Abstract
The development of sustainable seed coating formulations is essential to enhance crop performance while reducing reliance on synthetic inputs. This study evaluates biochar-enriched coatings incorporating olive pomace, buffalo digestate, and microbial consortia from Fagus, Quercus, and Pinus forest litters, including Trichoderma harzianum, for their effects on seed germination and plant growth. Four crops (Diplotaxis tenuifolia, Lactuca sativa, Solanum lycopersicum, and Zea mays) were tested through germination assays and field trials. Treatments containing digestate or pomace alone significantly reduced germination and seedling growth in D. tenuifolia and L. sativa (below 25%, compared to control), due to the phytotoxic effects of ammonia, salts, and polyphenols. In contrast, biochar-based coatings mitigated these effects, enhancing germination and root elongation. The addition of T. harzianum further improved seedling establishment, likely by enhancing nutrient uptake and suppressing soilborne pathogens, with increases exceeding 100% (compared to control). Field trials confirmed these findings, showing that biochar–T. harzianum combinations improved both shoot and root biomass, particularly in L. sativa and S. lycopersicum. Z. mays displayed greater tolerance to raw by-products, though biochar remained essential for optimal growth. While forest-derived microbial consortia supported microbial balance, their effect on biomass was less pronounced. These results highlight the potential of biochar-based coatings, especially when combined with T. harzianum, as sustainable alternatives to conventional seed treatments.
Keywords:
biochar; crops; seed coating; by-products; forest litter microbiome; Trichoderma harzianum 1. Introduction
The transition towards more sustainable agricultural practices is essential for minimizing environmental impact while enhancing crop productivity [1]. Conventional farming relies heavily on synthetic fertilizers and pesticides, which, despite their effectiveness, contribute to soil degradation, water contamination, biodiversity loss, and soil microbiome simplification [2,3]. The search for alternative solutions has led to the development of innovative approaches aimed at improving plant growth while reducing dependency on chemical inputs [4]. Among these strategies, seed coating has gained increasing attention as a promising method to support seed germination and early plant establishment [5].
By incorporating functional materials, this approach not only provides protection but also delivers essential nutrients, potentially decreasing the need for synthetic fertilizers and fungicides while improving plant resilience to environmental stressors [6]. One particularly promising material for soil amendment is biochar, a carbon-rich by-product derived from biomass pyrolysis [7]. Its highly porous structure confers excellent water retention and adsorption capacities, enabling the gradual release of nutrients essential for plant development [8]. These characteristics make biochar a valuable soil amendment, improving soil aeration, water-holding capacity, and microbial activity [9]. Its ability to adsorb and gradually release nutrients contributes to enhanced plant growth, particularly in low-fertility soils or those prone to nutrient leaching [10].
Recent studies have demonstrated that biochar plays a crucial role in modulating soil microbial communities by favouring beneficial microorganisms that enhance nutrient availability and support plant health [11,12]. The porous structure of biochar provides an optimal habitat for beneficial microbial communities, including nitrogen-fixing bacteria, mycorrhizal fungi, and phosphate-solubilizing microorganisms, which further contribute to soil fertility and plant growth [13]. Furthermore, biochar has been explored as a carrier for controlled-release systems, enabling the gradual delivery of bioactive compounds and beneficial microbes to the seed and rhizosphere [14]. In addition, one of the characteristics of biochar is its ability to maintain the viability of microorganisms with which it has been previously enriched during storage under certain conditions [15]. Moreover, biochar also exhibits this property under field conditions [16].
An emerging strategy involves combining biochar with agro-industrial by-products to create nutrient-enriched seed coatings that not only enhance plant growth but also contribute to sustainable waste management in agriculture. Numerous studies have explored the potential of biochar enrichment with fertilizers and organic by-products [17,18,19]. In southern Italy, two major agro-industrial residues—olive pomace, a by-product of olive oil extraction [20], and buffalo manure, a waste product from dairy farm operations [21]—pose a significant environmental challenge if not properly managed. Both materials contain high concentrations of organic matter, yet their disposal is often problematic due to their potential to cause water contamination and eutrophication [22,23].
