Abstract
Post-harvest quality and longevity of cut flowers are critical factors influencing customer satisfaction and purchase decisions. Maintaining post-harvest quality and ensuring a long vase life (VL) present significant challenges to the floriculture industry due to the perishable nature of cut flowers. Recent studies have demonstrated the potential of melatonin (MT), a multifunctional biomolecule, to improve the post-harvest quality and longevity of floricultural products. This review highlights recent advances in the application of MT to improve the longevity and post-harvest quality of cut flowers. The physiological, biochemical, and molecular mechanisms underlying MT’s effects, along with various application methods, are discussed. Furthermore, current challenges and knowledge gaps are identified, and future research directions are proposed to explore MT’s potential in diverse flower species and its practical application in the cut flower industry.
1. Introduction
Flower senescence is an irreversible process in nature that involves a series of metabolic, physiological, and biochemical changes, that lead to the death of flower, which include, flower wilting, shedding of flower parts, and fading of blossoms [1]. Early post-harvest deterioration in cut flowers is a significant challenge to the floricultural industry. Early senescence in cut flowers is often due to ethylene (ET)-induced senescence, oxidative stress, water stress, and disease infection during storage and transportation under conditions preventing adaptive management [2,3]. Therefore, improving the post-harvest quality of cut flowers is necessary to enhance the marketability and the economic value of the floricultural industry. To improve post-harvest quality and vase life (VL) of cut flowers, many traditional physical storage and transport methods, such as transportation without water supply (commonly referred to as ‘dry transportation’), controlled atmosphere storage, and low-temperature storage, have been applied [3,4,5,6]. Moreover, chemical reagents have been utilized to prolong VL of cut flowers [6,7,8]. Traditional chemical preservatives such as silver thiosulfate and sucrose have been used to prolong VL, but concerns about environmental impact and human health have driven the search for more sustainable alternatives [6,7].
Melatonin (MT, N-acetyl-5-methoxytryptamine), a small molecular indoleamine hormone derived from tryptophan, is commonly found across a wide range of plant species [9,10]. Endogenous MT is synthesized in the mitochondria and chloroplasts of leaves and roots and then transported to the fruits, flowers, and meristem in plants [11]. The regulation of the gene expression and signaling crosstalk of MT with other hormones such as ET, auxin, abscisic acid (ABA), cytokinin (CK), salicylic acid (SA), jasmonic acid (JA), and gibberellins (GA) has been characterized recently [12,13,14,15]. Recent studies have elucidated that MT interacts with these phytohormones, modulating diverse signaling pathways involved in growth, development, and stress responses. For example, MT promotes auxin biosynthesis and transport by inducing YUCCA and PIN gene expression, thereby enhancing root development and organogenesis [12]. Under abiotic stress, MT enhances ABA signaling by up-regulating ABA-responsive genes and activating SnRK2 kinases, contributing to improved stomatal regulation and stress adaptation [15]. In addition, MT can suppress ET biosynthesis by down-regulating ACS and ACO genes, thereby delaying senescence in ET-sensitive tissues such as cut flowers [16]. These hormonal interactions suggest that MT functions as a central regulator of hormonal cross-talk, influencing both development and post-harvest physiology.
Endogenous MT is involved in various biological processes in plants, including growth, seed germination, development, disease response, and senescence processes [17,18,19,20]. A previous study has shown that the amount of endogenous MT is generally low in plant tissues; thus, its low concentration may not be effective in improving post-harvest preservation [21]. Recently, exogenous MT has emerged as an eco-friendly preservative solution for horticultural crops such as vegetables, fruits, and flowers [21,22,23]. MT posed no harmful environmental effects, making it an attractive alternative to conventional preservative compounds used in the horticultural industry for prolonging shelf life in various crops [21,23,24]. The application of MT improved post-harvest quality and shelf life of various fruit and vegetable crops by reducing gray mold disease infection and increasing antioxidant enzymes for scavenging reactive oxygen species [21,25,26,27]. In the floricultural sector, MT treatment also delayed floral senescence and extended VL of cut flowers through enhancing the antioxidant system, improving stem strength and water uptake, and maintaining the membrane stability index [22,28,29,30].
Here, we systematically review the current application of MT as an effective preservative solution in floricultural crops. We summarize MT’s potential roles in improving the post-harvest quality of cut flowers at the physiological, biochemical, and molecular levels. Additionally, we propose challenges and future perspectives for exploring MT’s potential in diverse flower species and its integration into commercial practices.
2. Effects and Mechanisms of Exogenous MT in Post-Harvest Management of Cut Flowers
MT plays a crucial role in post-harvest physiology by regulating various biochemical and molecular pathways to extend VL of cut flowers [27,28,29]. The application of exogenous MT has been demonstrated to delay senescence, enhance WU and antioxidant defense, regulate hormone balance, maintain carbohydrate metabolism, and improve stress tolerance, ultimately preserving the post-harvest quality of cut flowers. As presented in Table 1, exogenous MT treatment significantly delayed flower senescence and extended VL of various cut flower species.

Table 1.
Effects of exogenous MT treatments on post-harvest quality and VL of different cut flower species. Treatment concentrations, methods, physiological effects, and VL extension are summarized from recent studies.
2.1. Anthurium Flowers
In cut anthurium flowers, low temperature storage is often applied to extend the storage life of cut flowers [31,41]. However, the disadvantage of this technique is causing chilling injury (CI), which is manifested as spathe browning in cut flowers [41,42]. After harvesting at opening of 40–50% spadix flowers, cut anthurium flowers were divided into five lots of 90 flowers for treatment with MT, applied in triplicate (30 flowers per replicate) by dipping of flower stem ends in 0, 1, 10, 100, and 1000 μM of MT for 15 min at 20 °C and storing at 4 °C, 85–90% relative humidity (RH) for 21 d. MT treatment reduced CI incidence rates in cut anthurium flowers by 11, 29, 51, and 31%, respectively, compared with that of non-treated flowers. Among the treated concentrations, 100 μM MT exhibited the highest effectiveness in reducing CI in anthurium flowers. Ameliorating CI in MT-treated flowers was accompanied by lower electrolyte leakage and malondialdehyde concentration during cold storage. MT treatments increased NADPH oxidase activity, proline, and phenolic concentrations in flowers compared to non-treated flowers. MT application also induced the expression levels of oxidase genes that may be responsible for ameliorating damaging H2O2 concentration in treated flowers compared to non-treated flowers during storage at 4 °C for 21 d. Overall, exogenous MT can ameliorate CI incidence and improve post-harvest quality in cut anthurium flowers during low-temperature storage [31] (Table 1).
