Ozonated Water for Enhancing Quality and Antioxidant Activity in Ready-to-Eat Table Grapes During Cold Storage
Abstract
:1. Introduction
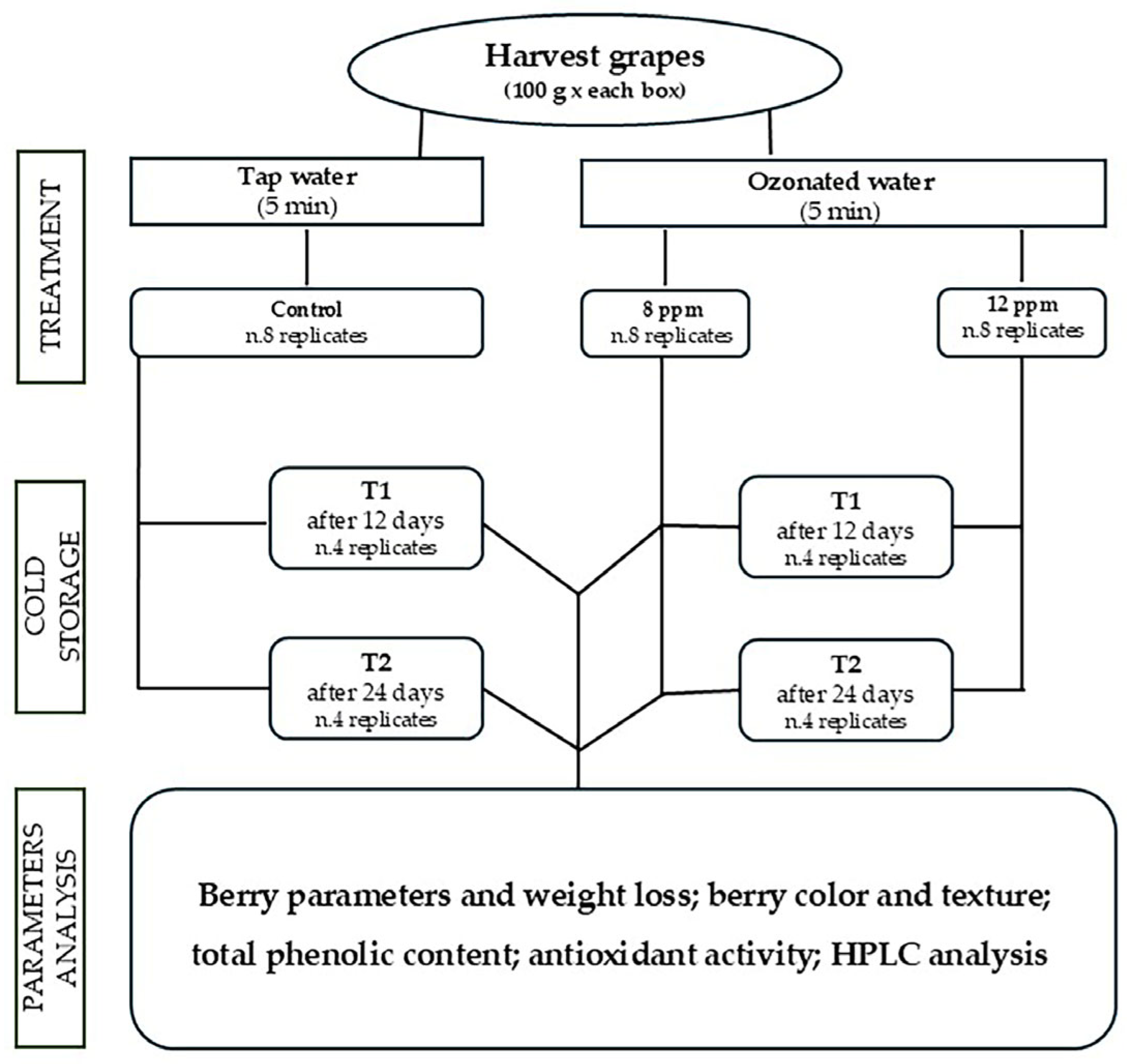
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Ozone Pre-Treatments
2.3. Berry Parameters and Weight Loss
2.4. Berry Color and Texture
2.5. Grape Samples and Preparation of Grape Skin Extracts (GSEs)
2.6. Total Phenolic Content (TPC)
2.7. Antioxidant Activity
2.8. HPLC Analysis
2.9. Statistical Analysis
3. Results
3.1. Berry Parameters and Weight Loss
3.2. Berry Color and Texture Parameters
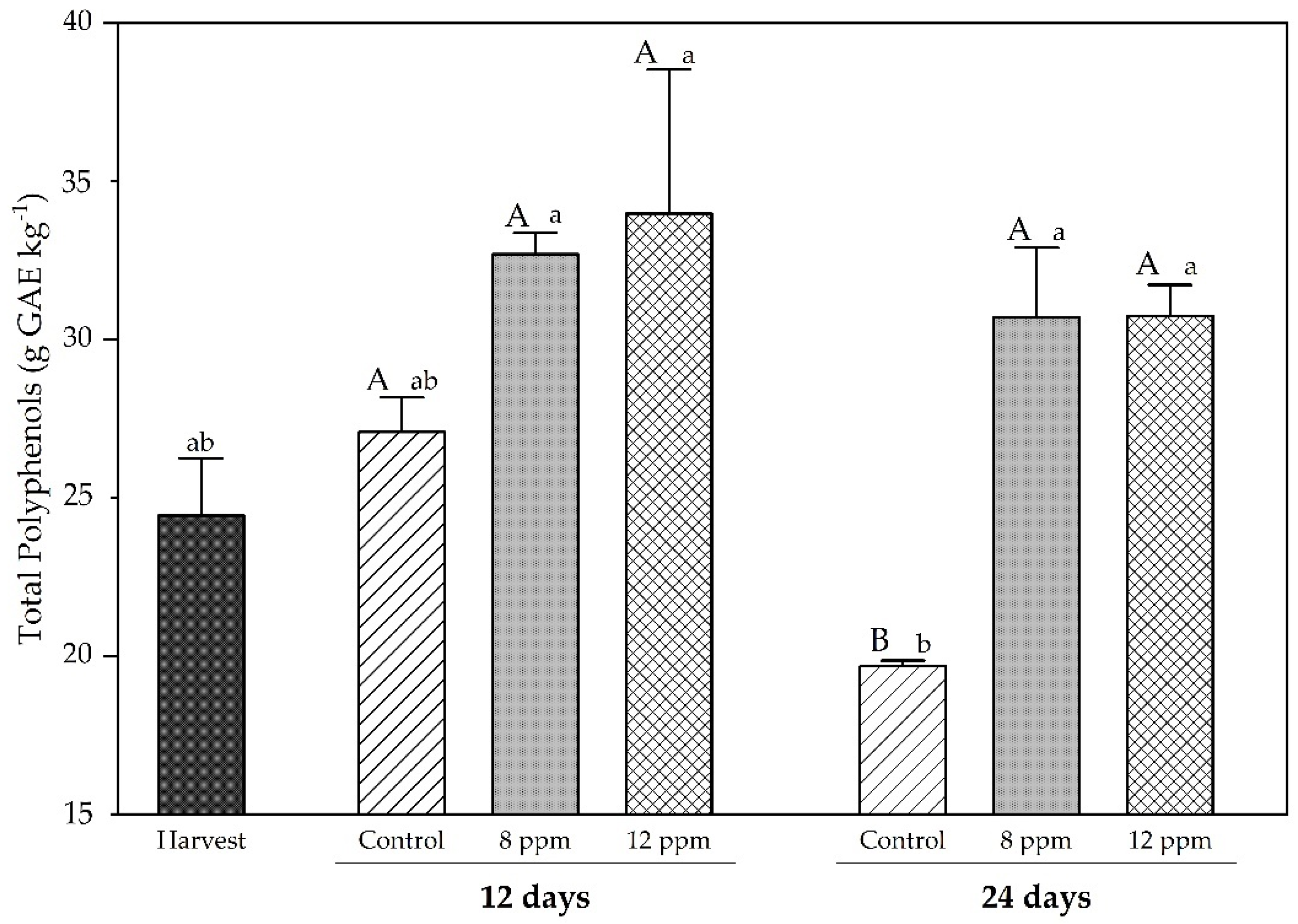
3.3. Total Phenolics
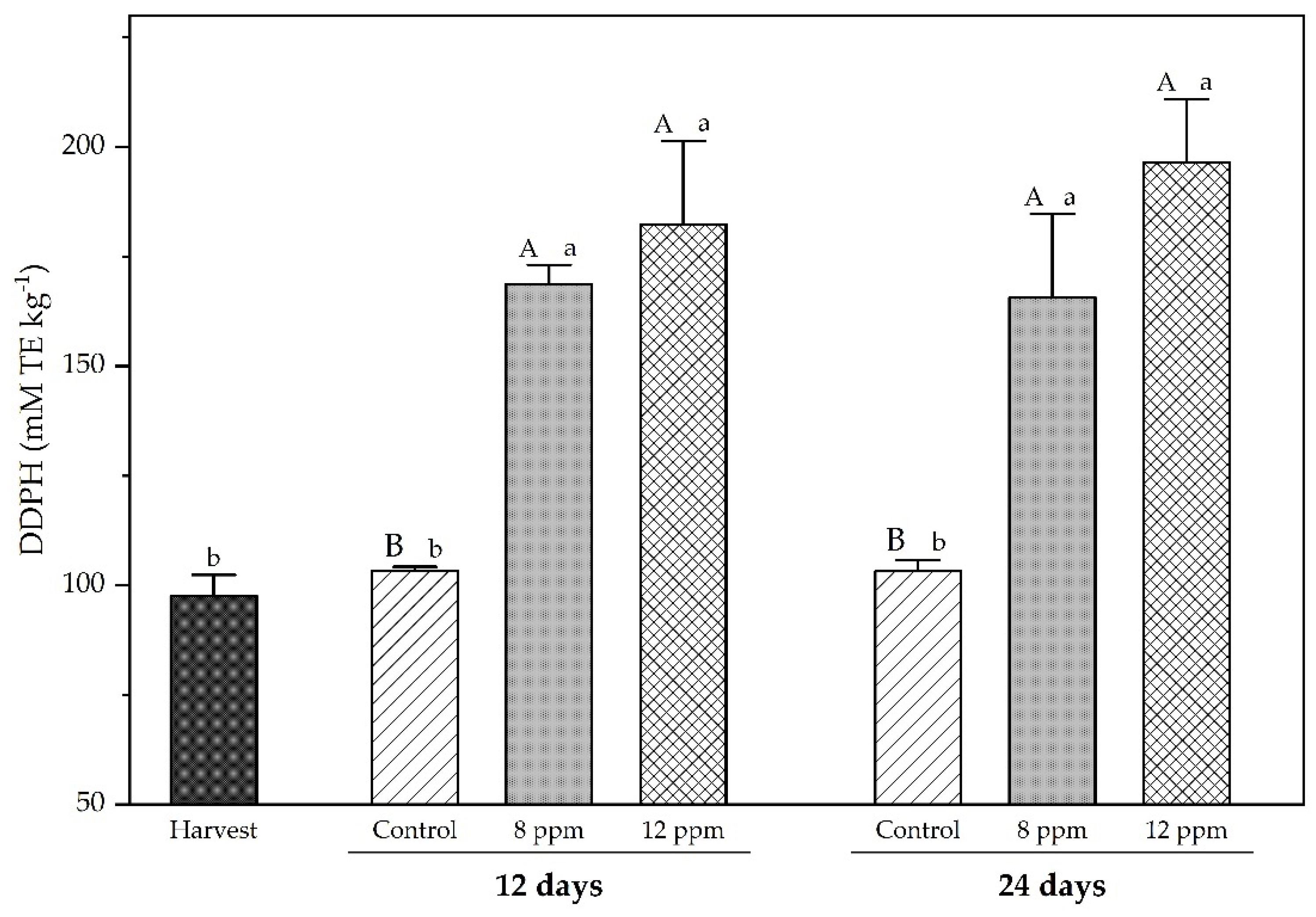
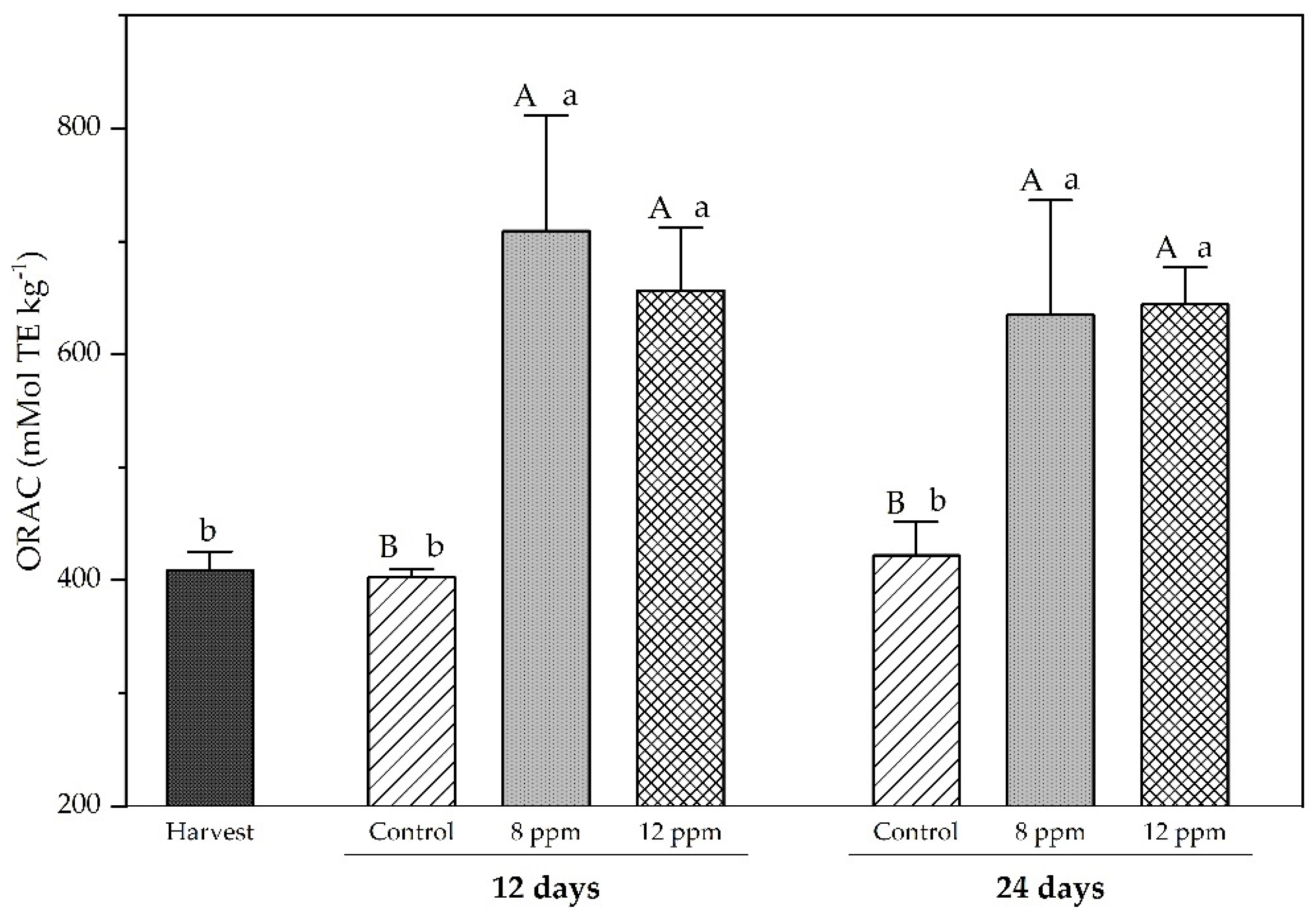
3.4. Antioxidants
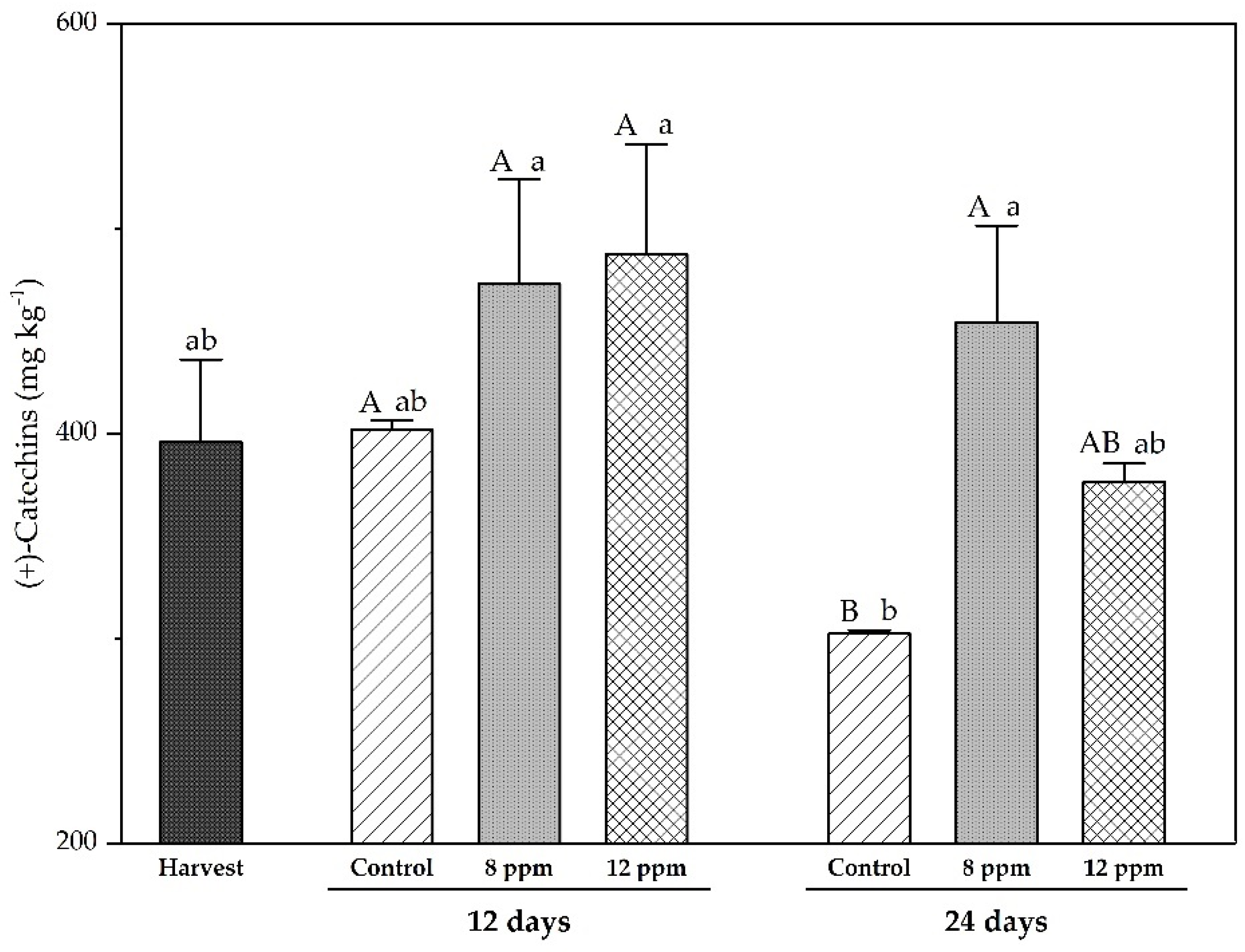
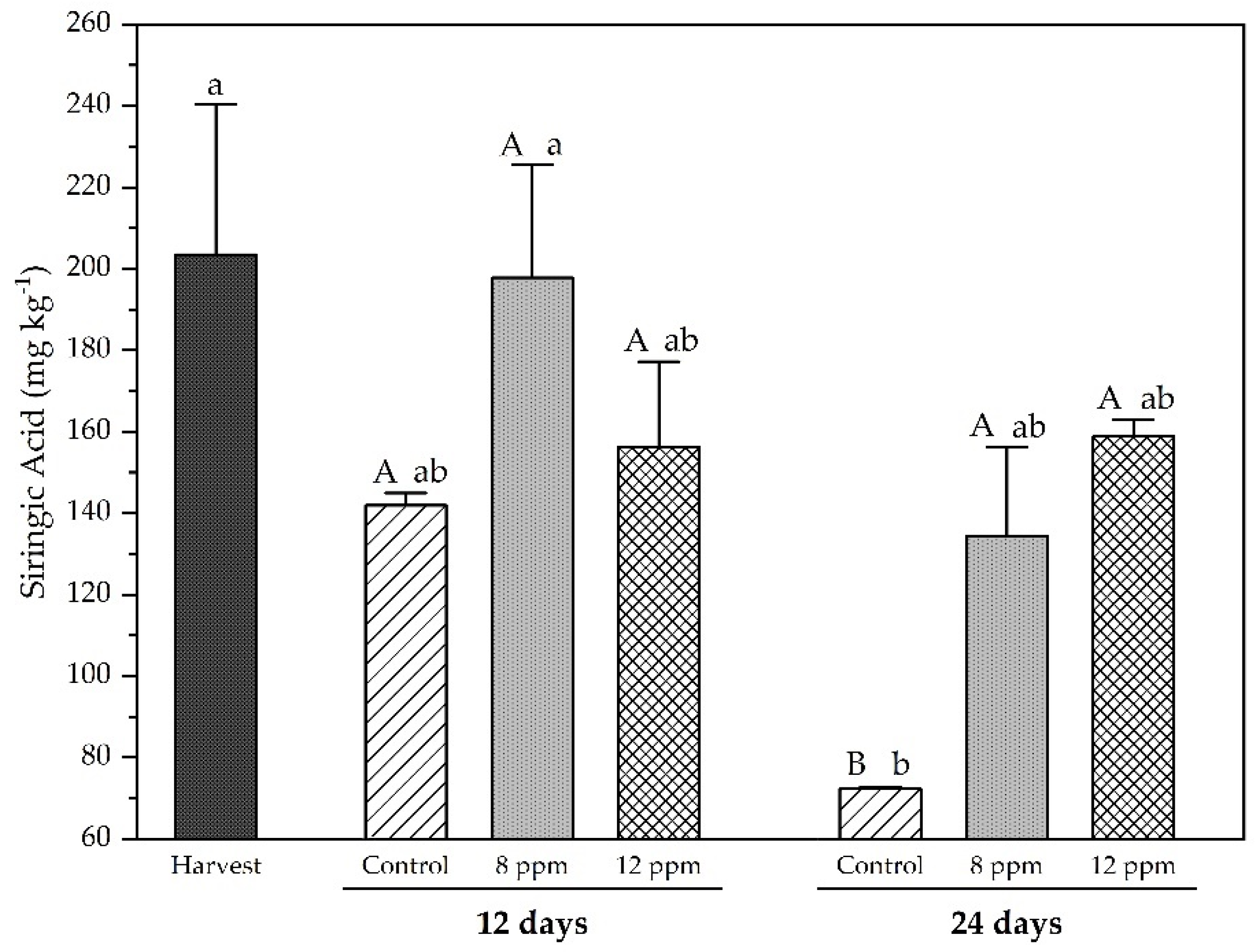
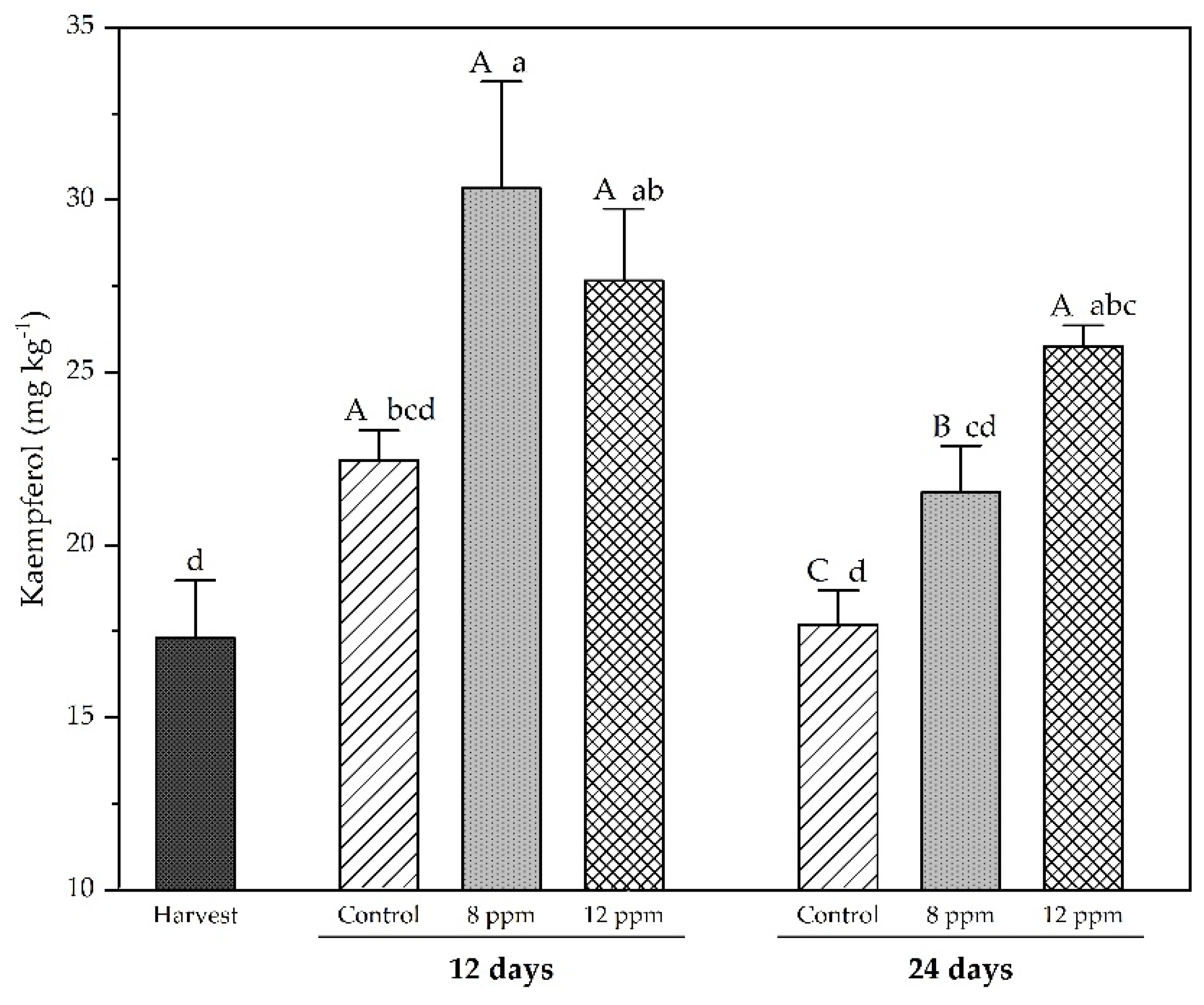
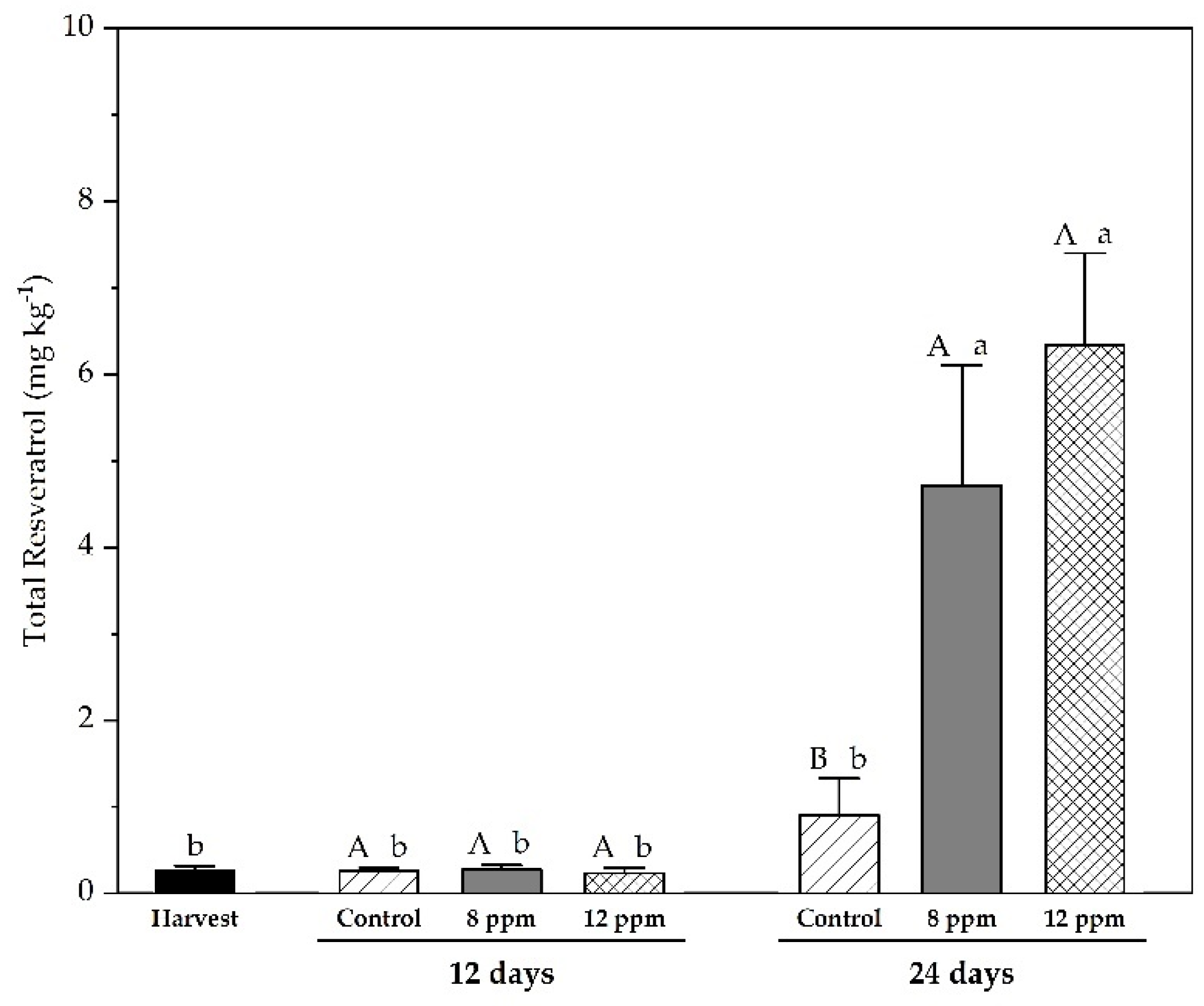
3.5. Analysis of Phenolic Compounds by HPLC
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Admane, N.; Genovese, F.; Altieri, G.; Tauriello, A.; Trani, A.; Gambacorta, G.; Verrastro, V.; Di Renzo, G.C. Effect of ozone or carbon dioxide pre-treatment during long-term storage of organic table grapes with modified atmosphere packaging. LWT Food Sci. Technol. 2018, 98, 170–178. [Google Scholar] [CrossRef]
- Pretel, M.T.; Martínez-Madrid, M.C.; Martínez, J.R.; Carreño, J.C.; Romojaro, F. Prolonged storage of ‘Aledo’ table grapes in a slightly CO2 enriched atmosphere in combination with generators of SO2. LWT Food Sci. Technol. 2006, 39, 1109–1116. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Li, X.; Wei, J.; Wu, B. Effect of nitrous oxide against Botrytis cinerea and phenylpropanoid pathway metabolism in table grapes. Sci. Hortic. 