Effects of Training Systems on Sugar Accumulation and Metabolism in ‘Kyoho’ Grape Berries
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
- (1)
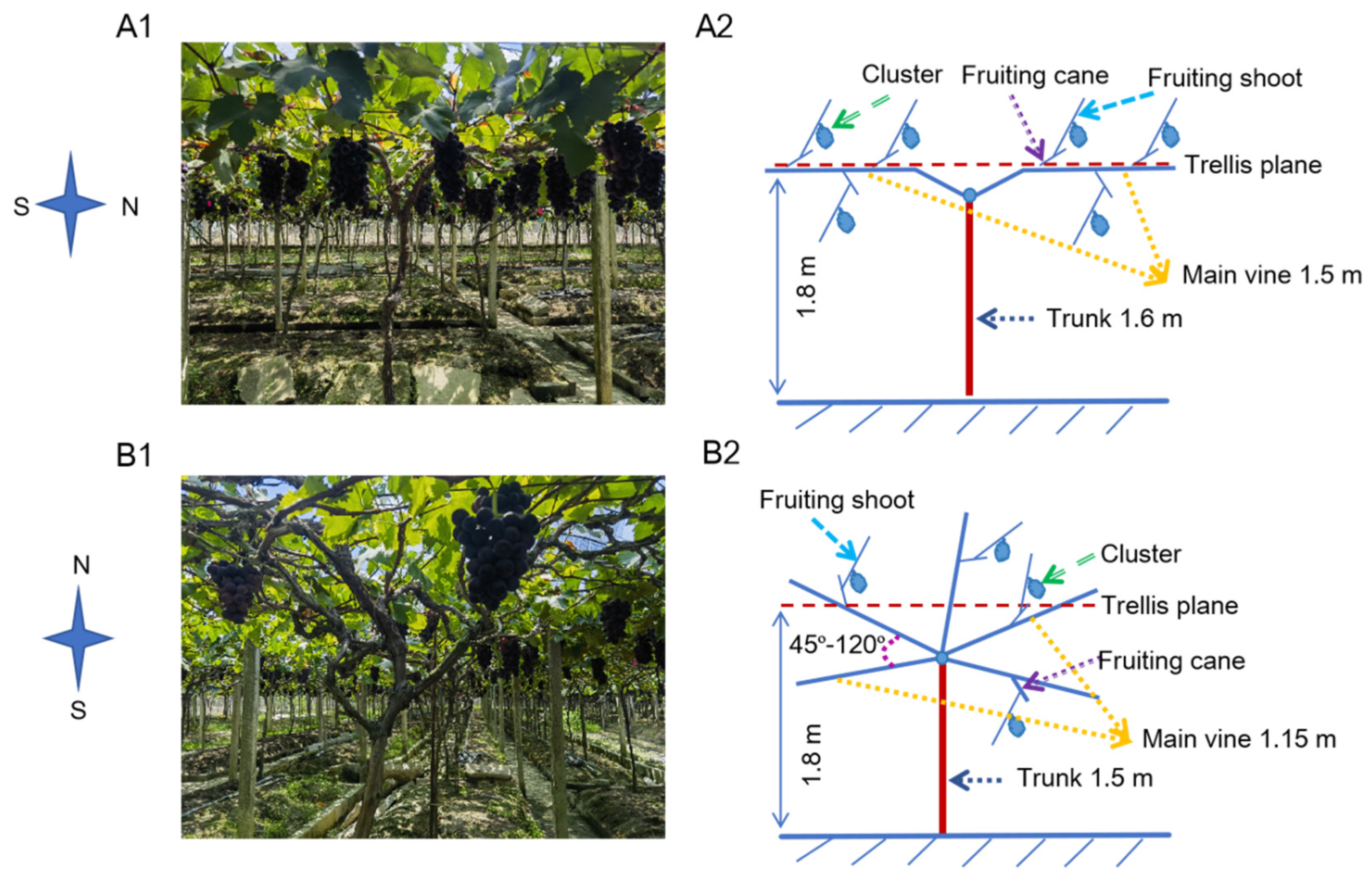
- The SL system, planted with 3.0 × 2.3 m spacing, featured a 1.6 m vertical trunk with two permanent horizontal cordons developed from opposing lateral shoots (Figure 1A). These cordons were spur-pruned, retaining fruiting units every 20 cm (2–3 buds per spur during winter pruning).
- (2)
- The IU system, planted with 1.5 × 2.3 m spacing, maintained a 1.5 m vertical trunk with multiple lateral branches trained radially at 45–120° angles to form an open-center canopy (Figure 1B). It similarly employed spur pruning (2–3 fruiting spurs per cordon, 2–3 buds retained).
2.2. Measurement Parameters and Methods
2.2.1. Determination of Fruit Phenotypic Traits, Color Parameters, and Biochemical Indices
- L*: lightness (0 = black, 100 = white);
- a*: green (−) to red (+) spectrum;
- b: blue (−) to yellow (+) spectrum;
- C: chroma (saturation intensity);
- h°: hue angle (0° = red, 90° = yellow, 180° = green).
