Chitin Oligomers from Alternaria alternata Induce Activation of Signal Transduction Pathways by Ethylene, Jasmonic Acid, and Salicylic Acid in Solanum lycopersicum Fruits
Abstract
1. Introduction
2. Materials and Methods
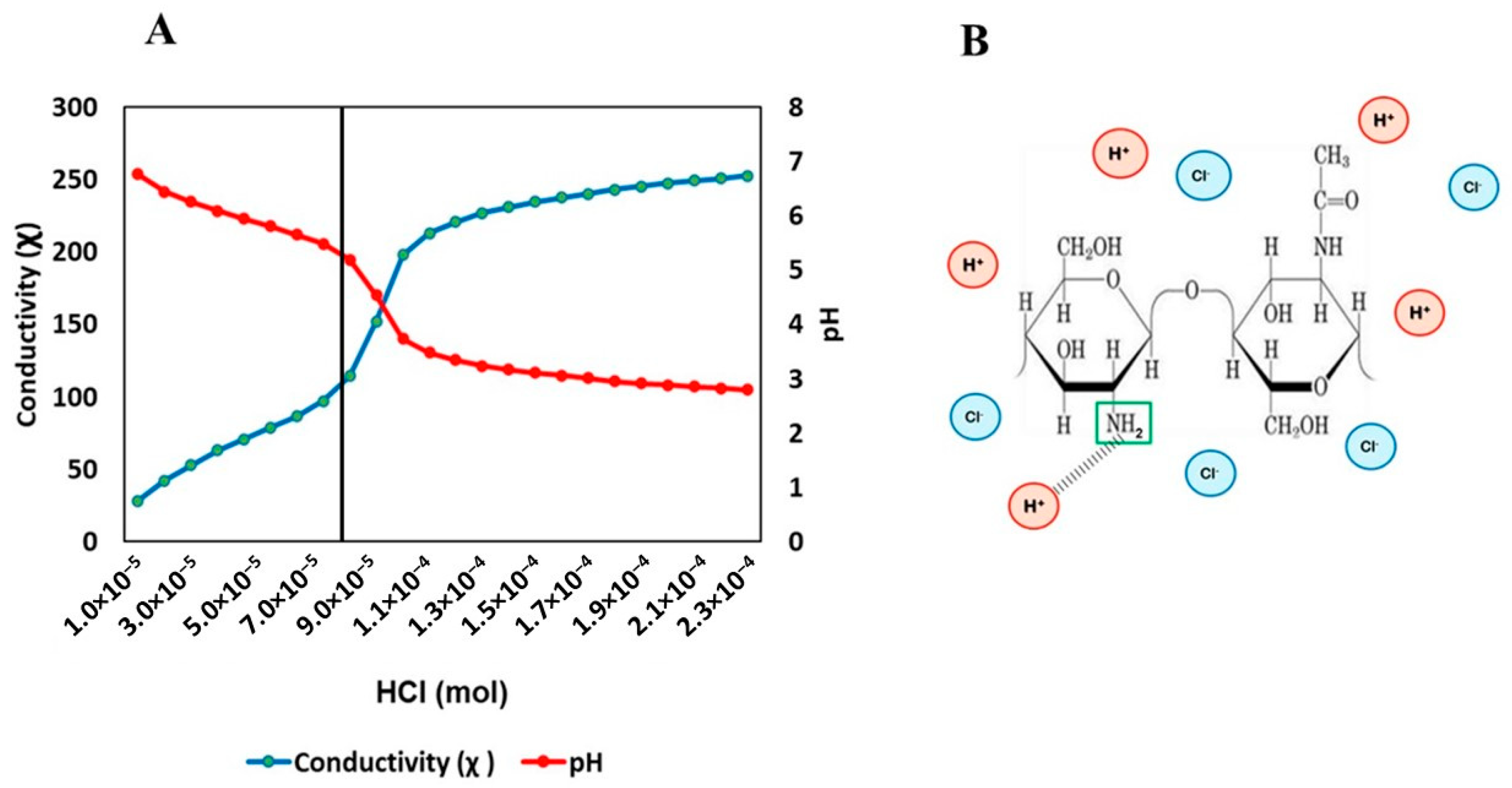
2.1. Extraction and Characterization of Chitin Oligomers from Alternaria alternata
2.2. Determination of the Biological Activity of Chitin Oligomers on Tomato Fruits
2.2.1. Plant Material
2.2.2. Postharvest Treatment of Tomato Fruits with Chitin Oligomers from Alternaria
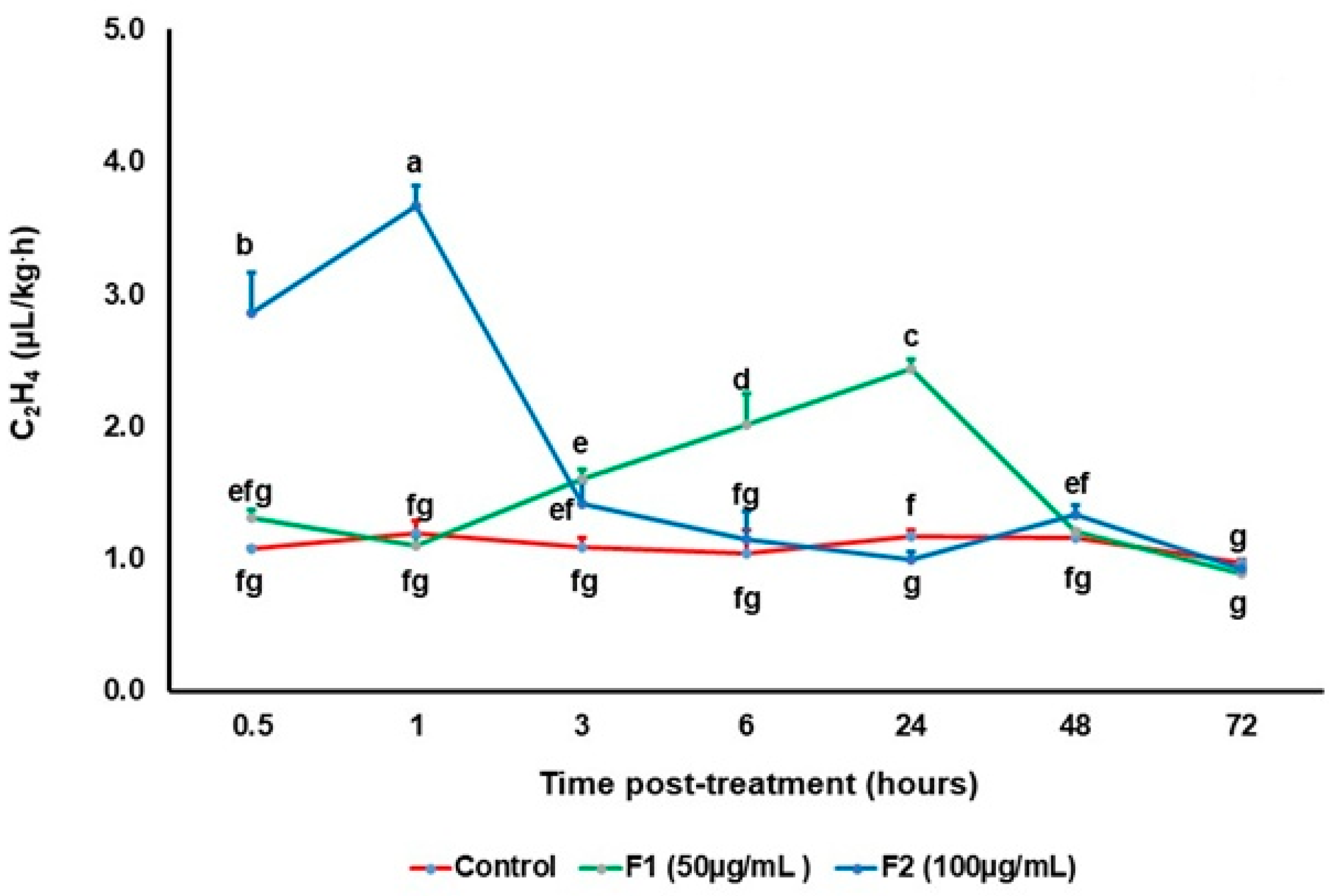
2.3. Quantification of Ethylene Production Using Gas Chromatography
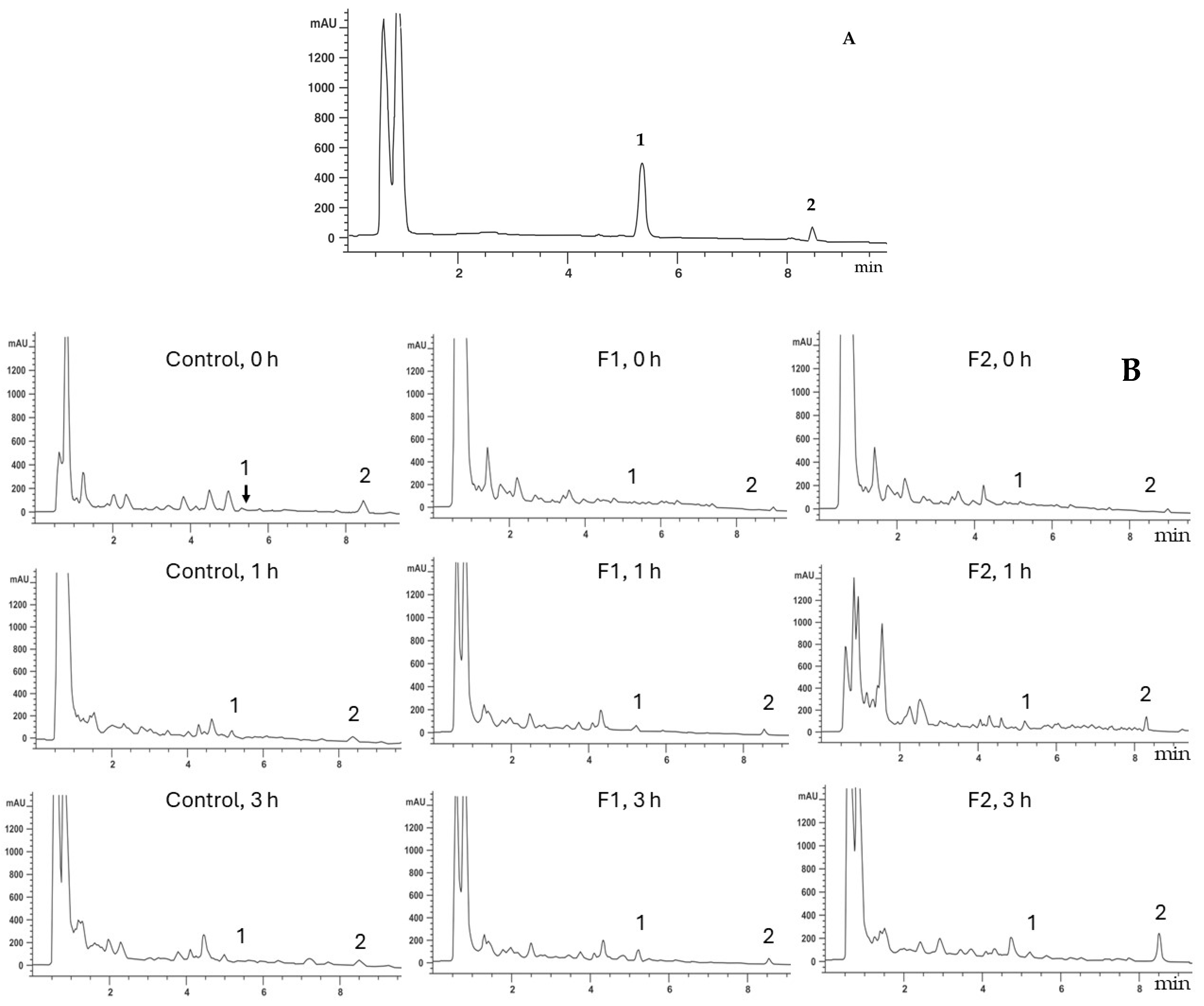
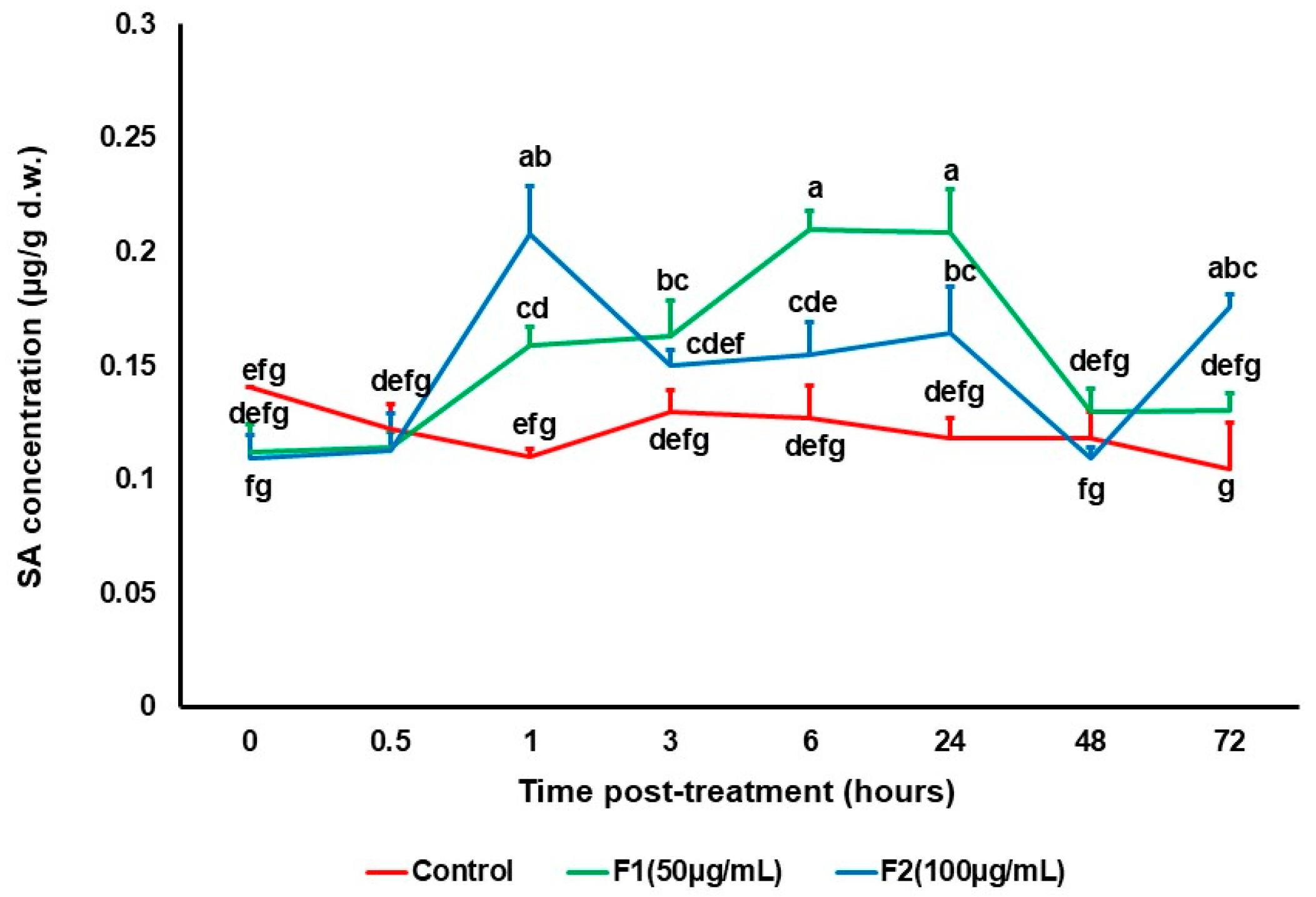
2.4. Jasmonic Acid and Salicylic Acid Quantification Using HPLC
2.5. RNA Extraction, First-Strand DNA Synthesis, and Quantification of Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Chemical Characteristics of Chitin Oligomers from A. alternata
3.2. Effect of Chitin Oligomers on the Production of Signaling Molecules
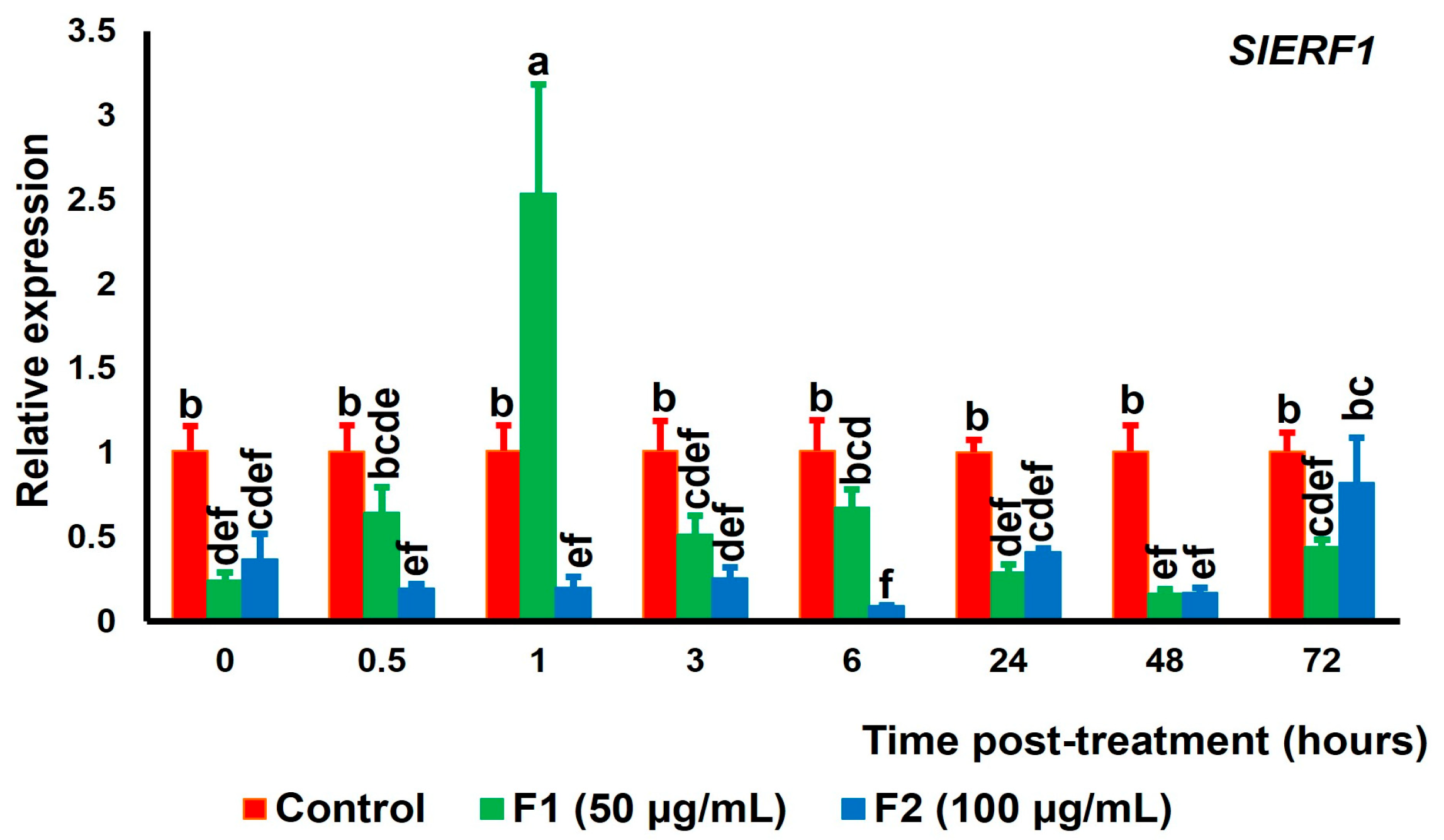
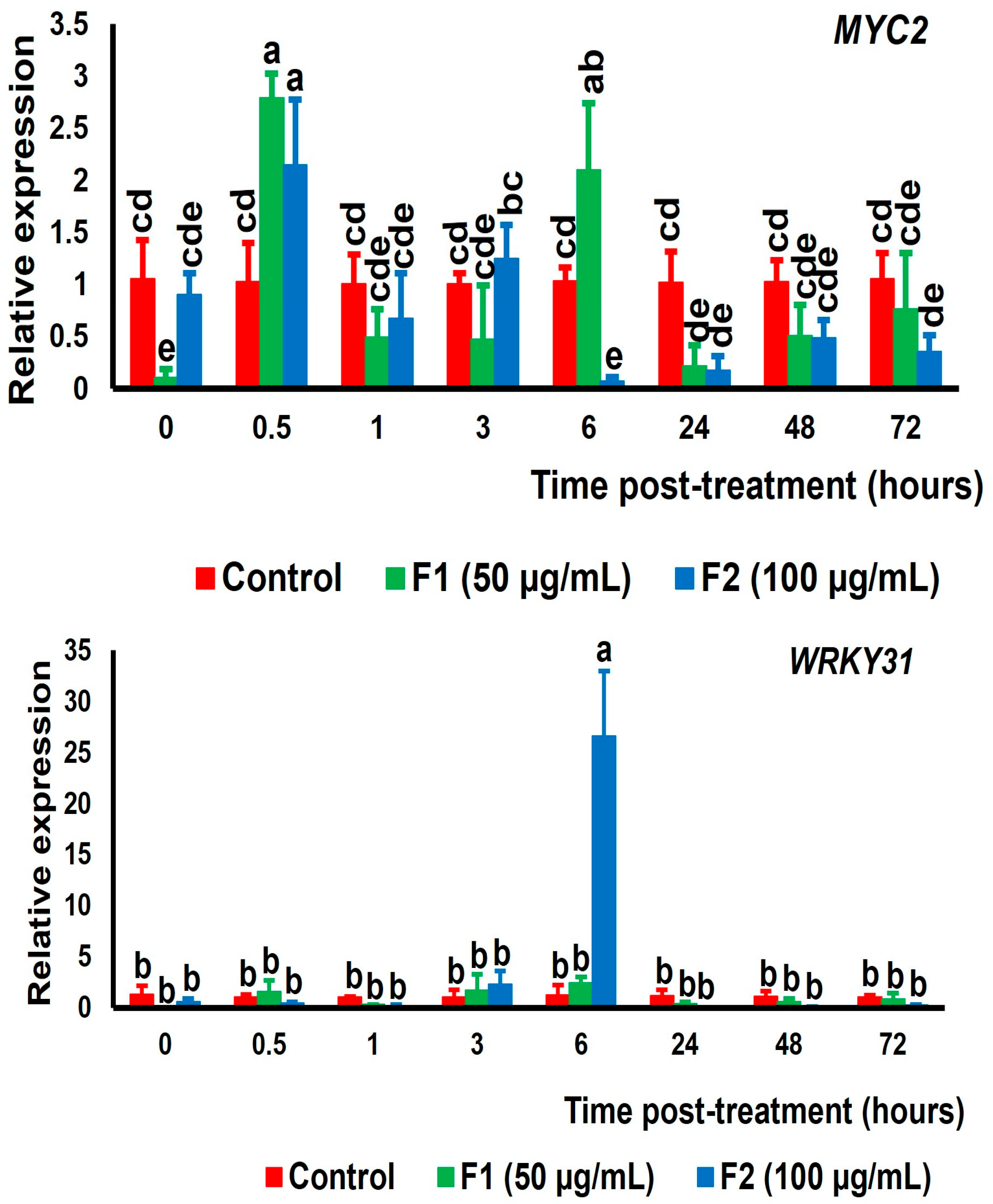
3.3. Expression of Genes Encoding Transcription Factors Related with Et, JA, and SA Signaling Pathways in Tomato Fruit Exposed to Chitin Oligomers
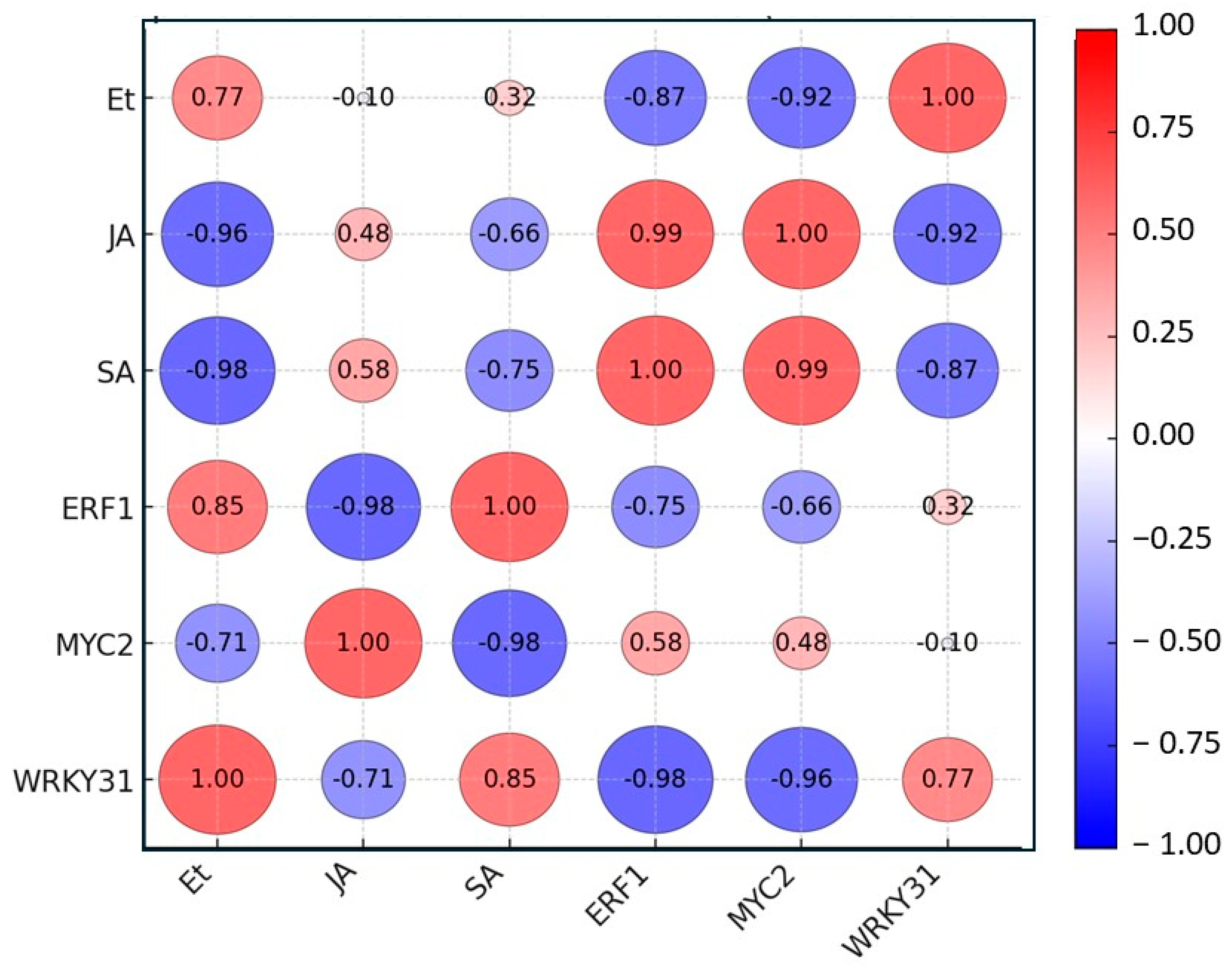
3.4. Correlation Analysis of Signaling Hormones
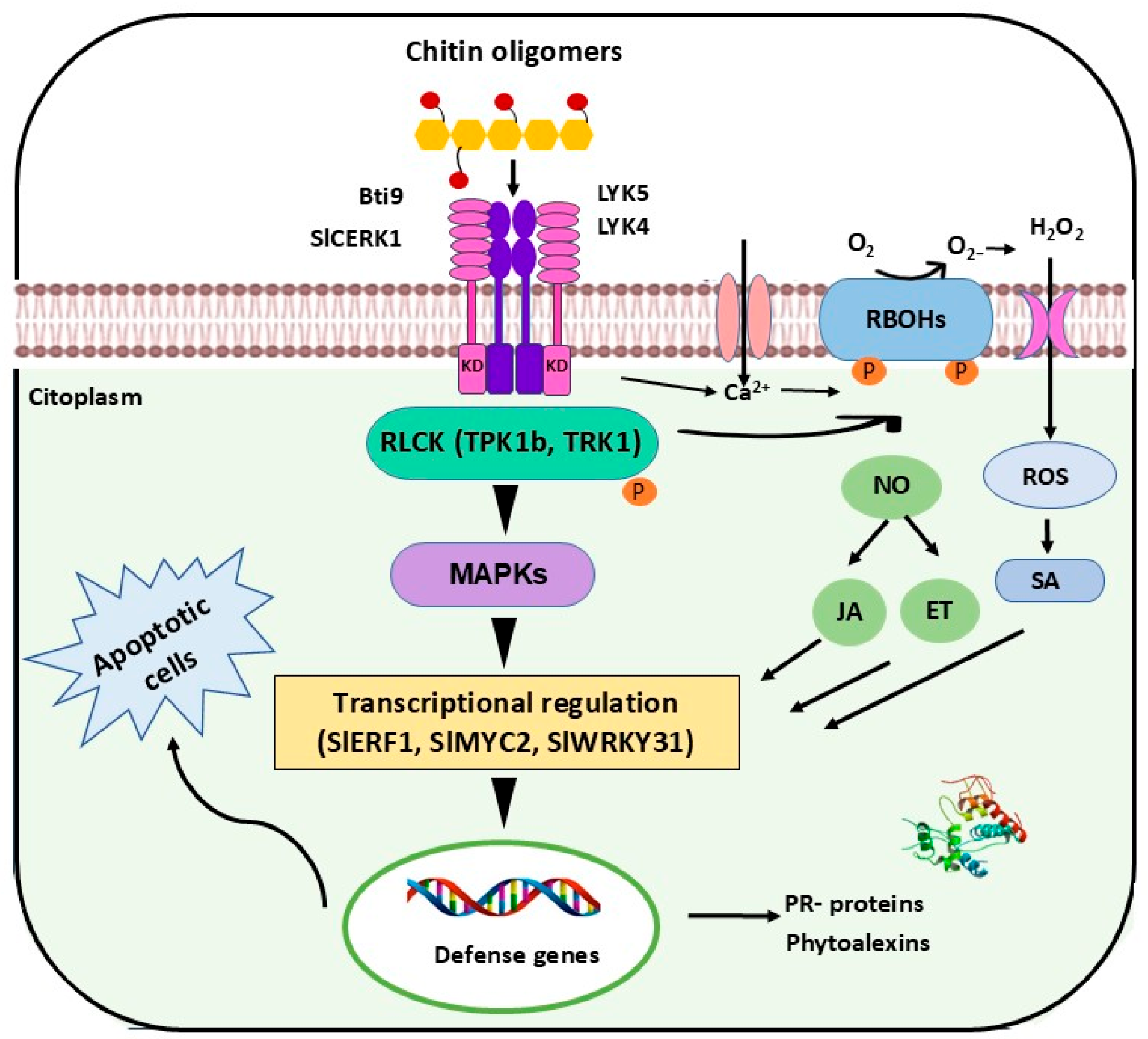
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/ (accessed on 11 December 2024).
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and necrotrophic lifestyle choice during postharvest disease development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Rojas, R.; Tiznado-Hernández, M.E. Chapter 5—Alternaria alternata (Black Rot, Black Spot). In Postharvest Decay; Bautista-Baños, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 147–187. [Google Scholar]
- Takao, K.; Akagi, Y.; Tsuge, T.; Kodama, M. Functional characterization of putative G protein-coupled receptors in the tomato pathotype of Alternaria alternata. J. Gen. Plant Pathol. 2016, 82, 82–88. [Google Scholar] [CrossRef]
- Pane, C.; Fratianni, F.; Parisi, M.; Nazzaro, F.; Zaccardelli, M. Control of Alternaria post-harvest infections on cherry tomato fruits by wild pepper phenolic-rich extracts. Crop Prot. 2016, 84, 81–87. [Google Scholar] [CrossRef]
- Kader, K. Small-Scale Postharvest Handling Practices: A Manual for Horticultural Crops; University of California, Davis, Postharvest Technology Research and Information Center: Berkeley, CA, USA, 2015. [Google Scholar]
- Islam, T.; Danishuddin; Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, S.K.; Singh, M.; Sharma, I.; Sharma, P.; Kamboj, P.; Saini, A.; Voraha, R.; Sharma, A.K.; Upadhyay, T.K.; et al. Chitin, Chitinases and Chitin Derivatives in Biopharmaceutical, Agricultural and Environmental Perspective. Biointerface Res. Appl. Chem. 2021, 11, 9985–10005. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef]