2019, 254, 99–105. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, J.; Xing, S.; Zhang, Z.; Wu, B.; Guan, J. Sulfur dioxide (SO2) accumulation in postharvest grape: The role of pedicels of four different varieties. Postharvest. Biol. Technol. 2022, 190, 111953. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Mohammadi, M.J.; Sulistiyani, S.; Ramírez-Coronel, A.A.; Kiani, F.; Jalil, A.T.; Almulla, A.F.; Asban, P.; Farhadi, M.; Derikondi, M. Effects of sulfur dioxide inhalation on human health: A review. Rev. Environ. Health 2024, 39, 331–337. [Google Scholar] [CrossRef]
- Xue, W.; Macleod, J.; Blaxland, J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods 2023, 12, 814. [Google Scholar] [CrossRef]
- Barthwal, R.; Negi, A.; Kathuria, D.; Singh, N. Ozonation: Post-harvest processing of different fruits and vegetables enhancing and preserving the quality. Food Chem. 2025, 463, 141489. [Google Scholar] [CrossRef]
- Sarron, E.; Gadonna-Widehem, P.; Aussenac, T. Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review. Foods 2021, 10, 605. [Google Scholar] [CrossRef]
- Prabha, V.; Barma, R.D.; Singh, R.; Madan, A. Ozone Technology in Food Processing: A Review. Trends Biosci. 2015, 8, 4031–4047. [Google Scholar]
- Shezi, S.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Changes in the Biochemistry of Fresh Produce in Response to Ozone Postharvest Treatment. Sci. Hortic. 2020, 269, 109397. [Google Scholar] [CrossRef]
- Caponio, G.; Vendemia, M.; Mallardi, D.; Marsico, A.D.; Alba, V.; Gentilesco, G.; Forte, G.; Velasco, R.; Coletta, A. Pesticide Residues and Berry Microbiome after Ozonated Water Washing in Table Grape Storage. Foods 2023, 12, 3144. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Pandiselvam, R.; Kothakota, A.; Padma Ishwarya, S.; Zalpouri, R.; Mahanti, N.K. Impact of ozone treatment on food polyphenols—A comprehensive review. Food Control. 2022, 142, 109207. [Google Scholar] [CrossRef]
