the synthesis of amino acids, which may accelerate biomass accumulation [19]. In addition, this process conserves metabolic energy within the plant because it requires less energy to synthesize amino acids from inorganic nitrogen absorbed by the roots; as a result, this conserved energy can be allocated to other essential physiological processes [20]. Moreover, they stimulate physiological and metabolic processes, such as increased nutrient uptake and stimulation of phytohormones like auxin, cytokinins, and gibberellins; act as a source of nitrogen reserves; activate the biosynthesis of numerous metabolites; participate in the synthesis of other essential amino acids, such as lysine, threonine, isoleucine, and methionine; and are an indispensable component for the synthesis of nucleotides [21]. Furthermore, the foliar application of amino acid mixtures can effectively enhance plant biomass accumulation by supplying vital components, facilitating nutrient transport and uptake, optimizing plant metabolism and physiological functions, and boosting resilience to stress.
The concentration of amino acids in foliar sprays is a critical factor determining their impact on plant growth and productivity. Therefore, determining the appropriate concentration for the specific crop, its growth stage, and the environmental conditions is crucial for maximizing the positive effects of foliar amino acid application [22]. Low concentrations of amino acids can act as signaling molecules, triggering the synthesis of essential compounds like chlorophyll, enhancing photosynthesis, and improving nutrient uptake [23]. Some studies suggest that low doses of specific amino acids can boost plant morphology, helping plants cope with environmental stresses like salinity or drought [15,17]. Conversely, applying excessively high concentrations of amino acids can be counterproductive and potentially harmful to plants, leading to phytotoxicity, causing leaf burn, or can infer some potential interactions based on their known roles and metabolic pathways [24]. For example, an imbalance between arginine and tryptophan levels could potentially affect overall nitrogen utilization in the plant [25]. Similarly, high levels of aspartic acid and arginine could potentially affect nitrogen flow and utilization [26]. Moreover, high concentrations of glycine might indirectly influence the carbon flow and availability of intermediates for aspartate-derived pathways [27]. In addition, exogenous glycine has been shown to hinder root elongation and reduce nitrate uptake in some plants, which affects other amino acid promoters [28]. Furthermore, an overabundance of one amino acid can sometimes lead to deficiencies or imbalances in others due to feedback regulation, competition for transport systems, or shifts in metabolic priorities [29].
Another important element affecting plant development and production is the frequency of foliar amino acid treatments. Applying more frequently helps maintain a steady supply of amino acids to meet the plant’s needs, particularly during periods of rapid growth or stress [16,30]. Timing the applications with critical growth stages can significantly enhance their effectiveness [17,18,31]. Several studies recommend spraying crops such as pechay every five to seven days, which promotes consistent growth and development by maintaining optimal metabolic processes [24,32,33]. Moreover, infrequent applications may not provide a consistent supply of amino acids when the plant needs them most, potentially limiting their impact on growth and productivity [33]. Therefore, the absorbed amino acids might be metabolized or translocated within the plant over time, and if not replenished, their beneficial effects may diminish. Furthermore, both the concentration of the amino acid solution and the application intervals are critical for optimizing plant growth and productivity due to several factors, including the plant species, its growth rate, the specific amino acid product used, and the prevailing environmental conditions.
Finding the right balance ensures that plants receive an adequate and timely supply of these essential compounds, leading to healthier growth and enhanced yields. Various alternatives can be applied to improve the quality of tomato seedling production, although the exact mechanisms activated by an amino acid mixture are still being investigated. The impact of amino acid combinations on enhancing the growth of tomato seedlings in conventional seedbeds has not been thoroughly examined in the existing literature. Consequently, the objective of this study was to evaluate the influence of the foliar application of an amino acid mixture at different doses and intervals on the growth of tomato seedlings under a traditional seedbed.
2. Materials and Methods
2.1. Growth Conditions and Tomato Seeds
The experiment was conducted in the experimental area of the collective farm “Rolando Reina Ramos” in Taguasco, Sancti Spíritus, Cuba, located between 22°01′01″ N and 79°19′04″ W. During the experimental period, the temperature (20−24 °C), relative humidity (80−87%), rainfall (35.60 mm), and 12 h photoperiod were monitored daily by the Meteorological Station of Cabaiguán, Cuba. The soil of the experimental field was Sialitic Brown, with pH = 5.9, organic matter (1.13 g kg−1), organic carbon (16.92 g kg−1), total nitrogen (1.58 g kg−1), available phosphorous (3.22 mg kg−1), and available potassium (98.16 mg kg−1). Additionally, during the experimental period, chemical and organic fertilizers, as well as pest control, were not used.
S. lycopersicum ESEN-2 seeds were obtained through the genetic program of the National Institute of Agricultural Sciences (INCA), Mayabeque, Cuba. This variety is designed for fresh consumption, with large, round, flat-based, orange-red fruits weighing around 200 g with a potential output of 100 t ha−1 [8]. Seeds with undamaged, full, and uniformly sized seed coats were selected and then rinsed with distilled water to remove dust and impurities. The surface was disinfected for 10 min with 2% sodium hypochlorite (v/v), followed by two washes with distilled water. After that, the seeds were placed on a paper towel to remove excess moisture, and then the seeds were subsequently utilized in the next experiment. Further, seeds were manually sown to a density of 4 gm−2, spaced 15 cm between lines, and deposited to a depth of approximately 3 cm in traditional seedbeds.