2.2. Carnations
Carnation flowers (Dianthus caryophyllus L.) are one of the most popular cut flowers in the floricultural industry due to their color, fragrance, and diversity of varieties [43]. The VL of cut carnations depends on the cultivar’s characteristics, such as the sensitivity to ethylene or the emission level of the scent [44,45]. During the post-harvest stage, petal wilting, browning, discolorations, bent neck, and the rise of reactive oxygen are typical indicators of ornamental product deterioration [44,45]. Recently, MT application significantly retarded flower senescence and prolonged VL of cut carnations. However, the effectiveness of MT treatments on cut carnations depends on the variety and application methods (single or combined with other techniques) [16,32,33]. Treatment with different MT concentrations (0.01, 0.1, and 1 mM) on the carnations ‘Baltico’ effectively extended VL of cut flowers. Among these concentrations, 0.1 mM MT was the most effective concentration with an increase in VL of 10.0 d compared to control flowers. MT at 0.1 mM significantly delayed petal senescence, and maintained initial levels of fresh weight, membrane stability index, bioactive compounds, and antioxidant activity for a longer time [32] (Table 1). Other research also showed that sole treatment or a combination of MT (1 mg L−1) with nana seleum (5 mg L−1) prolonged the vase life of cut carnation flowers by 3.0 d and 9.0 d compared to control groups (Table 1). In addition to delayed senescence and enhancing antioxidant ability, MT treatment induced the biosynthesis of hormonal compounds (SA, JA, and ABA), and increased the amounts of key lignin biosynthesis pathway metabolites in carnation petals [16] (Table 1). Previous study also indicated that holding cut carnations ‘White Liberty’ in 100 μM MT solution after exposure to vibration (open and dark columns for 0 and 10 hz, respectively) for 10 min prolonged VL of cut flowers by both improving water balance, boosting antioxidant activity, and mitigating the negative effects of simulated vibrational stress [33] (Table 1).
2.3. Chrysanthemums
Chrysanthemum (Chrysanthemum × morifolium) is an ethylene-insensitive flower where lipid peroxidation and membrane damage are strongly involved in flower deterioration during post-harvest handling [46,47]. The decline in the quality of cut chrysanthemums is mainly due to leaves wilting due to water stress or leaf yellowing [48,49]. This loss of turgidity, accompanied by chlorophyll degradation, results in the leaves senescing earlier than the inflorescences. During senescence, oxidative stress created by the production of reactive oxygen species (ROS) induces premature aging in cut flowers. Nucleic acids, membranes, pigment molecules, and proteins are damaged by ROS [47]. Two concentrations of MT (0.02 and 0.04 mM L−1) were applied on the cut chrysanthemum ‘Pingpong’ group (‘Yellow Pingpong’, ‘White Pingpong’, ‘Pink Pingpong’, and ‘Green Pingpong’ cultivars) [34]. MT maintained the best ornamental quality of the capitulum by decelerating fresh weight and flower diameter loss in terms of all varieties. MT at 0.04 mM L−1 showed more effectiveness than the lower applied concentration, with longer VL in all four cultivars (Table 1). Therefore, the application of 0.04 mM MT was suggested to improve the marketability of cut chrysanthemum ‘Pingpong’ [34]. This result implies that MT is considered an environmentally safer alternative to traditional preservatives such as silver thiosulfate. Other research also indicated that treatment with 5 µM MT enhanced water balance, improved fresh weight and flower diameter, and regulated the antioxidant system in petals, resulting in extending VL of cut Chrysanthemum morifolium R. flowers by 31.78% (~3.0 d) compared to untreated flowers [29] (Table 1).
2.4. Gerbera Flowers
Cut gerbera flowers (Gerbera jamesonii) are economically important cut flowers but have a short VL (5–8 d) due to petal senescence and stem bending [50]. Therefore, the preservation of cut gerbera flowers is crucial for maintaining their ornamental value and prolonging VL in the floriculture industry. Treatments with 0.1 mM and 0.5 mM MT and 0.1 mM and 0.5 mM encapsulated melatonin with nanochitosan (nCS-MT) significantly enhanced VL of cut gerbera ‘Terra kalina’ flowers by 5.0, 3.0, 8.7, and 5.3 d compared to untreated controls [35] (Table 1). Among these treatments, nCS-MT-treated flowers exhibited better preservation of membrane stability, reduced oxidative stress, and increased antioxidant enzyme activity. Carbohydrate content was maintained at higher levels in nCS-MT-treated petals, while total flavonoid and phenolic content increased. Cut flowers treated with nCS-MT showed reduced hydrogen peroxide accumulation and malondialdehyde levels, delaying petal senescence. Additionally, nCS-MT improved the stability and controlled release of MT, enhancing its efficacy in preserving floral quality [35]. The results highlight nCS-Mel as a sustainable alternative to synthetic preservatives, offering potential commercial applications in cut gerbera flowers.