- Goffi, V.; Modesti, M.; Forniti, R.; Botondi, R. Quality of green (Actinidia chinensis var. deliciosa ‘Hayward’) and yellow (A. chinensis var. chinensis ‘Soreli’) kiwifruit during cold storage at 0 °C in normal atmosphere and with gaseous ozone. Acta Hortic. 2018, 1218, 473–480. [Google Scholar] [CrossRef]
- Shah, N.; Sulaiman, N.; Sidek, A.; Supian, N.S.M. Quality assessment of ozone-treated citrus fruit juices. Int. Food Res. J. 2019, 26, 1405–1415. [Google Scholar]
- Augpole, I.; Rakejeva, T.; Dukalska, L.; Skudra, L. Providing Quality of Shredded Carrots during Storage by Treatment with Ozonated Water. Mater. Sci. Appl. Chem. 2014, 30, 10–17. [Google Scholar] [CrossRef]
- Botondi, R.; De Sanctis, F.; Moscatelli, N.; Vettraino, A.M.; Catelli, C.; Mencarelli, F. Ozone fumigation for safety and quality of wine grapes in postharvest dehydration. Food Chem. 2015, 188, 641–647. [Google Scholar] [CrossRef]
- Sarig, P.; Zahavi, T.; Zutkhi, Y.; Yannai, S. Ozone for control of post-harvest decay of table grapes caused by Rhizopus stolonifer. Physiol. Mol. Plant Pathol. 1996, 48, 403–415. [Google Scholar] [CrossRef]
- González-Barrio, R.; Beltrán, D.; Cantos, E.; Gil, M.I.; Espín, J.C.; Tomás-Barberán, F.A. Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. “Superior” white table grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Artés, F.; Tomás-Barberán, F.A. Quality and enhancement of bioactive phenolics in cv. Napoleon table grapes exposed to different postharvest gaseous treatments. J. Agric. Food Chem. 2003, 51, 5290–5295. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Aguayo, E.; Artés, F.; Tomás-Barberán, F.A. Enriched ozone atmosphere enhances bioactive phenolics in seedless table grapes after prolonged shelf life. J. Sci. Food Agric. 2007, 87, 824–831. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Vázquez, A.; Pérez, A.G.; García, J.M. Control of table grapes postharvest decay by ozone treatment and resveratrol induction. Food Sci. Technol. Int. 2009, 15, 495–502. [Google Scholar] [CrossRef]
- Silveira, A.C.; Oyarzún, D.; Escalona, V. Oxidative enzymes and functional quality of minimally processed grape berries sanitised with ozonated water. Int. J. Food Sci. Technol. 2018, 53, 1371–1380. [Google Scholar] [CrossRef]
- Geransayeh, M.; Mostofi, Y.; Vahid, A.; Nejatian, M.A. Effects of Ozonated Water on Storage Life and Postharvest Quality of Iranian Table Grape (cv. Bidaneh Qermez). J. Agric. Sci. 2012, 4, 31. [Google Scholar] [CrossRef]
- Laureano, J.; Giacosa, S.; Río Segade, S.; Torchio, F.; Cravero, F.; Gerbi, V.; Englezos, V.; Carboni, C.; Cocolin, L.; Rantsiou, K.; et al. Effects of Continuous Exposure to Ozone Gas and Electrolyzed Water on the Skin Hardness of Table and Wine Grape Varieties. J. Texture Stud. 2016, 47, 40–48. [Google Scholar] [CrossRef]
- Gao, C.C.; Lin, Q.; Dong, C.H.; Ji, H.P.; Yu, J.Z.; Chen, C.K.; Zhu, Z.Q.; Ban, Z.; Zhang, N.; Bao, Y.Y. Effects of ozone concentration on the postharvest quality and microbial diversity of Muscat Hamburg grapes. RSC Adv. 2020, 10, 9037–9045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vendemia, M.; Caponio, G.; Ferrulli, F.; Scarano, L.; Perniola, R.; Coletta, A.; Marsico, A.D. Preliminary studies on microbial management efficiency of ozonated water on Italian ready-to-eat table grape variety. BIO Web Conf. 2023, 68, 04009. [Google Scholar] [CrossRef]