2.2. Experimental Design
Under field conditions, treatments were adopted using a completely randomized block design, arranged in a factorial scheme with an additional treatment (3 × 2 + 1), with five replications. Three foliar doses of the amino acid mixture (0.25, 0.50, and 0.75 L ha−1) were combined with two application intervals (weekly and biweekly) and one additional control treatment (untreated). Combining foliar doses and intervals of application resulted in six combined treatments, which are represented as follows: applying 0.25 L ha−1 of AAM weekly (D1W1), applying 0.50 L ha−1 of AAM weekly (D2W1), applying 0.75 L ha−1 of AAM weekly (D3W1), applying 0.25 L ha−1 of AAM biweekly (D1W2), applying 0.50 L ha−1 of AAM biweekly (D2W2), and applying 0.75 L ha−1 of AAM biweekly (D3W2). The field plots were 3 m × 1 m (3 m2), and each plot was separated by a 2.0 m gap as a buffer. The useful area of the plot constituted the two central rows, scattering 0.5 m of the border.
2.3. Amino Acid Treatments and Composition
The foliar application was carried out between 9:00 and 10:00 a.m., avoiding the effects of high dew humidity and the evaporation risk due to increasing temperatures and wind conditions. Foliar spraying of tomato seedlings began seven days post-seeding, utilizing free doses of 0.25, 0.50, and 0.75 L ha−1 of the AAM at both weekly and biweekly intervals, according to Peña Calzada et al. [34]. A manual pressure back sprayer of 16 L capacity (Matabi, Goizper Group, Antzuola, Spain) was used to perform all foliar spraying. The declared composition of the amino acid-based growth promoter (commercialized as VIUSID® Agro) is as follows: aspartic acid (1.6% m/m), arginine (2.5% m/m), glycine (2.4% m/m), and tryptophan (0.5% m/m), with a pH of 6.80 and a net mass of 1.14 kg [15].
2.4. Determination of Growth Parameters
Twenty-five days after seeding (DAS), 50 tomato seedlings per treatment, totaling 300 plants, were collected in the useful area of each plot to determine the growth parameters. Stem length (SL, cm) was measured using a graduated rule from the plant base to the top visible dewlap insertion location. Stem thickness (ST, mm) was measured at a distance of 3 cm from the soil surface using calipers. The SL/ST ratio was calculated using the measured data for stem length and stem thickness. Leaf area index (LAI) was determined using Equation (1):
where l is the length of each leaflet; w is the width of each leaflet; and f is the constant factor equal to 0.66 previously determined by Kemp [35]. Root length (RL), root dry biomass (RB), shoot dry biomass (SB), and total dry biomass (TB) were measured after drying in a forced-air oven for 72 h at 60 °C, until a constant weight was determined using a digital microbalance (Sartorius MP3; Sartorius AG, Gottingen, Germany).
LAI = ∑ (l × w) × f
2.5. Data Analysis
The growth parameter data mentioned above were tested for normality and homogeneity of variance using the Shapiro–Wilk and Levene tests, respectively. Once these assumptions had been confirmed, a two-way ANOVA was conducted to assess the significance of doses (D), interval (I), and their interaction (D–I). If the D × I interaction was significant, the treatments were further analyzed; otherwise, comparisons of the individual factors were performed. In both cases, Tukey’s honest test (p < 0.05) was used to compare treatment means. All assumptions and statistical tests were executed using R software version 4.4.1 [36]. The figures were created using GraphPad Prism v8.0 (GraphPad Inc., San Diego, CA, USA).
3. Results
The findings indicated that control plants showed a significantly (p < 0.01) reduced stem thickness (ST) compared to the AAM-treated plants (Figure 1a). In addition, during 25 DAS, significant (p < 0.05) interactions between the dosage and application intervals of an amino acid mixture, as influenced by stem thickness, were validated (Figure 1a). The ST increased by 24%, 9%, and 10% with weekly applications of AAM at doses of 0.25, 0.50, and 0.75 L ha−1, respectively, compared to the biweekly applications of AAM (p < 0.01, Figure 1a). Additionally, in the combined D1W1 treatment, the ST was higher by 10% and 19% than the D2W1 and D3W1 treatments, respectively. However, the D2W1 treatment significantly (p < 0.01) increased the ST by 10% relative to the D3W1 treatment (Figure 1a). Similarly, the ST in the D1W2 treatment was 9% and 17% higher than in the D2W2 and D2W3 treatments, respectively. Additionally, the D2W2 treatment demonstrated notable differences in ST (11%) as compared to the D3W2 treatment (Figure 1a).
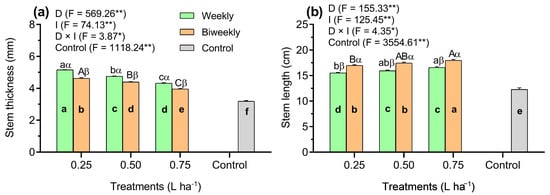
Figure 1.
Results observed in the stem thickness (a) and stem length (b) in tomato seedlings influenced by three doses of an amino acid mixture (0.25, 0.50, and 0.75 L ha−1), two application intervals (weekly and biweekly), and one additional control treatment (untreated). Data represented as means (n = 5) ± standard error (SE). Means followed by the same bold small letter among treatments are not significantly different. Means followed by the same letters, small in the weekly and capital in the biweekly, are not significantly different among the AAM doses. Different Greek letters (e.g., α, β) indicate significant differences at the same AAM doses, respectively, at p < 0.05 (Tukey’s Honest Significant Difference test). *, **: Significance levels at 0.05% and 0.01%, respectively. D: Doses of an amino acid mixture; I: application interval of an amino acid mixture; F: Fischer values; D × I: D–I interaction.