2.2.2. Sugar Component Analysis
2.2.3. Transcriptome Sequencing and Gene Expression Quantification
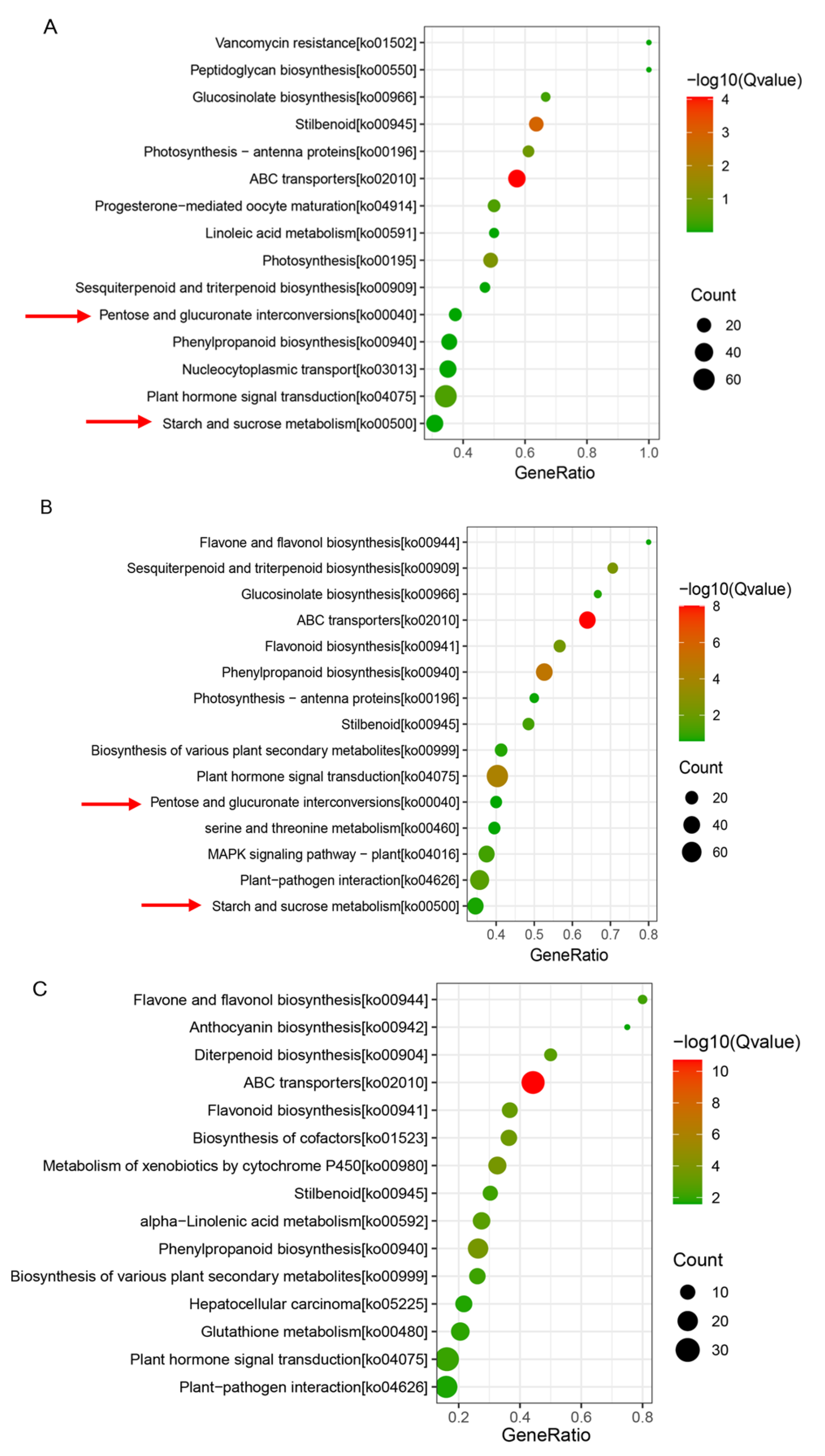
2.2.4. Metabolic Pathway Enrichment Analysis of DEGs
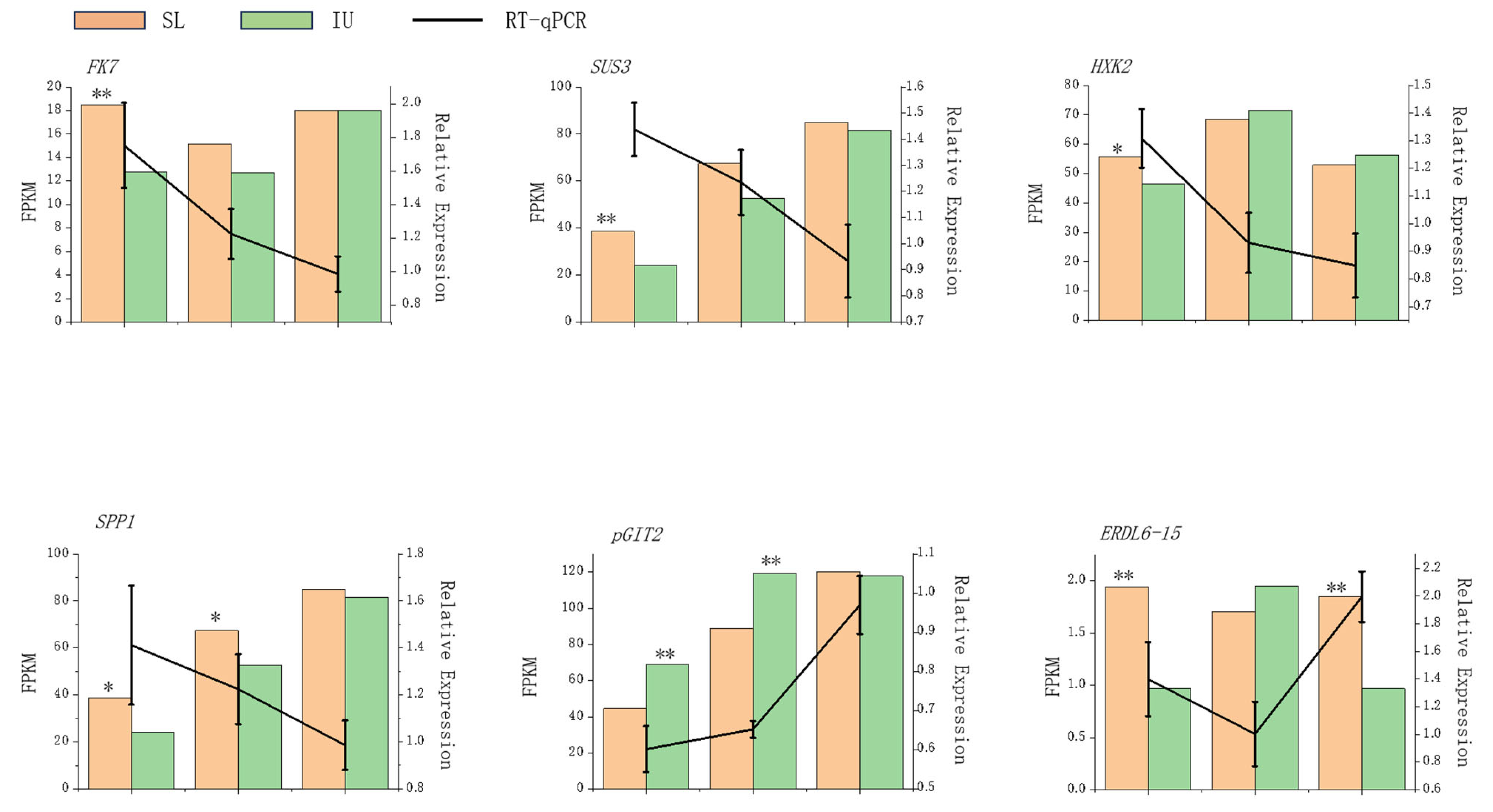
2.2.5. Quantitative Real-Time PCR (qRT-PCR)
2.3. Data Statistics and Analysis
3. Results
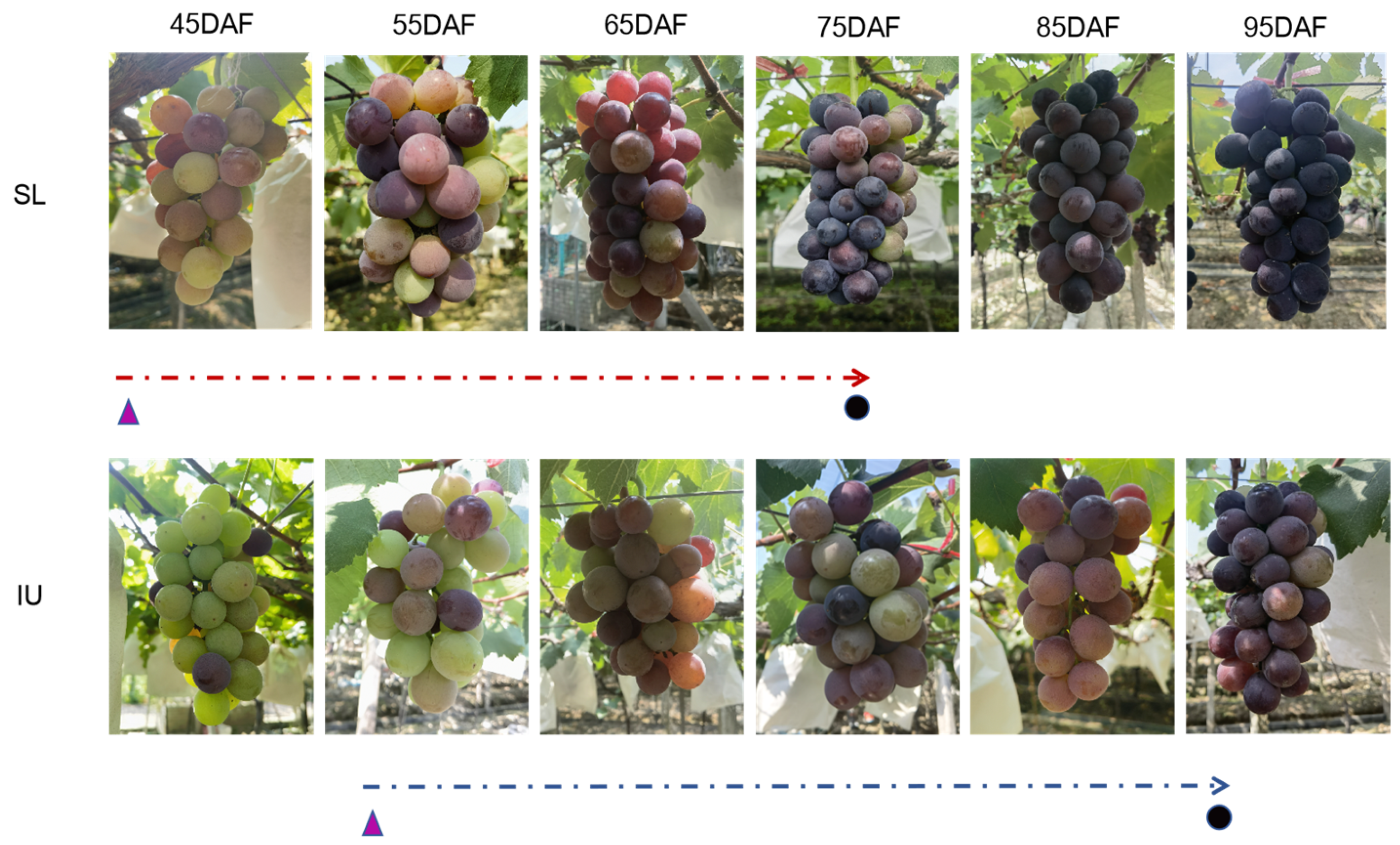
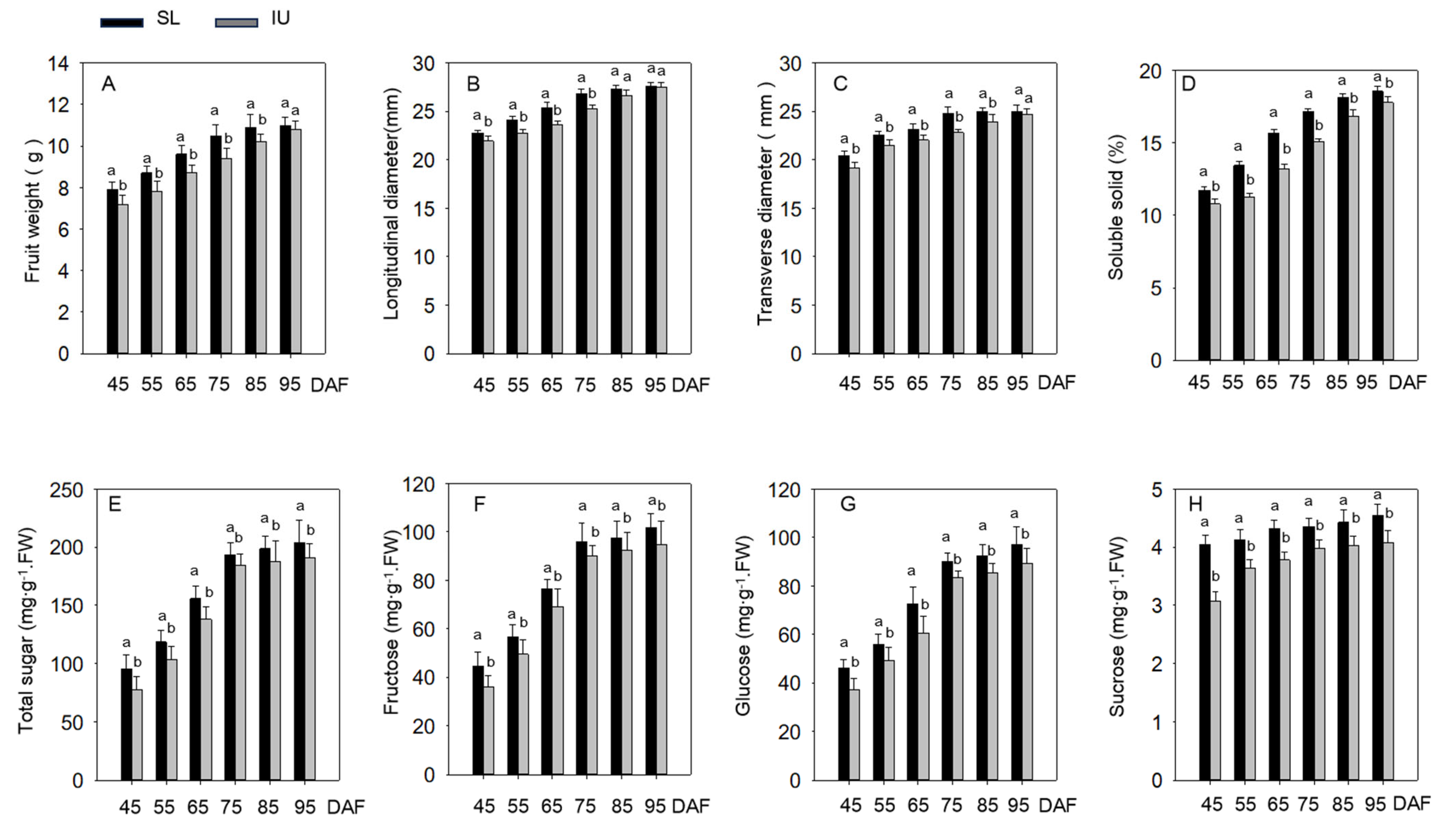
3.1. Effects of Training Systems on Grape Berry Development and Sugar Accumulation
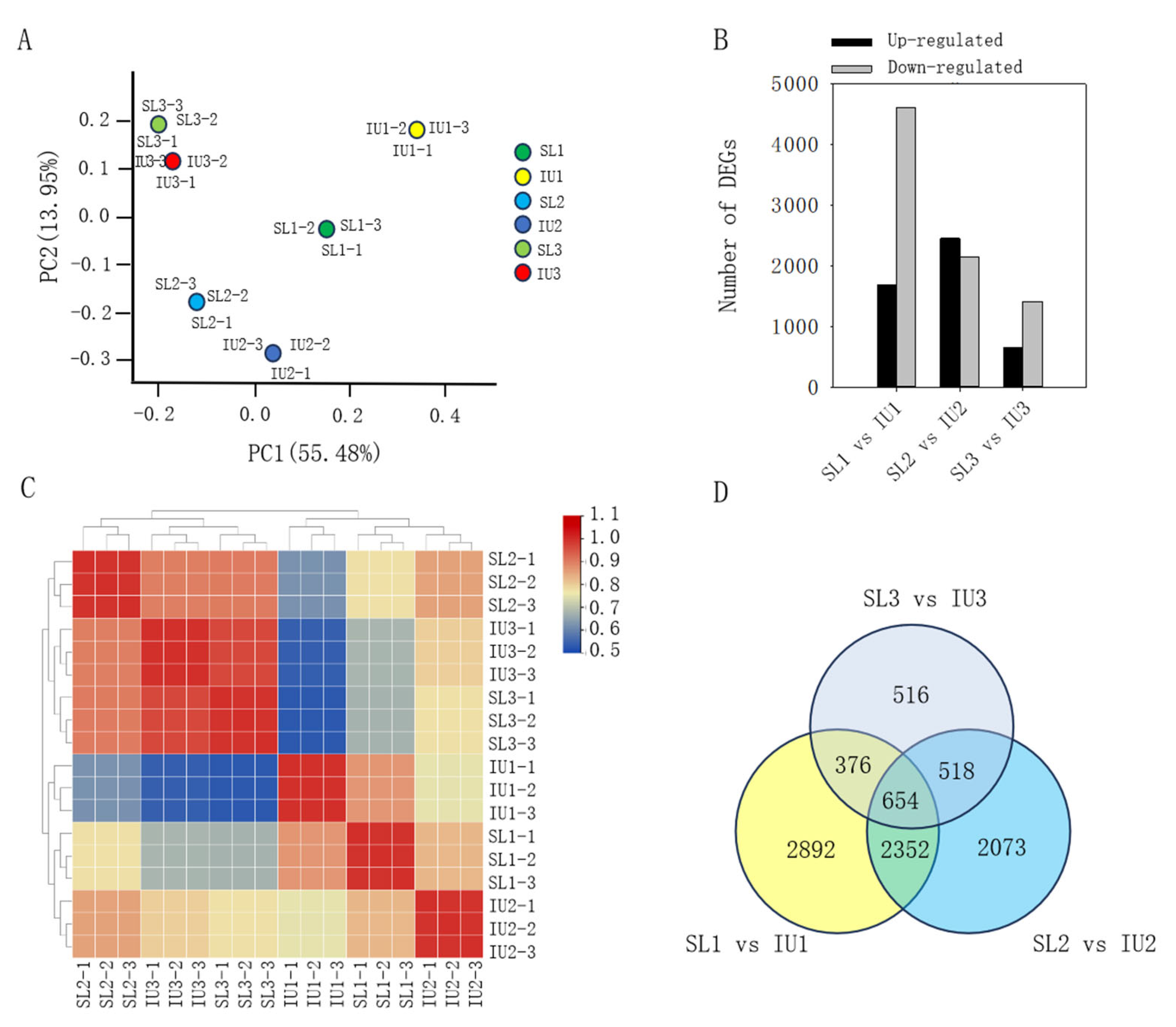
3.2. Transcriptome Profiling and Identification of DEGs
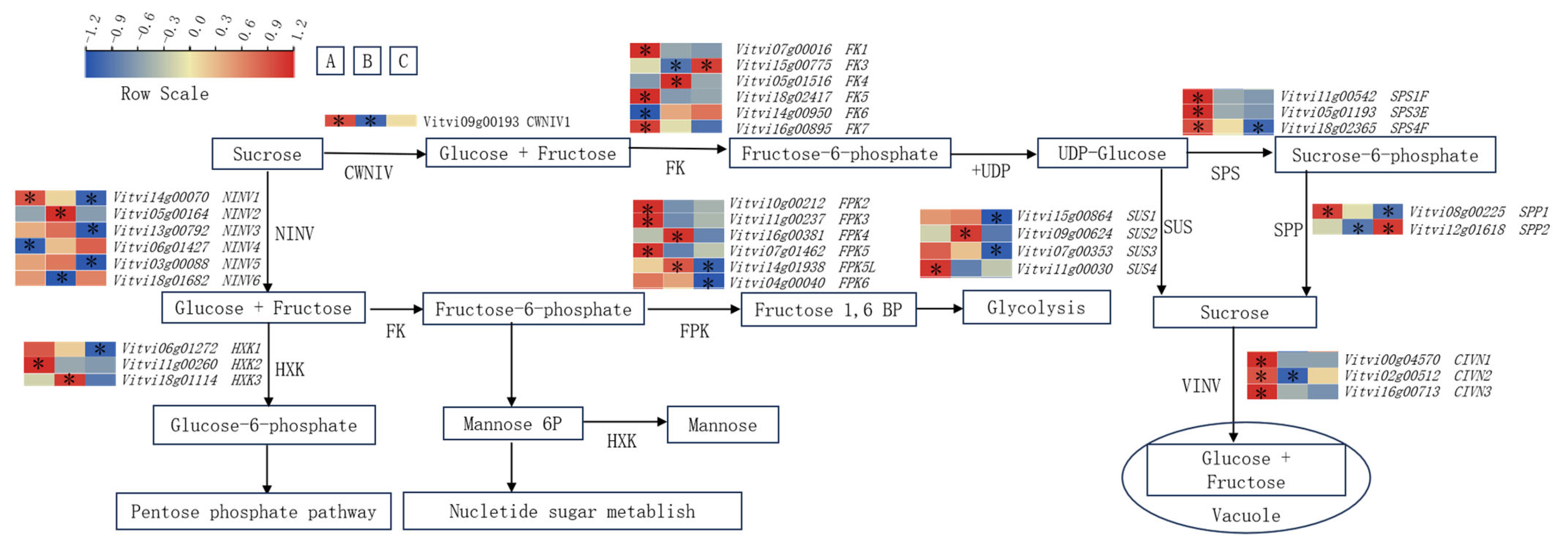
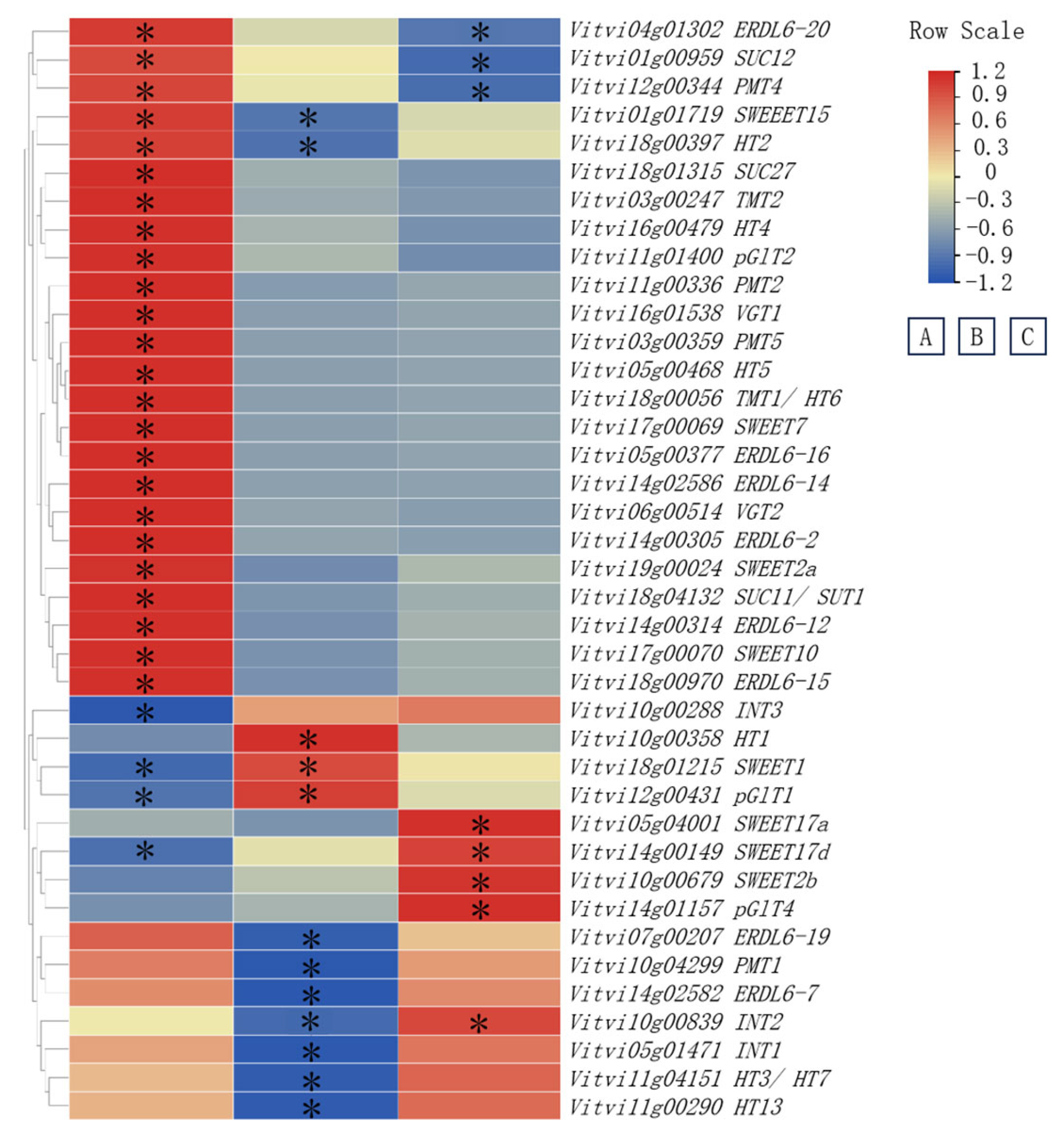
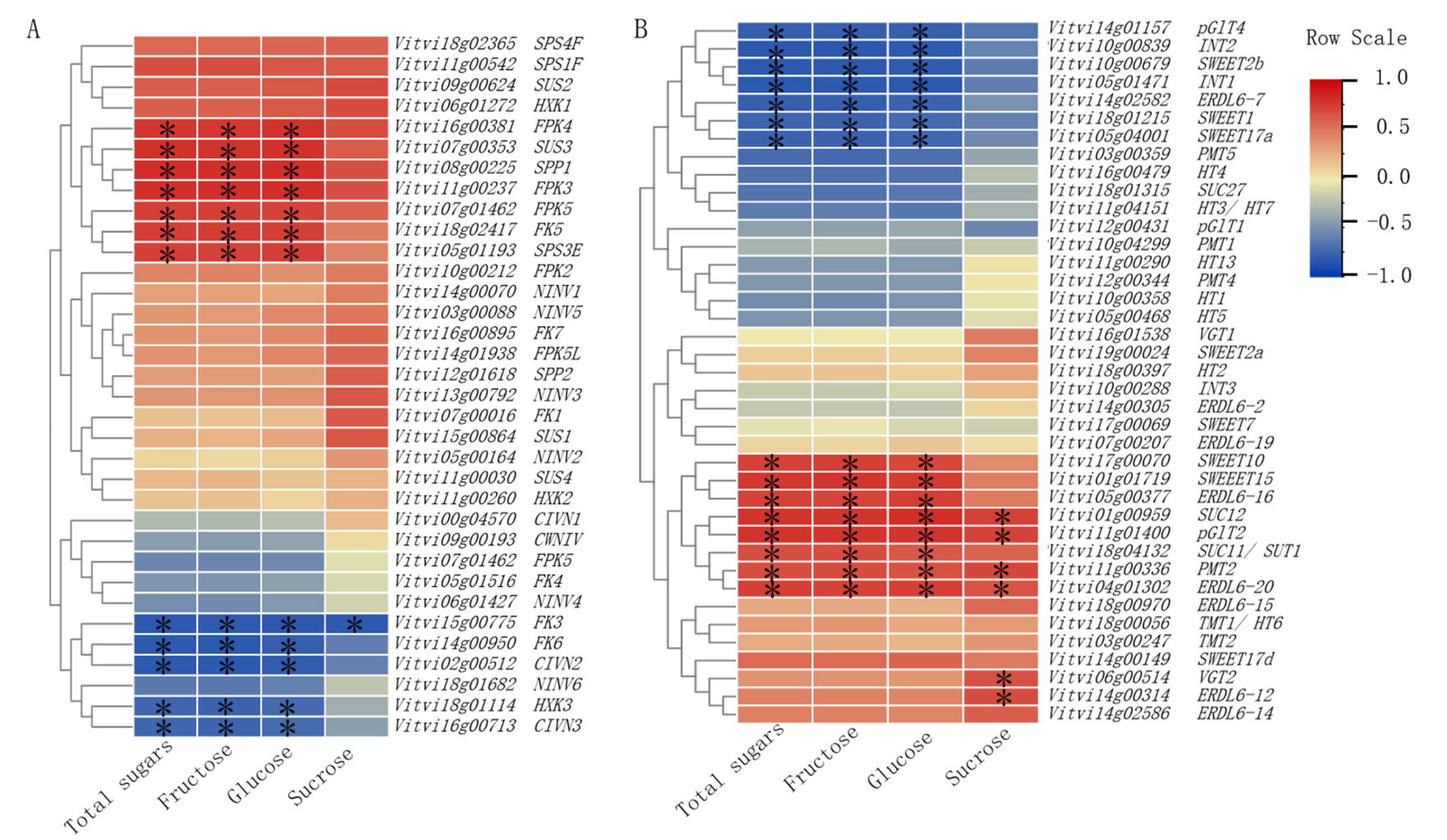
3.3. Impact of Training Systems on Sugar Metabolism and Transport-Related Genes in Grape Berries
3.4. Correlation Analysis Between Sugar Components and Genes Involved in Sugar Metabolism and Transport
4. Discussion
4.1. Relationship Between Sugar Accumulation and Berry Ripening
4.2. Impact of Training Systems on the Expression of Sugar Metabolism-Related Genes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yilmaz, T.; Ates, F.; Turan, M.; Hatterman-Valenti, H.; Kaya, O. Dynamics of sugars, organic acids, hormones, and antioxidants in grape varieties ‘italia’ and ‘bronx seedless’ during berry development and ripening. Horticulturae 2024, 10, 229. [Google Scholar] [CrossRef]
- Wang, X.X.; Fan, X.C.; Li, A.; Zhang, C.B.; Fang, J.G.; Liu, C.H.; Shangguan, L.F. Investigation and analysis on cracking trait in grape berry. Acta Hortic. Sin. 2016, 43, 2099–2108. (In Chinese) [Google Scholar] [CrossRef]
