Efficacy Evaluation of Different Mineral Clay Particles on Olive Production Traits and Olive Oil Quality of ‘Koroneiki’ Olive Cultivar Under Rainfed and Irrigated Conditions in Southern Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Site Location—Plant Material
- Surround® WP (kaolin—aluminum silicate 95% w/w) (Al2Si2O5(OH)4 95%) (Tessenderlo Kerley, Inc. Phoenix, AZ, USA), distributed in Greece by Hellafarm SA (Peania, Greece), at the recommended supplier dose rate of 3 Kg 100 L−1;
- Invelop® White Protect (Talc E553b) (Mg3Si4O21H20 100% w/w) (COMPO EXPERT GmbH, Münster/Westphalia, Germany), distributed in Greece by Compo Hellas (Marousi, Greece), at the recommended supplier dose rate of 3 Kg 100 L−1; and
- Aglev® SI 300 (attapulgite 100%) (Geohellas SA, Athens, Greece) at the recommended supplier dose rate of 2 Kg 100 L−1. The chemical formula of attapulgite is Mg5Si8O20(OH)2(OH2)44H2O [28].
2.2. Olive Oil Extraction Procedure
2.3. Olive Oil Analyses
2.4. Determination of Total Phenols
2.5. Antioxidant Capacity
2.6. Fatty Acids and Squalene Determination
2.7. a-Tocopherol Determination
2.8. Statistical Analysis
3. Results
3.1. Effects of the Treatments on Yield Components
3.2. Effects of the Treatments on Olive Oil Quality Characteristics
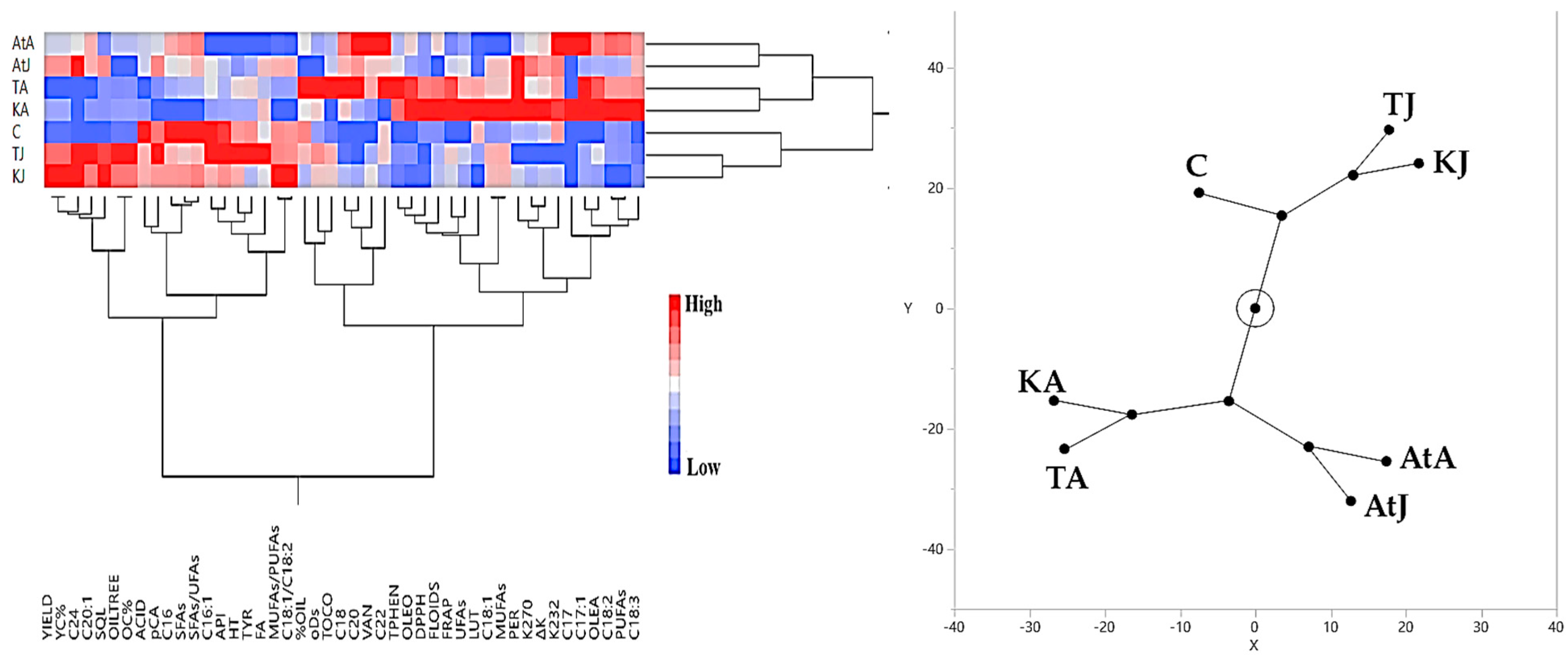
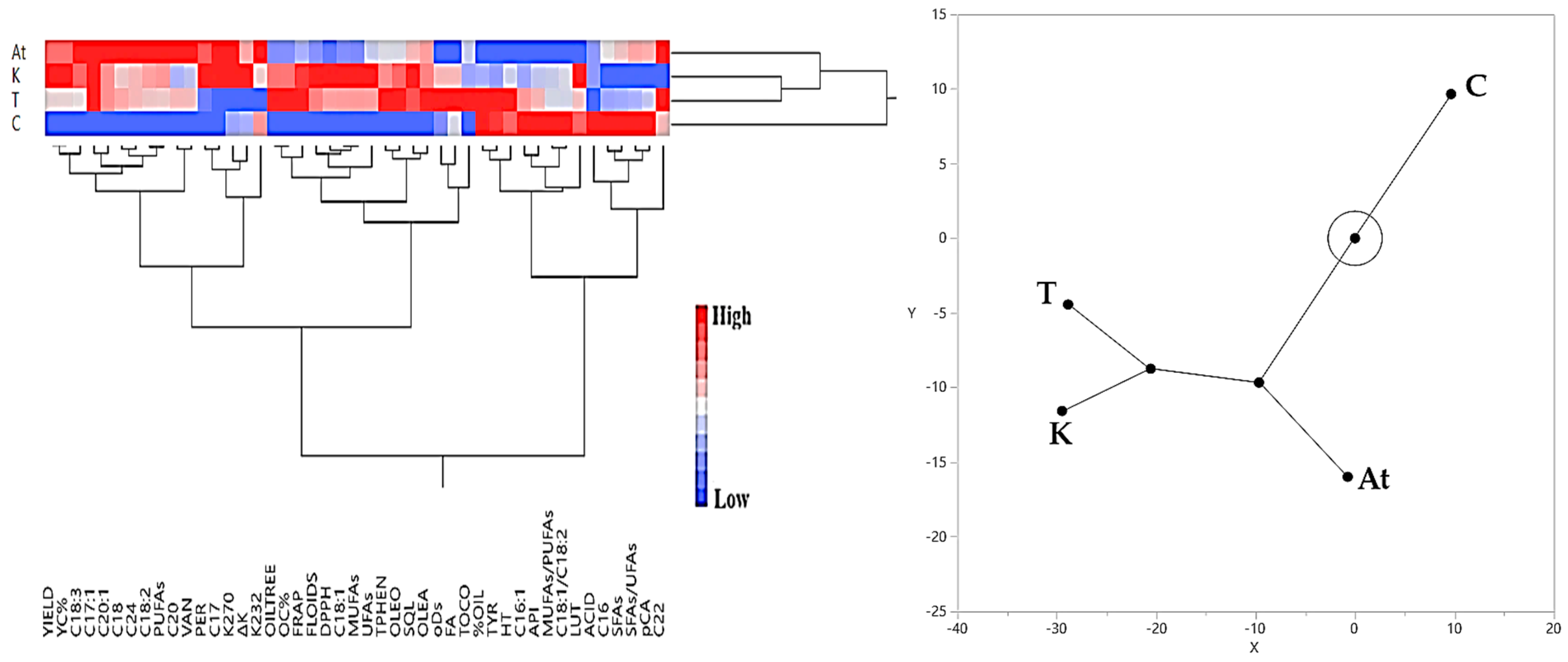
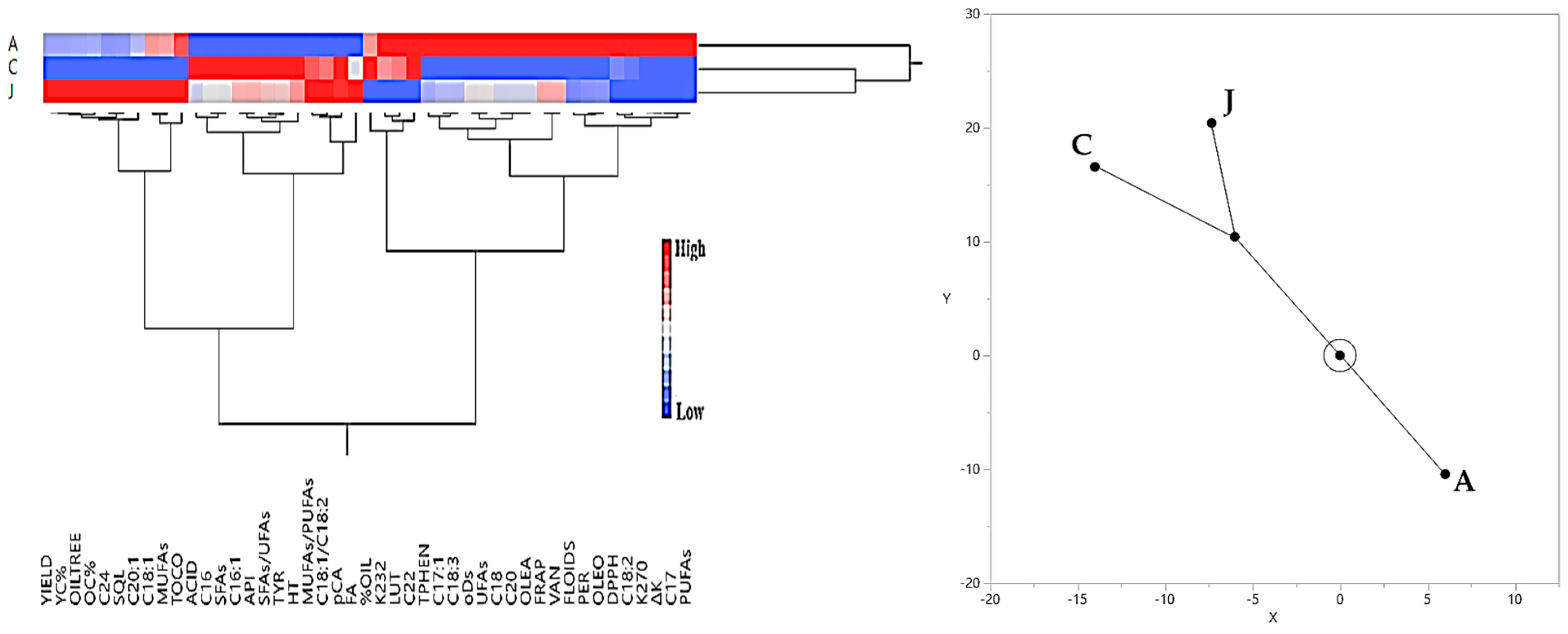
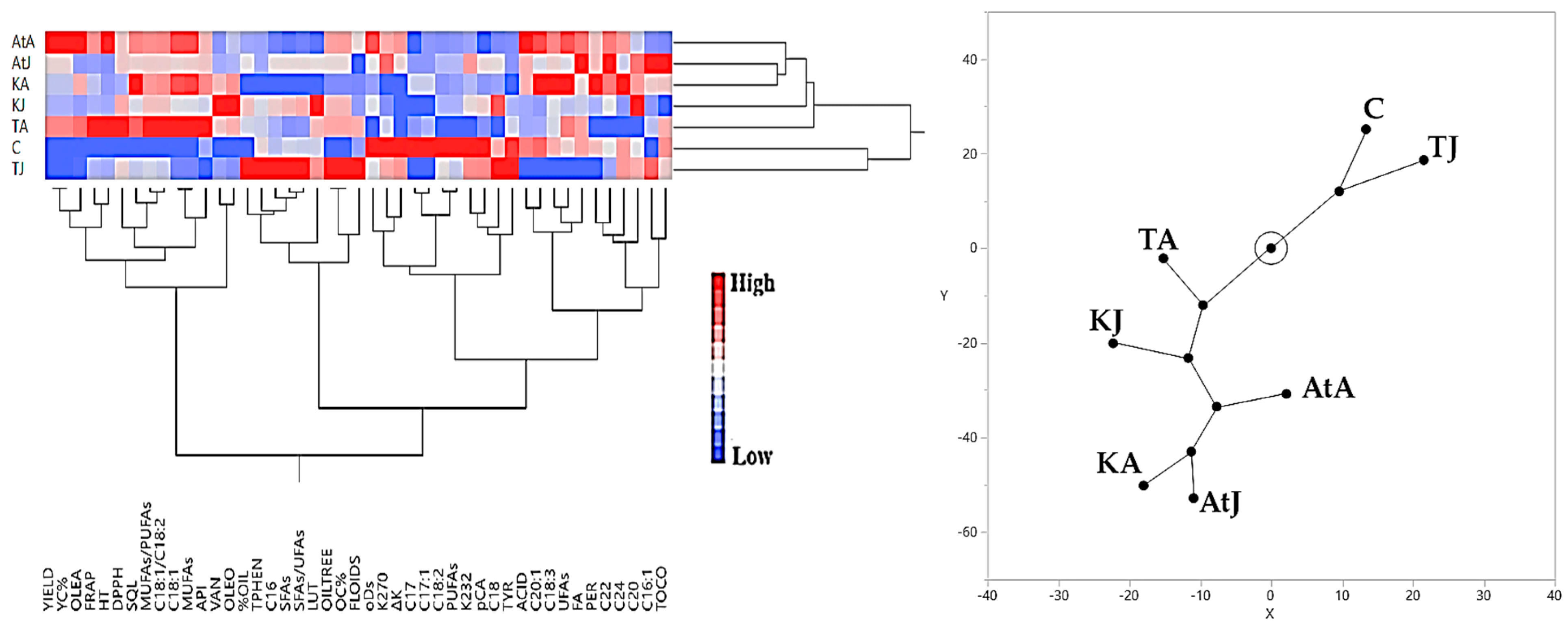
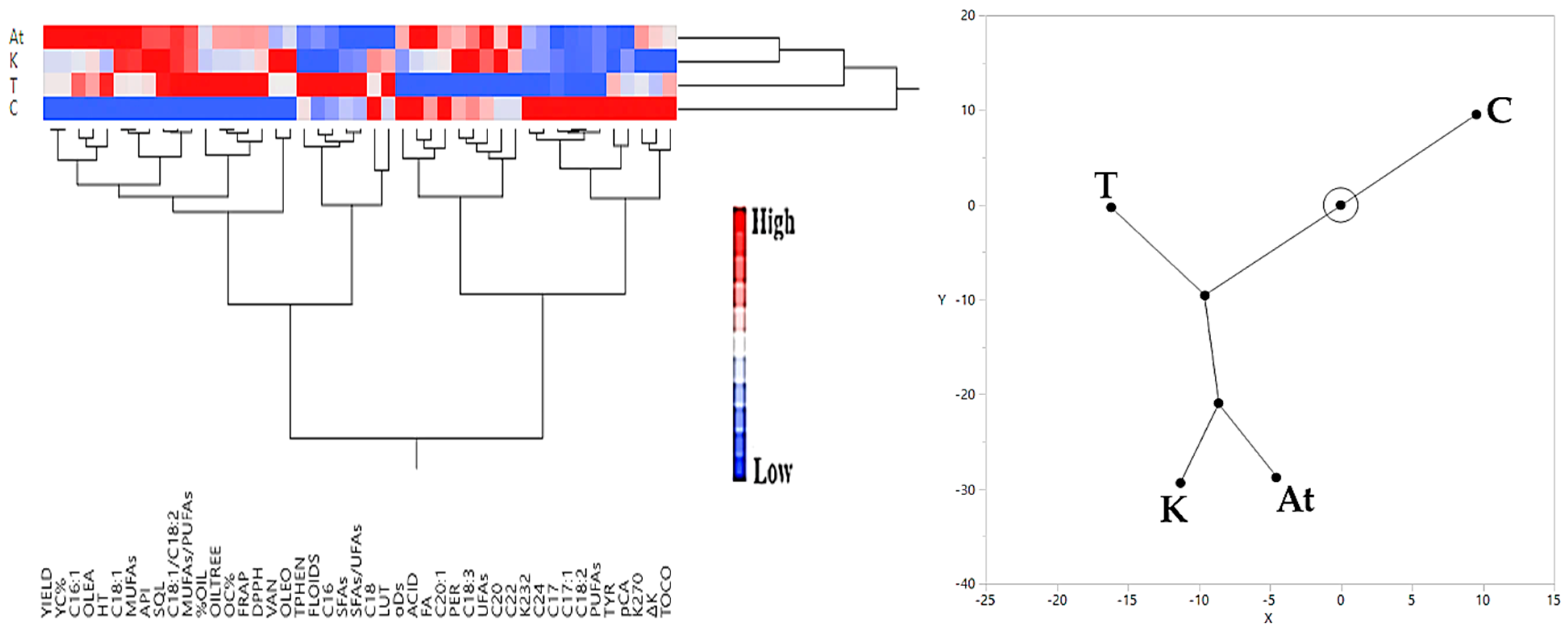
3.3. Hierarchical Cluster Analysis Results Regarding Olive Yield and Olive Oil Quality Characteristics
3.3.1. Irrigated Olive Grove
3.3.2. Rainfed Olive Grove
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, C.; Dinis, L.-T.; Silva, E.; Gonçalves, A.; Matos, C.; Rodrigues, M.A.; Moutinho-Pereira, J.; Barros, A.; Correia, C. Kaolin and salicylic acid foliar application modulate yield, quality and phytochemical composition of olive pulp and oil from rainfed trees. Sci. Hortic. 2018, 237, 176–183. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative effects of exogenous glycine betaine, kaolin clay particles and Ambiol on photosynthesis, leaf sclerophylly indexes and heat load of olive cv. Chondrolia Chalkidikis under drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Roussos, P.; Dimou, A.; Assimakopoulou, A.; Gasparatos, D.; Kostelenos, G.; Bouchaghier, P.; Argyrokastritis, I. Spatial distribution of nutrients and morpho-physiological indicators of salinity tolerance among five olive cultivars-The use of relative nutrient concentration as an efficient tolerance index. J. Plant Nutr. 2019, 42, 2269–2286. [Google Scholar] [CrossRef]
- Roussos, P.; Assimakopoulou, A.; Nikoloudi, A.; Salmas, I.; Nifakos, K.; Kalogeropoulos, P.; Kostelenos, G. Intra-and inter-cultivar impacts of salinity stress on leaf photosynthetic performance, carbohydrates and nutrient content of nine indigenous Greek olive cultivars. Acta Physiol. Plant. 2017, 39, 136. [Google Scholar] [CrossRef]
- Elloumi, O.; Benmoussa, H.; Feki, M.; Chaari, A.; Mimoun, M.B.; Ghrab, M. Assessing agroclimatic requirements and modeling olive phenophase events in warm and sub-arid climate areas. Theor. Appl. Climatol. 2024, 155, 8587–8598. [Google Scholar] [CrossRef]