- Parada, R.Y.; Egusa, M.; Aklog, Y.F.; Miura, C.; Ifuku, S.; Kaminaka, H. Optimization of nanofibrillation degree of chitin for induction of plant disease resistance: Elicitor activity and systemic resistance induced by chitin nanofiber in cabbage and strawberry. Int. J. Biol. Macromol. 2018, 118, 2185–2192. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, S.; Zhuang, M.; Zhang, Y.; Lv, H.; Ji, J.; Hou, X.; Fang, Z.; Wang, Y.; Yang, L. Genome-wide identification and expression analysis of the Brassica oleracea L. chitin-binding genes and response to pathogens infections. Planta 2021, 253, 80. [Google Scholar] [CrossRef]
- Um, E.A.; Nisar, N.; Tsuzuki, T.; Lowe, A.; Rossiter, J.T.; Javaid, A.; Powell, G.; Waseem, R.; Al-Mijalli, S.H.; Iqbal, M. Chitin nanofibers trigger membrane bound defense signaling and induce elicitor activity in plants. Int. J. Biol. Macromol. 2021, 178, 253–262. [Google Scholar] [CrossRef]
- Hao, G.; Tiley, H.; McCormick, S. Chitin Triggers Tissue-Specific Immunity in Wheat Associated with Fusarium Head Blight. Front. Plant Sci. 2022, 13, 832502. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, S.; Jiao, Y.; Wang, H. Synergistic effects of nanochitin on inhibition of tobacco root rot disease. Int. J. Biol. Macromol. 2017, 99, 205–212. [Google Scholar] [CrossRef]
- Sun, C.; Fu, D.; Jin, L.; Chen, M.; Zheng, X.; Yu, T. Chitin isolated from yeast cell wall induces the resistance of tomato fruit to Botrytis cinerea. Carbohydr. Polym. 2018, 199, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Valle-Sotelo, E.; Troncoso-Rojas, R.; Tiznado-Hernández, M.; Carvajal-Millan, E.; Estrada, A.; García, Y. Bioefficacy of fungal chitin oligomers in the control of postharvest decay in tomato fruit. Int. Food Res. J. 2022, 29, 1131–1142. [Google Scholar] [CrossRef]
- Henry García, Y.; Troncoso-Rojas, R.; Báez-Flores, M.E.; Hernández-Oñate, M.Á.; Tiznado-Hernández, M.E. RNA-Seq of Tomato Fruit-Alternaria Chitin Oligomer Interaction Reveals Genes Encoding Chitin Membrane Receptors and the Activation of the Defense Response. Horticulturae 2023, 9, 1064. [Google Scholar] [CrossRef]
- Iizasa, E.; Mitsutomi, M.; Nagano, Y. Direct binding of a plant LysM receptor-like kinase, LysM RLK1/CERK1, to chitin in vitro. J. Biol. Chem. 2010, 285, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Brulé, D.; Villano, C.; Davies, L.J.; Trdá, L.; Claverie, J.; Héloir, M.C.; Chiltz, A.; Adrian, M.; Darblade, B.; Tornero, P.; et al. The grapevine (Vitis vinifera) LysM receptor kinases VvLYK1-1 and VvLYK1-2 mediate chitooligosaccharide-triggered immunity. Plant Biotechnol. J. 2019, 17, 812–825. [Google Scholar] [CrossRef]