- Bian, L.; Guo, D.L.; Yu, K.K.; Wei, T.L.; Pei, M.S.; Liu, H.N.; Yu, Y.H. Cloning and expression analysis of the cytokinin response regulator VlRR5 in Kyoho grapevine. Acta Hortic. Sin. 2021, 48, 1437–1445. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Q.X.; Chen, T.; Xie, Q.; Lei, Y. Effects of training system modification on leaf photosynthetic characteristics and fruit quality in ‘Kyoho’ grapevines. North. Hortic. 2020, 17, 16–22. (In Chinese) [Google Scholar]
- Yin, H.; Wang, Z.; Wang, L.; Cao, J.; Wang, J.; Xi, Z. Effects of mesoclimate and microclimate variations mediated by high altitude and row orientation on sucrose metabolism and anthocyanin synthesis in grape berries. Hortic. Plant J. 2024, 10, 713–731. [Google Scholar] [CrossRef]
- Wu, B.H.; Liu, H.F.; Guan, L.; Fan, P.G.; Li, S.H. Carbohydrate metabolism in grape cultivars that differ in sucrose accumulation. Vitis 2011, 50, 51–57. [Google Scholar]
- Tian, L.; Jia, H.; Li, C.; Fan, P.; Xing, Y.; Shen, Y.Y. Sucrose accumulation during grape berry and strawberry fruit ripening is controlled predominantly by sucrose synthase activity. J. Hortic. Sci. Biotechnol. 2012, 87, 661–667. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Li, W.; He, B.J.; Gao, Y.-H.; Lu, J.X. Sucrose metabolism in grape (Vitis vinifera L.) branches under low temperature during overwintering covered with soil. Plant Growth Regul. 2014, 72, 229–238. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Y.; Feng, H. Colored plastic film mulching regulates light quality and sucrose metabolism in wine grape in an arid desert oasis. J. Plant Growth Regul. 