2.5. Peony Flowers
Herbaceous peony (Paeonia lactiflora Pall.) is a distinguished ornamental plant admired for its vibrant colors and graceful blooms [51]. Renowned for their exquisite beauty, peonies add elegance to gardens and are highly valued in the floral market [51]. However, cut peonies have a very short and poor-quality VL after long-term cold storage. The relatively short VL (5–6 d) of peonies, along with the tendency of their quality to decline, poses challenges for transportation and market promotion [52,53]. Treatments with three concentrations (25, 50, and 75 μM L−1) of MT significantly delayed flower senescence and extended VL of two peony cultivars ’Qi Hua Lu Shuang’ and ’Da Fu Gui’ [22] (Table 1). The optimal concentration of MT (50 μM L−1) prolonged VL of ’Qi Hua Lu Shuang’ and ’Da Fu Gui’ by 1.6 and 1.2 d, respectively, compared to control flowers [22]. MT applications effectively improved water balance and flower diameter, reduced relative electrical conductivity and malondialdehyde (MDA) content, and enhanced the activities of two antioxidant enzymes, superoxidase dismutase (SOD) and catalase (CAT), in petals of cut peonies [22]. Another previous study also showed that adding MT (0.1 and 0.5 mM) to a vase solution containing sucrose 0.5%, NaCl, citric acid, and 6-BA significantly prolonged VL of peony ‘Sarah’ after 30 d of cold storage at 2–3 °C [36]. The treatments effectively enhanced the absorption of water by inducing the expression of PlPIP1;3 and PlTIP1;1 in petals, delayed senescence by inhibiting PlSAG39-like gene, and alleviated the damage to cell membranes and enhanced the antioxidant capacity in cut flowers [36]. In addition, the treatment with exogenous MT effectively enhanced the accumulation of mRNA levels of lignin biosynthesis genes, such as PlPAL, PlCCR, PlCAD, PlCOMT, and PlPOD, resulting in improved flower stem strength by increasing the ratio of lignin content and the S/G lignin composition [54]. These results demonstrate that MT is an effective preservative for delaying senescence and preserving the post-harvest quality of cut peonies, offering valuable insights for the floral industry.
2.6. Rose Flowers
Rose flower (Rosa hybrida L.) senescence is characterized by water stress, ET damage, disease infection, and oxidative stress [3,55]. The oxidative stress is accelerated by metabolic processes occurring naturally after cutting from the mother plant. Furthermore, flower stem cutting itself causes oxidative injury and therefore overproduction of ROS that attack the cellular proteins, nucleic acids, and membrane lipids, leading to membrane deterioration [56]. During post-harvest deterioration of cut roses, ROS levels markedly increased, followed by activities related to the antioxidant system [56]. Pulsing treatment with MT (0.1, 0.2, and 0.3 mM) for 30 min effectively prolonged VL of cut roses ‘Frist Red’ and improved the relative water content of cut flowers compared to the control group. A 0.2 mM concentration of MT nearly doubled VL with respect to the control flowers [28] (Table 1). MT application significantly increased the phenol and glutathione content and CAT, ascorbate peroxidase (APX), and glutathione reductase (GR) enzyme activities compared to control flowers. In addition, the radical scavenging capacity in MT-treated flowers was higher than that of control and therefore MT treatment reduced H2O2 production and lipid peroxidation, which altogether were reflected in membrane stability maintenance, consequently retarding flower senescence and improving the post-harvest quality of cut roses [28].
2.7. Other Flowers
In addition to the major ornamental species discussed above, MT has been tested in a range of other cut flowers, including tuberose, snapdragon, ranunculus, amaryllis, and lisianthus, with variable outcomes depending on treatment method and concentration.
In tuberose cut flowers (Polianthes tuberosa L.), treatment with a solution containing MT 100 µM and arsenic 50 µM after twenty days of sprouting of tuberose bulbs effectively stimulated the oxidative defense-related antioxidants and prolonged VL of cut flowers by 60% compared to untreated flowers [37] (Table 1). However, the use of arsenic raises concerns about safety and environmental implications, suggesting a need for alternative synergists in future studies.
In snapdragon (Antirrhinum majus L.), spraying with MT solution (150 and 200 µM) after budding significantly increased the numbers and size of florets and extended VL of cut flowers ‘Snapshot White’ by 1.3 and 2.3 d compared to control flowers [38] (Table 1). This mild yet consistent improvement demonstrates the efficacy of foliar MT applications during pre-harvest phases in promoting ornamental value.
In ranunculus (Ranunculus asiaticus L.), a single 24 h pulsing treatment with 100 µM MT significantly delayed petal senescence and reduced ethylene biosynthesis or action, leading to a VL extension of 8.0–9.0 d. This highlights MT’s ET-interfering potential in ET-sensitive species and supports pulsing as an efficient short-term method [30] (Table 1).
In amaryllis bulbs (Hippeastrum hybridum Herb), single application of MT (0.01 mM) or combination with sucrose 0.4% delayed the degradation of anthocyanin and flavonoid pigments and maintained the stability of phenolic compounds and total carbohydrates in petals, and extended VL of cut flowers ‘Minerva’ by 2.0 d compared to control flowers [39] (Table 1).
Furthermore, spraying MT 1.5 µM on the shoots of flower buds on the shoots of lisianthus ‘Grand White’ flowers increased the relative weight gain compared to the control and increased vase life by 14 days. Also, MT-treated flowers showed the lowest ion leakage and less malondialdehyde accumulation, and also higher catalase enzyme activity compared to control flowers [40] (Table 1).
Across flower species, the exogenous application of MT significantly extended vase life by modulating key physiological responses. Quantitatively, MT treatments prolonged vase life by 1.2 to 14 days, depending on the flower type, treatment method, and concentration. Notably, low micromolar concentrations (1.5–100 μM) were sufficient to produce significant physiological effects in lisianthus, ranunculus, and chrysanthemum, while millimolar treatments were used more commonly in carnations and roses. Pulsing treatments (e.g., 24 h or 30 min exposure) were highly effective in ET-sensitive flowers like rose and ranunculus, while foliar spraying and vase solution applications provided gradual systemic benefits in gerbera, snapdragon, and lisianthus. Encapsulation techniques, such as nanochitosan-MT in gerbera, further enhanced stability and efficacy. These findings emphasize the necessity for species-specific optimization of MT concentration and delivery and highlight the untapped potential of MT as a practical post-harvest solution across floricultural crops.