The stem length (SL) exhibited a significantly lower (p < 0.01) value in the control plants than in the AAM-treated plants (Figure 1b). Moreover, the SL showed a significant (p < 0.05) interaction between the D and I treatments (Figure 1b). Biweekly AAM applications resulted in an increase of 9%, 10%, and 11% in ST at doses of 0.25, 0.50, and 0.75 L ha−1, respectively, compared to weekly AAM applications (Figure 1b). Additionally, the D1W3 treatment showed an 11% increase in RL compared to the D1W1 treatment. However, both treatments had a similar impact on RL at 25 DAS. Similarly, the ST in the D2W3 treatment was higher (10%) compared to the D2W1 treatment (p < 0.05). Additionally, the D2W2 treatment showed similar effects (p = 0.52) on ST when compared to both the D2W1 and D2W3 treatments (Figure 1b).
Results revealed that the control plants showed a significantly (p < 0.01) lower root length (RL) than in the AAM-treated plants (Figure 2a). Nonetheless, there was a significant (p < 0.01) interaction between D and I treatments on RL (Figure 2a). With weekly applications of AAM, the RL significantly (p < 0.01) increased by 12%, 9%, and 10% at doses of 0.25, 0.50, and 0.75 L ha−1 of AAM, respectively, compared to biweekly applications of AAM. Additionally, the RL in the D1W1 treatment was higher by 9% and 21% than the D1W2 and D1W3 treatments. Nonetheless, under D2W1 treatment, the RL was significantly (p < 0.01) increased by 11% compared to the D2W3 treatment (Figure 2a).
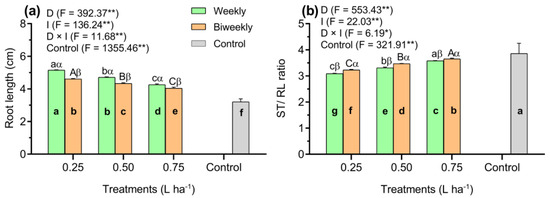
Figure 2.
Results observed in the root length (a) and the SL/RL ratio (b) in tomato seedlings influenced by three doses of an amino acid mixture (0.25, 0.50, and 0.75 L ha−1), two application intervals (weekly and biweekly), and one additional control treatment (untreated). Data represented as means (n = 5) ± SE. Means followed by the same bold small letter among treatments are not significantly different. Means followed by the same letters, small in the weekly and capital in the biweekly, are not significantly different among the AAM doses. Different Greek letters (e.g., α, β) indicate significant differences at the same AAM doses, respectively, at p < 0.05 (Tukey’s Honest Significant Difference test). *, **: Significance levels at 0.05% and 0.01%, respectively. D: Doses of an amino acid mixture; I: application interval of an amino acid mixture; F: Fischer values; D × I: D–I interaction.
Control plants revealed a higher SL/RL ratio than in the AAM-treated plants (p < 0.01; Figure 2b). However, the SL/RL ratio showed an interactive effect between the D and I treatments (p < 0.01), where the SL/RL ratio increased more with increased doses of AAM application in biweekly treatment compared to the weekly treatment (Figure 2b). In addition, the biweekly treatment significantly (p < 0.01) increased the SL/RL ratio in all AAM doses compared to the weekly treatment (Figure 2b). Moreover, at 25 days after sowing (DAS), the combined D3W1 treatment showed an increase of 16% and 8% compared to the D1W1 and D2W1 treatments, respectively (p < 0.01). Furthermore, the D3W2 treatment exhibited a greater SL/RL ratio, with increases of 17% and 8% over the D1W2 and D2W2 treatments, respectively (p < 0.01; Figure 2b).
Our results demonstrated that the leaf area index (LAI) was significantly (p < 0.01) decreased in the control plants compared to the AAM-treated plants (Figure 3a). Moreover, findings concerning the LAI indicated a significant (p < 0.01) D × I interaction (Figure 3a). In addition, a significantly (p < 0.01) reduced LAI was noted with biweekly applications of AAM compared to weekly applications of AAG. Additionally, in the combined D1W1 treatment, the LAI ratio was elevated by 37% and 20% compared to the D2W1 and D3W1 treatments, respectively. Similarly, the LAI ratio in the combined D3W2 treatment increased by 38% and 16% when compared to the D1W2 and D2W2 treatments. In particular, this last combined treatment also demonstrated significant effects on the LAI in relation to the D1W2 treatment (Figure 3a).
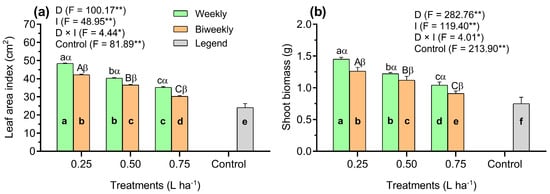
Figure 3.
Results observed of average growth parameters of the leaf area index (a) and shoot biomass (b) of tomato seedlings influenced by three doses of an amino acid mixture (0.25, 0.50, and 0.75 L ha−1), two application intervals (weekly and biweekly), and one additional control treatment (untreated). Data represented as means (n = 5) ± SE. Means followed by the same bold small letter among treatments are not significantly different. Means followed by the same letters, small in the weekly and capital in the biweekly, are not significantly different among AAM doses. Different Greek letters (e.g., α, β) indicate significant differences at the same AAM doses, respectively, at p < 0.05 (Tukey’s Honest Significant Difference test). *, **: Significance levels at 0.05% and 0.01%, respectively. D: Doses of an amino acid mixture; I: application interval of an amino acid mixture; F: Fischer values; D × I: D–I interaction.
Shoot biomass (SB) accumulation was significantly (p < 0.01) increased in AAM-treated plants compared to the control plants (Figure 3b). However, the SB accumulation showed a significant interaction (p < 0.01) between D and I treatments (Figure 3b). In addition, the SB in the combined D1W1 treatment was significantly (p < 0.01) higher by 19% and 39% than in the D2W1 and D3W1 treatments, respectively. Nonetheless, the D2W1 treatment showed an increase in SB of 17% in comparison with the D3W1 treatment (Figure 3b).
