A Rugulopteryx okamurae-Based Biostimulant Enhances Growth and Phytochemicals in Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Reagents
2.2. Biostimulant Obtainment
2.3. Biostimulants Analysis
2.4. Lettuce Samples
2.5. Fertigation Parameters and Crop Conditions
2.6. Growth Parameters and Sample for Chemical Analysis
2.7. Carotenoids Analysis
2.8. Tocopherol and Tocotrienol Analysis
2.9. Phytosterols and Squalene Analysis
2.10. Statistical Analysis
3. Results
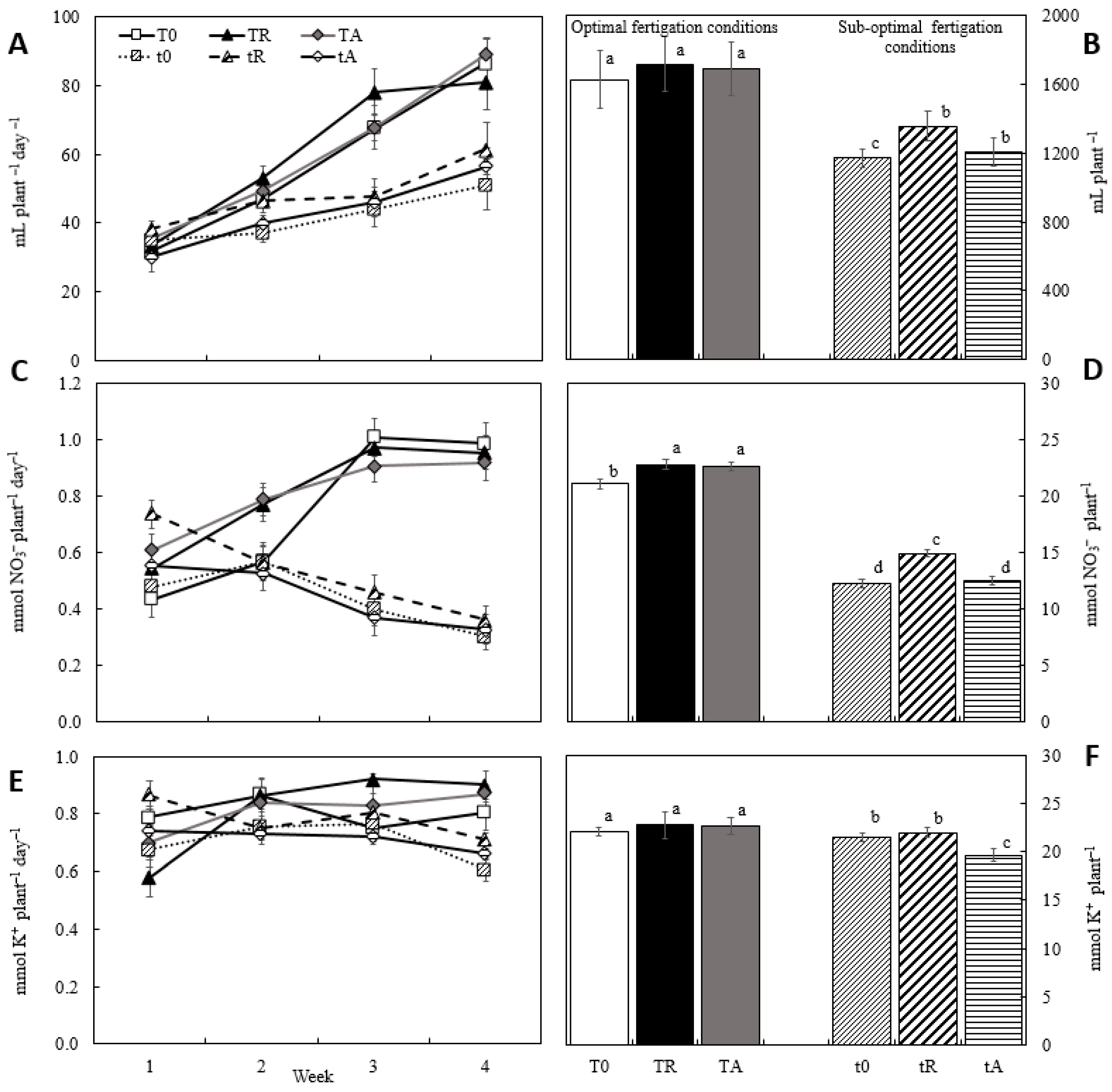
3.1. Effect of Biostimulant Application via Fertigation on Monitoring Parameters
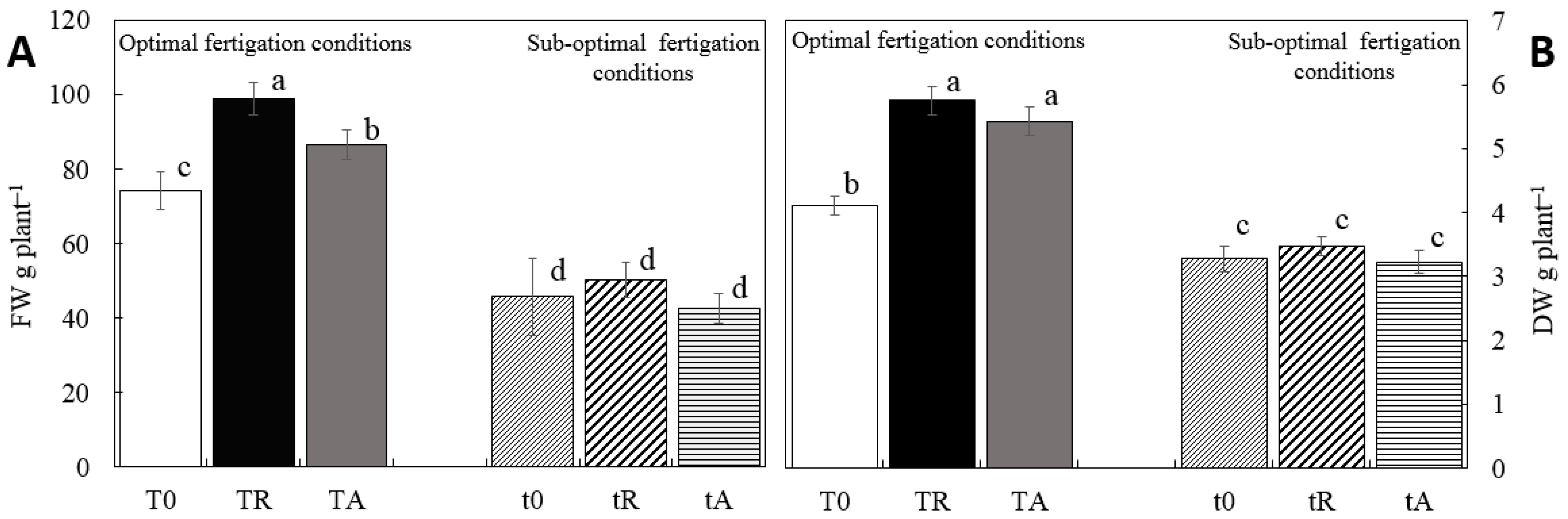
3.2. Effect of Biostimulants on Fertigation Uptake and Growth Parameters
3.3. Phytochemicals Content in Lettuce
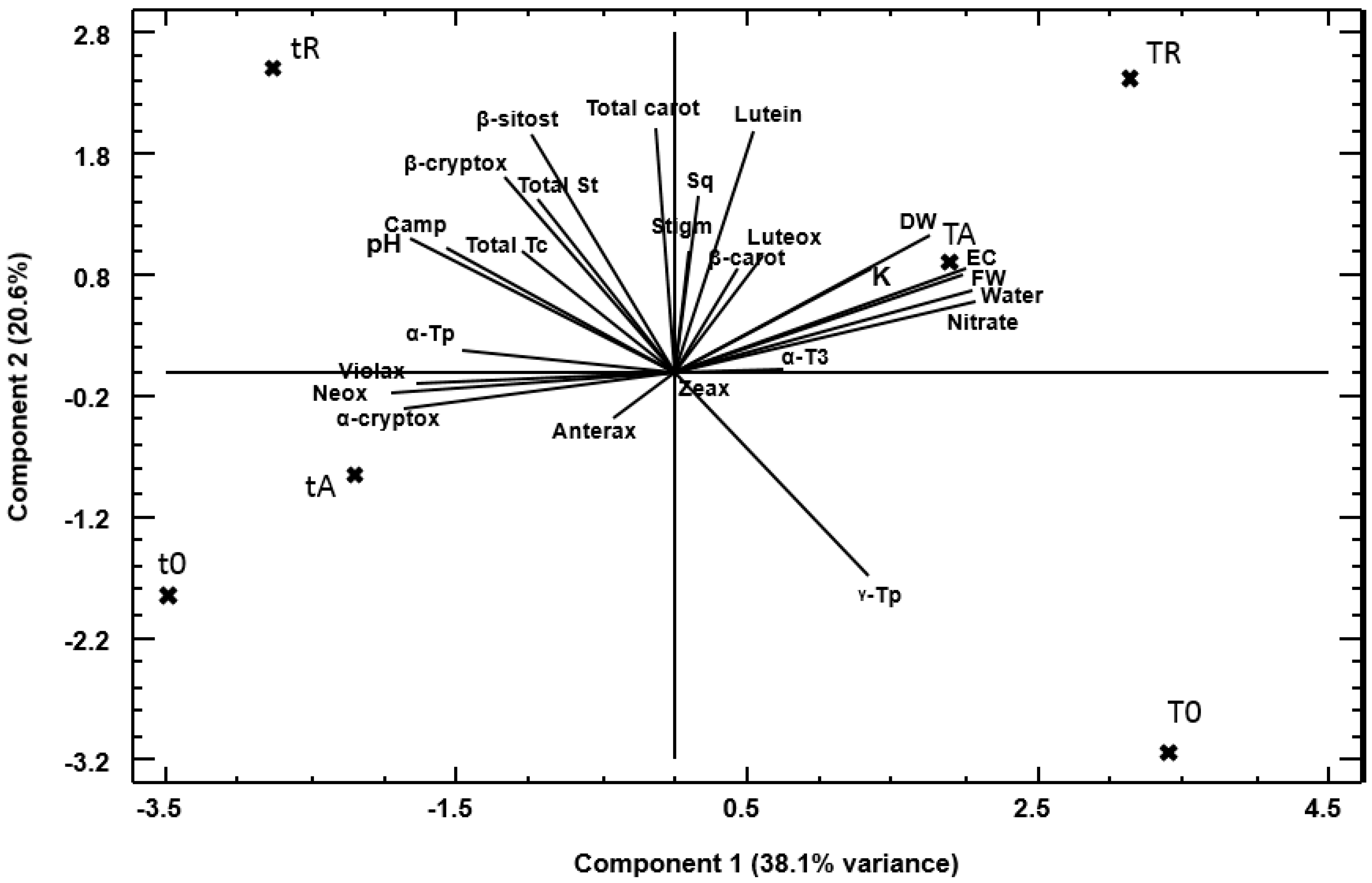
3.4. Principal Component Analysis
4. Discussion
4.1. Effect of Biostimulant Application via Fertigation on Monitoring Parameters
4.2. Effect of Biostimulants on Fertigation Uptake and Growth Parameters
4.3. Effect of Biostimulants on the Phytochemical Composition of Lettuce
4.4. Principal Component Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popescu, M. Agricultural uses of seaweeds extracts. Curr. Trends Nat. Sci. 2013, 2, 36–39. [Google Scholar]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (Vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar] [CrossRef]
- Cunha–Chiamolera, T.P.L.; Urrestarazu, M.; Morillas-España, A.; Ortega, R.; Miralles, I.; González–López, C.V.; Carbajal–Valenzuela, I.A. Evaluation of the reuse of regenerated water from microalgae–related wastewater treatment processes in horticulture. Agric. Water Manag. 2024, 292, 108660. [Google Scholar] [CrossRef]
- Raghunandan, B.L.; Vyas, R.V.; Patel, H.K.; Jhala, Y.K. Perspectives of seaweed as organic fertilizer in agriculture. In Soil Fertility Management for Sustainable Development; Springer: Singapore, 2019; pp. 267–289. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 29, 655. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Abkhoo, J.; Sabbagh, S.K. Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J. Appl. Phycol. 2016, 28, 1333–1342. [Google Scholar] [CrossRef]