- Jaiswal, N.; Liao, C.J.; Mengesha, B.; Han, H.; Lee, S.; Sharon, A.; Zhou, Y.; Mengiste, T. Regulation of plant immunity and growth by tomato receptor-like cytoplasmic kinase TRK1. New Phytol. 2022, 233, 458–478. [Google Scholar] [CrossRef]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef]
- Ai, Y.; Li, Q.; Li, C.; Wang, R.; Sun, X.; Chen, S.; Cai, X.Z.; Qi, X.; Liang, Y. Tomato LysM receptor kinase 4 mediates chitin-elicited fungal resistance in both leaves and fruit. Hortic. Res. 2023, 10, uhad082. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, J.; Fukui, K.; Seto, Y.; Takaoka, Y.; Okamoto, M. Ligand-receptor interactions in plant hormone signaling. Plant J. For. Cell Mol. Biol. 2021, 105, 290–306. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Li, B.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular mechanisms underlying multi-level defense responses of horticultural crops to fungal pathogens. Hortic. Res. 2022, 9, uhac066. [Google Scholar] [CrossRef] [PubMed]
- Reyes Zamora, O.; Troncoso-Rojas, R.; Báez-Flores, M.E.; Tiznado-Hernández, M.E.; Rascón-Chu, A. Signaling of Plant Defense Mediated by Receptor-like Kinases, Receptor-like Cytoplasmic Protein Kinases and MAPKs Triggered by Fungal Chitin in Horticultural Crops. Horticulturae 2024, 10, 361. [Google Scholar] [CrossRef]
- Sun, H.; Song, N.; Ma, L.; Li, J.; Ma, L.; Wu, J.; Wu, J. Ethylene signalling is essential for the resistance of Nicotiana attenuata against Alternaria alternata and phytoalexin scopoletin biosynthesis. Plant Pathol. 2017, 66, 277–284. [Google Scholar] [CrossRef]
- Chen, C.-l.; Yuan, F.; Li, X.-y.; Ma, R.-c.; Xie, H. Jasmonic acid and ethylene signaling pathways participate in the defense response of Chinese cabbage to Pectobacterium carotovorum infection. J. Integr. Agric. 2021, 20, 1314–1326. [Google Scholar] [CrossRef]
- Yu, X.; Feng, B.; He, P.; Shan, L. From Chaos to Harmony: Responses and Signaling upon Microbial Pattern Recognition. Annu. Rev. Phytopathol. 2017, 55, 109–137. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Park, Y.S.; Borrego, E.J.; Gao, X.; Christensen, S.A.; Schmelz, E.; Lanubile, A.; Drab, D.A.; Cody, W.; Yan, H.; Shim, W.B.; et al. Fusarium verticillioides Induces Maize-Derived Ethylene to Promote Virulence by Engaging Fungal G-Protein Signaling. Mol. Plant-Microbe Interact 2021, 34, 1157–1166. [Google Scholar] [CrossRef]
- Regmi, R.; Newman, T.E.; Kamphuis, L.G.; Derbyshire, M.C. Identification of B. napus small RNAs responsive to infection by a necrotrophic pathogen. BMC Plant Biol. 2021, 21, 366. [Google Scholar] [CrossRef]
- Li, S.; Wu, P.; Yu, X.; Cao, J.; Chen, X.; Gao, L.; Chen, K.; Grierson, D. Contrasting Roles of Ethylene Response Factors in Pathogen Response and Ripening in Fleshy Fruit. Cells 2022, 11, 2484. [Google Scholar] [CrossRef]