Our findings revealed that the root biomass (RB) production was significantly (p < 0.01) increased in the AAM-treated plants compared to the control plants (Figure 4a). However, the two-way ANOVA showed significant (p < 0.05) interaction between D and I treatments on RB accumulation (Figure 4a). Additionally, the RB accumulation was significantly (p < 0.01) higher in the weekly treatment than in the biweekly treatment in all AAM doses (Figure 4a). Similarly, applying the D1W1 treatment increased the RB accumulation by 19% and 11% compared to the D2W1 and D3W1 treatments; however, at the same time, the RB accumulation in the D1W2 treatment was higher by 23% than in the D3W1 treatment (p < 0.01). Furthermore, the RB showed a significant increase of 32% and 13% in the D2W2 treatment in comparison with the D2W2 and D3W2 treatments, respectively. Nevertheless, under D2W2 treatment, the RB was higher (16%) with respect to the D3W2 treatment (p < 0.01; Figure 4a).
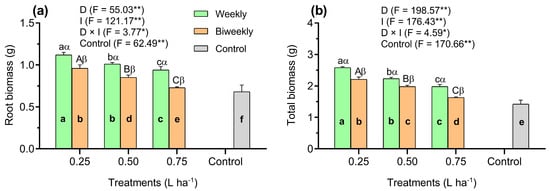
Figure 4.
Results observed of average growth parameters of root biomass (a) and total biomass (b) of tomato seedlings influenced by three doses of an amino acid mixture (0.25, 0.50, and 0.75 L ha−1), two application intervals (weekly and biweekly), and one additional control treatment (untreated). Data represented as means (n = 5) ± SE. Means followed by the same bold small letter among treatments are not significantly different. Means followed by the same letters, small in the weekly and capital in the biweekly, are not significantly different among AAM doses. Different Greek letters (e.g., α, β) indicate significant differences at the same AAM doses, respectively, at p < 0.05 (Tukey’s Honest Significant Difference test). *, **: Significance levels at 0.05% and 0.01%, respectively. D: Doses of an amino acid mixture; I: application interval of an amino acid mixture; F: Fischer values; D × I: D–I interaction.
The total biomass (TB) production was significantly (p < 0.01) decreased in the control plants in comparison with the AAM-treated plants (Figure 4b). Nonetheless, there was a significant (p < 0.05) interaction between D and I treatments on TB production (Figure 4b). Additionally, the TB accumulation was higher under weekly application than in the biweekly application in all AAM doses (p < 0.01, Figure 4b). Similarly, a significantly higher TB accumulation was shown in the D1W1 treatment, with increases of 30% and 16% over the D2W1 and D3W1 treatments, respectively. In addition, in the D2W1 treatment, the TB production was also 13% higher than in the D3W1 treatment (p < 0.01). On the other hand, the TB accumulation in the D2W1 and D2W2 treatments significantly (p < 0.01) increased by 36% and 12% compared to the D2W3 treatment, respectively. Further, the D2W2 treatment showed higher TB accumulation than in the D3W2 treatment (Figure 4b).
4. Discussion
Amino acids play an essential role in plant growth, acting as the building blocks of proteins, enzymes, photosynthesis, stress responses, nutrient uptake efficiency, and various metabolites essential for numerous physiological processes [32]. The foliar application of amino acids offers a direct and efficient way to supplement these crucial compounds, especially when plants are in critical developmental stages [29]. Studies indicate that adjusting the frequency of application according to the crop’s requirements and its particular growth phase can optimize the effectiveness of amino acid foliar sprays [16,19,33]. However, the optimal concentration and frequency of application depend on several factors, including the plant species, its growth rate, the individual or combination of amino acids used, and the prevailing environmental conditions [17,32].
Understanding the importance of amino acids in enhancing plant growth is essential, particularly in crops such as tomatoes, whose nutritional benefits and economic value are significant. Our findings demonstrate that the foliar-applied amino acid concentration solution and the application frequency are critical for optimizing tomato seedling growth. A positive impact of foliar-applied amino acid mixtures was observed on ST, LAI, SB, RB, and TB under the combined effects of D1W1 (applying 0.25 L ha−1 of AAM weekly) treatment. These results highlight the crucial roles of individual amino acids in plant physiology and development [37]. Similar response patterns were recently reported with the foliar of this same AAM in different plant species, such as maize [38], beets [31], radish [39], peanuts [40], and sunflower [17]. Determining the optimal concentration and application intervals for foliar-applied amino acid mixtures requires careful consideration of the specific plant, its developmental stage, and the environmental context [37]. Research indicates that lower to moderate concentrations, applied at suitable frequencies and tailored to the plant’s needs, are typically most effective in fostering healthy growth and boosting productivity [41]. In our study, we observed that weekly low doses of the mixture of amino acids promote tomato seedlings’ growth and vigor. These findings align with previous studies indicating that low amino acid doses are biologically and ecologically friendly, offering reproducible benefits in horticultural plant development [41].
The observed increase in shoot and root length following the mixture of amino acids can be attributed to several factors. Aspartic acid, as a precursor for other amino acids and involved in nitrogen assimilation, likely contributed to enhanced protein synthesis, a fundamental process for cell division and elongation in both shoot and root tissues [42]. The enhanced root growth observed could be particularly beneficial for nutrient and water uptake, laying a strong foundation for subsequent seedling establishment and vigor [43]. Moreover, aspartic acid is involved in the synthesis of asparagine, which is an important compound for nitrogen transport and storage, and enhances enzymatic activity [44]. Similarly, tryptophan is an important precursor for plant hormone indole-3-acetic acid (IAA), which stimulates optimal auxin levels and likely plays a significant role in promoting cell elongation and differentiation, leading to improved root development and architecture and overall seedling growth [45].