- Smoly, I.; Elbaz, H.; Engelen, C.; Wechsler, T.; Elbaz, G.; Ben-Ari, G.; Samach, A.; Friedlander, T. A model estimating the level of floral transition in olive trees exposed to warm periods during winter. J. Exp. Bot. 2024, 76, 1266–1284. [Google Scholar] [CrossRef]
- Wortmann, K.; Biton, I.; Zemach, H.; Many, Y.; Ben-Ari, G. Heat stress during reproductive development inhibits fertilization in olives. Sci. Hortic. 2024, 338, 113680. [Google Scholar] [CrossRef]
- Roussos, P.A. Climate Change Challenges in Temperate and Sub-Tropical Fruit Tree Cultivation. Encyclopedia 2024, 4, 558–582. [Google Scholar] [CrossRef]
- do Forno, B.C.B. Influence of Kaolin Application on Physiological Behavior of Olive Trees (Olea europaea L.) Submitted to Water Deficit. Master’s Thesis, Universidade de Tras-os-Montes e Alto Douro, Vila Real, Portugal, 2017; p. 51. [Google Scholar]
- Boari, F.; Donadio, A.; Schiattone, M.I.; Cantore, V. Particle film technology: A supplemental tool to save water. Agric. Water Manag. 2015, 147, 154–162. [Google Scholar] [CrossRef]
- Sharma, R.; Reddy, S.V.R.; Datta, S. Particle films and their applications in horticultural crops. Appl. Clay Sci. 2015, 116, 54–68. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J. Particle films: A new technology for agriculture. Hortic. Rev. 2010, 31, 1–44. [Google Scholar]
- Rotondi, A.; Bertazza, G.; Faccini, B.; Ferretti, G.; Morrone, L. Effect of different foliar particle films (kaolin and zeolitite) on chemical and sensory properties of olive oil. Agronomy 2022, 12, 3088. [Google Scholar] [CrossRef]
- Khaleghi, E.; Arzani, K.; Moallemi, N.; Barzegar, M. The efficacy of kaolin particle film on oil quality indices of olive trees (Olea europaea L.) cv ‘Zard’ grown under warm and semi-arid region of Iran. Food Chem. 2015, 166, 35–41. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin particle film modulates morphological, physiological and biochemical olive tree responses to drought and rewatering. Plant Physiol. Biochem. 2018, 133, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Conde, A.; Pimentel, D.; Neves, A.; Dinis, L.-T.; Bernardo, S.; Correia, C.M.; Gerós, H.; Moutinho-Pereira, J. Kaolin foliar application has a stimulatory effect on phenylpropanoid and flavonoid pathways in grape berries. Front. Plant Sci. 2016, 7, 1150. [Google Scholar] [CrossRef]
- Frioni, T.; Saracino, S.; Squeri, C.; Tombesi, S.; Palliotti, A.; Sabbatini, P.; Magnanini, E.; Poni, S. Understanding kaolin effects on grapevine leaf and whole-canopy physiology during water stress and re-watering. J. Plant Physiol. 2019, 242, 153020. [Google Scholar] [CrossRef]
- Santos, D.P.; da Rocha Sobrinho, M.; de Fátima de Castro Oliveira, M.; Costa, N.B.; Ferraz, T.M.; de Oliveira Reis, F.; Braum, H.; Campostrini, E.; de Assis Figueiredo, F.A.M.M. Effect of applying a calcined kaolin-based particle film on the photosynthetic capacity and growth of young eucalyptus plants. J. For. Res. 2021, 32, 2473–2484. [Google Scholar] [CrossRef]
- Chamchaiyaporn, T.; Jutamanee, K.; Kasemsap, P.; Vaithanomsat, P.; Henpitak, C. Effects of kaolin clay coating on mango leaf gas exchange, fruit yield and quality. Agric. Nat. Resour. 2013, 47, 479–491. [Google Scholar]
- Luciani, E.; Palliotti, A.; Frioni, T.; Tombesi, S.; Villa, F.; Zadra, C.; Farinelli, D. Kaolin treatments on Tonda Giffoni hazelnut (Corylus avellana L.) for the control of heat stress damages. Sci. Hortic. 2020, 263, 109097. [Google Scholar] [CrossRef]