- Gu, S.; Xie, L.; Guan, Q.; Sheng, X.; Fang, Y.; Wang, X. Effect of ethylene production by four pathogenic fungi on the postharvest diseases of green pepper (Capsicum annuum L.). Int. J. Food Microbiol. 2024, 418, 110729. [Google Scholar] [CrossRef]
- Wang, Q. Study on the expression regulation of the CTR1 gene in the ethylene signaling pathway. Biochem. Biophys. Res. Commun. 2024, 739, 150590. [Google Scholar] [CrossRef]
- Wang, X.; Wen, H.; Suprun, A.; Zhu, H. Ethylene Signaling in Regulating Plant Growth, Development, and Stress Responses. Plants 2025, 14, 309. [Google Scholar] [CrossRef]
- Wang, X.; Meng, H.; Tang, Y.; Zhang, Y.; He, Y.; Zhou, J.; Meng, X. Phosphorylation of an ethylene response factor by MPK3/MPK6 mediates negative feedback regulation of pathogen-induced ethylene biosynthesis in Arabidopsis. J. Genet. Genom. Yi Chuan Xue Bao 2022, 49, 810–822. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, K.; Shen, X.; Meng, J.; Huang, X.; Tan, Y.; Cardinale, F.; Liu, J.; Li, G.; Liu, J. Two interacting ethylene response factors negatively regulate peach resistance to Lasiodiplodia theobromae. Plant Physiol. 2023, 192, 3134–3151. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, B.; Luo, Y.; Fu, D. Unraveling the protein network of tomato fruit in response to necrotrophic phytopathogenic Rhizopus nigricans. PLoS ONE 2013, 8, e73034. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Jęcz, T.; Ciereszko, I.; Kononowicz, A.K. Jasmonic Acid as a Mediator in Plant Response to Necrotrophic Fungi. Cells 2023, 12, 1027. [Google Scholar] [CrossRef]
- Pandey, D.; Rajendran, S.R.C.K.; Gaur, M.; Sajeesh, P.K.; Kumar, A. Plant Defense Signaling and Responses Against Necrotrophic Fungal Pathogens. J. Plant Growth Regul. 2016, 35, 1159–1174. [Google Scholar] [CrossRef]
- Peian, Z.; Haifeng, J.; Peijie, G.; Sadeghnezhad, E.; Qianqian, P.; Tianyu, D.; Teng, L.; Huanchun, J.; Jinggui, F. Chitosan induces jasmonic acid production leading to resistance of ripened fruit against Botrytis cinerea infection. Food Chem. 2021, 337, 127772. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Tarighi, S. Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J. Plant Physiol. 2010, 167, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Hu, Y.; Tang, X.; Zhou, P.; Deng, X.; Wang, H.; Guo, Z. Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta 2012, 236, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Trang Nguyen, H.; Thi Mai To, H.; Lebrun, M.; Bellafiore, S.; Champion, A. Jasmonates—The Master Regulator of Rice Development, Adaptation and Defense. Plants 2019, 8, 339. [Google Scholar] [CrossRef]
- Song, S.; Huang, H.; Gao, H.; Wang, J.; Wu, D.; Liu, X.; Yang, S.; Zhai, Q.; Li, C.; Qi, T.; et al. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 2014, 26, 263–279. [Google Scholar] [CrossRef]
