Effect of Different Indole Butyric Acid (IBA) Concentrations in Various Rooting Media on the Rooting Success of Loropetalum chinense var. rubrum Yieh Cuttings and Its Modeling with Artificial Neural Networks
Abstract
1. Introduction
- To evaluate the rooting success of Loropetalum chinense var. rubrum Yieh cuttings in three different rooting media: 100% peat, 100% perlite, and a 50% peat–50% perlite mixture.
- To assess the effects of different indole-3-butyric acid (IBA) concentrations (1000 ppm, 3000 ppm, and 6000 ppm) on the rooting performance of the cuttings, alongside a control group with no hormone application.
- To measure the chlorophyll content (µmol/m2) of the cuttings’ leaves and analyze the relationship between chlorophyll synthesis and rooting success.
- To determine the responses of the cuttings to the physical and chemical characteristics of the rooting media, such as pH, moisture, temperature, and electrical conductivity.
- To model the rooting and chlorophyll response using artificial neural networks (ANNs) and validate experimental results through statistical analyses (ANOVA and t-test).
- To contribute to identifying the optimal propagation conditions for Loropetalum chinense in ecological restoration, commercial nursery production, and similar applications.
- To improve understanding of how leaf chlorophyll production interacts with root development in order to clarify metabolic processes involved in the rooting phase.
- To provide guiding data for future research and practical applications in the vegetative propagation of this and other ornamental plant species.
2. Materials and Methods
2.1. Material
2.2. Data Sets
2.3. Application Studies
2.4. Experimental Design
2.5. ANOVA and T Test
2.6. Artificial Neural Network
3. Result
3.1. Statistical Anaylses (ANOVA)
3.2. ANN
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBA | Indole butyric acid |
| ANN | Artificial neural network |
| SPSS | Statistical Package for the Social Sciences |
| °C | Degrees Celsius |
| EC | Electrical conductivity |
| mS/cm | MikroSiemens/santimetre |
| cm | Centimeter |
| μmol m−2 | Micromole per square meter |
| T | Temperature |
| P | pH |
| H | Hormone amount |
| E | EC |
| O | Environment |
| M | Humidity |
| C | Chlorophyll |
| R | Rooting |
References
- Zhang, L.; Yu, X.; Zhang, X.; Zhang, D.; Li, W.; Xiang, L.; Xu, L. Phenotypic Diversity Analysis of the Progeny Variation of a ‘Mosaic Leaf’ Loropetalum chinense var. rubrum Based on Flower Organ Characteristics. Diversity 2022, 14, 913. [Google Scholar] [CrossRef]
- Gong, W.; Liu, W.; Gu, L.; Kaneko, S.; Koch, M.A.; Zhang, D. From glacial refugia to wide distribution range: Demographic expansion of Loropetalum chinense (Hamamelidaceae) in Chinese subtropical evergreen broadleaved forest. Org. Divers. Evol. 2016, 16, 23–38. [Google Scholar] [CrossRef]
- Meholic, C.A. Assessment of the Hamamelidaceae in Global Living Collections. Ph.D. Thesis, The University of Florida, Gainesville, FL, USA, 2019. Available online: https://www.proquest.com/dissertations-theses/assessment-hamamelidaceae-global-living/docview/2307786433/se-2 (accessed on 12 March 2025).
- Zhang, X.; Zhang, L.; Zhang, D.; Liu, Y.; Lin, L.; Xiong, X.; Zhang, D.; Sun, M.; Cai, M.; Yu, X.; et al. Transcriptomic and Metabolomic Profiling Provides Insights into Flavonoid Biosynthesis and Flower Coloring in Loropetalum chinense and Loropetalum chinense var. rubrum. Agronomy 2023, 13, 1296. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, L.; Liu, Y.; Mo, Q.; Zhang, D.; Li, W.; Li, Y. Genome-Wide Analysis of the bHLH Gene Family in Loropetalum chinense var. rubrum: Identification, Classification, Evolution, and Diversity of Expression Patterns under Cultivation. Plants 2023, 12, 3392. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, W.; Zhang, X.; Li, W.; Zhou, Y.; Chen, Y.; Li, Y. Different Pruning Level Effects on Flowering Period and Chlorophyll Fluorescence Parameters of Loropetalum chinense var. rubrum. PeerJ 2022, 10, e13406. [Google Scholar] [CrossRef]
- Wu, E.; Zhang, D.M.; Li, L.; Wu, Y.W.; Yu, X.; Zhang, D.L.; Li, Y.L. ‘Xiangnong Xiangyun’: A New Variety of Loropetalum chinense. HortScience 2025, 60, 218–219. [Google Scholar]
- Wu, W.; Wen, S.; Feng, T.; Chen, G.; Yang, B. Review of Loropetalum chinense as an Industrial, Aesthetic, and Genetic Resource in China. HortScience 2021, 56, 1148–1153. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhong, Z.Z.; Li, D.; Yu, X.Y.; Chen, H.X.; Ding, J.N. Selection-Breeding of Variegated Bud Sports of Loropetalum chinense var. rubrum ‘Huayejimu 1’. J. Hunan Agric. Univ. 2011, 37, 31–33. [Google Scholar]
- Zhang, X.; Zhang, L.; Zhang, D.; Su, D.; Li, W.; Wang, X.; Chen, Q.; Cai, W.; Xu, L.; Cao, F.; et al. Comprehensive Analysis of Metabolome and Transcriptome Reveals the Mechanism of Color Formation in Different Leaves of Loropetalum chinense var. rubrum. BMC Plant Biol. 2023, 23, 133. [Google Scholar] [CrossRef]
- Oğuztürk, G.E.; Sipahi, M.; Çorbacı, Ö.L.; Oğuztürk, T. Çay Bitkisinin Kentsel Yeşil Alanlarda Kullanımı: Rize İli Ziraat Botanik Parkı Çay Bahçesi Uygulaması. Recep Tayyip Erdoğan Univ. J. Sci. Eng. 2023, 4, 208–218. [Google Scholar] [CrossRef]
- Yuan, N.; Comes, H.P.; Cao, Y.N.; Guo, R.; Zhang, Y.H.; Qiu, Y.X. A Comparative Study on Genetic Effects of Artificial and Natural Habitat Fragmentation on Loropetalum chinense (Hamamelidaceae) in Southeast China. Heredity 2015, 114, 544–551. [Google Scholar] [CrossRef][Green Version]
- Li, K.; Tan, B.; Liao, M.; Ni, J. Quadrat Soil Pollen Signal Reflects Plant Important Values in Forests and Shrublands from Subtropical China. Front. Plant Sci. 2024, 15, 1348182. [Google Scholar] [CrossRef]
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The Contribution of Ornamental Plants to Urban Ecosystem Services. Earth 2022, 3, 1258–1274. [Google Scholar] [CrossRef]
- Ren, P.; Didham, R.K.; Murphy, M.V.; Zeng, D.; Si, X.; Ding, P. Forest Edges Increase Pollinator Network Robustness to Extinction with Declining Area. Nat. Ecol. Evol. 2023, 7, 393–404. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.; Mou, H.; Liang, Z.; Ding, T.; Chen, S.; Li, F. Utilization of Indole Acetic Acid with Leucadendron rubrum and Rhododendron pulchrum for the Phytoremediation of Heavy Metals in the Artificial Soil Made of Municipal Sewage Sludge. Toxics 2022, 11, 43. [Google Scholar] [CrossRef]
- Dang, N.; Zhang, H.; Li, H.; Salam, M.M.A.; Chen, G. Comprehensive Evaluation of Dust Retention and Metal Accumulation by the Leaves of Roadside Plants in Hangzhou among Seasons. Forests 2022, 13, 1290. [Google Scholar] [CrossRef]
- Oğuztürk, G.E.; Murat, C.; Yurtseven, M.; Oğuztürk, T. The Effects of AI-Supported Autonomous Irrigation Systems on Water Efficiency and Plant Quality: A Case Study of Geranium psilostemon Ledeb. Plants 2025, 14, 770. [Google Scholar] [CrossRef]
- Andarwulan, N.; Yuliana, N.D.; Hasna, E.; Aziz, S.A.; Davis, T.D. Comparative Analysis of Three Torbangun Clones (Plectranthus amboinicus (Lour.) Spreng) Based on Phenotypic Characteristics and Phenolic Content. Am. J. Plant Sci. 2014, 5, 3673. [Google Scholar] [CrossRef]
- Yüksek, T.; Oğuztürk, T.; Çorbacı, Ö.L. The Effect of Worm Fertilizer and Peat Applications on the Development of Plectranthus amboinicus (Lour.) Plant in Different Pot Environment. J. Anatol. Environ. Anim. Sci. 2020, 5, 743–749. [Google Scholar] [CrossRef]
- Oğuztürk, T.; Acar, C. Farklı Yetişme Ortamlarında Toprak Sıcaklıklarının Değişimlerinin İncelenmesi, KTÜ Perennial Bahçe Örneği. J. Anatol. Environ. Anim. Sci. 2024, 9, 269–275. [Google Scholar]
- Zhang, D.; Chen, Q.; Zhang, X.; Lin, L.; Cai, M.; Cai, W.; Li, Y. Effects of Low Temperature on Flowering and the Expression of Related Genes in Loropetalum chinense var. rubrum. Front. Plant Sci. 2022, 13, 1000160. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ling, L.; Huo, W.; Li, Y.; Xu, L.; Xiang, L.; Yang, Y.; Xiong, X.; Zhang, D.; et al. Phenotypic, Physiological, and Molecular Response of Loropetalum chinense var. rubrum under Different Light Quality Treatments Based on Leaf Color Changes. Plants 2023, 12, 2169. [Google Scholar]
- Cai, W.; Zhang, D.; Zhang, X.; Chen, Q.; Liu, Y.; Lin, L.; Li, Y. Leaf Color Change and Photosystem Function Evaluation under Heat Treatment Revealed the Stress Resistance Variation between Loropetalum chinense and L. chinense var. rubrum. PeerJ 2023, 11, e14834. [Google Scholar] [CrossRef]
- Meteoroloji Genel Müdürlüğü. Türkiye’nin Yıllık Sıcaklık ve Yağış Raporu. Available online: https://www.mgm.gov.tr/veridegerlendirme/il-ve-ilceler-istatistik.aspx?k=H&m=RIZE (accessed on 12 March 2025).