- Antonakou, M.; Arapogiannis, T.; Roussos, P. Surround®(kaolin 95% w/w) wp crop protectant: A new broad spectrum crop protect antagainst insects, sun burn and heat stress on man ycrops. In Proceedings of the International Symposium on: Organic Agriculture in Mediterranean Problems and Perspectives, Chania, Greece, 4 July 2005; pp. 9–11. [Google Scholar]
- Oliveira, M.; Fernandes-Silva, A. Vineyard and Olive Orchard Management to Maintain Yield and Quality Under Abiotic Stress Conditions. In Modern Fruit Industry; Intech Open: Rijeka, Croatia, 2019. [Google Scholar]
- Nazeri, M.; Tabatabaei, S.J.; Mollayi, N. The effect of talc, kaolin, and zinc oxide on heat stress in pomegranate cv. Malas saveh. Iran. J. Plant Physiol. 2024, 1, 4809. [Google Scholar]
- Schrader, L.E.; Kahn, C.; Elfving, D.C. Sunburn browning decreases at-harvest internal fruit quality of apples (Malus domestica Borkh.). Int. J. Fruit Sci. 2009, 9, 425–437. [Google Scholar] [CrossRef]
- Paušic, A.; Lešnik, M. Controlling of spotted wing Drosophila (Drosophila suzukii Matsumura) on grapevine with the application of talc. In Proceedings of the Lectures and Papers from the 15th Slovenian Conference on Plant Protection, Portorož, Slovenia, 1–2 March 2022; pp. 355–360. [Google Scholar]
- Karyda, A.-G.; Christopoulou, V.; Gasparatos, D.; Roussos, P.A. The effects of attapoulgite alone plus olive mill waste on olive yield, olive oil quality, leaf nutrient status and soil properties. In Proceedings of the VI International Agricultural, Biological, Life Science Conference AGBIOL, Edirne, Turkey, 18–20 September 2024; p. 253. [Google Scholar]
- Liu, T.; Su, Y.; Niu, Z.; An, F. Attapulgite application improves maize yield, water, and fertilizer utilization efficiency in newly cultivated sandy farmland in northwestern China. Arid. Land Res. Manag. 2023, 37, 408–426. [Google Scholar] [CrossRef]
- Yang, T.; Xing, X.; Gao, Y.; Ma, X. An environmentally friendly soil amendment for enhancing soil water availability in drought-prone soils. Agronomy 2022, 12, 133. [Google Scholar] [CrossRef]
- Roussos, P.A.; Karabi, A.; Anastasiou, L.; Assimakopoulou, A.; Gasparatos, D. Apricot Tree Nutrient Uptake, Fruit Quality and Phytochemical Attributes, and Soil Fertility under Organic and Integrated Management. Appl. Sci. 2023, 13, 2596. [Google Scholar] [CrossRef]
- Azizifar, S.; Abdossi, V.; Gholami, R.; Ghavami, M.; Torkashvand, A.M. Effect of salicylic acid and kaolin on yield, physiological traits, and fatty acid composition in olive cultivars under regulated deficit irrigation. Acta Sci. Pol. Hortorum Cultus 2022, 21, 131–140. [Google Scholar] [CrossRef]
- Maletsika, P.; Nanos, G. Leaf and Fruit Responses to Kaolin Particle Film Applied onto Mature Olive Trees. J. Biol. Agric. Healthc. 2015, 5, 17–27. [Google Scholar]
- Saour, G.; Makee, H. Effects of kaolin particle film on olive fruit yield, oil content and quality. Adv. Hortic. Sci. 2003, 17, 204–206. [Google Scholar]
- Abdel Ghani, N.; Galal, M.; El-Sayed, M.; El-Marsafawy, S.M.; Omran, M. Effect of spraying kaolin and calcium carbonate on the productivity of “aggezi and picual” olive cvs. J. Plant Prod. 2013, 4, 1035–1050. [Google Scholar] [CrossRef]