- Zodape, S.T. Seaweeds as a biofertilizer. J. Sci. Ind. Res. 2001, 60, 378–382. [Google Scholar]
- Morillas-España, A.; Bermejo, R.; Abdala-Díaz, R.; Ruiz, Á.; Lafarga, T.; Acién, G.; Fernández-Sevilla, J.M. Biorefinery approach applied to the production of food colourants and biostimulants from Oscillatoria sp. Biology 2022, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Waaland, J. R Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J. Sci. Food Agric. 2012, 92, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Rápalo-Cruz, A.; Gómez-Serrano, C.; González-López, C.V.; Morillas-España, A.; Jiménez-Becker, S. Utilization of treated wastewater derived from microalgae production for the irrigation of horticultural crops. J. Appl. Phycol. 2024, 36, 1259–1268. [Google Scholar] [CrossRef]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Han, J.S.; Kim, H.J.; Jeon, Y.J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef]
- EC. REGULATION (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 24 January 2025).
- Julia, I.; Oscar, M.; Analía, L.; Virginia, L. Biofertilization with Macrocystis pyrifera algae extracts combined with PGPR-enhanced growth in Lactuca sativa seedlings. J. Appl. Phycol. 2020, 32, 4361–4371. [Google Scholar] [CrossRef]
- Ferruzca-Campos, E.A.; Rico-Chávez, A.K.; Guevara-González, R.G.; Urrestarazu, M.; Cunha-Chiamolera, T.P.L.; Reynoso-Camacho, R.; Guzmán-Cruz, R. Biostimulant and elicitor responses to cricket frass (Acheta domesticus) in tomato (Solanum lycopersicum L.) under protected conditions. Plants 2023, 12, 1327. [Google Scholar] [CrossRef]