- CIE. Colorimetry; Publication CIE no 15.2; CIE: Vienna, Austria, 1986. [Google Scholar]
- Di Stefano, R.; Cravero, M.C. Metodi per lo studio dei polifenoli dell’uva. Riv. Vitic. Enol. 1991, 44, 37–45, ISSN 0370-7865. [Google Scholar]
- Waterhouse, A.L. Determination of total phenolic. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; Wiley: New York, NY, USA, 2009; pp. I1.1.1–I1.1.8. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 1, 25–30. [Google Scholar] [CrossRef]
- Milella, R.A.; De Rosso, M.; Gasparro, M.; Gigante, I.; Debiase, G.; Forleo, L.R.; Marsico, A.D.; Perniola, R.; Tutino, V.; Notarnicola, M.; et al. Correlation between antioxidant and anticancer activity and phenolic profile of new Apulian table grape genotypes (V. vinifera L.). Front. Plant Sci. 2023, 14, 1187227. [Google Scholar] [CrossRef]
- Romero-Peréz, A.I.; Lamuela-Raventớs, R.M.; Andrés-Lacueva, C.; de la Torre-Boronat, M.C. Method for the Quantitative Extraction of Resveratrol and Piceid Isomers in Grape Berry Skins. Effect of Powdery Mildew on the Stilbene Content. J. Agric. Food Chem. 2001, 49, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Docimo, T.; Francese, G.; Ruggiero, A.; Batelli, G.; DePalma, M.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Mennella, G.; Tucci, M. Phenylpropanoids Accumulation in Eggplant Fruit: Characterization of Biosynthetic Genes and Regulation by a MYB Transcription Factor. Front. Plant Sci. 2016, 6, 1233. [Google Scholar] [CrossRef] [PubMed]
- Premjit, Y.; Sruthi, N.U.; Pandiselvam, R.; Kothakota, A. Aqueous ozone: Chemistry, physiochemical properties, microbial inactivation, factors influencing antimicrobial effectiveness, and application in food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1054–1085. [Google Scholar] [CrossRef]