- Azizifar, S.; Abdossi, V.; Gholami, R.; Ghavami, M.; Mohammadi Torkashvand, A. Evaluation of the effects of kaolin and salicylic acid on yield and some physiological responses of olives under drought stress. J. Hortic. Sci. 2022, 35, 605–619. [Google Scholar]
- Brito, C.; Dinis, L.-T.; Luzio, A.; Silva, E.; Gonçalves, A.; Meijón, M.; Escandón, M.; Arrobas, M.; Rodrigues, M.Â.; Moutinho-Pereira, J.; et al. Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical responses. Sci. Hortic. 2019, 246, 201–211. [Google Scholar] [CrossRef]
- Taskin, S.; Ertan, E. Exogenous applications of kaolin and glycine betaine increased the yield and quality of olive fruit and olive oil. Erwerbs-Obstbau 2023, 65, 337–346. [Google Scholar] [CrossRef]
- Cirillo, A.; Conti, S.; Graziani, G.; El-Nakhel, C.; Rouphael, Y.; Ritieni, A.; Di Vaio, C. Mitigation of high-temperature damage by application of kaolin and pinolene on young olive trees (Olea europaea L.): A preliminary experiment to assess biometric, eco-physiological and nutraceutical parameters. Agronomy 2021, 11, 1884. [Google Scholar] [CrossRef]
- Hamdy, A.E.; Abdel-Aziz, H.F.; El-Khamissi, H.; AlJwaizea, N.I.; El-Yazied, A.A.; Selim, S.; Tawfik, M.M.; AlHarbi, K.; Ali, M.S.; Elkelish, A. Kaolin improves photosynthetic pigments, and antioxidant content, and decreases sunburn of mangoes: Field study. Agronomy 2022, 12, 1535. [Google Scholar] [CrossRef]
- Brito, C.; Gonçalves, A.; Silva, E.; Martins, S.; Pinto, L.; Rocha, L.; Arrobas, M.; Rodrigues, M.Â.; Moutinho-Pereira, J.; Correia, C.M. Kaolin foliar spray improves olive tree performance and yield under sustained deficit irrigation. Sci. Hortic. 2021, 277, 109795. [Google Scholar] [CrossRef]
- Gharaghani, A.; Javarzari, A.M.; Rezaei, A.; Nejati, R. Kaolin spray improves growth, physiological functions, yield, and nut quality of ‘Tardy Nonpareil’ almond under deficit irrigation regimens. Erwerbs-Obstbau 2023, 65, 989–1001. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Brahmi, F.; Dabbou, S.; Hassine, K.B.; Taamali, A.; Chehab, H.; Ellouz, M.; Zarrouk, M.; Hammami, M. Effect of the growing area conditions on differentiation between Chemlali and Chétoui olive oils. Food Chem. 2010, 119, 220–225. [Google Scholar] [CrossRef]
- Rouina, Y.B.; Zouari, M.; Zouari, N.; Rouina, B.B.; Bouaziz, M. Olive tree (Olea europaea L. cv. Zelmati) grown in hot desert climate: Physio-biochemical responses and olive oil quality. Sci. Hortic. 2020, 261, 108915. [Google Scholar]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S.; Montedoro, G.; Serravalle, M.; Gucci, R. Irrigation effects on quality, phenolic composition, and selected volatiles of virgin olive oils cv. Leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef]
- Fernandes-Silva, A.; Gouveia, J.; Vasconcelos, P.; Ferreira, T.C.; Villalobos, F.J. Effect of different irrigation regimes on the quality attributes of monovarietal virgin olive oil from cv. “Cobrançosa”. Grasas Y Aceites 2013, 64, 41–49. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Chartzoulakis, K.; HarwooTd, J. Effect of irrigation on quality attributes of olive oil. J. Agric. Food Chem. 2009, 57, 7048–7055. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; Esti, M.; Notte, E.L. Evolution of phenolic compounds in virgin olive oil during storage. J. Am. Oil Chem. Soc. 1997, 74, 1259–1264. [Google Scholar] [CrossRef]