2.8. Mechanistic Synthesis of MT-Mediated Post-Harvest Regulation
Complex physiological and metabolic processes, including oxidative stress, hormone signaling, carbohydrate metabolism, water transport, and gene expression, govern floral senescence. After detachment from the parent plant, cut flowers undergo rapid metabolic changes, leading to decreased membrane integrity, cellular dehydration, and visible deterioration. Across diverse cut flower species, MT consistently exerts its preservative effects through several core physiological and molecular mechanisms during the post-harvest stage (Figure 1).
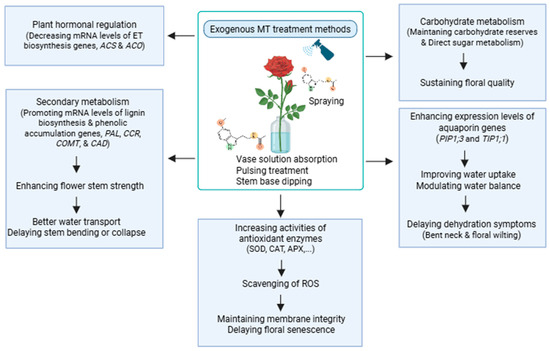
Figure 1.
Model of exogenous MT-mediated post-harvest preservation mechanism in prolonging the vase life of cut flowers. MT enhances antioxidant defense (CAT, SOD, and APX), reduces ROS accumulation, and maintains membrane stability. MT also regulates hormonal balance by downregulating ethylene biosynthesis (ACS and ACO), modulates water uptake through inducing aquaporins-related genes (PIP1;3 and TIP1;1), supports carbohydrate metabolism, and strengthens stem structure by inducing lignin biosynthesis genes (PAL, CCR, CAD, and COMT). Together, these effects delay senescence and improve post-harvest quality in cut flowers.
One of the central aspects of flower metabolism during senescence is the accumulation of ROS, which disrupts cellular homeostasis by damaging proteins, lipids, and nucleic acids. Thus, a primary mode of action is the scavenging of ROS by up-regulating enzymatic antioxidants such as CAT, SOD, APX, and GA, as seen in rose, peony, and carnation [27,28]. MT also enhances cell membrane integrity, often reflected by reduced electrolyte leakage and MDA levels, particularly under chilling or oxidative stress, as reported in anthurium, gerbera, and chrysanthemum.
Hormonal regulation also plays a pivotal role in post-harvest physiology. ET, ABA, JA, and SA are known to contribute to petal wilting, chlorophyll degradation, and senescence [3,17,20]. MT interacts with these phytohormones, often exhibiting antagonistic effects against senescence-promoting hormones like ET and ABA, while synergizing with stress-response hormones such as JA and SA [12,13,16]. MT treatment has been shown to suppress ET biosynthesis and signaling pathways in some species while simultaneously promoting endogenous levels of ABA or SA in others, indicating species- and context-specific crosstalk [16,30].
Carbohydrate metabolism is another important factor in flower longevity. Post-harvest depletion of soluble sugars leads to energy deficits and loss of turgor pressure. MT has been reported to maintain carbohydrate reserves and redirect sugar metabolism toward sustaining floral quality under stress [51,57,58].
In addition, MT modulates water balance via up-regulation of aquaporin genes (e.g., PlPIP1;3, PlTIP1;1 in peony), improving water uptake and delaying dehydration symptoms, such as bent neck or leaf wilting.
Furthermore, MT regulates secondary metabolism by promoting the expression levels of lignin biosynthesis and phenolic accumulation genes (PAL, CCR, COMT, and CAD), enhancing stem strength and structural resilience in cut flowers like peony and carnation [16,55]. This structural reinforcement contributes to better water transport and delayed stem bending or collapse, critical issues in commercial flower handling.
This comparative analysis highlights a multifaceted but convergent mechanistic model where MT acts as a central modulator of oxidative stress, water transport, hormonal balance, and structural maintenance, offering a promising foundation for developing universal and species-tailored MT-based preservative strategies in floriculture.
3. Limitations and Challenges for Application of MT in the Cut Flower Industry
Despite the promising role of MT in enhancing the post-harvest longevity of cut flowers, its widespread adoption in the floriculture industry faces several challenges. These limitations include issues related to optimal application protocols, species-specific variability, interactions with existing preservatives, stability concerns, and regulatory approvals. Addressing these challenges is essential for the effective commercial utilization of MT as a post-harvest treatment.
3.1. Standardization of Optimal Concentrations, Exposure Durations, and Application Methods
The efficacy of MT in extending VL is highly dependent on concentration, application method, and treatment duration. Various studies have reported species-specific optimal MT concentrations, ranging from nanomolar to millimolar levels. Many studies have shown that MT exhibits a hormetic dose–response relationship (low-concentration stimulation and high-concentration inhibition). For example, concentrations in the range of 10–100 μM are commonly effective in improving antioxidant activity, water balance, and membrane stability across multiple species, including rose, chrysanthemum, and ranunculus. However, doses exceeding 500 μM to 1 mM, as used in some carnation and peony studies, may not provide additional benefits and can potentially suppress growth or accelerate senescence in more sensitive species. In certain cases, MT concentrations above 1 mM have been associated with oxidative damage, impaired photosynthesis, or inhibited root elongation during vegetative growth, suggesting a threshold beyond which the compound may act as a stressor rather than a protective agent [21,23,59]. Moreover, the optimal concentration varies across species due to differences in cut flower metabolism, tissue permeability, and endogenous MT levels. For instance, lisianthus responded positively to just 1.5 μM, whereas carnations required 0.1–1.0 mM for significant effects. These differences underscore the need for species-specific titration studies to define both the efficacy window and toxicity threshold.