- Campayo, A.; Serrano de la Hoz, K.; García-Martínez, M.M.; Salinas, M.R.; Alonso, G.L. Spraying Ozonated Water on Bobal Grapevines: Effect on Wine Quality. Biomolecules 2020, 10, 213. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Application of Ozone for the Postharvest Treatment of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2013, 54, 312–339. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Ozone Applications in Fruit and Vegetable Processing. Food Rev. Int. 2007, 23, 91–106. [Google Scholar] [CrossRef]
- Alothman, M.; Kaur, B.; Fazilah, A.; Bhat, R.; Karim, A.A. Ozone-induced changes of antioxidant capacity of fresh-cut tropical fruits. IFSET 2010, 11, 666–671. [Google Scholar] [CrossRef]
- Rodoni, L.; Casadei, N.; Concell’on, A.; Chaves Alicia, A.R.; Vicente, A.R. Effect of short-term ozone treatments on tomato (Solanum lycopersicum L.) fruit quality and cell wall degradation. J. Agric. Food Chem. 2010, 58, 594–599. [Google Scholar] [CrossRef]
- Carbone, K.; Mencarelli, F. Influence of Short-Term Postharvest Ozone Treatments in Nitrogen or Air Atmosphere on the Metabolic Response of White Wine Grapes. Food Bioprocess Technol. 2015, 8, 1739–1749. [Google Scholar] [CrossRef]
- Foy, C.D.; Lee, E.H.; Rowland, R.; Devine, T.E.; Buzzell, R.I. Ozone tolerance related to flavonol glycoside genes in soybean. J. Plant Nutr. 1995, 18, 637–647. [Google Scholar] [CrossRef]
- Modesti, M.; Macaluso, M.; Taglieri, I.; Bellincontro, A.; Sanmartin, C. Ozone and Bioactive Compounds in Grapes and Wine. Foods 2021, 10, 2934. [Google Scholar] [CrossRef]

| Parameter | Harvest | 12 Days | 24 Days |
|---|---|---|---|