- Papanikolaou, C.; Melliou, E.; Magiatis, P. Olive oil phenols. In Functional Foods; Intech Open: Rijeka, Croatia, 2019. [Google Scholar]
- Petoumenou, D.G. Enhancing yield and physiological performance by foliar applications of chemically inert mineral particles in a rainfed vineyard under Mediterranean conditions. Plants 2023, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Patumi, M.; d’Andria, R.; Marsilio, V.; Fontanazza, G.; Morelli, G.; Lanza, B. Olive and olive oil quality after intensive monocone olive growing (Olea europaea L., cv. Kalamata) in different irrigation regimes. Food Chem. 2002, 77, 27–34. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Bernardo, S.; Conde, A.; Pimentel, D.; Ferreira, H.; Félix, L.; Gerós, H.; Correia, C.; Moutinho-Pereira, J. Kaolin exogenous application boosts antioxidant capacity and phenolic content in berries and leaves of grapevine under summer stress. J. Plant Physiol. 2016, 191, 45–53. [Google Scholar] [CrossRef]
- Sharma, R.; Datta, S.; Varghese, E. Effect of Surround WP®, a kaolin-based particle film on sunburn, fruit cracking and postharvest quality of ‘Kandhari’ pomegranates. Crop Protection 2018, 114, 18–22. [Google Scholar] [CrossRef]
- Bongiorno, D.; Di Stefano, V.; Indelicato, S.; Avellone, G.; Ceraulo, L. Bio-phenols determination in olive oils: Recent mass spectrometry approaches. Mass. Spectrom. Rev. 2023, 42, 1462–1502. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Díaz, T.; López, Ó.; Fernández-Bolaños, J.G.; Sánchez, J.; De Miguel, C.; Gil, M.V.; Martín-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef]
- Servili, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Tsolis, T.; Kyriakou, D.; Sifnaiou, E.; Thomos, D.; Glykos, D.; Tsiafoulis, C.G.; Garoufis, A. NMR Analysis of Extra Virgin Olive Oil of the Epirus Region of Greece with Emphasis on Selected Phenolic Compounds. Molecules 2024, 29, 1111. [Google Scholar] [CrossRef]
- Kritikou, E.; Kalogiouri, N.P.; Kostakis, M.; Kanakis, D.-C.; Martakos, I.; Lazarou, C.; Pentogennis, M.; Thomaidis, N.S. Geographical characterization of olive oils from the North Aegean region based on the analysis of biophenols with UHPLC-QTOF-MS. Foods 2021, 10, 2102. [Google Scholar] [CrossRef] [PubMed]
- Diamantakos, P.; Ioannidis, K.; Papanikolaou, C.; Tsolakou, A.; Rigakou, A.; Melliou, E.; Magiatis, P. A new definition of the term “high-phenolic olive oil” based on large scale statistical data of Greek olive oils analyzed by qNMR. Molecules 2021, 26, 1115. [Google Scholar] [CrossRef] [PubMed]
- Faghim, J.; Mohamed, M.B.; Bagues, M.; Guasmi, F.; Triki, T.; Nagaz, K. Irrigation effects on phenolic profile and extra virgin olive oil quality of “Chemlali” variety grown in south Tunisia. S. Afr. J. Bot. 2021, 141, 322–329. [Google Scholar] [CrossRef]
- Hernández, M.L.; Padilla, M.N.; Sicardo, M.D.; Mancha, M.; Martínez-Rivas, J.M. Effect of different environmental stresses on the expression of oleate desaturase genes and fatty acid composition in olive fruit. Phytochemistry 2011, 72, 178–187. [Google Scholar] [CrossRef]
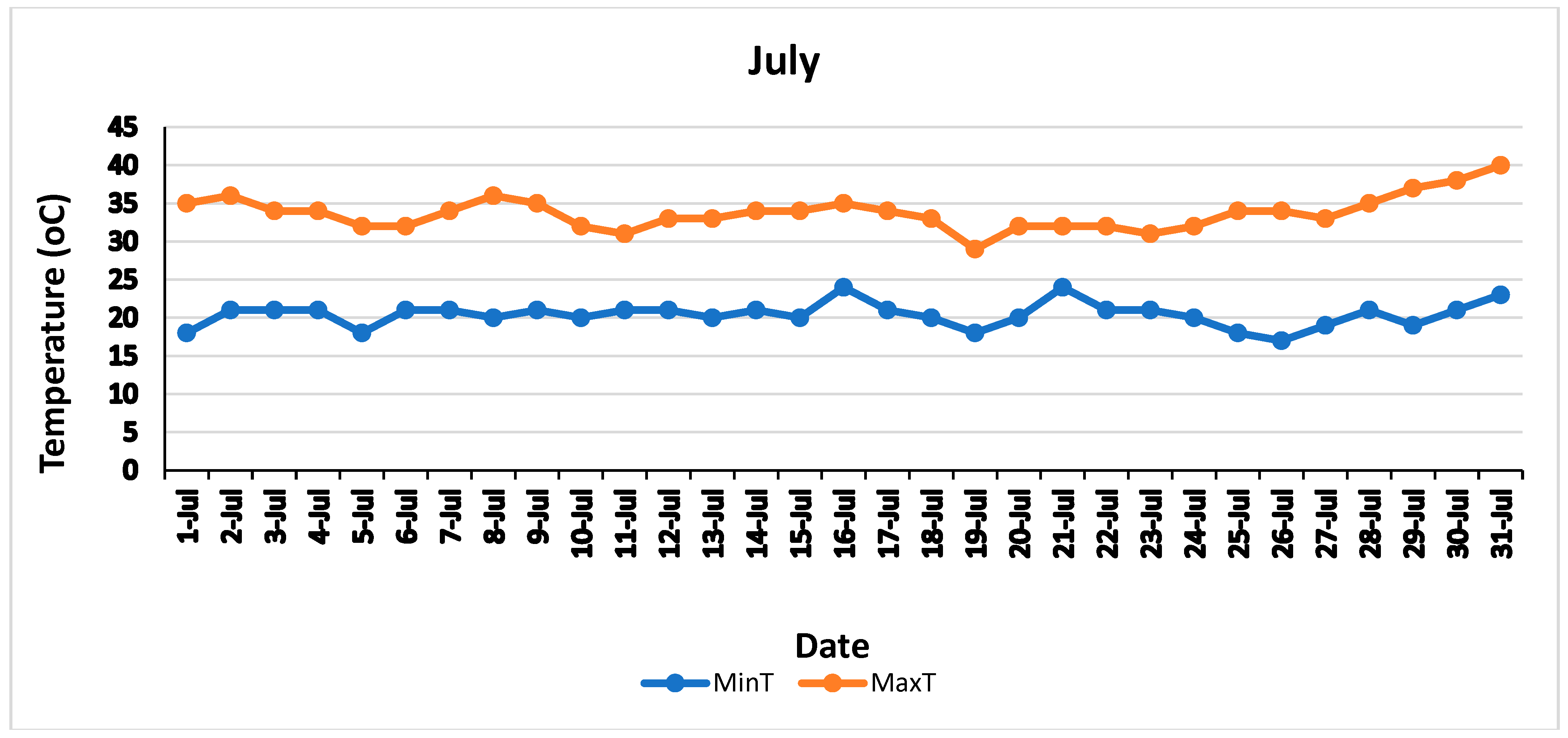
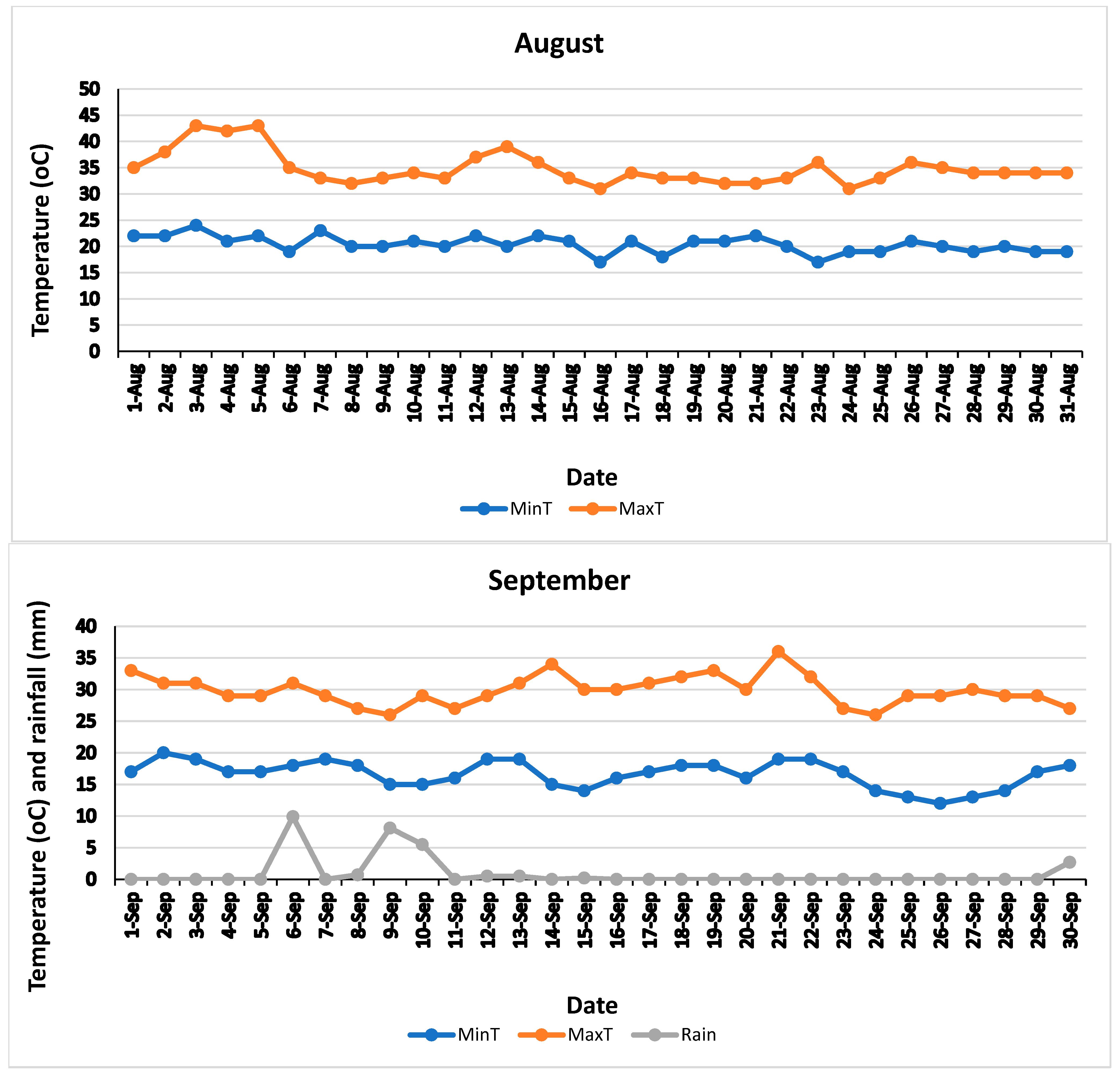

| Treatment | Yield (kg/Tree) | Oil Percentage Per Fruit | Oil Per Tree (Kg) | Yield Change (%) | Oil Per Tree Change (%) |