- Cunha-Chiamolera, T.P.L.; Urrestarazu, M.; Carbajal-Valenzuela, I.A.; Ramos, J.B.; Ortega, R.; Miralles, I.; Guevara-González, R.G. Extracellular fragmented self-DNA displays biostimulation of lettuce in soilless culture. Horticulturae 2024, 10, 964. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.A.; de Burgos-Navarro, I.; Chileh-Chelh, T.; Belarbi, E.H.; Álvarez-Corral, M.; Carmona-Fernández, M.; Ezzaitouni, M.; Guil-Guerrero, J.L. The agronomic potential of the invasive brown seaweed Rugulopteryx okamurae: Optimisation of alginate, mannitol, and phlorotannin extraction. Plants 2024, 13, 3539. [Google Scholar] [CrossRef] [PubMed]
- González, V.; Díez-Ortiz, M.; Simón, M.; van Gestel, C.A. Application of bioassays with Enchytraeus crypticus and Folsomia candida to evaluate the toxicity of a metal-contaminated soil, before and after remediation. J. Soils Sediments 2011, 11, 1199–1208. [Google Scholar] [CrossRef]
- Peçanha, D.A.; Cunha-Chiamolera, T.P.L.; Chourak, Y.; Martínez-Rivera, E.Y.; Urrestarazu, M. Effect of the matric potential on growth and water, nitrate and potassium absorption of vegetables under soilless culture. J. Soil Sci. Plant Nutr. 2021, 21, 3493–3501. [Google Scholar] [CrossRef]
- Kimura, M.; Rodríguez-Amaya, D.B. A scheme for obtaining standards and HPLC quantification of leafy vegetable carotenoids. Food Chem. 2002, 78, 389–398. [Google Scholar] [CrossRef]
- Fabrikov, D.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Rodríguez-García, I.; Gómez-Mercado, F.; Urrestarazu, M.; Lao, M.T.; Rincón-Cervera, M.A.; Álvaro, J.E.; Lyashenko, S. Borage oil: Tocopherols, sterols and squalene in farmed and endemic-wild Borago species. J. Food Compos. Anal. 2019, 83, 103299. [Google Scholar] [CrossRef]
- Petersen, R.G. Agricultural Field Experiments; Marcel Dekker: New York, NY, USA, 1994; 409p, ISBN 0-8247-8912-1. [Google Scholar]