- Gautam, J.K.; Giri, M.K.; Singh, D.; Chattopadhyay, S.; Nandi, A.K. MYC2 influences salicylic acid biosynthesis and defense against bacterial pathogens in Arabidopsis thaliana. Physiol. Plant. 2021, 173, 2248–2261. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Qiu, L.; Han, X.; Zhu, Y.; Liu, L.; Man, M.; Li, F.; Ren, M.; Xing, Y. MYC2: A Master Switch for Plant Physiological Processes and Specialized Metabolite Synthesis. Int. J. Mol. Sci. 2023, 24, 3511. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, H.; Yue, Y.; Zhou, T.; Zhu, Z.; Wang, C. Integrated transcriptomic and transgenic analyses reveal potential mechanisms of poplar resistance to Alternaria alternata infection. BMC Plant Biol. 2022, 22, 413. [Google Scholar] [CrossRef]
- Zhu, L.; Ni, W.; Liu, S.; Cai, B.; Xing, H.; Wang, S. Transcriptomics Analysis of Apple Leaves in Response to Alternaria alternata Apple Pathotype Infection. Front. Plant Sci. 2017, 8, 22. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Chen, P.; Zhou, K.; Xue, W.; Abid, K.; Chen, S. Redox Status, JA and ET Signaling Pathway Regulating Responses to Botrytis cinerea Infection Between the Resistant Cucumber Genotype and Its Susceptible Mutant. Front. Plant Sci. 2020, 11, 559070. [Google Scholar] [CrossRef]
- Ullah, C.; Chen, Y.H.; Ortega, M.A.; Tsai, C.J. The diversity of salicylic acid biosynthesis and defense signaling in plants: Knowledge gaps and future opportunities. Curr. Opin. Plant Biol. 2023, 72, 102349. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cao, H.; Wang, J.; Zhang, H. Recent advances in functional assays of WRKY transcription factors in plant immunity against pathogens. Front. Plant Sci. 2024, 15, 1517595. [Google Scholar] [CrossRef]
- Javed, T.; Gao, S.J. WRKY transcription factors in plant defense. Trends Genet. 2023, 39, 787–801. [Google Scholar] [CrossRef]
- Yin, W.; Wang, X.; Liu, H.; Wang, Y.; Nocker, S.; Tu, M.; Fang, J.; Guo, J.; Li, Z.; Wang, X. Overexpression of VqWRKY31 enhances powdery mildew resistance in grapevine by promoting salicylic acid signaling and specific metabolite synthesis. Hortic. Res. 2022, 9, uhab064. [Google Scholar] [CrossRef]
- Luo, D.; Cai, J.; Sun, W.; Yang, Q.; Hu, G.; Wang, T. Tomato SlWRKY3 Negatively Regulates Botrytis cinerea Resistance via TPK1b. Plants 2024, 13, 1597. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Ahmad, P. Salicylic acid interacts with other plant growth regulators and signal molecules in response to stressful environments in plants. Plant Physiol. Biochem. 2023, 196, 431–443. [Google Scholar] [CrossRef]
- Mishra, S.; Roychowdhury, R.; Ray, S.; Hada, A.; Kumar, A.; Sarker, U.; Aftab, T.; Das, R. Salicylic acid (SA)-mediated plant immunity against biotic stresses: An insight on molecular components and signaling mechanism. Plant Stress 2024, 11, 100427. [Google Scholar] [CrossRef]