|---|---|---|---|---|---|
| Irrigated olive orchard | |||||
| Control | 40.4 b | 17.75 a | 7.20 a | 100.0 a | 100.0 a |
| At-J | 48.3 ab | 14.89 a | 7.14 a | 119.5 a | 99.1 a |
| At-A | 44.9 ab | 16.90 a | 7.57 a | 111.1 a | 105.1 a |
| K-J | 51.5 a * | 16.32 a | 8.43 a | 127.3 a | 117.0 a |
| K-A | 43.0 ab | 16.87 a | 7.42 a | 106.4 a | 103.1 a |
| T-J | 49.9 a * | 17.18 a | 8.76 a | 123.6 a | 121.6 a |
| T-A | 39.9 b | 18.33 a | 7.34 a | 98.9 a | 101.9 a |
| Rainfed olive orchard | |||||
| Control | 21.3 b | 13.46 b | 2.87 c | 100.0 b | 100.0 c |
| At-J | 24.7 ab | 16.89 b | 4.12 ab * | 115.7 ab | 144.1 ab * |
| At-A | 29.0 a * | 15.46 b | 4.47 a * | 136.1 a * | 156.6 a * |
| K-J | 23.4 ab | 18.04 b* | 4.30 a * | 109.8 ab | 150.7 a * |
| K-A | 23.8 ab | 13.46 b | 3.25 bc | 111.9 ab | 113.0 bc |
| T-J | 21.3 b | 24.05 a * | 5.12 a * | 100.0 b | 178.5 a * |
| T-A | 27.0 ab * | 16.61 b | 4.42 a * | 126.7 ab * | 154.4 a * |
| Treatment | Free Acidity (g Oleic Acid 100 g−1) | Peroxides (meq O2 Kg−1) | K232 | K270 | ΔK |
|---|---|---|---|---|---|
| Irrigated olive orchard | |||||
| Control | 0.30 a | 9.75 a | 1.46 a | 0.107 bc | −0.03 a |
| At-J | 0.25 a | 11.0 a | 1.50 a | 0.140 ab | −0.01 a |
| At-A | 0.25 a | 9.87 a | 1.55 a | 0.122 abc | −0.02 a |
| K-J | 0.26 a | 10.00 a | 1.31 ab | 0.105 bc | −0.03 a |
| K-A | 0.23 a | 11.00 a | 1.54 a | 0.152 a * | −0.01 a |
| T-J | 0.26 a | 8.63 a | 1.04 b * | 0.085 c | −0.06 a |
| T-A | 0.21 a | 11.00 a | 1.47 a | 0.120 abc | −0.02 a |
| Rainfed olive orchard | |||||
| Control | 0.31 ab | 12.50 a | 1.65 a | 0.150 a | 0.020 a |
| At-J | 0.29 ab | 12.50 a | 1.52 ab * | 0.135 ab | −0.030 ab |
| At-A | 0.33 a | 12.37 a | 1.30 bc | 0.122 ab | −0.010 ab |
| K-J | 0.26 ab | 11.25 a | 1.43 ab * | 0.067 cd * | −0.072 b * |
| K-A | 0.30 ab | 15.00 a | 1.34 bc | 0.050 d * | −0.067 b * |
| T-J | 0.23 b * | 9.87 a | 1.54 ab * | 0.122 ab | −0.005 ab |
| T-A | 0.26 ab | 9.87 a | 1.14 c | 0.095 bc * | −0.072 b * |
| Treatment | Total Phenols (mg GAE Kg−1) | o-Diphenols (mg CAE/Kg−1) | Total Flavonoids (mg CtE Kg−1) | FRAP (μmol Trolox Equiv. Kg−1) | DPPH (μmol Trolox Equiv. Kg−1) |
|---|---|---|---|---|---|
| Irrigated olive orchard | |||||
| Control | 60.72 c | 19.91 ab | 52.49 ab | 1036.4 a | 319.6 a |
| At-J | 77.13 abc | 15.96 b | 47.74 b | 1454.0 a | 322.2 a |
| At-A | 73.26 abc | 20.67 ab | 63.07 ab | 1168.0 a | 323.5 a |
| K-J | 63.51 c | 24.96 ab | 58.40 ab | 1471.6 a | 305.3 a |
| K-A | 98.33 ab * | 24.79 ab | 77.98 a | 1968.0 a * | 472.1 a |
| T-J | 66.29 bc | 28.32 ab | 57.40 ab | 1555.6 a | 301.4 a |
| T-A | 105.29 a * | 32.43 a * | 73.23 ab | 1913.7 a | 426.5 a |
| Rainfed olive orchard | |||||
| Control | 69.08 b | 25.69 a | 53.07 a | 1073.2 b | 266.2 c |
| At-J | 54.69 b | 20.33 a | 49.41 a | 1201.9 ab | 666.2 ab * |
| At-A | 53.29 b | 25.21 a | 57.48 a | 1499.6 ab | 667.5 ab * |
| K-J | 64.91 b | 20.16 a | 52.90 a | 1243.0 ab | 718.3 ab * |
| K-A | 37.51 b | 16.97 a | 51.40 a | 1277.2 ab | 507.3 bc |
| T-J | 120.62 a * | 20.17 a | 72.31 a | 1250.0 ab | 651.9 ab * |
| T-A | 63.04 b | 13.53 a * | 60.98 a | 1700.1 a * | 933.3 a * |
| Treatment | Hydroxytyrosol | Tyrosol | Vanillin | p-coumaric Acid | Ferulic Acid | Oleacein | Oleocanthal | Luteolin | Apigenin | a-Tocopherol |
|---|---|---|---|---|---|---|---|---|---|---|