2023, 42, 7705–7714. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, W.; Zhang, B.; Qin, H.; Wang, Y.; Yang, Y.; Xu, P.; Wang, Y.; Fan, S.; Li, C.; et al. Effects of different shaping methods and loading on fruit quality and volatile compounds in ‘Beibinghong’ grapes. Foods 2025, 14, 772. [Google Scholar] [CrossRef]
- Du, W.; Li, S.; Du, T.; Huang, W.; Zhang, Y.; Kang, H.; Yao, Y.; Gao, Z.; Du, Y. ‘Miguang’ grape response to pergola and single-curtain training systems. Horticulturae 2023, 9, 113. [Google Scholar] [CrossRef]
- Xia, H.Y.; Liu, Q.; Peng, J.Q.; Wu, W.; Gong, L.Z. Effects of f-shaped tree shape on photosynthetic characteristics and fruit quality in‘shine muscat’grapevines with rain-shelter cultivation. Acta Hortic. Sin. 2024, 51, 560–570. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.M.; You, J.L.; Nie, W.F.; Sun, M.H.; Xie, Z.S. Transcription profiles reveal age-dependent variations of photosynthetic properties and sugar metabolism in grape leaves (Vitis vinifera L.). Int. J. Mol. Sci. 2022, 23, 2243. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Chi, Y.H.; Wang, J.Z.; Zhou, J.X.; Cheng, Y.S.; Zhang, B.L.; Ma, A.; Vanitha, J.; Ramachandran, S. Sucrose metabolism gene families and their biological functions. Sci. Rep. 2015, 5, 17583. [Google Scholar] [CrossRef]
- Fang, F.; Liu, B.; Fu, L.; Tang, H.; Li, Y.; Pang, X.; Zhang, Z. Water Supply via Pedicel Reduces Postharvest Pericarp Browning of Litchi (Litchi chinensis) Fruit. Foods 2024, 13, 814. [Google Scholar] [CrossRef]