- Vasconcelos, R.G.; Medeiros, R.D.S.; Silva, D.D.; Sampaio, P.D.T.B. Vegetative Propagation of Himatanthus sucuuba (Spruce ex Müll. Arg.) Woodson by Stem Cuttings. J. Exp. Agric. Int. 2020, 42, 8–15. [Google Scholar] [CrossRef]
- Oğuztürk, T. Doğal ve Egzotik Bazı Perennial Bitkilerin Trabzon Koşullarında Gelişimlerinin Belirlenmesi. Ph.D. Thesis, Karadeniz Technical University, Trabzon, Turkey, 2023. [Google Scholar]
- Doungous, O.; Minyaka, E.; Medza-Mve, S.D.; Medueghue, A.F.; Ngone, M.A.; Simo, C.; Nsimi, A.M. Improving Propagation Methods of Gnetum africanum and G. buchholzianum from Cuttings for Rapid Multiplication, Domestication and Conservation. Agrofor. Syst. 2019, 93, 1557–1565. [Google Scholar] [CrossRef]
- Prakash, A.; Paul, P. Recent Trends in Propagation of Horticulture Crops and Application of Genomics Tools in Plant Breeding. In Recent Trends in Propagation of Horticulture Crops; Taran Publication: New Delhi, India, 2021; pp. 581–602. [Google Scholar]
- Shanthi, K.; Bachpai, V.K.W.; Anisha, S.; Ganesan, M.; Anithaa, R.G.; Subashini, V.; Chakravarthi, M.; Sivakumar, V.; Yasodha, R. Micropropagation of Eucalyptus camaldulensis for the Production of Rejuvenated Stock Plants for Microcuttings Propagation and Genetic Fidelity Assessment. New For. 2015, 46, 357–371. [Google Scholar] [CrossRef]
- Cochran, D.R.; Gilliam, C.H.; Eakes, D.J.; Wehtje, G.R.; Knight, P.R. Herbicide Use in Propagation of Loropetalum chinense ‘Ruby’. J. Environ. Hortic. 2008, 26, 139–143. [Google Scholar] [CrossRef]
- Rezende, T.; Carvalho, S.; Filho, J.; Filho, C.; Simões, L.; Paulino, R.; Oliveira, L.; Nascimento, T. Vegetative Propagation of Coffee by Mini-Cutting. Coffee Sci. 2017, 12, 91–99. [Google Scholar] [CrossRef]
- Doğan, M. Zeatin ve Farklı Oksin Kombinasyonlarının Önemli Tıbbi Bitki Limnophila aromatica (Lamk.) Merr.’nın İn Vitro Mikroçoğaltımı Üzerine Etkisi. Kahramanmaraş Sütçü İmam Univ. Tarım Doğa Derg. 2019, 22, 323–329. [Google Scholar] [CrossRef]
- Kayın, N.; Turan, F. Bitki Doku Kültürünün Biyoteknolojik Olarak Kullanımı. J. Agric. Biotechnol. 2023, 4, 1–10. [Google Scholar] [CrossRef]
- Aminah, H. Vegetative Propagation of Endospermum malaccense by Leafy Stem Cuttings: Effects of Indole Butyric Acid (IBA) Concentrations and Propagation Systems (Mist and Non-Mist). J. Trop. For. Sci. 2003, 15, 249–258. [Google Scholar]
- Babaie, H.; Zarei, H.; Nikdel, K.; Firoozjai, M.N. Effect of Different Concentrations of IBA and Time of Taking Cutting on Rooting, Growth and Survival of Ficus binnendijkii ‘Amstel Queen’ Cuttings. Not. Sci. Biol. 2014, 6, 163–166. [Google Scholar] [CrossRef]
- Al-Zebari, S.M.K.; Al-Brifkany, A.A.A.M. Effect of Cutting Type and IBA on Rooting and Growth of Citron (Citrus medica L.). Am. J. Exp. Agric. 2015, 5, 134–138. [Google Scholar] [CrossRef]