- Arnon, D.I.; Johnson, C.M. Influence of hydrogen ion concentration on the growth of higher plants under controlled conditions. Plant Physiol. 1942, 17, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Atero-Calvo, S.; Izquierdo-Ramos, M.J.; García-Huertas, C.; Rodríguez-Alcántara, M.; Navarro-Morillo, I.; Navarro-León, E. An Evaluation of the effectivity of the green leaves biostimulant on lettuce growth, nutritional quality, and mineral element efficiencies under optimal growth conditions. Plants 2024, 13, 917. [Google Scholar] [CrossRef] [PubMed]
- Ergun, O.; Dasgan, H.Y.; Isık, O. Effects of microalgae Chlorella vulgaris on hydroponically grown lettuce. In Proceedings of the XXX International Horticultural Congress IHC2018: II International Symposium on Soilless Culture and VIII International 1273, Istanbul, Turkey, 12–16 August 2018; pp. 169–176. [Google Scholar]
- Ammaturo, C.; Pacheco, D.; Cotas, J.; Formisano, L.; Ciriello, M.; Pereira, L.; Bahcevandziev, K. Use of Chlorella vulgaris and Ulva lactuca as biostimulant on lettuce. Appl. Sci. 2023, 13, 9046. [Google Scholar] [CrossRef]
- Kopta, T.; Pavlikova, M.; Sękara, A.; Pokluda, R.; Maršálek, B. Effect of bacterial-algal biostimulant on the yield and internal quality of lettuce (Lactuca sativa L.) produced for spring and summer crop. Not. Bot. Horti Agrobot. 2018, 46, 615–621. [Google Scholar] [CrossRef]
- Chen, X.; Song, M.; Zhao, J.; Yin, D.; Ye, X.; Yu, J. Excessive composite pollution carbon sources enhance the bio-fertilizer efficiency of Tetradesmus obliquus: Focused on cultivation period. Environ. Sci. Pollut. Res. Int. 2024, 31, 6054–6066. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Carotenes and xanthophylls as antioxidants. In Handbook of Antioxidants For food Preservation; Woodhead Publishing: Sawston, UK, 2015; pp. 17–50. [Google Scholar]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ishar, M.P.S. Mechanism of antioxidant action of vitamin-E: Role of charge transfer/electron transfer in free radical quenching by alpha-tocopherol and its oxidative transformations—A review. Proc. Natl. Acad. Sci. India Sect. A 2006, 76, 265. [Google Scholar]