Olive pomace, rich in polyphenols and fatty acids, has traditionally been used as an organic amendment or composted to improve soil structure and fertility [24]. However, the presence of phenolic compounds can exert phytotoxic effects, inhibiting seed germination and plant growth when applied in excessive amounts [25]. Similarly, buffalo digestate, a by-product of anaerobic digestion of buffalo manure, is commonly used as an organic fertilizer due to its high nitrogen and phosphorus content [26]. However, the presence of ammonia, salts, and other residual organic compounds can induce phytotoxicity, necessitating careful management to ensure its safe agricultural application [27]. Given these limitations, incorporating these by-products into biochar-based seed coatings offer an opportunity to mitigate their toxic effects while harnessing their beneficial properties as nutrient sources. To further enhance the effectiveness of seed coatings enriched with biochar and agro-industrial by-products, the introduction of beneficial microbiomes has been proposed as an additional strategy. Although biochar can reduce some of the phytotoxicity associated with these by-products, it does not fully eliminate it. Microbial inoculants have gained increasing attention for their ability to enhance nutrient cycling, improve soil structure, and suppress plant pathogens [28]. Natural microbial consortia, such as those found in natural forest, may play a key role in organic matter decomposition [29] and nutrient cycling, contributing to improved soil fertility. These microorganisms, including decomposer fungi and nitrogen-fixing bacteria, can enhance plant growth by facilitating nutrient availability and improving root-soil interactions [30]. Integrating beneficial microorganisms into seed coatings can also enhance seedling vigour, stimulate root proliferation, and improve water and nutrient uptake efficiency [31]. Microbial consortia were selected from forest leaf litters, as microbial communities in such environments tend to form complex and stable networks due to the presence of diverse microhabitats and minimal environmental disturbances. These networks support efficient nutrient cycling and promote the decomposition of organic matter [32]. In addition to naturally occurring microbial consortia, industrial microbial strains such as T. harzianum have been widely studied for their ability to promote plant growth and suppress pathogens [33]. Trichoderma species stimulate root development, enhance nutrient uptake, and protect plants from soilborne diseases through antagonistic mechanisms such as mycoparasitism and the production of antimicrobial compounds [34]. Their ability to degrade complex organic materials makes them particularly effective in seed coatings containing agro-industrial by-products, as they can accelerate the decomposition of potentially toxic compounds while facilitating nutrient release [35]. In particular, T. harzianum was selected for its well-documented role in promoting plant growth, nutrient uptake, and pathogen suppression [36,37,38]. Additionally, microbial consortia derived from Fagus, Quercus, and Pinus forest litter were included due to their ecological adaptation to organic-rich substrates and their potential to support plant establishment through enhanced microbial diversity and soil health [32]. Inoculants based on Trichoderma spp. and other microorganisms classified as plant growth-promoting rhizobacteria (PGPR) have also been shown to enhance plant resilience to abiotic stress factors such as drought and soil salinity, further increasing their potential for use in sustainable seed treatments [33]. Moreover, several authors [39,40,41] have demonstrated the effective synergy between biochar and Trichoderma spp. when applied together, as this combination improves germination performance, plant growth, and pathogen suppression.
Understanding the interaction between biochar, phytotoxic agro-industrial by-products, and microbial inoculants on the seed surface is crucial to elucidating their combined effects on seed germination and early seedling development. This study aims to investigate the effects of biochar-enriched seed coatings containing olive pomace and/or buffalo digestate on the in vitro germination and open-field growth performance of four key agricultural crops: wild rocket (Diplotaxis Tenuifolia), lactuca (Lactuca sativa), tomato (Solanum lycopersicum), and corn (Zea mays). Additionally, the potential of natural and industrial microbial consortia to enhance plant performance and mitigate the phytotoxic effects of these by-products will be explored. Given the growing interest in effective seed treatments, understanding the interactions between biochar, agro-industrial by-products, microbial inoculants derived from Fagus, Quercus, and Pinus forest soils, and the industrial microbial inoculant Trichoderma harzianum may contribute to the development of innovative formulations that enhance crop productivity while reducing dependence on synthetic agricultural inputs. Based on previous findings, this research will test the following hypotheses:
- The combination of biochar with agro-industrial by-products (buffalo digestate and/or olive pomace) mitigates their phytotoxic effects while simultaneously enhancing plant growth.
- The synergistic action of beneficial microbiomes and agro-industrial by-products reduces the phytotoxicity of these materials, while biochar further improves plant resilience and growth by mediating these interactions.