The ameliorative effect on plant growth, such as leaf area, by foliar-applied AAM can be an important, accessible strategy. In this sense, glycine plays a vital role in chlorophyll biosynthesis, which contributes to improved leaf size and photosynthetic efficiency, leading to increased carbohydrate production and subsequent biomass accumulation [20]. Aspartic acid plays a crucial role in metabolic activation, nucleotide synthesis, and the production of nicotinamide adenine dinucleotide and is also a precursor to the tricarboxylic acid cycle, which contributes to the synthesis of other essential amino acids, such as lysine, threonine, isoleucine, and methionine [46].
Arginine can enhance carbon utilization, activate vital metabolic pathways, and upregulate genes related to carotenoids and lipids, including arginine decarboxylase, carnitine transporter, arginine succinate synthase, nitric oxide synthase, and ornithine aminotransferase [19]. Arginine also influences gene transcription involved in carbon fixation and pyruvate metabolism, which are essential for energy production in plants, as seen in the Krebs cycle [47]. Furthermore, arginine acts as the primary substrate for the synthesis of osmolytes and signaling molecules, including proline, nitric oxide, and polyamines, and is recognized for its ability to effectively enhance the activity of antioxidant enzymes [47]. The individual effects of these specific amino acids are well documented and can yield significant synergistic results when combined, serving as an effective and environmentally friendly strategy to enhance plant growth in a relatively short time.
Effective management of cultivation practices is essential for generating sufficient biomass, which serves as a fundamental foundation for plant growth and seed production. A combination of effects of these particular amino acids, selected for their various functions in plant metabolism, probably played a significant role in enhancing seedling growth. In our study, we found significantly higher root, shoot, and total biomass accumulation, especially under weekly low doses of the mixture of amino acids. This strategy could explain the significant enhancements observed in a relatively short period. These mixtures provide readily available building blocks for protein synthesis, vital for rapid cell division and tissue development in young plants [48]. Certain amino acids can stimulate root growth, improving water and nutrient uptake, while others encourage shoot elongation and leaf expansion, thereby maximizing photosynthetic capacity [49]. Furthermore, amino acids can function as signaling molecules, initiating beneficial physiological responses and enhancing the seedling’s resilience to unfavorable environmental conditions [20]. The enhanced vigor of seedlings from the foliar application of AAM often results in healthier, more resilient seedlings that yield more and adapt better in their subsequent life cycle.
Recent studies have indicated that the optimal tomato seedling characteristics produced under traditional seedbeds are 5 mm of stem thickness, 15 cm of stem length, and healthy roots and leaves [8,9]. These same authors indicated that the production cycle of tomato seedlings in traditional seedbeds is 30 to 35 days. In our study, only the D1W1 treatment reached the aforementioned ST and SL characteristics at 25 DAS, indicating a five-day advance in their production cycle under these conditions. In addition, higher LAI, SB, RB, and TB indicate that the foliar application of AMM at low doses weekly represents an economic strategy for robust and higher-vigor tomato seedling production under traditional seedbed conditions. As a result, these alternatives are vital for achieving successful stand establishment and productive tomato cultivation, leading to faster, more uniform, and consistent plant growth, which is crucial for maximizing light interception, nutrient uptake, and overall yield [9]. Moreover, robust seedlings that have well-developed root systems and sturdy stems are better equipped to adapt during transplanting, leading to the effective absorption of water and nutrients from the soil, which promotes vigorous vegetative growth and a higher ability to support a heavy fruit load and an increase in overall yield [8]. Additionally, vigorous plants utilize water and fertilizers more efficiently, reducing input costs and minimizing environmental impact, and a higher yield of good-quality fruits translates to better returns in the market [50]. Furthermore, starting with robust and high-quality tomato seedlings can set the stage for a healthy, productive, and profitable tomato crop, which can minimize risks, maximize resource utilization, and ultimately lead to better yields and higher-quality fruits.
Although foliar-applied AAM at 0.50 and 0.75 L ha−1 weekly or biweekly increased SL, the ST did not reach the standard conditions for the production of tomato seedlings. Nonetheless, RL, LAI, SB, RB, and TB were also inferior to the D1W1 treatment. These findings indicate that a possible antagonistic effect occurred with the foliar application of the amino acid mixture that affected the growth of tomato seedlings, which could imply some potential interactions based on their known functions and metabolic pathways. Furthermore, overly high amino acid concentrations can induce phytotoxicity, leaf burn, and other problems, and plants may be unable to absorb and use such large amounts of amino acids, resulting in resource waste without adding value [29]. Tryptophan and arginine can compete for precursors through the shikimate pathway or shared metabolic intermediates, potentially limiting the availability of precursors for each other [25]. Also, an imbalance in the plant’s arginine, aspartic acid, glycine, and tryptophan levels might potentially impact nitrogen metabolism [24,26]. In addition, high aspartic acid and glycine levels may indirectly alter carbon transport and the availability of intermediates for aspartate-derived pathways [27]. Furthermore, changes in glycine and aspartic acid levels can influence root development and nutrient uptake (particularly nitrate), suggesting that they are involved in plant growth promotion [28]. Therefore, an excess of one amino acid can occasionally cause deficits or imbalances in others due to feedback regulation, competition for transport systems, or alterations in metabolic priority [29]. As a result, our data suggest that foliar-applied AMM at high concentrations decreased tomato seedling growth while boosting cycle production.