| Irrigated olive orchard | ||||||||||
| Control | 0.95 a | 5.84 ab | 0.12 a | 0.42 a | 0.08 a | 23.99 a | 72.48 a | 7.32 a | 1.56 a | 31.26 a |
| At-J | 0.39 a | 3.97 ab | 0.15 a | 0.42 a | 0.08 a | 26.47 a | 76.36 a | 6.55 a | 1.27 a | 33.62 a |
| At-A | 0.19 a | 2.83 b * | 0.17 a * | 0.39 a | 0.07 a | 28.98 a | 80.71 a | 6.51 a | 1.05 a | 33.30 a |
| K-J | 0.86 a | 4.17 ab | 0.14 a | 0.41 a | 0.08 a | 25.79 a | 72.89 a | 6.50 a | 1.45 a | 34.79 a |
| K-A | 0.51 a | 3.94 ab | 0.13 a | 0.32 a | 0.08 a | 30.39 a * | 90.65 a | 8.26 a | 1.21 a | 30.65 a |
| T-J | 1.28 a | 6.95 a | 0.14 a | 0.44 a | 0.09 a | 27.40 a | 75.19 a | 6.80 a | 1.57 a | 35.34 a |
| T-A | 0.78 a | 4.84 ab | 0.15 a | 0.34 a | 0.08 a | 29.96 a | 90.00 a | 7.50 a | 1.25 a | 39.37 a |
| Rainfed olive orchard | ||||||||||
| Control | 0.13 b | 9.73 a | 0.13 b | 0.38 a | 0.06 a | 21.97 c | 101.78 b | 11.06 ab | 1.32 a | 44.10 a |
| At-J | 0.27 b | 4.62 ab | 0.14 b | 0.32 ab | 0.06 a | 25.20 bc | 120.93 ab | 11.43 ab | 1.61 a | 46.60 a |
| At-A | 1.00 a * | 4.42 ab * | 0.14 b | 0.27 b * | 0.06 a | 39.49 a * | 123.82 ab | 8.66 b | 1.65 a | 37.66 a |
| K-J | 0.35 ab | 5.41 ab | 0.20 a * | 0.33 ab | 0.06 a | 25.54 bc | 160.56 a * | 14.85 a | 1.50 a | 35.92 a |
| K-A | 0.31 b | 3.55 b * | 0.16 ab | 0.29 ab | 0.06 a | 31.89 ab * | 143.81 ab * | 8.38 b | 1.80 a | 42.41 a |
| T-J | 0.36 ab | 9.95 a | 0.14 b | 0.35 ab | 0.04 a | 22.23 c | 111.90 b | 13.02 ab | 1.01 a | 42.23 a |
| T-A | 1.01 a * | 5.99 ab | 0.16 ab | 0.29 ab * | 0.06 a | 36.77 a * | 137.10 ab | 12.08 ab | 1.99 a | 43.04 a |
| Treatment | C16 | C16:1 | C17 | C17:1 | C18 | C18:1 | C18:2 | C20 | C18:3 | C20:1 | C22 | C24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irrigated olive orchard | ||||||||||||
| Control | 8.54 a | 0.85 a | 0.03 a | 0.06 a | 2.47 b | 80.39 a | 5.83 ab | 0.47 a | 0.76 d | 0.37 a | 0.16 a | 0.04 a |
| At-J | 7.90 ab | 0.84 a | 0.03 a | 0.06 a | 2.58 ab | 80.79 a | 5.90 ab | 0.49 a | 0.82 a–d * | 0.37 a | 0.16 a | 0.05 a |
| At-A | 8.10 a | 0.83 a | 0.03 a | 0.07 a | 2.61 ab * | 80.17 a | 6.29 a | 0.49 a | 0.84 abc * | 0.37 a | 0.16 a | 0.05 a |
| K-J | 8.19 a | 0.84 a | 0.03 a | 0.07 a | 2.53 ab | 80.81 a | 5.68 b | 0.48 a | 0.78 bcd | 0.37 a | 0.16 a | 0.05 a |
| K-A | 7.02 b * | 0.84 a | 0.03 a | 0.07 a | 2.57 ab | 81.18 a | 6.37 a * | 0.48 a | 0.87 a * | 0.37 a | 0.15 a | 0.05 a |
| T-J | 8.06 a | 0.86 a | 0.03 a | 0.07 a | 2.48 ab | 80.83 a | 5.84 ab | 0.47 a | 0.77 cd | 0.38 a | 0.16 a | 0.05 a |
| T-A | 7.57 b * | 0.84 a | 0.03 a | 0.07 a | 2.64 a * | 80.77 a | 6.16 ab | 0.49 a | 0.84 ab * | 0.36 a | 0.16 a | 0.05 a |
| Rainfed olive orchard | ||||||||||||
| Control | 8.47 b | 0.86 a | 0.06 a | 0.12 a | 2.52 ab | 77.41 b | 8.69 a | 0.44 a | 0.82 a | 0.37 ab | 0.15 a | 0.04 a |
| At-J | 8.68 b | 0.94 a * | 0.04 b * | 0.08 b * | 2.47 abc | 79.61 a * | 6.35 b * | 0.45 a | 0.84 a | 0.35 abc | 0.16 a | 0.04 a |
| At-A | 8.08 b | 0.88 a | 0.03 b * | 0.08 b * | 2.31 bc * | 80.67 a * | 6.07 b * | 0.43 a | 0.87 a | 0.37 ab | 0.15 a | 0.04 a |
| K-J | 8.78 b | 0.88 a | 0.03 b * | 0.07 b * | 2.64 a | 79.48 a * | 6.31 b * | 0.46 a | 0.73 a | 0.33 bc * | 0.15 a | 0.04 a |
| K-A | 7.91 b | 0.91 a | 0.04 b * | 0.08 b * | 2.36 bc | 80.68 a * | 6.10 b * | 0.45 a | 0.91 a | 0.38 a | 0.15 a | 0.04 a |