| TSS (°Brix) | |||
| Control | 18.38 ± 0.19 b | 18.76 ± 0.08 ab A | 19.50 ± 0.06 a A |
| 8 ppm | 18.37 ± 0.38 b A | 18.03 ± 0.15 b B | |
| 12 ppm | 18.44 ± 0.09 b A | 18.20 ± 0.25 b B | |
| TA (g tartaric acid 100 mL−1) | |||
| Control | 3.54 ± 0.08 a | 3.42 ± 0.02 a B | 3.59 ± 0.04 a A |
| 8 ppm | 3.57 ± 0.05 a A | 3.59 ± 0.01 a A | |
| 12 ppm | 3.42 ± 0.03 a B | 3.58 ± 0.02 a A | |
| pH | |||
| Control | 4.07 ± 0.04 a | 4.19 ± 0.08 a A | 4.24 ± 0.06 a A |
| 8 ppm | 4.08 ± 0.08 a A | 4.00 ± 0.03 a B | |
| 12 ppm | 4.06 ± 0.04 a A | 4.21 ± 0.06 a AB | |
| Weight loss (%) | |||
| Control | 0.30 ± 0.007 c A | 1.06 ± 0.005 a A | |
| 8 ppm | 0.26 ± 0.009 d B | 0.86 ± 0.009 b B | |
| 12 ppm | 0.13 ± 0.005 e C | 0.83 ± 0.005 b B |
| Parameter | Harvest | 12 Days | 24 Days |
|---|---|---|---|
| L* Control | 37.28 ± 0.72 a | 31.82 ± 0.45 b A | 32.46 ± 0.56 b AB |
| 8 ppm | 31.30 ± 0.56 b A | 31.37 ± 0.36 b B | |
| 12 ppm | 32.06 ± 0.39 b A | 32.85 ± 0.41 b A | |
| a* Control | −2.09 ± 0.11 c | −1.70 ± 0.08 ab A | −1.51 ± 0.07 a A |
| 8 ppm | −1.69 ± 0.11 ab A | −1.50 ± 0.08 a A | |
| 12 ppm | −1.66 ± 0.08 ab A | −1.87 ± 0.07 bc B | |
| b* Control | 3.77 ± 0.22 b | 4.92 ± 0.30 a A | 5.09 ± 0.36 a A |
| 8 ppm | 4.88 ± 0.26 ab A | 5.56 ± 0.37 a A | |
| 12 ppm | 4.90 ± 0.26 a A | 5.31 ± 0.39 a A | |
| C* Control | 7.06 ± 0.31 a | 5.18 ± 0.33 b A | 5.05 ± 0.36 b A |
| 8 ppm | 5.49 ± 0.32 ab A | 5.88 ± 0.44 ab A | |
| 12 ppm | 5.64 ± 0.31 ab A | 5.30 ± 0.19 ab A | |
| H (°) Control | 117.50 ± 2.12 a | 107.64 ± 1.45 b AB | 107.18 ± 1.10 b AB |
| 8 ppm | 109.70 ± 1.16 b A | 108.79 ± 1.38 b A | |
| 12 ppm | 105.61 ± 1.13 b B | 104.52 ± 0.73 b B |
| Treatments | Hardness (N) | Cohesiveness (−) | Gumminess (N) | Elasticity (-) | Chewiness (N) |