- Meulmeester, F.L.; Luo, J.; Martens, L.G.; Mills, K.; van Heemst, D.; Noordam, R. Antioxidant supplementation in oxidative stress-related diseases: What have we learned from studies on alpha-tocopherol? Antioxidants 2022, 11, 2322. [Google Scholar] [CrossRef]
- Gupta, A.K.; Savopoulos, C.G.; Ahuja, J.; Hatzitolios, A.I. Role of phytosterols in lipid-lowering: Current perspectives. QJM Int. J. Med. 2011, 104, 301–308. [Google Scholar] [CrossRef] [PubMed]
- AbuMweis, S.S.; Jones, P.J. Cholesterol-lowering effect of plant sterols. Curr. Atheroscler. Rep. 2008, 10, 467–472. [Google Scholar] [CrossRef]
- Kelly, G.S. Squalene and its potential clinical uses. Altern. Med. Rev. 1999, 4, 29–36. [Google Scholar] [PubMed]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermúdez, B.; Muriana, F.J.; Alarcón-de-la-Lastra, C. Squalene targets pro-and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Rodríguez-Concepción, M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef]
- Caliandro, R.; Nagel, K.A.; Kastenholz, B.; Bassi, R.; Li, Z.; Niyogi, K.K.; Pogson, B.J.; Schurr, U.; Matsubara, S. Effects of altered α-and β-branch carotenoid biosynthesis on photoprotection and whole-plant acclimation of Arabidopsis to photo-oxidative stress. Plant Cell Environ. 2013, 36, 438–453. [Google Scholar] [CrossRef]
- Maldini, M.; Natella, F.; Baima, S.; Morelli, G.; Scaccini, C.; Langridge, J.; Astarita, G. Untargeted metabolomics reveals predominant alterations in lipid metabolism following light exposure in broccoli sprouts. Int. J. Mol. Sci. 2015, 16, 13678–13691. [Google Scholar] [CrossRef]
- Randhir, A.; Laird, D.W.; Maker, G.; Trengove, R.; Moheimani, N.R. Microalgae: A potential sustainable commercial source of sterols. Algal Res. 2020, 46, 101772. [Google Scholar] [CrossRef]
- Zandvakili, O.R.; Barker, A.V.; Hashemi, M.; Etemadi, F.; Autio, W.R. Comparisons of commercial organic and chemical fertilizer solutions on growth and composition of lettuce. J. Plant Nutr. 2019, 42, 990–1000. [Google Scholar] [CrossRef]