- Nan, X.; Li, W.; Shao, M.; Cui, Z.; Wang, H.; Huo, J.; Chen, L.; Chen, B.; Ma, Z. Shading treatment reduces grape sugar content by suppressing photosynthesis-antenna protein pathway gene expression in grape berries. Int. J. Mol. Sci. 2024, 25, 5029. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Liang, Y.; Han, Y.; Han, Y.; Tan, J. High potassium application rate increased grain yield of shading-stressed winter wheat by improving photosynthesis and photosynthate translocation. Front. Plant Sci. 2020, 11, 134. [Google Scholar] [CrossRef]
- Song, Y.; Hanner, R.H.; Meng, B. Genome-wide screening of novel RT-qPCR reference genes for study of GLRaV-3 infection in wine grapes and refinement of an RNA isolation protocol for grape berries. Plant Methods 2021, 17, 110. [Google Scholar] [CrossRef]
- Kong, Q.S. Chinese Grape Varieties; China Agric. Sci. Technol. Press: Beijing, China, 2004; p. 254. (In Chinese) [Google Scholar]
- Liu, C.H.; Shen, Y.J.; Chen, J. Descriptors and Data Standard for Grape (Vitis L.); China Agric. Press: Beijing, China, 2006; p. 96. (In Chinese) [Google Scholar]
- Zhong, H.; Yadav, V.; Wen, Z.; Zhou, X.; Wang, M.; Han, S.; Pan, M.; Zhang, C.; Zhang, F.; Wu, X. Comprehensive metabolomics-based analysis of sugar composition and content in berries of 18 grape varieties. Front. Plant Sci. 2023, 14, 1200071. [Google Scholar] [CrossRef]
- Lu, L.; Delrot, S.; Liang, Z. From acidity to sweetness: A comprehensive review of carbon accumulation in grape berries. Mol. Hortic. 2024, 4, 22. [Google Scholar] [CrossRef]