Furthermore, multiple application methods have been explored, including pulsing treatments (short-term exposure to high concentrations), continuous vase solution absorption, foliar spraying, and stem base dipping. However, there is no universal standard for its application across different flower species. Different flower species have been found to respond differently to MT treatment methods. Therefore, the application method should be tailored to the flower species, uptake rate, transport to target flowers, and intended purpose. Further studies are required to optimize concentration and exposure time to maximize MT’s effectiveness without adverse side-effects. MT can be applied through several techniques, including vase solution absorption, pulsing treatments, stem base dipping, and foliar spraying. Each method has distinct advantages based on the species’ physiology and handling context. For example, pulsing treatments provide a high-dose, short-duration exposure useful for quick treatment during processing, whereas spraying is suitable for pre-harvest or immediate post-harvest use, especially in field-grown crops. Dipping treatments, often used for cold-sensitive flowers like anthuriums, allow uniform uptake and are less dependent on xylem function.
While vase solution applications are most commonly reported in the literature, spraying and dipping may be more scalable and economical for large-volume processing. However, these require careful control of dosage and formulation to avoid MT photodegradation and ensure even distribution. Nanoformulations (e.g., nCS-MT) have shown promise in improving delivery efficiency and shelf life [35], but further work is needed to translate these into practical tools for floriculture industries.
3.2. Interaction with Other Preservative Solutions
MT may interact synergistically or antagonistically with conventional post-harvest treatments, potentially affecting its efficacy [60]. Cut flowers are often treated with sucrose, antimicrobial agents, and ET inhibitors to extend VL [61]. While MT has been shown to enhance stress tolerance [62], its compatibility with these existing treatments remains unclear. Some studies have suggested that MT may enhance carbohydrate metabolism and WU, complementing sucrose-based preservatives [57,63], while others indicate potential interference with ET signaling pathways [58,64], requiring further investigation into possible antagonistic effects. Given the limited inherent antimicrobial activity of MT, its co-application with traditional antimicrobials (e.g., silver nanoparticles or chlorine dioxide) may offer synergistic effects. Future studies should evaluate the compatibility and effectiveness of such combined strategies in prolonging VL. However, these interaction effects are still largely hypothetical, and experimental validation is needed to determine the nature and extent of such synergistic or antagonistic outcomes in diverse floral species and treatment contexts.
3.3. Flower Species-Specific Responses and Physiological Variability
As shown in Table 1, MT exhibits highly variable physiological effects among different cut flower species, limiting its broad applicability. While some species, such as carnation and rose flowers, respond positively to MT treatment with extended VL and delayed senescence, others exhibit minimal or inconsistent responses. This variability may be attributed to differences in intrinsic MT biosynthesis and signaling pathways, variation in cut flower metabolism and WU efficiency, and environmental factors influencing post-harvest physiology [10,65]. These inconsistencies make it challenging to develop a generalized MT-based post-harvest treatment applicable to all flower species, necessitating species-specific optimization studies.
3.4. Stability and Degradation of MT in Vase Solutions
The stability of MT in aqueous environments is a critical factor influencing its commercial viability. MT is a light-sensitive molecule prone to oxidation and degradation under prolonged exposure to air, high temperatures, and variable pH conditions [66,67]. Additionally, microbial activity in vase solutions may accelerate MT breakdown, reducing its effectiveness over time [68]. Developing stabilized MT formulations, such as nano-encapsulation and controlled-release systems, may enhance their longevity and efficacy in real-world applications.
3.5. Limited Field Validation and Large-Scale Cut Flower Industry Trials
The data in Table 1 show that most studies investigating MT’s effects on cut flower longevity have been conducted under controlled laboratory conditions, limiting their practical applicability in commercial settings. The effectiveness of MT under real-world supply chain conditions, including cold storage, long-distance transport, and retail display, remains largely untested. Large-scale industry trials are necessary to evaluate the effectiveness of MT treatments under commercial storage and transport conditions, their influence on consumer preference and marketability, and economic feasibility compared to existing post-harvest solutions. Currently, industrial-scale case studies of MT application remain scarce, and estimates regarding its cost-effectiveness or implementation logistics are largely lacking. To address this gap, further research is required to establish practical benchmarks and field-tested protocols for commercial use.
3.6. Industrial-Scale Production and Cost Considerations
Widespread adoption of MT in the cut flower industry is partly limited by cost and availability. Although MT can be chemically synthesized or extracted from natural sources (e.g., plants or microbes), chemical synthesis remains the dominant method for large-scale production due to its high yield and purity. However, this method may involve hazardous solvents or reagents, raising concerns about environmental sustainability and regulatory approval for use in edible or ornamental crops.
To reduce production costs, recent advances have focused on microbial biosynthesis of MT using genetically engineered bacteria or yeast strains [e.g., E. coli expressing plant MT biosynthetic genes], which offer a potentially low-cost and eco-friendly alternative. These fermentation-based platforms can use inexpensive feedstocks and reduce reliance on petrochemicals.
Furthermore, encapsulation technologies, such as nanochitosan or biodegradable polymers, can extend MT’s stability and reduce dosage requirements, thereby lowering overall treatment cost per flower unit. Optimizing these systems for commercial scale remains a key area for future development. However, actual economic data, production costs, or detailed life cycle assessments for MT-based formulations are still limited in the literature, and these knowledge gaps need to be addressed through future techno-economic analyses and industry-specific trials.
4. Future Prospects and Conclusions
MT has emerged as a promising eco-friendly preservative for extending VL and improving the post-harvest quality of cut flowers. Its antioxidant properties, regulation of water balance, hormonal modulation, and stress resistance mechanisms provide a viable alternative to conventional chemical preservatives. However, despite its demonstrated effectiveness, the widespread adoption of MT in the floriculture industry is hindered by optimal application methods, species-specific responses, stability concerns, interactions with existing preservatives, and regulatory challenges. Addressing these limitations through targeted research and technological advancements will be essential for the successful integration of MT into commercial post-harvest management practices. To fully realize the potential of MT in the floriculture industry, future research should focus on several key areas:
- (i)
- Optimization of MT application strategies
- Establishing standardized MT concentrations, treatment durations, and application methods, such as pulsing, vase solutions, and foliar sprays tailored to different cut flower species.
- Developing slow-release or encapsulated MT formulations to enhance stability and prolong their effectiveness under commercial storage and handling conditions.