- Henry García, Y.; Troncoso-Rojas, R.; Tiznado-Hernández, M.E.; Báez-Flores, M.E.; Carvajal-Millan, E.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Martínez-Robinson, K.G. Enzymatic treatments as alternative to produce chitin fragments of low molecular weight from Alternaria alternata. J. Appl. Polym. Sci. 2019, 136, 47339. [Google Scholar] [CrossRef]
- Pryor, B.M.; Michailides, T.J. Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with Alternaria late blight of pistachio. Phytopathology 2002, 92, 406–416. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Gannibal, P.B.; Peever, T.L.; Pryor, B.M. The sections of Alternaria: Formalizing species-group concepts. Mycologia 2013, 105, 530–546. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.; Mora, L.; Capretti, G.; Piergiovanni, L. Charge Density Quantification of Polyelectrolyte Polysaccharides by Conductometric Titration: An Analytical Chemistry Experiment. J. Chem. Educ. 2012, 89, 121–124. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Rojas, R.; Sánchez-Estrada, A.; Ruelas, C.; García, H.S.; Tiznado-Hernández, M. Effect of benzyl isothiocyanate on tomato fruit infection development by Alternaria alternata. J. Sci. Food Agric. 2005, 85, 1427–1434. [Google Scholar] [CrossRef]
- Durgbanshi, A.; Arbona, V.; Pozo, O.; Miersch, O.; Sancho, J.V.; Gómez-Cadenas, A. Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 8437–8442. [Google Scholar] [CrossRef]
- López-Gómez, R.; Gómez-Lim, M.A. A Method for Extracting Intact RNA from Fruits Rich in Polysaccharides using Ripe Mango Mesocarp. HortScience 1992, 27, 440–442. [Google Scholar] [CrossRef]
- Feng, L.; Lintula, S.; Ho, T.H.; Anastasina, M.; Paju, A.; Haglund, C.; Stenman, U.H.; Hotakainen, K.; Orpana, A.; Kainov, D.; et al. Technique for strand-specific gene-expression analysis and monitoring of primer-independent cDNA synthesis in reverse transcription. BioTechniques 2012, 52, 263–270. [Google Scholar] [CrossRef]
- Kõressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Izadi, H.; Asadi, H.; Bemani, M. Chitin: A comparison between its main sources. Front. Mater. 2025, 12, 1537067. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Draughon, F.A.; Conway, W.S.; Sams, C.E. Physicochemical properties and bioactivity of fungal chitin and chitosan. J. Agric. Food Chem. 2005, 53, 3888–3894. [Google Scholar] [CrossRef] [PubMed]
- Percot, A.; Viton, C.; Domard, A. Optimization of chitin extraction from shrimp shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hongkulsup, C.; Khutoryanskiy, V.V.; Niranjan, K. Enzyme assisted extraction of chitin from shrimp shells (Litopenaeus vannamei). J. Chem. Technol. Biotechnol. 2016, 91, 1250–1256. [Google Scholar] [CrossRef]