| T-J | 10.13 a * | 0.93 a | 0.03 b * | 0.07 b * | 2.67 a | 77.94 b | 6.58 b * | 0.45 a | 0.69 b * | 0.32 c * | 0.14 a | 0.04 a |
| T-A | 8.40 b | 0.89 a | 0.04 b * | 0.08 b * | 2.24 c * | 80.85 a * | 5.78 b * | 0.41 a | 0.82 a | 0.34 abc | 0.14 a | 0.03 a |
| Treatment | SFAs | MUFAs | PUFAs | UFAs | MUFAs/PUFAs | SFA s/UFAs | C18:1/C18:2 | Squalene (mg/100 g) |
|---|---|---|---|---|---|---|---|---|
| Irrigated olive orchard | ||||||||
| Control | 11.72 a | 81.67 a | 6.59 bc | 88.27 b | 12.40 ab | 0.132 a | 13.82 ab | 499.3 a |
| At-J | 11.20 ab | 82.07 a | 6.72 abc | 88.74 ab | 12.21 ab | 0.127 ab | 13.70 ab | 518.9 a |
| At-A | 11.44 a | 81.43 a | 7.12 ab | 88.56 b | 11.45 b | 0.130 a | 12.78 b | 502.9 a |
| K-J | 11.44 a | 82.10 a | 6.46 c | 88.56 b | 12.72 a | 0.130 a | 14.24 a | 525.3 a |
| K-A | 10.30 b * | 82.46 a | 7.23 a* | 89.70 a * | 11.40 b | 0.112 b | 12.75 b | 504.3 a |
| T-J | 11.26 ab | 82.13 a | 6.61 bc | 88.74 ab | 12.43 ab | 0.127 ab | 13.85 ab | 524.2 a |
| T-A | 10.94 ab | 82.05 a | 7.00 abc | 89.05 ab | 11.73 ab | 0.122 ab | 12.75 b | 504.2 a |
| Rainfed olive orchard | ||||||||
| Control | 11.69 bc | 78.76 c | 9.54 a | 88.30 ab | 8.36 b | 0.132 bc | 9.06 c | 412.9 b |
| At-J | 11.82 bc | 80.98 a * | 7.19 b * | 88.17 ab | 11.26 a * | 0.135 bc | 12.54 ab * | 529.4 ab |
| At-A | 11.04 c | 82.00 a * | 6.95 b * | 88.95 a | 11.81 a * | 0.125 bc | 13.29 ab * | 557.6 ab * |
| * K-J | 12.09 b | 80.77 ab * | 7.14 b * | 87.90 b | 11.33 a * | 0.140 ab | 12.63 ab * | 519.8 ab |
| K-A | 10.94 c | 82.04 a * | 7.01 b * | 89.06 a | 11.69 a * | 0.122 c | 13.21 ab * | 579.4 a * |
| T-J | 13.46 a * | 79.26 bc | 7.28 b * | 86.54 c * | 10.92 a * | 0.155 a * | 11.89 b * | 508.3 ab |
| T-A | 11.25 bc | 82.15 a * | 6.61 b * | 88.76 ab | 12.47 a * | 0.127 bc | 14.03 a * | 568.8 a * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roussos, P.A.; Karyda, A.-G.; Kapasouris, P.; Kosmadaki, P.G.; Kotsi, C.; Zoti, M. Efficacy Evaluation of Different Mineral Clay Particles on Olive Production Traits and Olive Oil Quality of ‘Koroneiki’ Olive Cultivar Under Rainfed and Irrigated Conditions in Southern Greece. Horticulturae 2025, 11, 579. https://doi.org/10.3390/horticulturae11060579
Roussos PA, Karyda A-G, Kapasouris P, Kosmadaki PG, Kotsi C, Zoti M. Efficacy Evaluation of Different Mineral Clay Particles on Olive Production Traits and Olive Oil Quality of ‘Koroneiki’ Olive Cultivar Under Rainfed and Irrigated Conditions in Southern Greece. Horticulturae. 2025; 11(6):579. https://doi.org/10.3390/horticulturae11060579
Chicago/Turabian StyleRoussos, Petros Anargyrou, Asimina-Georgia Karyda, Panagiotis Kapasouris, Panagiota G. Kosmadaki, Chrysa Kotsi, and Maria Zoti. 2025. "Efficacy Evaluation of Different Mineral Clay Particles on Olive Production Traits and Olive Oil Quality of ‘Koroneiki’ Olive Cultivar Under Rainfed and Irrigated Conditions in Southern Greece" Horticulturae 11, no. 6: 579. https://doi.org/10.3390/horticulturae11060579
APA StyleRoussos, P. A., Karyda, A.-G., Kapasouris, P., Kosmadaki, P. G., Kotsi, C., & Zoti, M. (2025). Efficacy Evaluation of Different Mineral Clay Particles on Olive Production Traits and Olive Oil Quality of ‘Koroneiki’ Olive Cultivar Under Rainfed and Irrigated Conditions in Southern Greece. Horticulturae, 11(6), 579. https://doi.org/10.3390/horticulturae11060579