- (ii)
- Mechanistic insights into MT’s role in post-harvest physiology
- Investigating the molecular and genetic mechanisms underlying MT-induced senescence delay, WU enhancement, and oxidative stress mitigation.
- Identifying key MT-responsive genes and signaling pathways involved in postharvest longevity to enhance understanding and optimize application methods.
- (iii)
- Integration with commercial post-harvest treatments
- Evaluating synergistic or antagonistic effects of MT with widely used post-harvest treatments, such as sucrose supplementation, ethylene inhibitors, and antimicrobial agents.
- Assessing its effectiveness under cold storage, modified atmosphere packaging, and long-distance transport conditions to ensure its practical applicability.
- (iv)
- Commercialization and large-scale validation
- Conducting extensive field trials to validate MT’s efficacy under real-world supply chain conditions, including storage, transport, and retail environments.
- Investigating cost-effective production methods to improve the affordability of MT-based preservatives and enhance their commercial viability.
- Addressing regulatory approvals and ensuring compliance with industry standards to facilitate MT’s adoption as a safe and sustainable post-harvest preservative. While MT is considered an eco-friendly preservative due to its natural occurrence in plants and relatively low toxicity, comprehensive life cycle assessments and comparative toxicity studies are still limited and warrant further investigation.
In conclusion, the application of exogenous MT offers a sustainable and innovative approach to improving the post-harvest longevity of cut flowers. By mitigating oxidative stress, delaying senescence, enhancing WU, and maintaining cellular integrity, MT has demonstrated significant potential in preserving flower freshness and extending VL of cut flowers. However, its economic feasibility, species-dependent effectiveness, stability, and regulatory constraints must be addressed before it can be widely adopted in commercial floriculture. Future advancements in biotechnology, formulation science, and post-harvest technology will be crucial for overcoming these challenges. Large-scale industry validation and regulatory approval will further determine the feasibility of MT as a mainstream preservative. If these obstacles are effectively managed, MT has the potential to revolutionize post-harvest flower preservation, offering a more sustainable and environmentally friendly alternative to existing chemical treatments.
Author Contributions
T.N.: writing—original draft; S.H.: writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tripathi, S.K.; Tuteja, N. Integrated Signaling in Flower Senescence. Plant Signal. Behav. 2007, 2, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Jiang, C.-Z. Postharvest Biology and Technology of Cut Flowers and Potted Plants. Hortic. Rev. 2012, 4, 1–54. [Google Scholar]
- Fanourakis, D.; Pieruschka, R.; Savvides, A.; Macnish, A.J.; Sarlikioti, V.; Woltering, E.J. Sources of vase life variation in cut roses: A review. Postharvest Biol. Technol. 2013, 78, 1–15. [Google Scholar] [CrossRef]
- Shinde, S.P.; Chaudhari, S.R.; Matche, R.S. A way forward for a sustainable active packaging solution for prolonging the freshness and shelf life of Rosa hybrida L. cut flowers. Postharvest Biol. Technol. 2023, 204, 112475. [Google Scholar] [CrossRef]
- Sun, J.; Guo, H.; Tao, J. Effects of Harvest Stage, Storage, and Preservation Technology on Postharvest Ornamental Value of Cut Peony (Paeonia lactiflora) Flowers. Agronomy 2022, 12, 230. [Google Scholar] [CrossRef]
- Thakur, N. A review on the effect of storage methods and packaging material on the post-harvest longevity of cut flowers. Int. J. Chem. Stud. 2020, 8, 2375–2379. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Lim, J.H. Do Eco-Friendly Floral Preservative Solutions Prolong Vase Life Better than Chemical Solutions? Horticulturae 2021, 7, 415. [Google Scholar] [CrossRef]
- Chen, S. Review of Recent Advances in Chemical Preservatives for Cut Flowers. Br. J. Biol. Stud. 2024, 4, 1–8. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting Plants to Survive and Thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827. [Google Scholar] [CrossRef]
- Teng, Z.; Zheng, W.; Jiang, S.; Hong, S.-B.; Zhu, Z.; Zang, Y. Role of melatonin in promoting plant growth by regulating carbon assimilation and ATP accumulation. Plant Sci. 2022, 319, 111276. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin Function and Crosstalk with Other Phytohormones under Normal and Stressful Conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef]
- Huang, S.; Jin, S. Melatonin Interaction with Other Phytohormones in the Regulation of Abiotic Stresses in Horticultural Plants. Antioxidants 2024, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jia, M.; Wang, Y.; Lu, H.; Wang, X. The complexity of melatonin and other phytohormones crosstalk with other signaling molecules for drought tolerance in horticultural crops. Sci. Hortic. 2023, 321, 112348. [Google Scholar] [CrossRef]