- García Gómez, J.C.; Sempere Valverde, J.; Ostalé Valriberas, E.; Martínez, M.; Olaya Ponzone, L.; Roi González, A.; Espinosa Torre, F.; Sánchez Moyano, J.E.; Megina Martínez, C.; Parada, J.A. Rugulopteryx okamurae (EY Dawson) IK Hwang, WJ Lee & HS Kim (Dictyotales, Ochrophyta), alga exótica “explosiva” en el estrecho de Gibraltar. Observaciones preliminares de su distribución e impacto. Almoraima. Rev. Estud. Campogibraltareños 2018, 49, 97–113. [Google Scholar]
- Ortíz, C.L.; Moya, M.P.; Navarro, V.B. A rapid chromatographic method for simultaneous determination of β-sitosterol and tocopherol homologues in vegetable oils. J. Food Compos. Anal. 2006, 19, 141–149. [Google Scholar] [CrossRef]
- González, V.; García, I.; Del Moral, F.; De Haro, S.; Sánchez, J.A.; Simón, M. Impact of unconfined sulphur-mine waste on a semi-arid environment (Almería, SE Spain). J. Environ. Manag. 2011, 92, 1509–1519. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, C.; Straver, N.B. Nutrient Solution for Vegetables and Flowers Grown in Water or Substrates, 10th ed.; Glasshouse Crops Research Station: Naaldwijk, The Netherlands, 1994; pp. 1–33. [Google Scholar]
| A. nodosum (Jospalga 25) a | R. okamurae Biofertilizer b | Regulation (EU) 2019/1009 [16] | |
|---|---|---|---|
| Alginate (g 100 g−1 dw) | 14.5 | 19.4 ± 1.4 | - |
| Mannitol (g 100 g−1 dw) | 5.6 | 7.9 ± 0.5 | - |
| Phlorotannins (g 100 g−1 dw) | 0.56 | 0.35 ± 0.0 | - |
| Arsenic (As) (mg kg−1 dw) | 25 | 19 ± 1 | 40 mg kg−1 dw |
| Cadmium (Cd) (mg kg−1 dw) | 0.5 | 0.7 ± 0.2 | 1.5 mg kg−1 dw |
| Lead (Pb) (mg kg−1 dw) | 60 | 46 ± 4 | 120 mg kg−1 dw |
| Nutrient Solution | Biostimulant Extracted From: | |||
|---|---|---|---|---|
| Macronutrient | Micronutrient | R. okamurae | A. nodosum | |
| T0 | 100% | √ | - | - |
| TR | 100% | √ | √ | - |
| TA | 100% | √ | - | √ |
| t0 | 50% | √ | - | - |
| tR | 50% | √ | √ | - |
| tA | 50% | √ | - | √ |
| Standard Optimum Fertigation | Suboptimal Fertigation | ||||
|---|---|---|---|---|---|