- Liao, C.J.; Hailemariam, S.; Sharon, A.; Mengiste, T. Pathogenic strategies and immune mechanisms to necrotrophs: Differences and similarities to biotrophs and hemibiotrophs. Curr. Opin. Plant Biol. 2022, 69, 102291. [Google Scholar] [CrossRef]
- Gong, B.Q.; Wang, F.Z.; Li, J.F. Hide-and-Seek: Chitin-Triggered Plant Immunity and Fungal Counterstrategies. Trends Plant Sci. 2020, 25, 805–816. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Gholizadeh Vazvani, M.; Vatankhah, M.; Kennedy, J.F. Chitin-induced disease resistance in plants: A review. Int. J. Biol. Macromol. 2024, 266, 131105. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, N.; Li, C.; Wang, X.; Xu, X.; Chen, W.; Xing, G.; Zheng, W. The early response during the interaction of fungal phytopathogen and host plant. Open Biol. 2017, 7, 170057. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, R.; Sheng, J.; Shen, L. SlERF2 Is Associated with Methyl Jasmonate-Mediated Defense Response against Botrytis cinerea in Tomato Fruit. J. Agric. Food Chem. 2018, 66, 9923–9932. [Google Scholar] [CrossRef]
- Dong, T.; Zheng, T.; Fu, W.; Guan, L.; Jia, H.; Fang, J. The Effect of Ethylene on the Color Change and Resistance to Botrytis cinerea Infection in ‘Kyoho’ Grape Fruits. Foods 2020, 9, 892. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Vincenti, E.; Powell, A.L.; Cantu, D. Tomato transcriptome and mutant analyses suggest a role for plant stress hormones in the interaction between fruit and Botrytis cinerea. Front. Plant Sci. 2013, 4, 142. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, A.; Zhu, S.; Zhang, L. Expression of ACO1, ERS1 and ERF1 genes in harvested bananas in relation to heat-induced defense against Colletotrichum musae. J. Plant Physiol. 2011, 168, 1634–1640. [Google Scholar] [CrossRef]
- Czékus, Z.; Iqbal, N.; Pollák, B.; Martics, A.; Ördög, A.; Poór, P. Role of ethylene and light in chitosan-induced local and systemic defence responses of tomato plants. J. Plant Physiol. 2021, 263, 153461. [Google Scholar] [CrossRef]
- Veselova, S.; Nuzhnaya, T.; Maksimov, I. The Role of Salicylic, Jasmonic Acid and Ethylene in the Development of the Resistance/Susceptibility of Wheat to the SnTox1-Producing Isolate of the Pathogenic Fungus Stagonospora nodorum (Berk.). Plants 2024, 13, 2546. [Google Scholar] [CrossRef]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef] [PubMed]
- Hickman, R.; Van Verk, M.C.; Van Dijken, A.J.H.; Mendes, M.P.; Vroegop-Vos, I.A.; Caarls, L.; Steenbergen, M.; Van der Nagel, I.; Wesselink, G.J.; Jironkin, A.; et al. Architecture and Dynamics of the Jasmonic Acid Gene Regulatory Network. Plant Cell 2017, 29, 2086–2105. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.M.; Sánchez-Parra, B.; Ortiz-García, P.; Santamaría, M.E.; Díaz, I.; Pollmann, S. Jasmonic Acid-Dependent MYC Transcription Factors Bind to a Tandem G-Box Motif in the YUCCA8 and YUCCA9 Promoters to Regulate Biotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 9768. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cai, T.; Wu, Y.; Chen, S.; Chen, J. Physiological and transcriptomics analysis of the effect of recombinant serine protease on the preservation of loquat. Genomics 2021, 113, 3750–3761. [Google Scholar] [CrossRef]