However, it is important to consider potential limitations and future research directions. The optimal concentration and frequency of application for each amino acid and their combinations may vary depending on the cultivar, plant growth stage, soil type, and environmental conditions. Using a mixture of amino acids provides a more complete and balanced nutritional approach, supporting various aspects of plant growth and development. Further studies are warranted to investigate the underlying molecular mechanisms through which these amino acids exert their growth-promoting effects, including gene expression analysis and hormonal profiling. Additionally, evaluating the long-term impact of these foliar applications on subsequent plant development, yield, and fruit quality would provide a more comprehensive understanding of their agricultural significance. Exploring the economic feasibility and scalability of this approach for commercial tomato production is also crucial for its practical application.
5. Conclusions
The results provide compelling evidence for the positive effects of the foliar application of an amino acid mixture on improving the growth of tomato seedlings in areas like stem thickness, stem length, leaf area index, and root, shoot, and total dry biomass production. Therefore, the foliar application of an amino acid mixture at 0.25 L ha−1 weekly is an environmentally sound best management practice for tomato seedling development under traditional seedbed conditions. These results highlight the possibility that foliar spraying of an amino acid mixture is an effective strategy for producing robust and vigorous tomato seedlings, which in turn can enhance crop establishment in subsequent stages.
Author Contributions
Conceptualization, A.C.H. and J.F.M.R.; methodology, J.F.M.R.; software, A.C.H.; validation, A.C.H., J.F.M.R. and K.P.C.; formal analysis, A.C.H. and Y.P.D.; investigation, A.C.H., J.F.M.R., K.P.C., Y.P.D. and A.J.M.; resources, J.F.M.R.; data curation, A.C.H., J.F.M.R., K.P.C., Y.P.D. and A.J.M.; writing—original draft preparation, A.C.H.; writing—review and editing, A.C.H., J.F.M.R. and K.P.C.; visualization, A.C.H.; supervision, J.F.M.R.; project administration, J.F.M.R.; funding acquisition, A.C.H. and K.P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Sancti Spiritus José Marti Perez (UNISS) through the institutional project “University, urban agriculture, and rural communities: an interdisciplinary approach to promote food sovereignty and gender equality” (AgroFuturo), grant number NA223SS500-035. The APC was subsidized by Catalysis S.A. Enterprise, Spain.
Data Availability Statement
All relevant data are within the paper.
Acknowledgments
The authors are grateful to the University of Sancti Spiritus José Marti Perez (UNISS) and the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES—Grant no. 88887.975003/2024-00) for providing the necessary facilities for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SI | Stem length |
| ST | Stem thickness |
| RL | Root length |
| RB | Root biomass |
| SB | Shoot biomass |
| TB | Total biomass |
| LAI | Leaf area index |
| DAS | Days after seeding |
| D1W1 | Applying 0.25 L ha−1 of AAM weekly |
| D2W1 | Applying 0.50 L ha−1 of AAM weekly |
| D3W1 | Applying 0.75 L ha−1 of AAM weekly |
| D1W2 | Applying 0.25 L ha−1 of AAM biweekly |
| D2W2 | Applying 0.50 L ha−1 of AAM biweekly |
| D3W2 | Applying 0.75 L ha−1 of AAM biweekly |
| D | Doses of a mixture of amino acids |
| I | Application interval of a mixture of amino acids |
| D × I | D–I interaction |
| F | Fischer values |
References
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Janni, M.; Maestri, E.; Gullì, M.; Marmiroli, M.; Marmiroli, N. Plant Responses to Climate Change, How Global Warming May Impact on Food Security: A Critical Review. Front. Plant Sci. 2024, 14, 1297569. [Google Scholar] [CrossRef] [PubMed]
- Nimma, D.; Devi, O.R.; Laishram, B.; Ramesh, J.V.N.; Boddupalli, S.; Ayyasamy, R.; Tirth, V.; Arabil, A. Implications of Climate Change on Freshwater Ecosystems and Their Biodiversity. Desalination Water Treat. 2025, 321, 100889. [Google Scholar] [CrossRef]
- Rodríguez, D.J.; Ramírez-Pérez, C.; Ramírez-Rodríguez, H.; Villarreal-Quintanilla, J.Á.; Hernández-Pérez, A.; Díaz-Jimenez, M.L.V.; Peña-Ramos, F.M. Bioestimulantes de plantas del semidesierto en el crecimiento radicular y aéreo de plántulas de tomate. Ecosistemas Y Recur. Agropecu. 2024, 11, e4145. [Google Scholar] [CrossRef]
- Domínguez, I.; del Río, J.L.; Ortiz-Somovilla, V.; Cantos-Villar, E. Technological Innovations for Reducing Tomato Loss in the Agri-Food Industry. Food Res. Int. 2025, 203, 115798. [Google Scholar] [CrossRef]
- Goh, Y.S.; Hum, Y.C.; Lee, Y.L.; Lai, K.W.; Yap, W.-S.; Tee, Y.K. A Meta-Analysis: Food Production and Vegetable Crop Yields of Hydroponics. Sci. Hortic. 2023, 321, 112339. [Google Scholar] [CrossRef]