| TR | TA | tR | tA | ||
| Uptake | Water | 6.12 | 3.82 | 15.95 | 3.06 |
| Nitrate | 8.02 | 7.27 | 21.56 | 2.04 | |
| Potassium | 3.03 | 2.76 | 1.99 | −8.79 | |
| Yield | Fresh weight | 33.29 | 16.39 | 9.79 | −6.69 |
| Dry weight | 39.90 | 32.12 | 6.09 | −1.83 | |
| Mean | 18.07 | 12.47 | 11.08 | −2.44 | |
| Samples d | All-trans- Violaxanthin | 9′-cis-Neoxanthin | Luteoxanthin | Antheraxanthin | Lutein | All-trans-Zeaxanthin | α-Cryptoxanthin | β-Cryptoxanthin | All-trans-β-Carotene | Total Carotenoids mg 100 g−1 fw |
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | 0.9 ± 0.1 d | 1.8 ± 0.1 d | 0.3 ± 0.0 b | 0.1 ± 0.0 a | 6.3 ± 0.3 c | 0.1 ± 0.0 a | traces | traces | 1.2 ± 0.1 b | 10.8 ± 0.4 b |
| TA | 1.6 ± 0.3 b | 2.3 ± 0.5 bc | 0.4 ± 0.1 b | 0.2 ± 0.0 a | 8.8 ± 0.4 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 2.3 ± 0.3 a | 15.8 ± 0.8 a |
| TR | 1.1 ± 0.1 cd | 1.9 ± 0.0 cd | 0.8 ± 0.0 a | 0.1 ± 0.0 a | 9.8 ± 0.3 a | 0.1 ± 0.0 a | traces | 0.1 ± 0.0 a | 1.2 ± 0.2 b | 15.1 ± 0.3 a |
| t0 | 2.1 ± 0.0 a | 2.7 ± 0.1 a | 0.5 ± 0.1 ab | 0.2 ± 0.0 a | 4.4 ± 0.5 c | 0.1 ± 0.0 a | 0.2 ± 0.0 a | 0.1 ± 0.0 a | 1.1 ± 0.1 b | 11.4 ± 0.5 b |
| tA | 1.6 ± 0.1 b | 2.8 ± 0.3 a | 0.4 ± 0.1 b | 0.1 ± 0.0 a | 8.8 ± 0.4 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 1.3 ± 0.2 b | 15.3 ± 0.6 a |
| tR | 1.5 ± 0.1 bc | 2.4 ± 0.2 ab | 0.3 ± 0.1 b | 0.1 ± 0.0 a | 9.4 ± 0.2 ab | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 1.5 ± 0.2 b | 15.5 ± 0.4 a |
| Species /Codes | Tocols (mg 100−1 g fw) | Total Tc mg 100 g−1 fw | Sterols (mg 100−1 g fw) | Total St mg 100 g−1 fw | Squalene mg 100 g−1 fw | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| α-T3 | α-Tp | γ-Tp | δ-Tp | Stigmasterol | Campesterol | β-Sitosterol | ||||
| T0 | 0.02 ± 0.00 b | 0.15 ± 0.01 bc | 0.26 ± 0.00 b | traces | 0.44 ± 0.01 b | 20.9 ± 2.8 bc | 2.2 ± 0.2 d | 0.2 ± 0.0 b | 23.3 ± 2.8 cd | 0.1 ± 0.0 a |