|---|---|---|---|---|---|
| Harvest | 8.30 ± 0.34 a | 0.26 ± 0.006 c | 2.15 ± 0.09 b | 0.62 ± 0.006 a | 1.15 ± 0.04 b |
| Control | 5.70 ± 0.13 c | 0.26 ± 0.005 c | 1.50 ± 0.04 c | 0.32 ± 0.009 b | 0.96 ± 0.03 c |
| 8 ppm | 6.99 ± 0.26 b | 0.36 ± 0.007 a | 2.52 ± 0.10 a | 0.59 ± 0.007 a | 1.46 ± 0.04 a |
| 12 ppm | 7.01 ± 0.26 b | 0.30 ± 0.006 b | 2.13 ± 0.01 b | 0.60 ± 0.011 a | 1.05 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milella, R.A.; Forte, G.; Gentilesco, G.; Caponio, G.; Francese, G.; D’Alessandro, A.; Giannandrea, M.A.; Coletta, A. Ozonated Water for Enhancing Quality and Antioxidant Activity in Ready-to-Eat Table Grapes During Cold Storage. Horticulturae 2025, 11, 555. https://doi.org/10.3390/horticulturae11050555
Milella RA, Forte G, Gentilesco G, Caponio G, Francese G, D’Alessandro A, Giannandrea MA, Coletta A. Ozonated Water for Enhancing Quality and Antioxidant Activity in Ready-to-Eat Table Grapes During Cold Storage. Horticulturae. 2025; 11(5):555. https://doi.org/10.3390/horticulturae11050555
Chicago/Turabian StyleMilella, Rosa Anna, Giovanna Forte, Giovanni Gentilesco, Gabriele Caponio, Gianluca Francese, Antonietta D’Alessandro, Maria Angela Giannandrea, and Antonio Coletta. 2025. "Ozonated Water for Enhancing Quality and Antioxidant Activity in Ready-to-Eat Table Grapes During Cold Storage" Horticulturae 11, no. 5: 555. https://doi.org/10.3390/horticulturae11050555
APA StyleMilella, R. A., Forte, G., Gentilesco, G., Caponio, G., Francese, G., D’Alessandro, A., Giannandrea, M. A., & Coletta, A. (2025). Ozonated Water for Enhancing Quality and Antioxidant Activity in Ready-to-Eat Table Grapes During Cold Storage. Horticulturae, 11(5), 555. https://doi.org/10.3390/horticulturae11050555