- Štefančič, M.; Stampar, F.; Osterc, G. Influence of IAA and IBA on Root Development and Quality of Prunus ‘GiSelA 5’ Leafy Cuttings. HortScience 2005, 40, 2052–2055. [Google Scholar] [CrossRef]
- Mehraj, H.; Shiam, I.; Taufique, T.; Shahrin, S.; Uddin, A. Influence of Indole-3-Butyric Acid (IBA) on Sprouting and Rooting Potential of Bougainvillea spectabilis Cuttings. Bangladesh Res. Publ. J. 2013, 9, 44–49. [Google Scholar]
- Meraklı, N.; Memon, A. Bitki Büyümesinde ve Gelişiminde Bitki Büyümesini Teşvik Eden Rizobakterinin (PGPR) Rolü: Toprak-Bitki İlişkisi. Turk. J. Agric. Food Sci. Technol. 2020, 8, 2590–2602. [Google Scholar] [CrossRef]
- Yıldırım, N.; Pulatkan, M.; Oğuztürk, G.E. GA₃ Treatments on Seed Germination in Rhodothamnus sessilifolius, an Endangered Species in Turkey. Caldasia 2022, 44, 241–247. [Google Scholar] [CrossRef]
- Karimi, H.R.; Afzalifar, M.; Mansouri, M.Z. The Effect of IBA and Salicylic Acid on Rooting and Vegetative Parameters of Pomegranate Cuttings. J. Appl. Hortic. 2012, 2, 1085–1091. [Google Scholar]
- AI Makhmari, S.M. Effect of Substrates, IBA and Types of Cuttings on Rooting. Master’s Thesis, United Arab Emirates University, Al Ain, UAE, 2016. Available online: https://scholarworks.uaeu.ac.ae/all_theses/722 (accessed on 12 March 2025).
- Braun, L.; Wyse, D. Optimizing IBA Concentration and Stem and Segment Size for Rooting of Hybrid Hazelnuts from Hardwood Stem Cuttings. J. Environ. Hortic. 2019, 37, 1–8. [Google Scholar] [CrossRef]
- Bahru, T.; Derero, A. Effects of Cutting Source and IBA Concentration on Shooting and Rooting Ability of Pouteria adolfi-friederici Stem Cuttings at Polypropagator. Cell Biol. Dev. 2023, 7, 56–66. [Google Scholar] [CrossRef]
- Tiwari, J.K.; Ameen, G.; Sandilya, V.K.; Kumar, A.; Singh, A.K. Effect of IBA on Root Development and Associated Biochemical Changes in Single- and Double-Node Stem-Cuttings of Spine Gourd. Veg. Sci. 2022, 49, 33–40. [Google Scholar] [CrossRef]
- Sevik, H.; Guney, K. Effects of IAA, IBA, NAA, and GA3 on Rooting and Morphological Features of Melissa officinalis L. Stem Cuttings. Sci. World J. 2013, 2013, 909507. [Google Scholar] [CrossRef]
- Lin, L.; Ma, Q.; Wang, J.; Lv, X.; Liao, M.A.; Xia, H.; Liang, D. Effects of Indole-3-Butyric Acid (IBA) on Growth and Cadmium Accumulation in the Accumulator Plant Stellaria media. Environ. Prog. Sustain. Energy 2018, 37, 733–737. [Google Scholar] [CrossRef]
- Parmar, J.P.; Tiwari, R.; Gautam, K.K.; Yadav, L.; Upadhyay, N. Effect of Indole-3-Butyric Acid (IBA), Rooting Media and Their Interaction on Different Rooting and Growth Characteristics of Air-Layers in Guava (Psidium guajava L. cv. L-49). J. Appl. Nat. Sci. 2018, 10, 241. [Google Scholar] [CrossRef]
- Parlakova Karagöz, F. Impact of PGPR Formulations Combined with Exogenous IBA Levels to Enhance Root Capacity in Poinsettia Cuttings. Agronomy 2023, 13, 878. [Google Scholar] [CrossRef]