| TA | 0.08 ± 0.02 a | 0.12 ± 0.00 c | 0.24 ± 0.08 b | traces | 0.45 ± 0.08 b | 17.9 ± 2.6 c | 3.9 ± 0.4 c | 0.4 ± 0.1 a | 22.2 ± 2.6 d | 0.1 ± 0.0 a |
| TR | 0.03 ± 0.01 b | 0.14 ± 0.01 bc | 0.25 ± 0.04 b | traces | 0.42 ± 0.04 b | 25.6 ± 0.7 a | 3.2 ± 0.1 c | 0.4 ± 0.0 a | 29.1 ± 0.7 ab | 0.1 ± 0.0 a |
| t0 | 0.03 ± 0.01 b | 0.18 ± 0.03 ab | 0.26 ± 0.05 b | traces | 0.46 ± 0.06 b | 21.1 ± 2.1 b | 5.9 ± 0.6 b | 0.4 ± 0.1 a | 27.4 ± 1.2 bc | traces |
| tA | 0.03 ± 0.01 b | 0.14 ± 0.00 bc | 0.26 ± 0.02 b | traces | 0.42 ± 0.02 b | 21.8 ± 2.1 b | 3.0 ± 0.4 cd | 0.3 ± 0.0 ab | 25.1 ± 2.2 c | 0.1 ± 0.0 a |
| tR | 0.02 ± 0.00 b | 0.20 ± 0.01 a | 0.35 ± 0.03 a | traces | 0.57 ± 0.03 a | 22.6 ± 1.3 ab | 7.7 ± 0.6 a | 0.5 ± 0.0 a | 30.8 ± 1.0 a | 0.2 ± 0.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha-Chiamolera, T.P.L.; Chileh-Chelh, T.; Ezzaitouni, M.; Urrestarazu, M.; Carrillo Montalbán, J.d.D.; Guil-Guerrero, J.L. A Rugulopteryx okamurae-Based Biostimulant Enhances Growth and Phytochemicals in Lettuce. Horticulturae 2025, 11, 558. https://doi.org/10.3390/horticulturae11050558
Cunha-Chiamolera TPL, Chileh-Chelh T, Ezzaitouni M, Urrestarazu M, Carrillo Montalbán JdD, Guil-Guerrero JL. A Rugulopteryx okamurae-Based Biostimulant Enhances Growth and Phytochemicals in Lettuce. Horticulturae. 2025; 11(5):558. https://doi.org/10.3390/horticulturae11050558
Chicago/Turabian StyleCunha-Chiamolera, Tatiana P. L., Tarik Chileh-Chelh, Mohamed Ezzaitouni, Miguel Urrestarazu, Juan de Dios Carrillo Montalbán, and José Luis Guil-Guerrero. 2025. "A Rugulopteryx okamurae-Based Biostimulant Enhances Growth and Phytochemicals in Lettuce" Horticulturae 11, no. 5: 558. https://doi.org/10.3390/horticulturae11050558
APA StyleCunha-Chiamolera, T. P. L., Chileh-Chelh, T., Ezzaitouni, M., Urrestarazu, M., Carrillo Montalbán, J. d. D., & Guil-Guerrero, J. L. (2025). A Rugulopteryx okamurae-Based Biostimulant Enhances Growth and Phytochemicals in Lettuce. Horticulturae, 11(5), 558. https://doi.org/10.3390/horticulturae11050558