- Nojiri, H.; Sugimori, M.; Yamane, H.; Nishimura, Y.; Yamada, A.; Shibuya, N.; Kodama, O.; Murofushi, N.; Omori, T. Involvement of Jasmonic Acid in Elicitor-Induced Phytoalexin Production in Suspension-Cultured Rice Cells. Plant Physiol. 1996, 110, 387–392. [Google Scholar] [CrossRef]
- Doares, S.H.; Syrovets, T.; Weiler, E.W.; Ryan, C.A. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 4095–4098. [Google Scholar] [CrossRef]
- Zhang, B.; Ramonell, K.; Somerville, S.; Stacey, G. Characterization of early, chitin-induced gene expression in Arabidopsis. Mol. Plant Microbe Interact 2002, 15, 963–970. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequence | Size (bp) | Tm (°C) |
|---|---|---|---|
| ERF1 | Fw-GACAAGGCGGCTTTGAGAAT Rv-GATCCTCCTCCATGCTTCTCA | 20 21 | 55.8 56.2 |
| MYC2 | Fw-TACTTCCAGGGGAAGCAATG Rv-GACGTGATTCAATGGCTCCT | 20 20 | 54.6 55.0 |
| WRKY31 | Fw-AATTGATCAGGGGCAGCAAG Rv-TCTGCCCGTATTTCCTCCAA | 20 20 | 55.7 56.1 |
| GAPDH | Fw-GTGGCTGTTAACGATCCCTT Rv-GTGACTGGCTTCTCATCGAA | 20 20 | 55.0 54.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Zamora, O.; Tiznado-Hernández, M.E.; Báez-Flores, M.E.; Rascón-Chu, A.; Troncoso-Rojas, R. Chitin Oligomers from Alternaria alternata Induce Activation of Signal Transduction Pathways by Ethylene, Jasmonic Acid, and Salicylic Acid in Solanum lycopersicum Fruits. Horticulturae 2025, 11, 565. https://doi.org/10.3390/horticulturae11060565
Reyes-Zamora O, Tiznado-Hernández ME, Báez-Flores ME, Rascón-Chu A, Troncoso-Rojas R. Chitin Oligomers from Alternaria alternata Induce Activation of Signal Transduction Pathways by Ethylene, Jasmonic Acid, and Salicylic Acid in Solanum lycopersicum Fruits. Horticulturae. 2025; 11(6):565. https://doi.org/10.3390/horticulturae11060565
Chicago/Turabian StyleReyes-Zamora, Orlando, Martín Ernesto Tiznado-Hernández, María Elena Báez-Flores, Agustín Rascón-Chu, and Rosalba Troncoso-Rojas. 2025. "Chitin Oligomers from Alternaria alternata Induce Activation of Signal Transduction Pathways by Ethylene, Jasmonic Acid, and Salicylic Acid in Solanum lycopersicum Fruits" Horticulturae 11, no. 6: 565. https://doi.org/10.3390/horticulturae11060565
APA StyleReyes-Zamora, O., Tiznado-Hernández, M. E., Báez-Flores, M. E., Rascón-Chu, A., & Troncoso-Rojas, R. (2025). Chitin Oligomers from Alternaria alternata Induce Activation of Signal Transduction Pathways by Ethylene, Jasmonic Acid, and Salicylic Acid in Solanum lycopersicum Fruits. Horticulturae, 11(6), 565. https://doi.org/10.3390/horticulturae11060565