- İşçi, B.; Kacar, E.; Altındişli, A. Effects of IBA and Plant Growth-Promoting Rhizobacteria (PGPR) on Rooting of Ramsey American Grapevine Rootstock. Appl. Ecol. Environ. Res. 2019, 17, 4693–4705. [Google Scholar] [CrossRef]
- Tosun, N.; Onan, E. Bitki Hastalıklarının Entegre Yönetiminde Bitki İmmunitesi Uyarıcılarının Potansiyel Kullanımı. Ege Univ. J. Agric. Fac. 2020, 57, 145–156. [Google Scholar]
- Azad, M.S.; Alam, M.J.; Mollick, A.S.; Matin, M.A. Responses of IBA on Rooting, Biomass Production and Survival of Branch Cuttings of Santalum album L., a Wild Threatened Tropical Medicinal Tree Species. J. Sci. Technol. Environ. Inf. 2016, 2, 195–206. [Google Scholar] [CrossRef]
- Song, W.; Song, Y.; Liu, X.; Zhang, X.; Xin, R.; Duan, S.; Guan, S.; Sun, X. Improvement of Culture Conditions and Plant Growth Regulators for In Vitro Callus Induction and Plant Regeneration in Paeonia lactiflora Pall. Plants 2023, 12, 3968. [Google Scholar] [CrossRef]
- Ma, J.; Fan, J.; Zhang, W.; Zhou, R.; Shen, Y.; Peng, Q.; Li, H.; Lei, C. Tissue Culture Response and In Vitro Plant Regeneration of Malus ‘Baiyun’ (a New Cultivar of Ornamental Crabapple). Plants 2024, 13, 2080. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Yin, J.; Liu, Y.; Cai, X. Plant Regeneration via Organogenesis in Jerusalem Artichokes and Comparative Analysis of Endogenous Hormones and Antioxidant Enzymes in Typical and Atypical Shoots. Plants 2023, 12, 3789. [Google Scholar] [CrossRef]
- Lancashire, L.J.; Lemetre, C.; Ball, G.R. An Introduction to Artificial Neural Networks in Bioinformatics—Application to Complex Microarray and Mass Spectrometry Datasets in Cancer Studies. Brief. Bioinform. 2009, 10, 315–329. [Google Scholar] [CrossRef]
- Abiodun, O.I.; Jantan, A.; Omolara, A.E.; Dada, K.V.; Mohamed, N.A.; Arshad, H. State-of-the-Art in Artificial Neural Network Applications: A Survey. Heliyon 2018, 4, e00938. [Google Scholar] [CrossRef]
- Tekin, T.G. Geniş Ekonomik Grupların İhracat Değerinin Yapay Sinir Ağı Yöntemiyle Tahminlenmesi. EKEV Akad. Derg. 2023, 95, 319–335. [Google Scholar] [CrossRef]
- Paliwal, M.; Kumar, U.A. Neural Networks and Statistical Techniques: A Review of Applications. Expert Syst. Appl. 2009, 36, 2–17. [Google Scholar] [CrossRef]
- Almeida, J.S. Predictive Non-Linear Modeling of Complex Data by Artificial Neural Networks. Curr. Opin. Biotechnol. 2002, 13, 72–76. [Google Scholar] [CrossRef]
- Erdoğan, H.; Terzioğlu, M.; Kayakuş, M. Almanya’dan Konaklama Amacıyla Türkiye’ye Gelen Turist Sayısının Yapay Zekâ Teknikleri Kullanılarak Tahmin Edilmesi. Avrupa Bilim Teknoloji Dergisi 2021, 27, 961–971. [Google Scholar] [CrossRef]
- Bayraktar, Ş.; Alparslan, C.; Salihoğlu, N.; Sarıkaya, M. A Holistic Research Based on RSM and ANN for Improving Drilling Outcomes in Al–Si–Cu–Mg (C355) Alloy. J. Mater. Res. Technol. 2025, 35, 1596–1607. [Google Scholar] [CrossRef]