- Ali, M.; Pan, Y.; Liu, H.; Cheng, Z. Melatonin interaction with abscisic acid in the regulation of abiotic stress in Solanaceae family plants. Front. Plant Sci. 2023, 14, 1271137. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, L.; Miao, P.; Dong, Q.; Cheng, H.; Wang, Y.; Li, D.; Pan, C. A novel perspective to investigate how nanoselenium and melatonin lengthen the cut carnation vase shelf. Plant Physiol. Biochem. 2023, 196, 982–992. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Y.; Wang, Y.; Zhang, L.; Li, L.; Looi, L.J.; Zhang, Z. The potential of melatonin and its crosstalk with other hormones in the fight against stress. Front. Plant Sci. 2024, 15, 1492036. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A.; et al. Melatonin Enhances Seed Germination and Seedling Growth of Medicago sativa Under Salinity via a Putative Melatonin Receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jing, T.; Wang, Y.; Ai, X.; Bi, H. Melatonin delays leaf senescence and improves cucumber yield by modulating chlorophyll degradation and photoinhibition of PSII and PSI. Environ. Exp. Bot. 2022, 200, 104915. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2017, 121, 195–207. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin Is a Potential Target for Improving Post-Harvest Preservation of Fruits and Vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Sun, M.; Zhu, W.; Zheng, Y.; Zhu, S.; Chen, L.; Chen, X.; Teixeira da Silva, J.A.; Dong, G.; et al. Melatonin enhances vase life and alters physiological responses in peony (Paeonia lactiflora Pall.) cut flowers. Postharvest Biol. Technol. 2024, 212, 112896. [Google Scholar] [CrossRef]
- Gao, T.; Liu, X.; Tan, K.; Zhang, D.; Zhu, B.; Ma, F.; Li, C. Introducing melatonin to the horticultural industry: Physiological roles, potential applications, and challenges. Hortic. Res. 2022, 9, uhac094. [Google Scholar] [CrossRef] [PubMed]
- Jayarajan, S.; Sharma, R. Melatonin: A blooming biomolecule for postharvest management of perishable fruits and vegetables. Trends Food Sci. Technol. 2021, 116, 318–328. [Google Scholar] [CrossRef]
- Mandal, M.K.; Suren, H.; Ward, B.; Boroujerdi, A.; Kousik, C. Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J. Pineal Res. 2018, 65, e12505. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Melatonin improved tomato fruit quality by promoting ripening and reducing chilling injury. Postharvest Biol. Technol. 2016, 117, 68–75. [Google Scholar]
- Mazrou, R.M.; Hassan, S.; Yang, M.; Hassan, F.A.S. Melatonin Preserves the Postharvest Quality of Cut Roses through Enhancing the Antioxidant System. Plants 2022, 11, 2713. [Google Scholar] [CrossRef]
- Wang, B.; Huang, A.; Liu, L.; Li, Y.; Zhang, H.; Wang, L. Effects of an Exogenous Melatonin Treatment on the Physiological Indexes and Storage Duration of Cut Chrysanthemum Flowers. Hortic. Sci. Technol. 2024, 42, 533–548. [Google Scholar] [CrossRef]
- Trivellini, A.; Ferrante, A.; Mensuali, A.; Incrocci, L. Pulse-treatments with thidiazuron and melatonin improve quality and prolong vase-life of Ranunculus asiaticus L. cut flowers. Act. Hortic. 2024, 1397, 31–34. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Nojadeh, M.S.; Ebrahimzadeh, A. Exogenous melatonin ameliorates chilling injury in cut anthurium flowers during low temperature storage. Postharvest Biol. Technol. 2019, 148, 184–191. [Google Scholar] [CrossRef]
- Lezoul, N.E.H.; Serrano, M.; Ruiz-Aracil, M.C.; Belkadi, M.; Castillo, S.; Valero, D.; Guillén, F. Melatonin as a new postharvest treatment for increasing cut carnation (Dianthus caryophyllus L.) vase life. Postharvest Biol. Technol. 2022, 184, 111759. [Google Scholar] [CrossRef]
- Safaei Far, A.; Sadegh, M.-F.; Abdolhossein, R.N.; Feizollah, S.; Masoumeh, A.-M.; Fanourakis, D. Nano Silver and melatonin effectively delay the senescence of cut carnation flowers under simulated vibrational stress. J. Hortic. Sci. Biotechnol. 2024, 99, 597–608. [Google Scholar] [CrossRef]
- Yu, Z.; Li, S.; Hong, Y. Influence of Tea Polyphenols, Chitosan, and Melatonin as the Eco-Friendly Post-Harvest Treatments on the Vase Life of the Cut Chrysanthemum ‘Pingpong’ Group. Agriculture 2024, 14, 1507. [Google Scholar] [CrossRef]
- SeyedHajizadeh, H.; FarajiChelanolya, A.; Zahedi, S.M.; Moghadam, A.; Mahdavinia, G.; Kaya, O. Nanochitosan-encapsulated melatonin: An eco-friendly strategy to delay petal senescence in cut gerbera flowers. BMC Plant Biol. 2024, 24, 1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tian, C.; Ren, X.; Zhang, X.; Xue, J.; Hao, R. A composite vase solution improved vase quality of cut peony after long-term cold storage by maintaining better physiological activities and enhancing the absorption of water. Postharvest Biol. Technol. 2024, 213, 112945. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Darras, A.; Nafees, M.; Ferrante, A.; Siddique, K.H.M. Preharvest melatonin foliar treatments enhance postharvest longevity of cut tuberose via altering physio-biochemical traits. Front. Plant Sci. 2023, 14, 1151722. [Google Scholar] [CrossRef]
- Xiang, D.; Nguyen, C.D.; Felter, L.; Clark, D.; Huo, H. The effects of preharvest LED light, melatonin and AVG treatments on the quality of postharvest snapdragon and vase life. J. Floric. Landsc. 2020, 6, 14–19. [Google Scholar] [CrossRef]
- Louredo de Brito, F.; Júnior, N.; Martins, L.; Martim, M.; Silva, L.; Guerra, T.; Simões, A. Application of melatonin and sucrose in prolonging the vase life of amaryllis cut flowers (Hippeastrum Hybridum Herb). Ornam. Hortic. 2023, 29, 489–499. [Google Scholar] [CrossRef]
- Zeinali Pour, N. Impact of Melatonin Treatment on Vase Life and Postharvest Quality of Eustomna grandiflorum cv. Grand white. In Proceedings of the First National Conference on Production and Postharvest Technology of Horticultural Plants, Birjand, Iran, 25 May 2022. [Google Scholar]
- Soleimani Aghdam, M.; Naderi, R.; Sarcheshmeh, M.A.A.; Babalar, M. Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol. Technol. 2015, 110, 70–76. [Google Scholar] [CrossRef]