- Lizazo, I.C.; Hurtado, A.C.; Hernández, M.G.R.; Casas, A.P.; Balmori, D.M.; Díaz, Y.P. Potentialities of two biostimulants on germination and growth of tomato seedlings. Cienc. Tecnol. Agropecu. 2022, 23, e2343. [Google Scholar] [CrossRef]
- Calero, A.; Quintero, E.; Pérez, Y.; Olivera, D.; Peña, K.; Castro, I.; Jiménez, J. Evaluation of efficient microorganisms in the tomato seedling production (Solanum lycopersicum L.). Rev. De Cienc. Agrícolas 2019, 36, 67–78. [Google Scholar] [CrossRef]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of Biostimulants in Tomato Plants (Solanum lycopersicum) to Enhance Plant Growth and Salt Stress Tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Jeong, S.J.; Masabni, J.; Niu, G. Biostimulants Applied in Seedling Stage Can Improve Onion Early Bulb Growth: Cultivar- and Fertilizer-Type-Specific Positive Effects. Horticulturae 2025, 11, 402. [Google Scholar] [CrossRef]
- Bell, J.C.; Bound, S.A.; Buntain, M. Biostimulants in Agricultural and Horticultural Production. In Horticultural Reviews; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 35–95. ISBN 978-1-119-85198-1. [Google Scholar]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Kisvarga, S.; Farkas, D.; Boronkay, G.; Neményi, A.; Orlóci, L. Effects of Biostimulants in Horticulture, with Emphasis on Ornamental Plant Production. Agronomy 2022, 12, 1043. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Olivera Viciedo, D.; Habermann, E.; Calero Hurtado, A.; Lupino Gratão, P.; De Mello Prado, R.; Lata-Tenesaca, L.F.; Martinez, C.A.; Ajila Celi, G.E.; Rodríguez, J.C. Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants. Agronomy 2022, 12, 2014. [Google Scholar] [CrossRef]
- Peña-Calzada, K.; Calero-Hurtado, A.; Meléndrez-Rodríguez, J.F.; Rodríguez-Fernández, J.C.; Gutiérrez-Cádenas, O.G.; García-González, M.T.; Madrigal-Carmona, L.; Jiménez-Medina, A. Impacts of the Biostimulant VIUSID® Agro on Growth, Productivity, and Tolerance to Salt Stress in Crops: A Systematic Review. Horticulturae 2025, 11, 407. [Google Scholar] [CrossRef]
- Calero Hurtado, A.; Peña Calzada, K.; Fasoli, J.V.B.; Jiménez, J.; Sánchez López, L. Synergic Effects of the Microbial Consortium and Amino Acid-Based Growth Promoter in Sunflower Productivity Under Water-Deficit Conditions. Water 2025, 17, 1365. [Google Scholar] [CrossRef]
- Díaz, Y.P.; Hurtado, A.C.; Calzada, K.P.; Díaz, J.L.G.; González, V.R. Plant densities and foliar application of amino acids increasing sesame yield. Temas Agrar. 2024, 29, 100–112. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Saleh, A.A.; Ali, M.M.; Sas-Paszt, L.; Abada, H.S.; Mosa, W.F.A. Growth Performance of Guava Trees after the Exogenous Application of Amino Acids Glutamic Acid, Arginine, and Glycine. Horticulturae 2022, 8, 1110. [Google Scholar] [CrossRef]
- Ban, Y.J.; Song, Y.H.; Kim, J.Y.; Cha, J.Y.; Ali, I.; Baiseitova, A.; Shah, A.B.; Kim, W.-Y.; Park, K.H. A Significant Change in Free Amino Acids of Soybean (Glycine max L. Merr) through Ethylene Application. Molecules 2021, 26, 1128. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yi, J.Q.; Piao, X.S.; Li, P.F.; Zeng, Z.K.; Wang, D.; Liu, L.; Wang, G.Q.; Han, X. The Metabolizable Energy Value, Standardized Ileal Digestibility of Amino Acids in Soybean Meal, Soy Protein Concentrate and Fermented Soybean Meal, and the Application of These Products in Early-Weaned Piglets. Asian-Australas J. Anim. Sci. 2013, 26, 691–699. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I.; Bocianowski, J.; Sladkovska, T.; Wolski, K. The Effect of Foliar Application of an Amino Acid-Based Biostimulant on Lawn Functional Value. Agronomy 2020, 10, 1656. [Google Scholar] [CrossRef]
- Navarro-León, E.; Borda, E.; Marín, C.; Sierras, N.; Blasco, B.; Ruiz, J.M. Application of an Enzymatic Hydrolysed L-α-Amino Acid Based Biostimulant to Improve Sunflower Tolerance to Imazamox. Plants 2022, 11, 2761. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Borrego, E.J.; Savka, M.A.; Dobson, R.C.J.; Hudson, A.O. Amino Acid–Derived Defense Metabolites from Plants: A Potential Source to Facilitate Novel Antimicrobial Development. J. Biol. Chem. 2021, 296, 100438. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.S.; Ryu, K.W.; Thompson, C.B. Arginine: At the Crossroads of Nitrogen Metabolism. EMBO J. 2025, 44, 1275–1293. [Google Scholar] [CrossRef]
- Lei, S.; Rossi, S.; Huang, B. Metabolic and Physiological Regulation of Aspartic Acid-Mediated Enhancement of Heat Stress Tolerance in Perennial Ryegrass. Plants 2022, 11, 199. [Google Scholar] [CrossRef]
- Oogai, Y.; Yamaguchi, M.; Kawada-Matsuo, M.; Sumitomo, T.; Kawabata, S.; Komatsuzawa, H. Lysine and Threonine Biosynthesis from Aspartate Contributes to Staphylococcus Aureus Growth in Calf Serum. Appl. Environ. Microbiol. 2016, 82, 6150–6157. [Google Scholar] [CrossRef]
- Han, R.; Khalid, M.; Juan, J.; Huang, D. Exogenous Glycine Inhibits Root Elongation and Reduces Nitrate-N Uptake in Pak Choi (Brassica campestris ssp. Chinensis L.). PLoS ONE 2018, 13, e0204488. [Google Scholar] [CrossRef]