- Kolesnyk, V.; Peterka, J.; Alekseev, O.; Neshta, A.; Xu, J.; Lysenko, B.; Hrbal, J. Application of ANN for Analysis of Hole Accuracy and Drilling Temperature When Drilling CFRP/Ti Alloy Stacks. Materials 2022, 15, 1940. [Google Scholar] [CrossRef]
- Alparslan, C.; Bayraktar, Ş. Experimental Research and Optimization Based on Response Surface Methodology on Machining Characteristics of Cast Al-7Si-0.6Mg Alloy: Effects of Cutting Parameters and Heat Treatment. Measurement 2024, 236, 115111. [Google Scholar] [CrossRef]
- Cottrell, M.; Olteanu, M.; Rossi, F.; Rynkiewicz, J.; Villa-Vialaneix, N. Neural Networks for Complex Data. KI–Künstl. Intell. 2012, 26, 373–380. [Google Scholar] [CrossRef][Green Version]
- Özcan, C.; Köprü, E.Y. Yapay Sinir Ağları ile Sıvı Ham Demir Tahmini ve 5. Yüksek Fırın Uygulaması. Avrupa Bilim Teknoloji Dergisi 2020, 155–162. [Google Scholar] [CrossRef]
- Bao, X.; Zheng, S.; Li, L.; Wang, K.; Zhang, W.; Wang, W.; Wang, Y.; Ou, Y.; Yang, W. Effects of Matrices and Hormones on Rooting Traits of Alnus nepalensis Rooted Cutting. Guangxi For. Sci. 2015, 4, 370–375. [Google Scholar]
- Wang, W.; Zhou, X.; Wu, S.; Zhao, M.; Jin, Z.; Bei, K.; Zheng, X.; Fan, C. Vertical Green Wall Systems for Rainwater and Sewage Treatment. Sustainability 2024, 16, 7593. [Google Scholar] [CrossRef]
- Mao, J.; Chen, H.; Zhou, H.; Qi, X.; Chen, S.; Feng, J.; Jin, Y.; Li, C.; Deng, Y.; Zhang, H. Effects of Steel Slag Used as Substrate on the Growth of Hydrangea macrophylla Cuttings. Horticulturae 2024, 10, 1053. [Google Scholar] [CrossRef]
- Tyson, R.; Simonne, E.; Davis, M.; Lamb, E.; White, J.; Treadwell, D. Effect of Nutrient Solution, Nitrate-Nitrogen Concentration, and pH on Nitrification Rate in Perlite Medium. J. Plant Nutr. 2007, 30, 901–913. [Google Scholar] [CrossRef]
- Silber, A.; Bar-Yosef, B.; Levkovitch, I.; Soryano, S. pH-Dependent Surface Properties of Perlite: Effects of Plant Growth. Geoderma 2010, 158, 275–281. [Google Scholar] [CrossRef]
- Merhaut, D.J.; Blythe, E.; Newman, J.P.; Albano, J. Nutrient Release from Controlled-Release Fertilizers in a Neutral-pH Substrate in an Outdoor Environment: I. Leachate Electrical Conductivity, pH, and Nitrogen, Phosphorus, and Potassium Concentrations. HortScience 2006, 41, 780. [Google Scholar] [CrossRef]
- Gachukia, M.; Evans, M. Root Substrate pH, Electrical Conductivity, and Macroelement Concentration of Sphagnum Peat-Based Substrates Amended with Parboiled Fresh Rice Hulls or Perlite. HortTechnology 2008, 18, 644–649. [Google Scholar] [CrossRef]
- Silber, A.; Bar-Yosef, B.; Suryano, S.; Levkovitch, I. Zinc Adsorption by Perlite: Effects of pH, Ionic Strength, Temperature, and Pre-Use as Growth Substrate. Geoderma 2012, 170, 159–167. [Google Scholar] [CrossRef]