- Acharya, T.P.; Malladi, A.; Nambeesan, S.U. Sustained carbon import supports sugar accumulation and anthocyanin biosynthesis during fruit development and ripening in blueberry (Vaccinium ashei). Sci. Rep. 2024, 14, 24964. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, S.; Luo, J.; Wang, X.; Shi, C. QTL mapping and transcriptome analysis of sugar content during fruit ripening of Pyrus pyrifolia. Front. Plant Sci. 2023, 14, 1137104. [Google Scholar] [CrossRef]
- Liu, M.; Ji, H.; Jiang, Q.; Liu, T.; Cao, H.; Zhang, Z. Effects of full shading of clusters from véraison to ripeness on fruit quality and volatile compounds in Cabernet Sauvignon grapes. Food Chem. X. 2024, 21, 101232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, X.B.; Liu, F.Z.; Cheng, C.G.; Wang, X.D.; Wang, B.L.; Zheng, X.C.; Wang, H.B. Effects of canopy shapes of grape on canopy microenvironment, leaf and fruit quality in greenhouse. J. Appl. Ecol. 2015, 26, 3730–3736. [Google Scholar]
- Zhang, Z.; Zou, L.; Ren, C.; Ren, F.; Wang, Y.; Fan, P.; Li, S.; Liang, Z. VvSWEET10 mediates sugar accumulation in grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef]
- Lu, L.; Delrot, S.; Fan, P.; Zhang, Z.; Wu, D.; Dong, F.; García-Caparros, P.; Li, S.; Dai, Z.; Liang, Z. The transcription factors ERF105 and NAC72 regulate expression of a sugar transporter gene and hexose accumulation in grape. Plant Cell 2025, 37, koae326. [Google Scholar] [CrossRef]
- Bai, Q.; Chen, X.; Zheng, Z.; Feng, J.; Zhang, Y.; Shen, Y.; Huang, Y. Vacuolar Phosphate Transporter1 (VPT1) may transport sugar in response to soluble sugar status of grape fruits. Hortic. Res. 2023, 10, uhac260. [Google Scholar] [CrossRef]