- Liang, L.; Deng, Y.; Sun, X.; Jia, X.; Su, J. Exogenous Nitric Oxide Pretreatment Enhances Chilling Tolerance of Anthurium. J. Amer Soc. Hort. Sci. 2018, 143, 3–13. [Google Scholar] [CrossRef]
- Ranjbar, A.; Ahmadi, N. Effects of 1-MCP and ethylene on antioxidant enzymes activity and postharvest physio-biochemical characterics of cut carnation flower cv. Fortune. Adv. Hortic. Sci. 2015, 29, 192–198. [Google Scholar]
- In, B.-C.; Ha, S.T.T.; Kim, Y.-T.; Lim, J.H. Relationship among floral scent intensity, ethylene sensitivity, and longevity of carnation flowers. Hortic. Environ. Biotechnol. 2021, 62, 907–916. [Google Scholar] [CrossRef]
- Yangkhamman, P.; Fukai, S.; Ichimura, K. Ethylene Production and Vase Life of Cut Carnation Flowers under High Temperature Conditions. Engei Gakkai Zasshi 2005, 74, 337–341. [Google Scholar] [CrossRef]
- Williams, M.H.; Nell, T.A.; Barrett, J.E. Investigation of proteins in petals of potted chrysanthemum as a potential indicator of longevity. Postharvest Biol. Technol. 1995, 5, 91–100. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Montaldi, E.R.; Puntarulo, S. Oxidants and antioxidants during aging of chrysanthemum petals. Plant Sci. 1997, 129, 157–165. [Google Scholar] [CrossRef]
- Satoh, S.; Narumi, T.; Watanabe, M.; Aida, R.; Ohmiya, A. Reduced leaf senescence in chrysanthemum transformed with mutated ethylene receptor genes. Acta Hortic. 2007, 764, 89–94. [Google Scholar] [CrossRef]
- Liu, R.; Zuo, X.; Chen, Y.; Qian, Z.; Xu, C.; Wang, L.; Chen, S. Transcriptional Regulation in Leaves of Cut Chrysanthemum (Chrysanthemum morifolium) ‘FenDante’ in Response to Post-Harvest Ethylene Treatment. Horticulturae 2022, 8, 573. [Google Scholar] [CrossRef]
- Perik, R.R.J.; Razé, D.; Harkema, H.; Zhong, Y.; van Doorn, W.G. Bending in cut Gerbera jamesonii flowers relates to adverse water relations and lack of stem sclerenchyma development, not to expansion of the stem central cavity or stem elongation. Postharvest Biol. Technol. 2012, 74, 11–18. [Google Scholar] [CrossRef]
- Kamenetsky-Goldstein, R.; Yu, X. Cut peony industry: The first 30 years of research and new horizons. Acta Hortic. 2022, 9, uhac079. [Google Scholar] [CrossRef]
- Zhao, D.; Cheng, M.; Tang, W.; Liu, D.; Zhou, S.; Meng, J.; Tao, J. Nano-silver modifies the vase life of cut herbaceous peony (Paeonia lactiflora Pall.) flowers. Protoplasma 2018, 255, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, J.; Jeong, B.R. Synergistic Effects of Silicon and Preservative on Promoting Postharvest Performance of Cut Flowers of Peony (Paeonia lactiflora Pall.). Int. J. Mol. Sci. 2022, 23, 13211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Luan, Y.; Shi, W.; Tang, Y.; Huang, X.; Tao, J. Melatonin enhances stem strength by increasing lignin content and secondary cell wall thickness in herbaceous peony. J. Exp. Bot. 2022, 73, 5974–5991. [Google Scholar] [CrossRef]
- Thi Ha, S.T.; Ham, J.Y.; Kim, Y.-T.; In, B.-C. Interaction between plant hormones in response to Botrytis cinerea infection in cut flowers with differential tissue sensitivity to ethylene. Postharvest Biol. Technol. 2025, 223, 113441. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, G.; Dixit, K. Senescence in rose (Rosa hybrida L.): Role of the endogenous antioxidant system. J. Hortic. Sci. Biotechnol. 2008, 83, 125–131. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2014, 66, 657–668. [Google Scholar] [CrossRef]
- Mubarok, S.; Suminar, E.; Abidat, A.H.; Setyawati, C.A.; Setiawan, E.; Buswar, A.S. Overview of Melatonin’s Impact on Postharvest Physiology and Quality of Fruits. Horticulturae 2023, 9, 586. [Google Scholar] [CrossRef]
- Samanta, S.; Roychoudhury, A. Crosstalk of melatonin with major phytohormones and growth regulators in mediating abiotic stress tolerance in plants. S. Afr. J. Bot. 2023, 163, 201–216. [Google Scholar] [CrossRef]
- Malakar, M.; Paiva, P.D.O.; Beruto, M.; da Cunha Neto, A.R. Review of recent advances in post-harvest techniques for tropical cut flowers and future prospects: Heliconia as a case-study. Front. Plant Sci. 2023, 14, 1221346. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef] [PubMed]
- Kobylińska, A.; Borek, S.; Posmyk, M.M. Melatonin redirects carbohydrates metabolism during sugar starvation in plant cells. J. Pineal Res. 2018, 64, e12466. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Mehran, M.; Rehman, H.U.; Ullah, S.; Bakhsh, M.Z.M.; Tahira, M.; Maqsood, M.F.K.; Rauf, A.; Ghafar, S.; Haider, K.; et al. Mechanistic review of melatonin metabolism and signaling pathways in plants: Biosynthesis, regulation, and roles under abiotic stress. Plant Stress 2024, 14, 100685. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Huang, X.; Zheng, X.; Tian, Y.; Shi, L.; Dong, P.; Li, Z. Melatonin: Biosynthesis, content, and function in horticultural plants and potential application. Sci. Hortic. 2021, 288, 110392. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Loypimai, P. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon 2020, 6, e03648. [Google Scholar] [CrossRef]
- Canizo, B.V.; Jofré, M.F.; Mammana, S.B.; Dazat, R.E.; Silva, M.F.; Gomez, F.J.V. Stability of melatonin in eutectic systems: New avenues in therapeutic product development. J. Ion. Liq. 2024, 4, 100112. [Google Scholar] [CrossRef]
- Zhen, L.; Huang, Y.; Bi, X.; Gao, A.; Peng, L.; Chen, Y. Melatonin feeding changed the microbial diversity and metabolism of the broiler cecum. Front. Microbiol. 2024, 15, 1422272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).