- Markoska, V.; Spalevic, V.; Gulaboski, R. A Research on the Influence of Porosity on Perlite Substrate and Its Interaction on Porosity of Two Types of Soil and Peat Substrate. Poljopr. I Sumar. 2018, 64, 15–29. [Google Scholar] [CrossRef]
- Cannavo, P.; Hafdhi, H.; Michel, J. Impact of Root Growth on the Physical Properties of Peat Substrate under a Constant Water Regimen. HortScience 2011, 46, 1394–1399. [Google Scholar] [CrossRef]
- Yafuso, E.J. The Relationship Between Water and Oxygen in Plant Propagation. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2019. [Google Scholar]
- Yu, X.; Li, L.; Li, Y.; Liu, Z.; Yang, N.; Zhang, M. Screening of Composite Substrates Suitable for Tissue-Cultured Plantlets Growth of Asparagus cochinchinensis (Lour.) Merr. Folia Hortic. 2022, 34, 39–50. [Google Scholar] [CrossRef]
- Hajiaghaei Kamrani, M.; Rahimi Chegeni, A.; Hosseinniya, H. Effects of Different Growing Media on Yield and Growth Parameters of Potato Minitubers (Solanum tuberosum L.). Commun. Soil Sci. Plant Anal. 2019, 50, 1838–1853. [Google Scholar] [CrossRef]
- Abass, M.M.; Thabet, R.S.; Lasheen, F.F.; Abdelhamid, A.N.; Hassan, K.M.; Saudy, H.S.; Boghdady, M.S. Modulating the Rhizosphere Medium and Indole−3−Butyric Acid Supply Influence Rooting, Nutrients and Biochemical Constituents and Histological Features of Pedilanthus tithymaloides. J. Soil Sci. Plant Nutr. 2024, 24, 6880–6892. [Google Scholar] [CrossRef]
- Michel, J. The Physical Properties of Peat: A Key Factor for Modern Growing Media. Mires Peat 2010, 6, 2. [Google Scholar]
- Latrach, L.; Farissi, M.; Mouradi, M.; Makoudi, B.; Bouizgaren, A.; Ghoulam, C. Growth and Nodulation of Alfalfa–Rhizobia Symbiosis under Salinity: Electrolyte Leakage, Stomatal Conductance, and Chlorophyll Fluorescence. Turk. J. Agric. For. 2014, 38, 320–326. [Google Scholar] [CrossRef]
- Tan, U. Application of Indole-3-Butyric Acid (IBA) Enhances Agronomic, Physiological and Antioxidant Traits of Salvia fruticosa under Saline Conditions: A Practical Approach. PeerJ 2025, 13, e18846. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Xu, N.; He, B. Effects of IBA on Rooting Characteristics and Changes in the Relevant Oxidases during Rooting of Scaevola crassifolia. Pratacult. Sci. 2011, 5, 777–782. [Google Scholar]
- Karataş, İ. Production of rosmarinic acid and biomass from adventitious root cultures of Ocimum basilicum by optimization of medium components in airlift bioreactors. Plant Cell Tissue Organ Cult. 2022, 151, 235–251. [Google Scholar] [CrossRef]
- Aydin, E.; Er, E. The Effect of Different IBA Doses on Rooting in Soft-Wood Cuttings of Rootstock Candidate Sweet Cherry, Sour Cherry and Mahaleb Genotypes. Turk. J. Food Agric. Sci. 2023, 5, 48–54. [Google Scholar] [CrossRef]