- Li, J.C.; Duan, L.S.; Zhang, X.S. Effect of rainproof cultivation on grape disease incidence and light intensity under the shelter. J. Fruit Sci. 2009, 26, 847–850. (In Chinese) [Google Scholar]
- Yu, H.; Zhou, S.H.; Li, H.Y.; Huang, Y.; Cheng, G.; Liu, J.B.; Lan, Z.F. Investigation and analysis on resistance to gray mold of grape inflorescences under rain-shelter cultivation. Sino-Overseas Grapevine Wine 2024, 2, 22–27. (In Chinese) [Google Scholar] [CrossRef]
| Primer | Gene ID | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| Actin | Vitvi11g00056 | ATCAGGAAGGACCTCTATGG | AACGACCTTAATCTTCATGCTGC |
| pGlT2 | Vitvi11g01400 | GGGTACTTATGGCAGCTTCA | TGCGCCACCATCCAATAA |
| ERDL6-15 | Vitvi18g00970 | GGAGTTGTGGCTATGGTAGTTG | CCCTGCAAGGCCCTTTATATT |
| FK7 | Vitvi16g00895 | CCGTAATCCAAGCGCTGATA | GAGTTGACCTACATGGTTCCTC |
| SUS3 | Vitvi07g00353 | CAAGGAATGGGTTTCGAGAGAG | GAGCCTGAAGAATGTCCAAGAG |
| SPP1 | Vitvi08g00225 | CCGGATAATGGTTGGGTTCA | GGGTCGTTGCTCTGTTTCT |
| HXK2 | Vitvi11g00260 | CATCGGGCTCCCTCATTAAA | CGCGCATATCAAGACCAATTC |
| Training System | Days After Flowering (DAF) | L* | a* | b* | C | h° |
|---|---|---|---|---|---|---|
| SL | 45 | 32.45 ± 2.67 a | 4.02 ± 1.12 a | −0.93 ± 0.64 b | 4.17 ± 1.78 a | 310.62 ± 22.15 a |
| 55 | 32.02 ± 2.32 b | 4.32 ± 1.46 a | −1.08 ± 0.87 b | 4.35 ± 1.43 a | 317.86 ± 15.66 a | |
| 65 | 31.74 ± 3.05 b | 4.91 ± 2.07 a | −1.36 ± 0.63 b | 4.95 ± 1.06 a | 326.87 ± 18.73 a | |
| 75 | 30.43 ± 1.72 b | 5.22 ± 2.54 a | −1.63 ± 0.85 b | 5.26 ± 1.51 a | 331.03 ± 20.18 a | |
| 85 | 29.75 ± 2.33 b | 5.84 ± 1.86 a | −1.81 ± 1.02 b | 5.87 ± 2.60 a | 338.67 ± 19.26 a | |