- Kawade, K.; Tabeta, H.; Ferjani, A.; Hirai, M.Y. The Roles of Functional Amino Acids in Plant Growth and Development. Plant Cell Physiol. 2023, 64, 1482–1493. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Junior, G.d.S.S.; Prado, R.d.M.; Peña Calzada, K.; Olivera Viciedo, D. Silicon Induces Salt Stress Amelioration in Sunflower Plants by Improving Photosynthetic Pigments and Mineral Status. Stresses 2024, 4, 860–869. [Google Scholar] [CrossRef]
- Calzada, K.P.; Hurtado, A.C.; Peistrup, V.; Mühlmann, I.; Miranda, D.R.; Coca, L.I.R.; González, M.R.; Fernández, J.C.R. Physiological and productive responses of sugar beet plants treated with amino acid solution. Temas Agrar. 2024, 29, 113–125. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and Economical Effect of Application Biostimulants Containing Seaweed Extracts and Amino Acids as an Element of Agroecological Management of Bean Cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef] [PubMed]
- Jacomassi, L.M.; Pacola, M.; Momesso, L.; Viveiros, J.; Júnior, O.A.; de Siqueira, G.F.; de Campos, M.; Crusciol, C.A.C. Foliar Application of Amino Acids and Nutrients as a Tool to Mitigate Water Stress and Stabilize Sugarcane Yield and Bioenergy Generation. Plants 2024, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Calzada, K.P.; Fernández, J.C.R.; Meléndrez, J.F. EL VIUSID agro una alternativa en el incremento de la producción. Rev. Caribeña De Cienc. Soc. 2016, 15, 1–6. [Google Scholar]
- Kemp, C.D. Methods of Estimating the Leaf Area of Grasses from Linear Measurements. Ann. Bot. 1960, 24, 491–499. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Zhang, C.; Zhang, J.; Liu, W.; Ji, J.; Zhang, K.; Li, H.; Feng, Y.; Xue, J.; Ji, C.; Zhang, L.; et al. Mechanisms of Branched Chain Amino Acids Promoting Growth and Lipid Accumulation in Camelina sativa Seedlings under Drought and Salt Stress. Sustain. Energy Technol. Assess. 2025, 75, 104201. [Google Scholar] [CrossRef]
- Peña, K.; Calero Hurtado, A.; Viciedo, D.; Rodríguez, J.; Fernandes, T.; García, R.; Ajila, G. Agroproductive Response of Zea Mayz L. with the Foliar Application of VIUSID Agro®. Rev. De La Fac. De Agron. Univ. Del Zulia 2021, 38, 573–584. [Google Scholar] [CrossRef]
- Calzada, K.P.; Rodríguez, J.C.; Viciedo, D.O.; Hurtado, A.C.; Meléndrez, J.F.; Valdez, R.G. VIUSID Agro® dose effect on the morpho-physiological and productive behavior of radish (Raphanus sativus L.). Rev. De La Fac. De Agron. Univ. Del Zulia 2018, 35, 293–317. [Google Scholar]
- González, Y.A.; Díaz, Y.P.; Hurtado, A.C.; Calzada, K.P. Plant densities and fertilizers improving sustainable peanut production. Rev. De La Fac. De Cienc. 2025, 14, 23–38. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Lea, P.J.; Miflin, B.J. Glutamate Synthase and the Synthesis of Glutamate in Plants. Plant Physiol. Biochem. 2003, 41, 555–564. [Google Scholar] [CrossRef]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of Polyamines in Plant Response to Abiotic Stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-Aspartate: An Essential Metabolite for Plant Growth and Stress Acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhuang, S.; Zhang, W. Advances in Plant Auxin Biology: Synthesis, Metabolism, Signaling, Interaction with Other Hormones, and Roles under Abiotic Stress. Plants 2024, 13, 2523. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Sekara, A.; Al-ashkar, I.; Habib-ur-Rahman, M.; Skalicky, M.; Brestic, M.; Kumar, A.; Sabagh, A.E.; Abdelhamid, M.T. Exogenous Aspartic Acid Alleviates Salt Stress-Induced Decline in Growth by Enhancing Antioxidants and Compatible Solutes While Reducing Reactive Oxygen Species in Wheat. Front. Plant Sci. 2022, 13, 987641. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Hatamnia, A.A.; Tiznado-Hernández, M.E. Arginine Catabolism Induced by Exogenous Arginine Treatment Reduces the Loss of Green Color Rate in Broccoli Florets. Physiol. Mol. Plant Pathol. 2023, 124, 101973. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Cheng, Q.; Sun, W. The Effects of Amino Acids, Phenols and Protein Hydrolysates as Biostimulants on Sustainable Crop Production and Alleviated Stress. Recent Pat. Biotechnol. 2022, 16, 319–328. [Google Scholar] [CrossRef]
- Kheir, A.M.S.; Ding, Z.; Gawish, M.S.; Abou El Ghit, H.M.; Hashim, T.A.; Ali, E.F.; Eissa, M.A.; Zhou, Z.; Al-Harbi, M.S.; El-Gioushy, S.F. The Exogenous Application of Micro-Nutrient Elements and Amino Acids Improved the Yield, Nutritional Status and Quality of Mango in Arid Regions. Plants 2021, 10, 2057. [Google Scholar] [CrossRef]
- Fusco, G.M.; Burato, A.; Pentangelo, A.; Cardarelli, M.; Nicastro, R.; Carillo, P.; Parisi, M. Can Microbial Consortium Applications Affect Yield and Quality of Conventionally Managed Processing Tomato? Plants 2023, 12, 14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).