- Pacholczak, A.; Zajączkowska, M.; Nowakowska, K. The Effect of Brassinosteroids on Rooting of Stem Cuttings in Two Barberry (Berberis thunbergii L.) Cultivars. Agronomy 2021, 11, 699. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I. Effects of Exogenous L-Arginine on In Vitro Rooting, Chlorophyll, Carbohydrate, and Proline Concentrations in the Sweet Cherry Rootstock M × M 14 (Prunus avium L. × Prunus mahaleb L.). Plant Biotechnol. Rep. 2013, 7, 457–465. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Kim, S.H.; Yu, C.Y.; Chung, I.M. Biochemical and Physiological Changes during Early Adventitious Root Formation in Chrysanthemum indicum Linné Cuttings. Plants 2022, 11, 1440. [Google Scholar] [CrossRef] [PubMed]
- Suvorova, G.G.; Oskorbina, M.V.; Kopytova, L.D.; Yan’kova, L.S.; Popova, E.V. Seasonal Changes in Photosynthetic Activity and Chlorophylls in the Scots Pine and Siberian Spruce with Optimal or Insufficient Moistening. Contemp. Probl. Ecol. 2011, 4, 626–633. [Google Scholar] [CrossRef]
- Percival, D.; Kaur, J.; Hainstock, L.J.; Privé, J.P. Seasonal Changes in Photochemistry, Light Use Efficiency and Net Photosynthetic Rates of Wild Blueberry (Vaccinium angustifolium Ait.). Can. J. Plant Sci. 2012, 92, 1135–1143. [Google Scholar] [CrossRef][Green Version]
- Ruchika; Csintalan, Z.; Péli, E.R. Seasonality and Small Spatial-Scale Variation of Chlorophyll a Fluorescence in Bryophyte Syntrichia ruralis [Hedw.] in Semi-Arid Sandy Grassland, Hungary. Plants 2020, 9, 92. [Google Scholar] [CrossRef]
- Yu, L.; Luo, X.; Croft, H.; Rogers, C.A.; Chen, J.M. Seasonal Variation in the Relationship between Leaf Chlorophyll Content and Photosynthetic Capacity. Plant Cell Environ. 2024, 47, 3953–3965. [Google Scholar] [CrossRef]
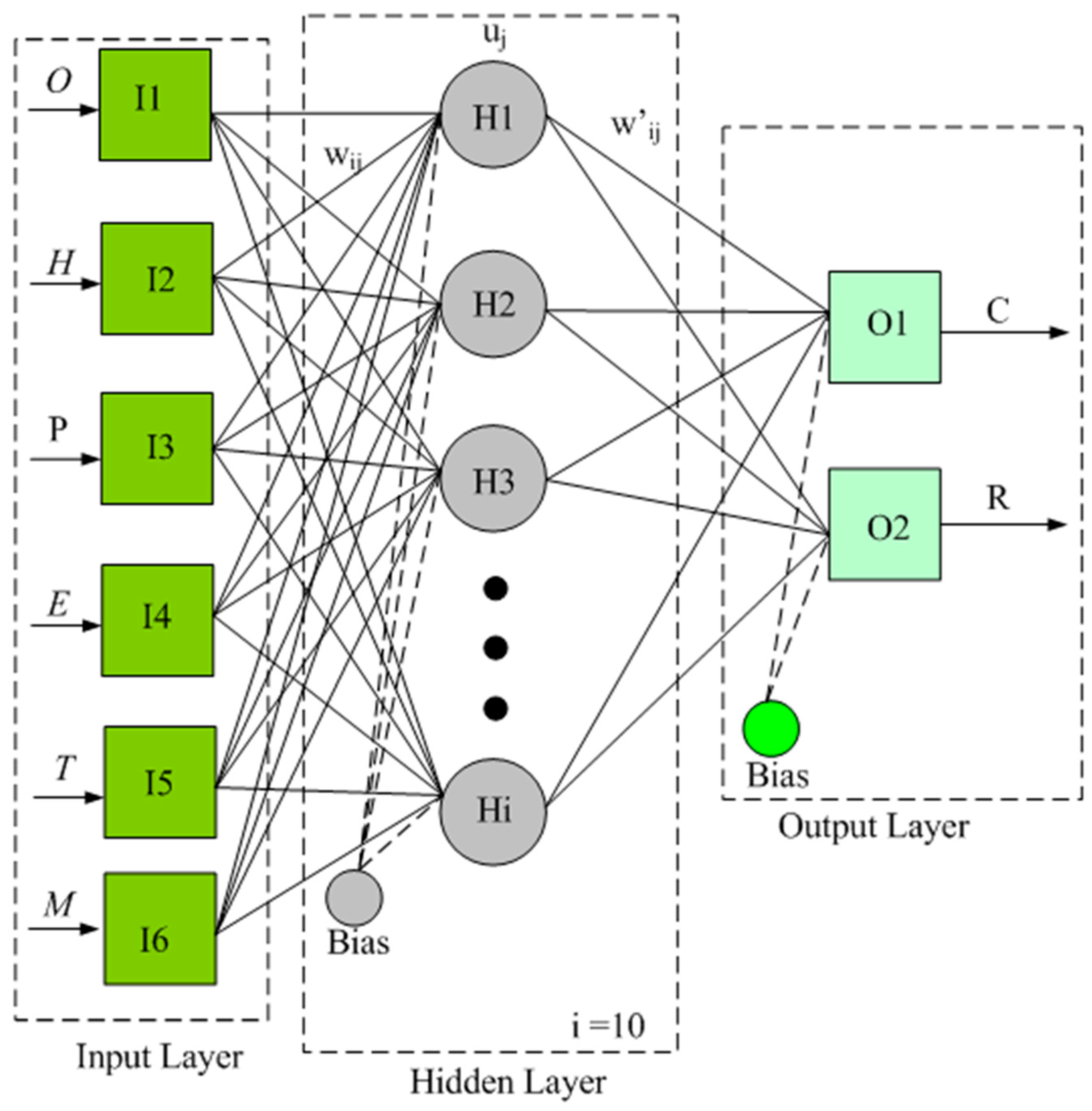

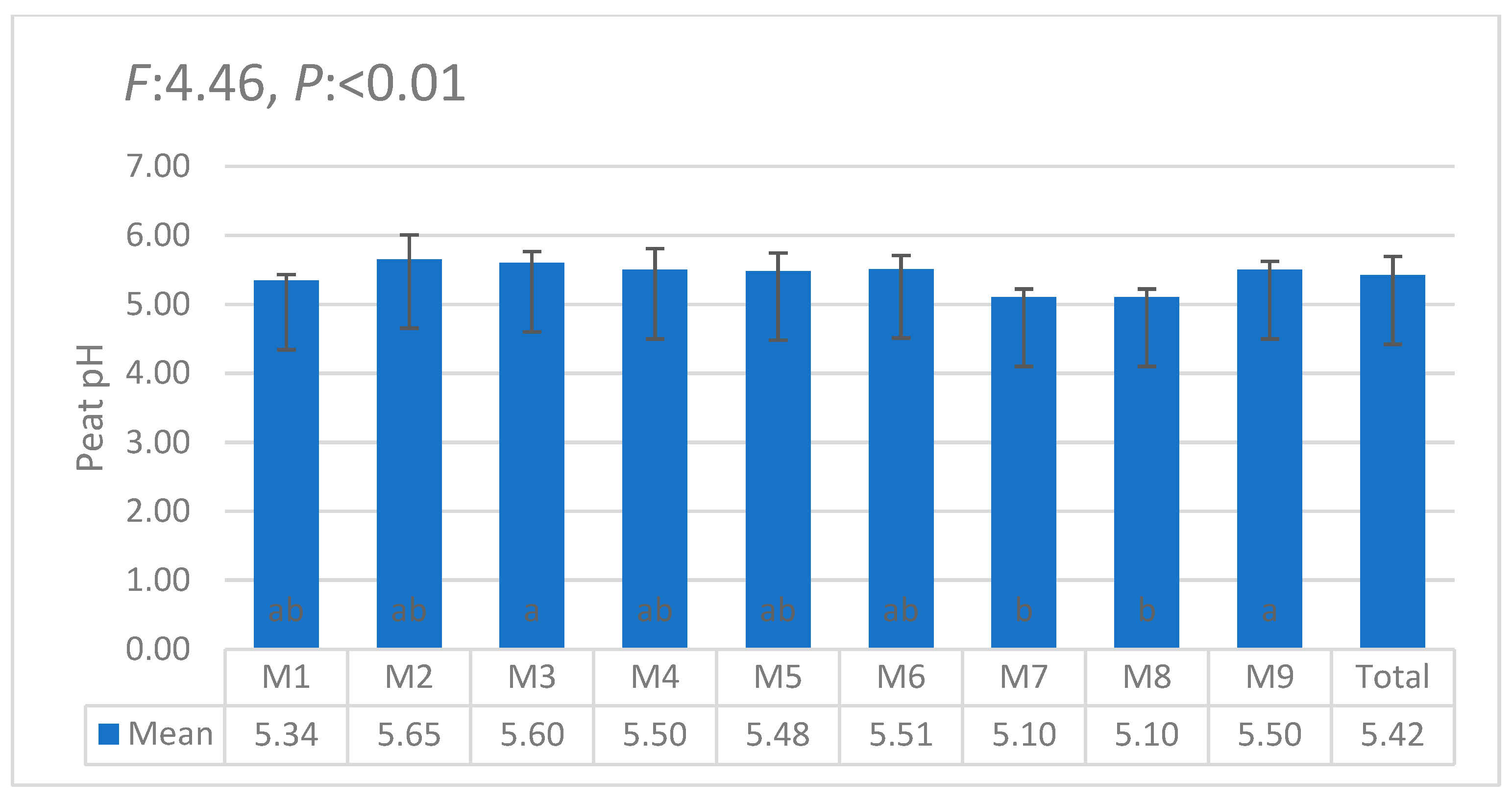

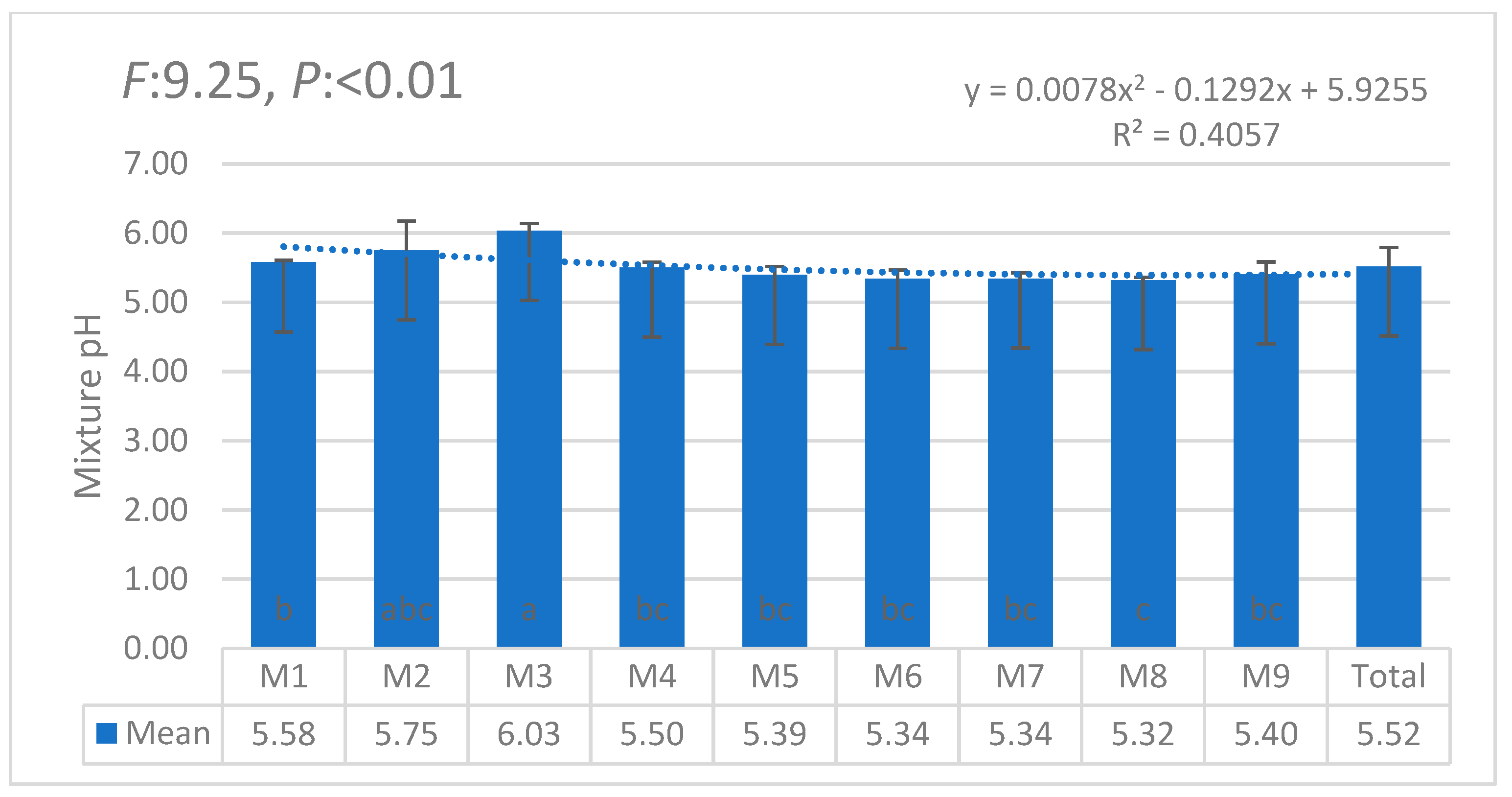


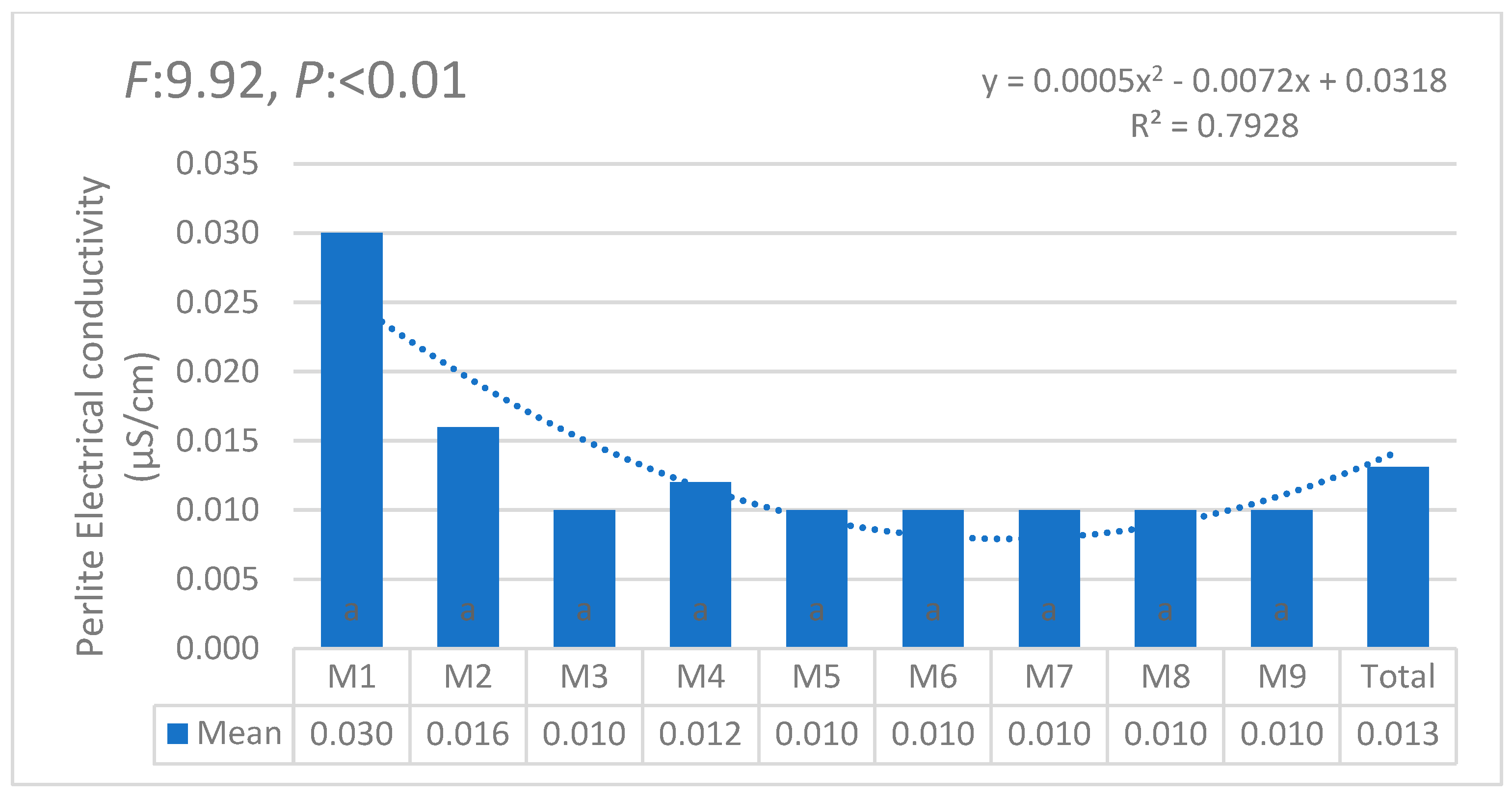
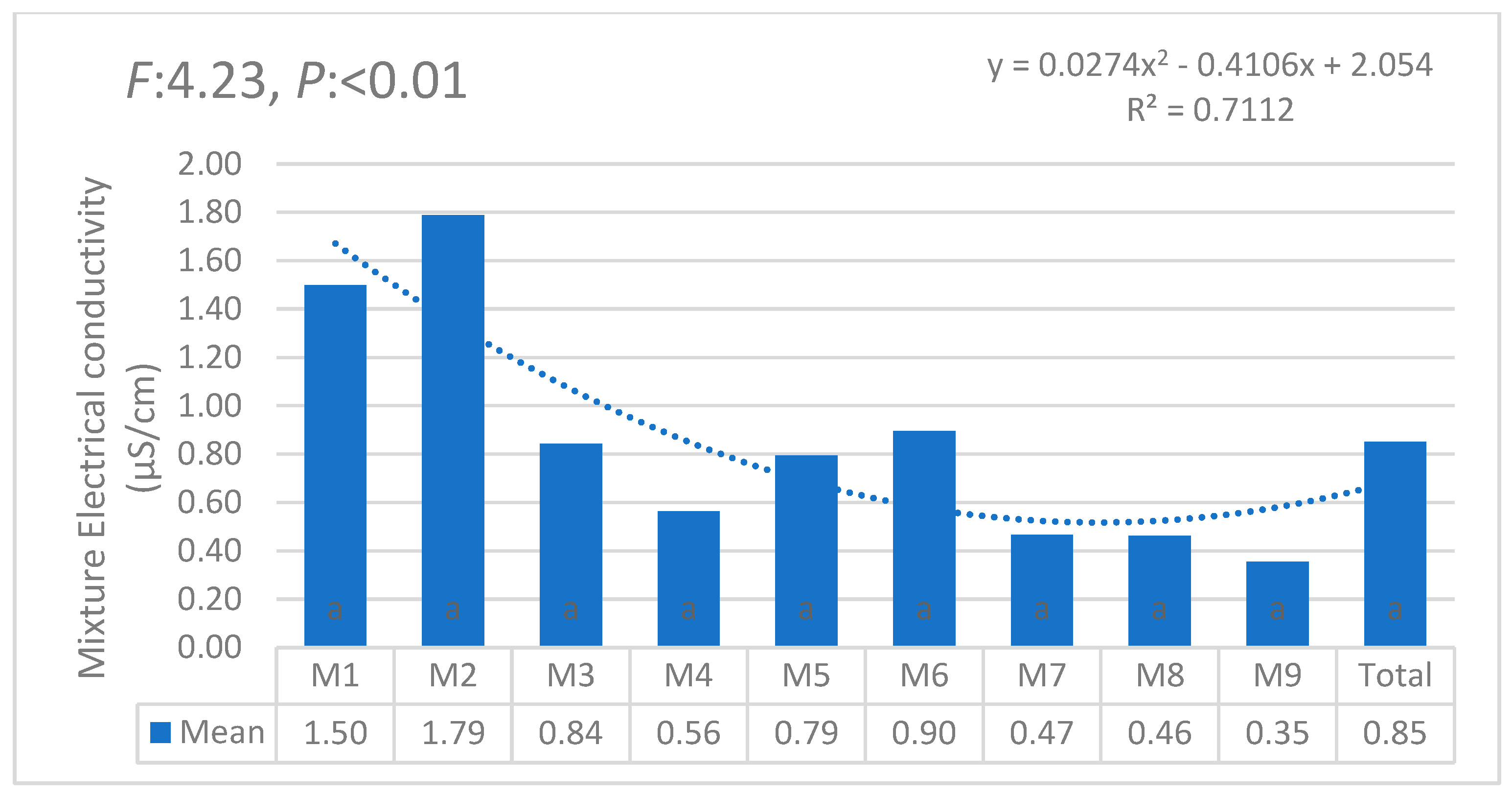
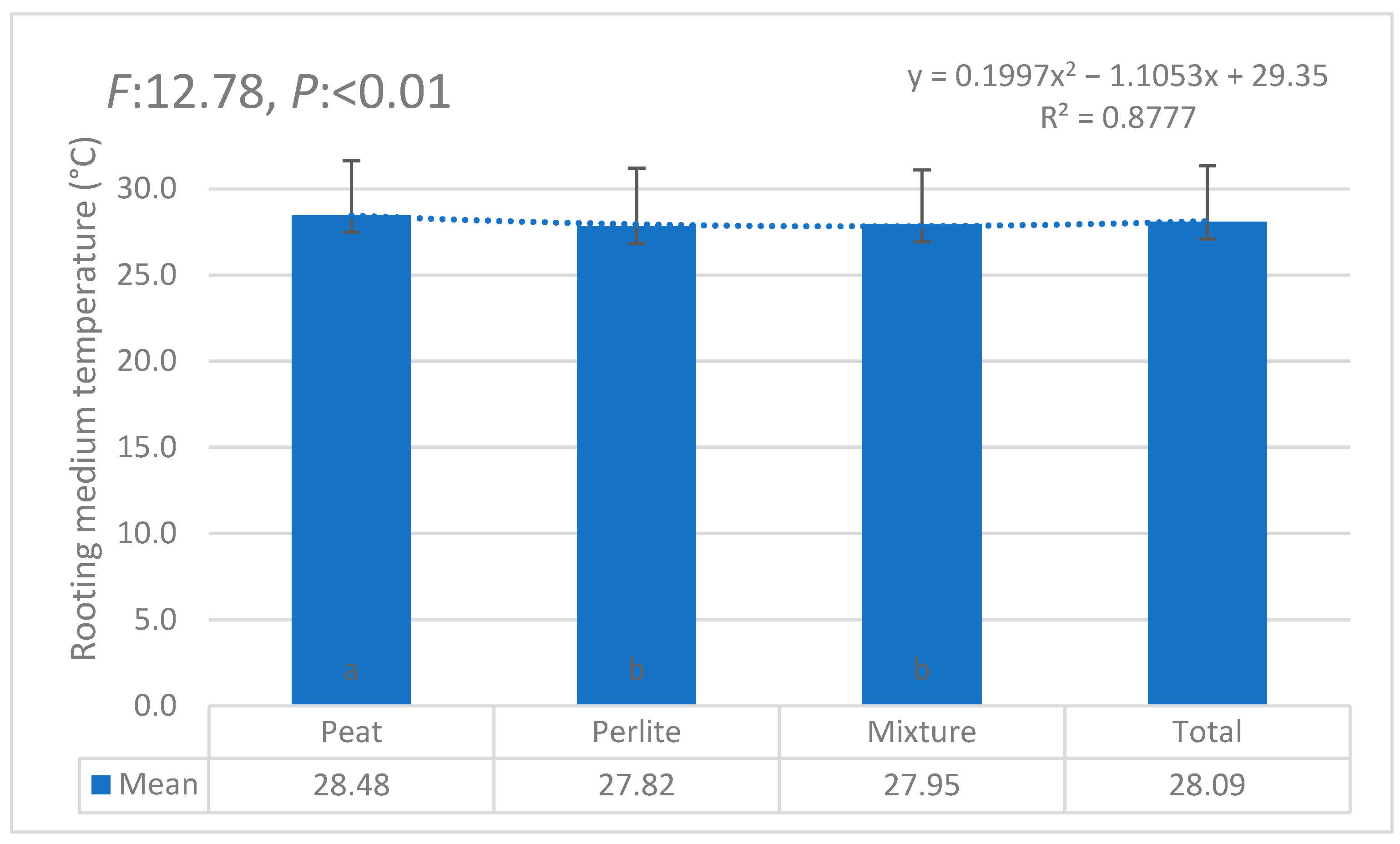
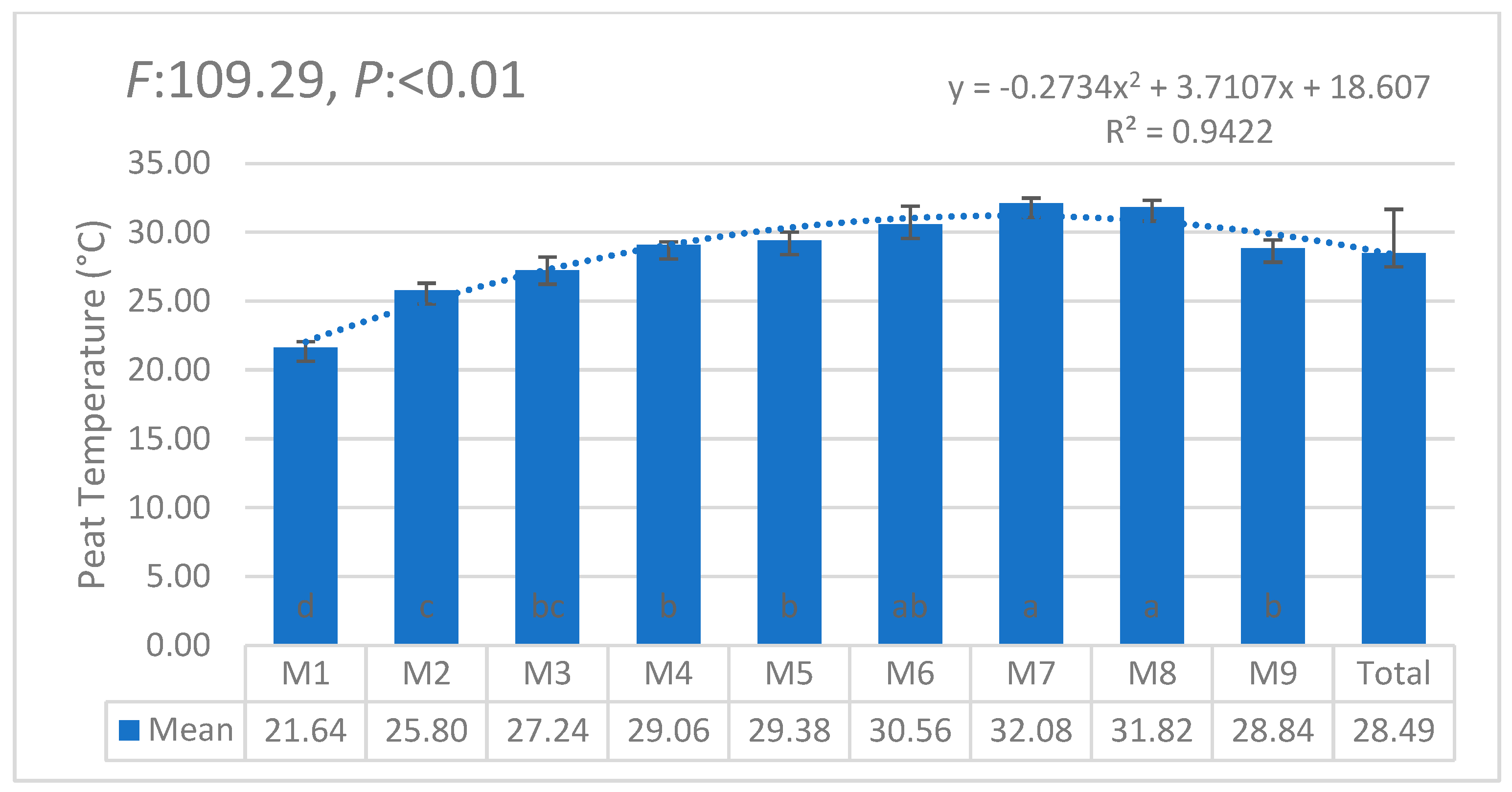
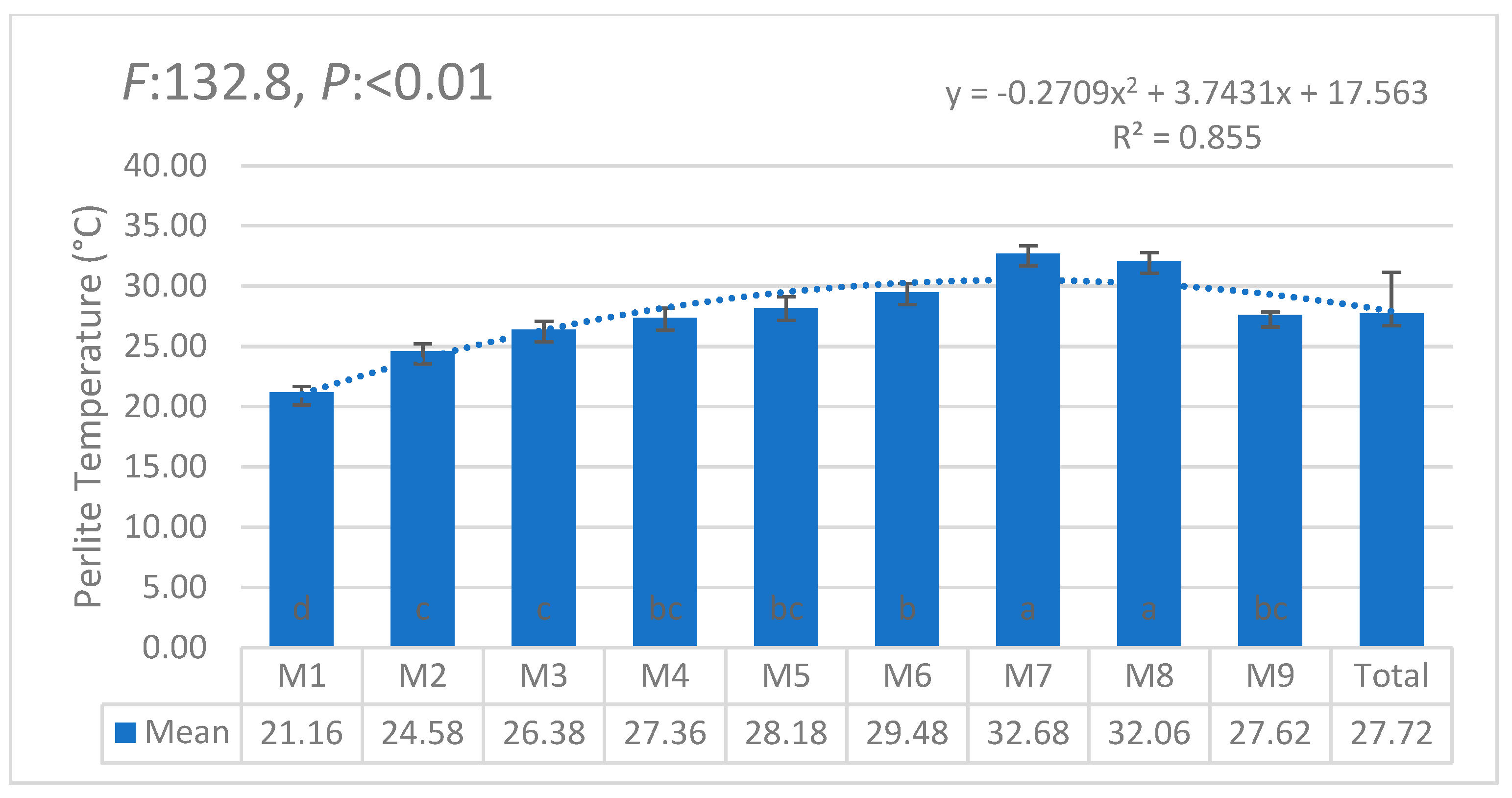
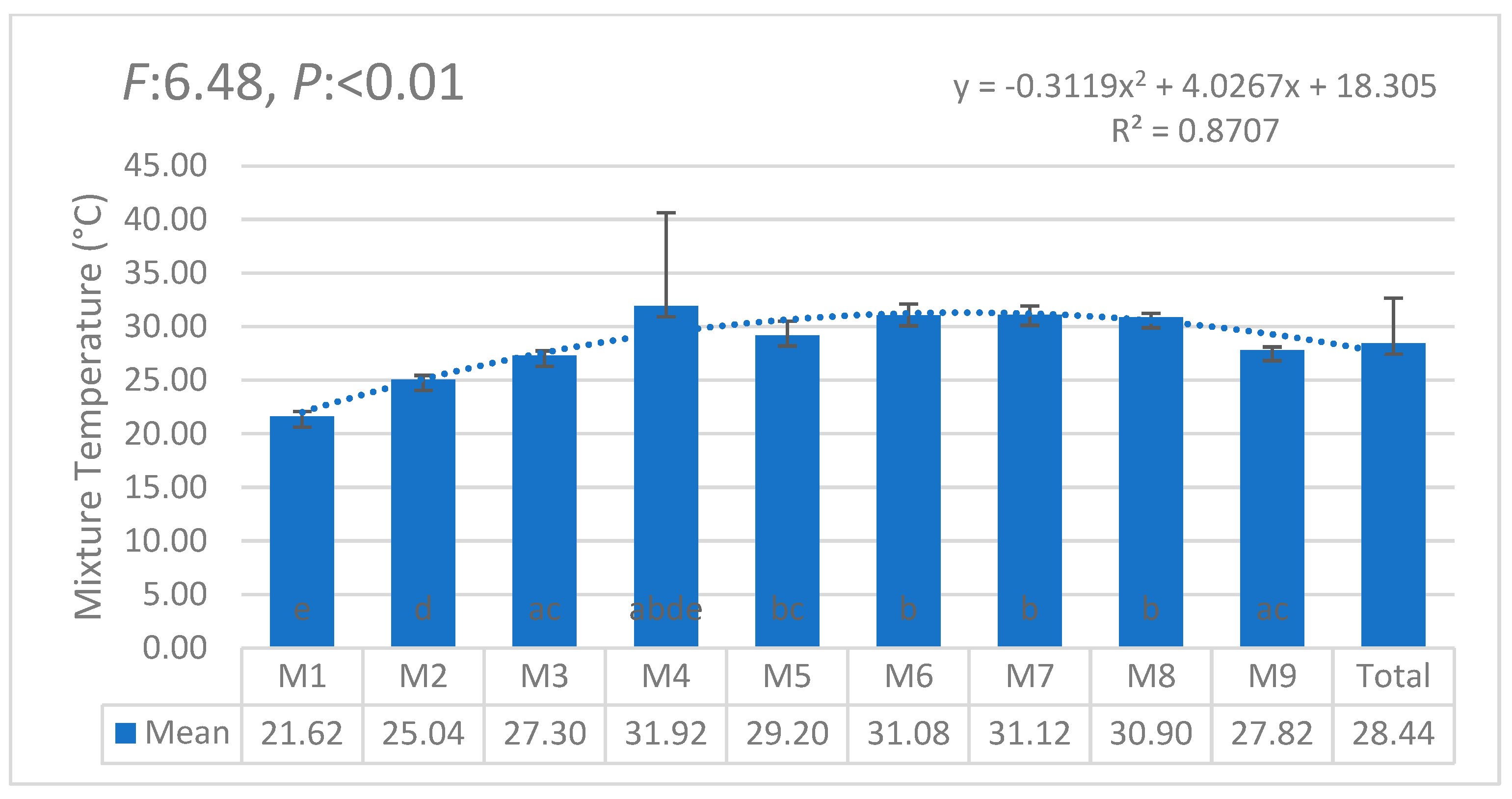

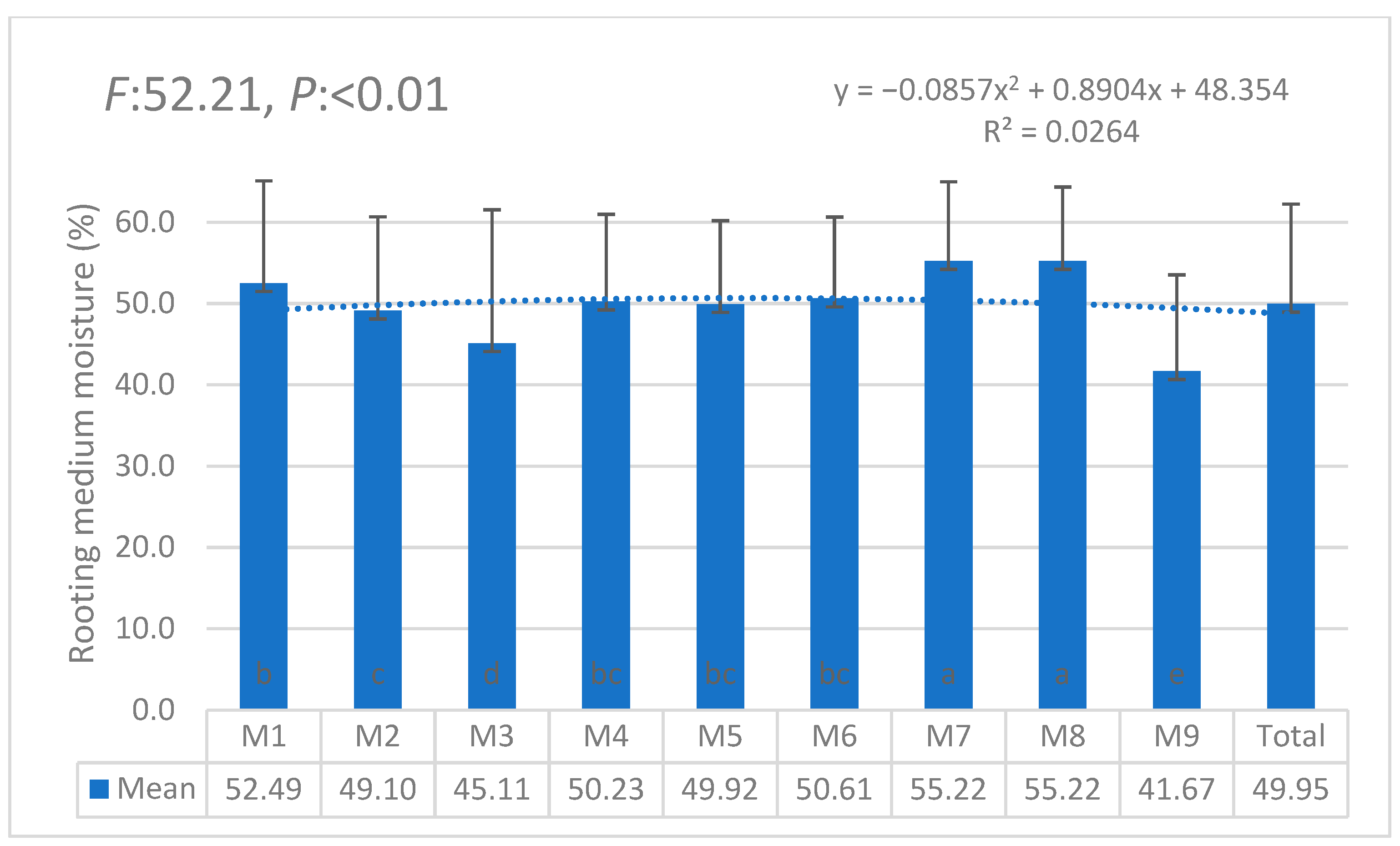
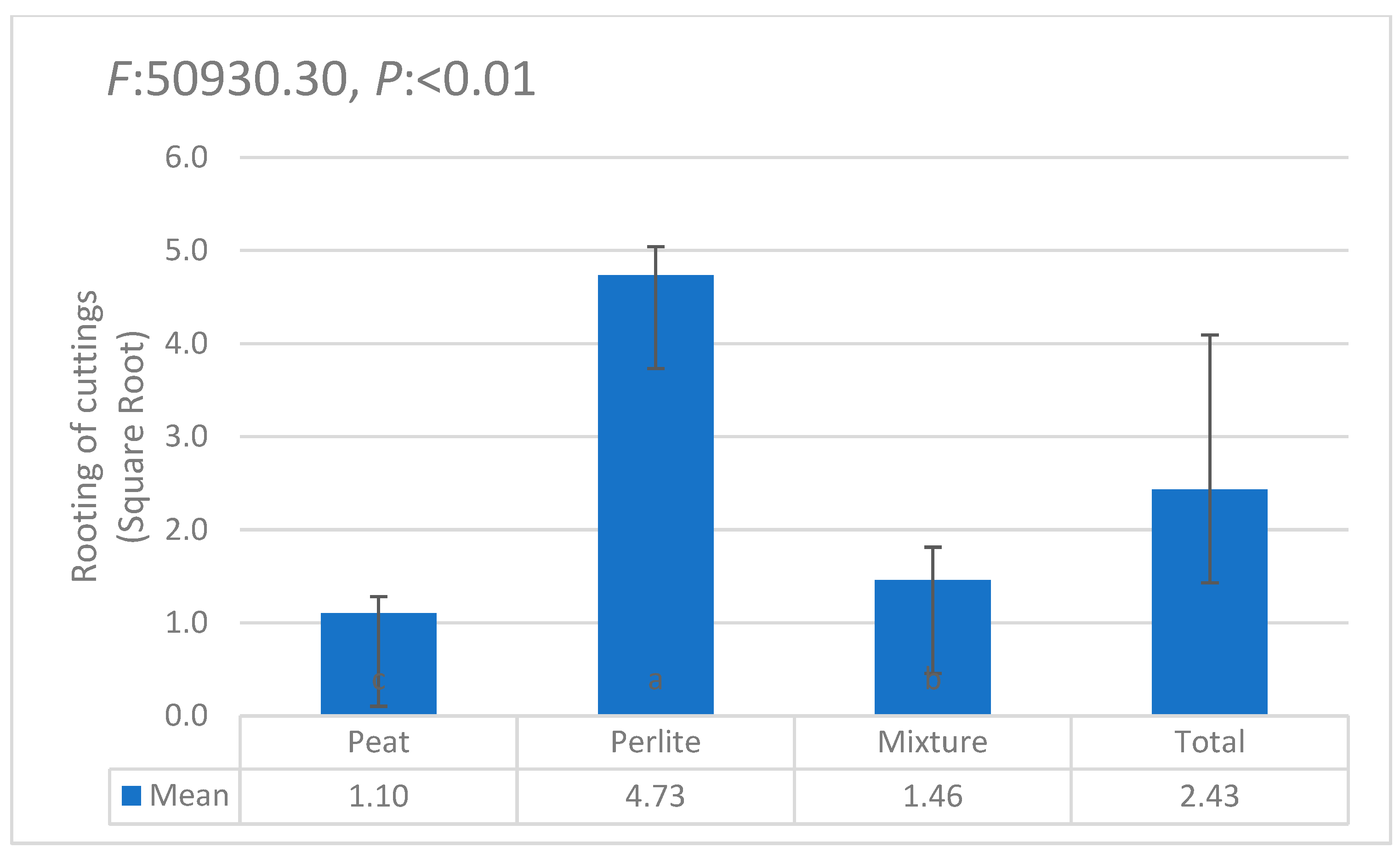
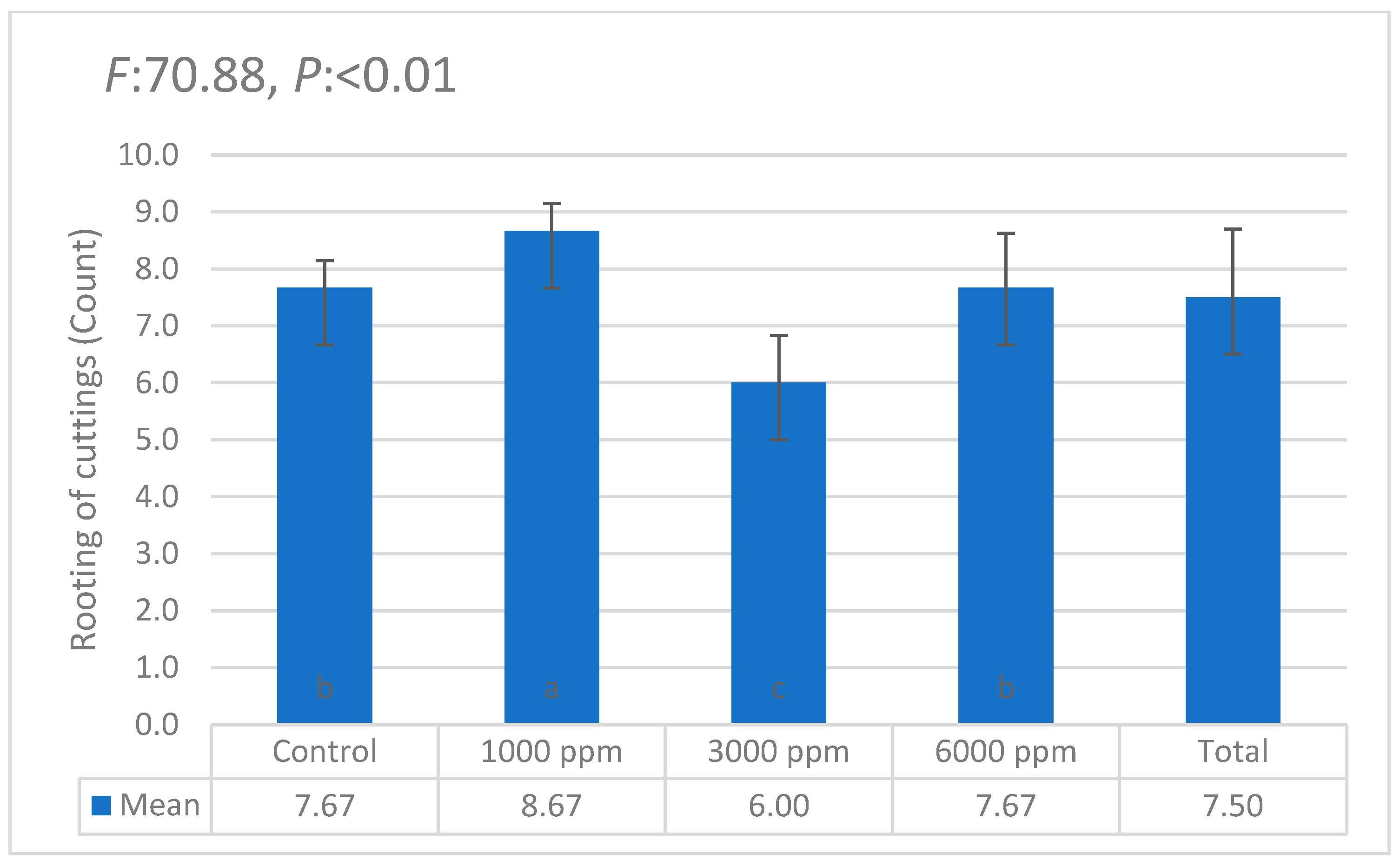
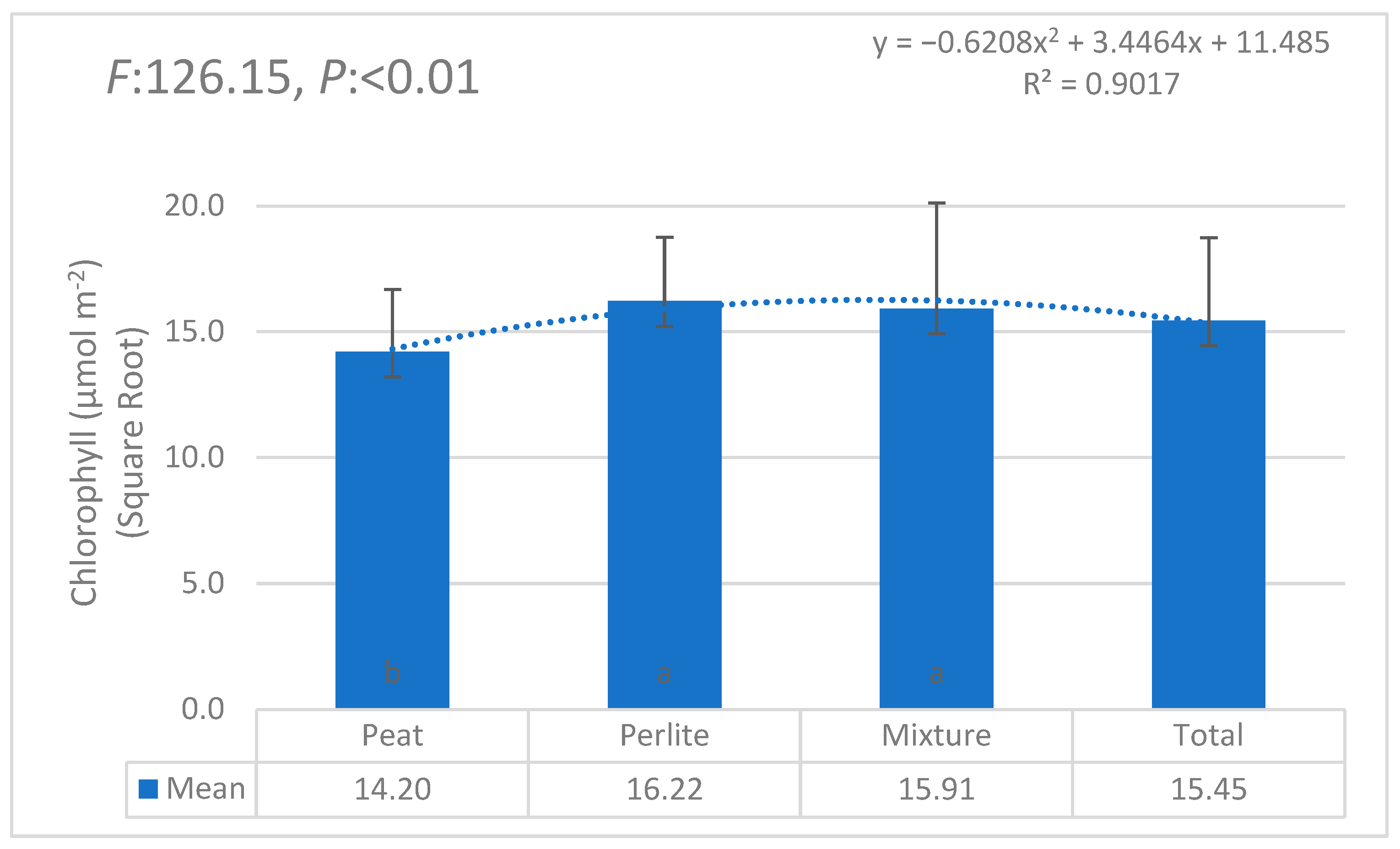

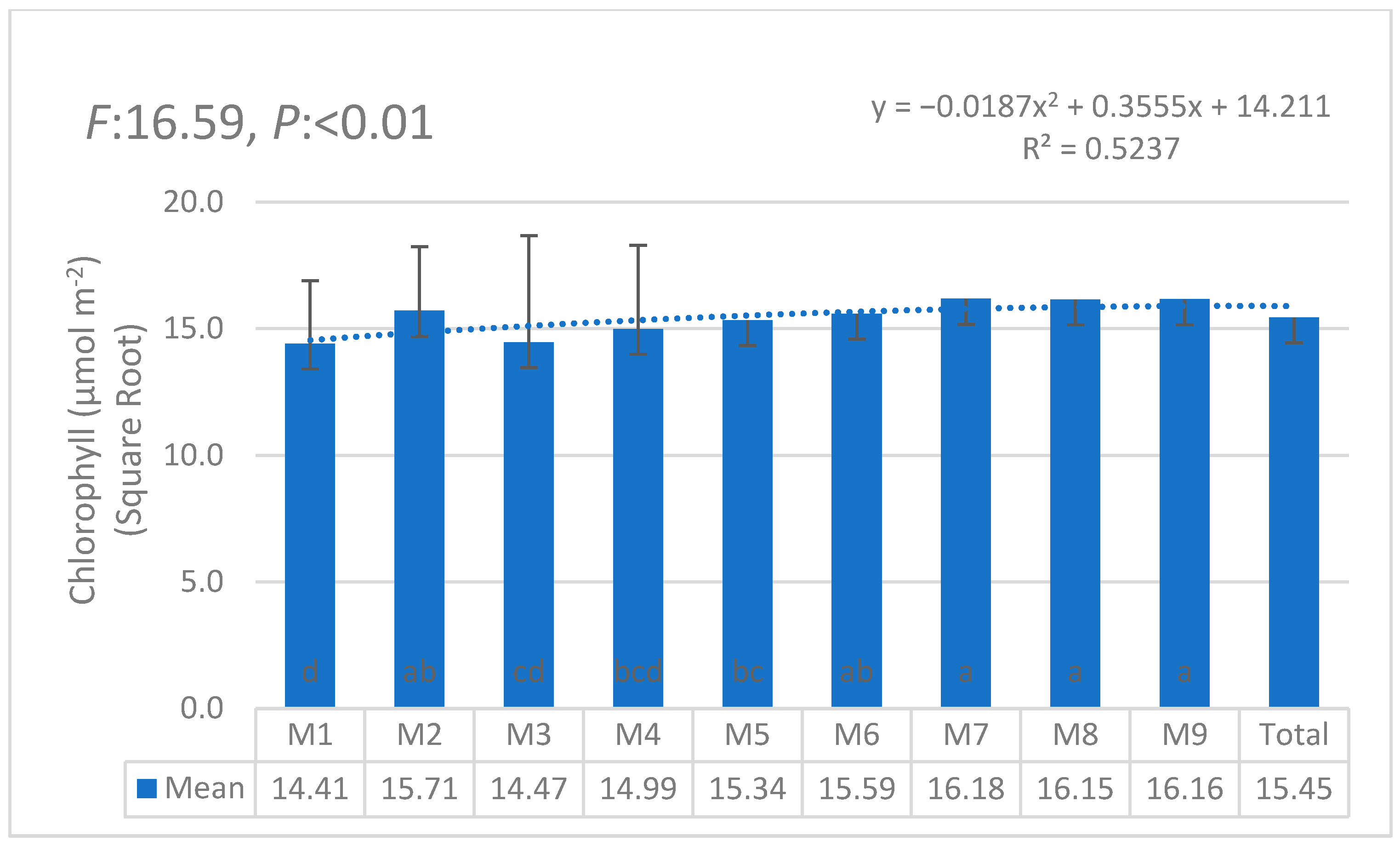
| i | W1 | W2 | W3 | W4 | W5 | W6 | θi | Lw | θ11 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −8.8872 | −1.0549 | −47.336 | −37.8254 | 84.0242 | 3.0384 | 1.2183 | 47.6539 | 217.1088 |
| 2 | 8.1794 | 1.0004 | −152.61 | −133.963 | −40.0058 | −53.3014 | −31.7692 | 45.2017 | |
| 3 | −13.0838 | −0.0832 | 136.53 | 54.0415 | 25.812 | −5.2988 | −3.9067 | 62.9046 | |
| 4 | −14.029 | −6.0072 | 61.144 | 186.788 | 97.2744 | −9.0497 | 5.1317 | −20.7534 | |
| 5 | 82.012 | −17.422 | 15.732 | −65.9827 | 363.886 | 532.292 | 6.1269 | −49.1541 | |