| 95 | 28.96 ± 1.25 b | 6.21 ± 1.72 a | −1.97 ± 0.84 b | 6.23 ± 2.25 a | 347.62 ± 17.65 a | |
| IU | 45 | 33.96 ± 2.14 a | 3.25 ± 2.76 b | −0.57 ± 0.25 a | 3.25 ± 2.57 b | 283.78 ± 24.53 b |
| 55 | 33.24 ± 0.97 a | 3.74 ± 2.48 b | −0.72 ± 0.65 a | 3.77 ± 1.29 b | 294.38 ± 21.37 b | |
| 65 | 32.65 ± 2.33 a | 4.2 ± 1.59 b | −0.96 ± 0.79 a | 4.24 ± 1.32 b | 308.06 ± 18.75 b | |
| 75 | 31.89 ± 1.88 a | 4.79 ± 1.66 b | −1.25 ± 0.82 a | 4.79 ± 2.13 b | 317.56 ± 16.43 a | |
| 85 | 30.79 ± 2.26 a | 5.02 ± 1.28 b | −1.42 ± 1.05 a | 5.05 ± 2.45 b | 324.12 ± 20.91 a | |
| 95 | 29.74 ± 2.59 a | 5.08 ± 2.43 b | −1.6 ± 0.89 a | 5.12 ± 1.64 b | 339.71 ± 22.67 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lin, J.; Chen, T.; Chen, K.; Lei, Y. Effects of Training Systems on Sugar Accumulation and Metabolism in ‘Kyoho’ Grape Berries. Horticulturae 2025, 11, 578. https://doi.org/10.3390/horticulturae11060578
Liu X, Lin J, Chen T, Chen K, Lei Y. Effects of Training Systems on Sugar Accumulation and Metabolism in ‘Kyoho’ Grape Berries. Horticulturae. 2025; 11(6):578. https://doi.org/10.3390/horticulturae11060578
Chicago/Turabian StyleLiu, Xinming, Jinhui Lin, Ting Chen, Keyang Chen, and Yan Lei. 2025. "Effects of Training Systems on Sugar Accumulation and Metabolism in ‘Kyoho’ Grape Berries" Horticulturae 11, no. 6: 578. https://doi.org/10.3390/horticulturae11060578
APA StyleLiu, X., Lin, J., Chen, T., Chen, K., & Lei, Y. (2025). Effects of Training Systems on Sugar Accumulation and Metabolism in ‘Kyoho’ Grape Berries. Horticulturae, 11(6), 578. https://doi.org/10.3390/horticulturae11060578