| 6 | −0.481 | 1.7411 | −17.012 | 2.7393 | −86.6964 | −116.583 | −13.1457 | −18.978 | |
| 7 | −27.592 | 0.0778 | −157.65 | −23.1222 | 14.9759 | 19.6133 | −45.9056 | −51.7034 | |
| 8 | 45.8897 | 26.604 | 100.19 | 39.1366 | −58.38 | −54.3333 | −16.812 | −29.8601 | |
| 9 | 1.9125 | −0.7573 | −42.293 | −79.6553 | 1.0574 | −0.9486 | −24.224 | −6.6785 | |
| 10 | 43.7316 | 0.5859 | 28.071 | 5.5977 | −92.6572 | −42.025 | 11.6064 | 62.5415 |
| i | W1 | W2 | W3 | W4 | W5 | W6 | θi | Lw | θ11 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.7403 | −0.028 | 4.1406 | −1.0125 | −0.2986 | 0.6381 | 8.4076 | 5 | 9.9929 |
| 2 | 5.7677 | 0.0099 | 0.2313 | 1.327 | −1.4458 | −1.8879 | −14.3251 | −1.4187 | |
| 3 | 2.252 | 0.0401 | −10.3116 | −1.152 | −0.323 | −0.8172 | −22.7695 | 2 | |
| 4 | 1.4877 | −0.0742 | 1.6346 | 1.3536 | −0.5111 | −0.2602 | −13.177 | 7 | |
| 5 | −0.2448 | −7.9738 × 10−11 | −1.41 × 10−6 | −5.91 × 10−7 | 1.22 × 10−7 | 1.13 × 10−7 | 13.731 | −20.8338 | |
| 6 | 9.3115 | −0.3131 | −0.4306 | −0.4948 | 5.0959 | 10.7002 | −2.0956 | −5 | |
| 7 | 0.6823 | 0.0345 | −6.137 | −2.2646 | −0.8864 | −1.7388 | −17.1508 | −1 | |
| 8 | 1.2562 | 0.0072 | 1.9285 | −0.1133 | −1.034 | 1.8899 | −17.6737 | −5.4262 | |
| 9 | −3.1606 | −0.0019 | −2.856 | 1.9153 | −1.2155 | 1.4472 | 14 | 3.4146 | |
| 10 | −0.5993 | 0.1124 | 0.799 | −0.0701 | 0.0176 | 0.1962 | −0.706 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oğuztürk, T.; Alparslan, C.; Aydın, Y.; Öztatar, U.; Oğuztürk, G.E. Effect of Different Indole Butyric Acid (IBA) Concentrations in Various Rooting Media on the Rooting Success of Loropetalum chinense var. rubrum Yieh Cuttings and Its Modeling with Artificial Neural Networks. Horticulturae 2025, 11, 564. https://doi.org/10.3390/horticulturae11060564
Oğuztürk T, Alparslan C, Aydın Y, Öztatar U, Oğuztürk GE. Effect of Different Indole Butyric Acid (IBA) Concentrations in Various Rooting Media on the Rooting Success of Loropetalum chinense var. rubrum Yieh Cuttings and Its Modeling with Artificial Neural Networks. Horticulturae. 2025; 11(6):564. https://doi.org/10.3390/horticulturae11060564
Chicago/Turabian StyleOğuztürk, Türker, Cem Alparslan, Yusuf Aydın, Umut Öztatar, and Gülcay Ercan Oğuztürk. 2025. "Effect of Different Indole Butyric Acid (IBA) Concentrations in Various Rooting Media on the Rooting Success of Loropetalum chinense var. rubrum Yieh Cuttings and Its Modeling with Artificial Neural Networks" Horticulturae 11, no. 6: 564. https://doi.org/10.3390/horticulturae11060564
APA StyleOğuztürk, T., Alparslan, C., Aydın, Y., Öztatar, U., & Oğuztürk, G. E. (2025). Effect of Different Indole Butyric Acid (IBA) Concentrations in Various Rooting Media on the Rooting Success of Loropetalum chinense var. rubrum Yieh Cuttings and Its Modeling with Artificial Neural Networks. Horticulturae, 11(6), 564. https://doi.org/10.3390/horticulturae11060564