2. Materials and Methods
2.1. Study Site, Soil and Material Analyses
The field experiment was conducted over a period of five months, from March to July 2024, in the phytopathology greenhouses of the Department of Agricultural Sciences in Portici (Naples, Southern Italy; 40°48′41″ N, 14°20′34″ E; 65 m a.s.l.), located near Mount Vesuvius. This area is characterized by a Mediterranean climate, with an annual mean temperature of 16.5 °C, reaching an average high of 28.5 °C in July and a low of 9.5 °C in January. Environmental conditions of greenhouse were monitored throughout the experiment using an Opi evja-sensor, which recorded daily average parameters. Temperature ranged between 13 °C and 38 °C, with a daylight intensity of 1220 μmol·s−1·m−2. These values were calculated as the average from measurements taken at noon on five sunny days. The daily light integral (DLI) over the experimental period was estimated at 22 moles·m−2·day−1. The soil used in the study was classified as a volcanic Andosol, consisting of 47.0% sand, 32.0% silt, and 21.0% clay, with a pH of 7.95. Chemical analyses showed an organic carbon content of 20.94 g/kg, total nitrogen of 1.33 g/kg, and a C/N ratio of 15.74. The soil contained 119 g/kg of total CaCO3 and 26.9 mg/kg of available phosphorus (P2O5), while exchangeable cations included potassium (0.13 meq/100 g), magnesium (4.09 meq/100 g), calcium (13.7 meq/100 g), and sodium (0.08 meq/100 g). The electrical conductivity (EC) was measured at 0.3189 dS/m. Soil hydraulic properties were estimated based on the average of two independent analyses using Rawls and Brakensiek pedotransfer functions, with a bulk density of 1.4 g/cm3. Field capacity was determined at 24.2%, wilting point at 9%, and available water capacity at 15.2%.
The biochar used in this study was supplied by BiokW s.r.l. and obtained through up-draft gasification of plant-based waste, primarily residues from pear and apple orchards. Chemical analyses confirmed the absence of significant contaminants, including polycyclic aromatic hydrocarbons (PAHs), dioxins, and furans. The biochar was also analysed by the company for its chemical and physical properties. It contained 55.7% total carbon (C), 0.49% nitrogen (N), 0.08% phosphorus (P), 0.92% potassium (K), 2.95% calcium (Ca), 0.43% magnesium (Mg), and 1258 mg/kg sodium (Na). The material had a pH of 7.9 and an electrical conductivity of 29 mS/m. Ash content was 26.51%, moisture content was 59.2%, and water retention capacity reached 74.85% (Table 1). Before application, the biochar was ground into a fine powder to facilitate the preparation of treatment suspensions (particles size < 1 mm). Olive pomace, obtained from a two-phase extraction plant in the province of Salerno, was air dried for approximately 20 days to reduce its initial moisture content of 20% and sieved at 1 mm to remove inert solid fractions. Elemental analysis was performed using an NA 1500 Fison 1108 (Thermo Fisher Scientific) to determine carbon, nitrogen, and hydrogen content. The pH and electrical conductivity were measured with a CRISON Basic 20 pH-meter and conductimeter. Polyphenol characterization was carried out via LC-MS analysis, identifying O-alkyl C (61–90 ppm) and di-O-alkyl C (91–110 ppm) fractions associated with mono and polysaccharides. For the analysis, the biochar was dried at 60 °C, finely ground, and sieved to obtain particles smaller than 500 µm. A sequential extraction was then performed using a methanol:water:formic acid mixture (80:19:1, v/v/v). Other compounds detected included fatty acids, dicarboxylic acids, carboxylic acid derivatives, phenolic compounds, and triterpenic acids, in accordance with previous findings (Table 1). The digestate used in the study was primarily derived from buffalo slurry collected from a local farm in Battipaglia. Chemical composition analysis indicated a total solids (TS) content of 43.96 g/kg, with volatile solids (VS) accounting for 64.9%. Total Kjeldahl nitrogen (TKN) was measured at 6.3%, while ammoniacal nitrogen (TAN) was found at 3.7%, resulting in a TAN/TKN ratio of 58.3% (Table 1).

Table 1.
Chemical analysis of biochar (a) (pH, CE, ashes, UR, water retention, C, N, P, K, Ca, Mg, Na). TS total solids; VS: volatile solids; TKN: total Kjeldahl nitrogen; TAN: ammoniacal nitrogen, olive pomace (b) (C, N, C/N, H/C, pH, CE) and buffalo digestate (c) (ST, SV, TKN, TAN, TAN/TKN).
2.2. Coating Preparation and Application
Seed coating experiments were carried out to assess germination and plant growth under open-field conditions. Before coating application all seeds were surface-sterilized by immersion in a 0.1% sodium hypochlorite solution for 5 min, then thoroughly rinsed with deionized water. Both trials employed same coating materials, including powdered biochar, dried buffalo digestate, natural microbial inocula derived from Quercus, Fagus, and Pinus litter extracts, T. harzianum, and Arabidòl® (Arabic gum) as a binder. The seed coatings were prepared using a volumetric approach, following a standard formulation consisting of 8 parts of biochar, 2 parts of buffalo digestate and/or olive pomace, 8 parts of water, 1 part of Arabidòl® as a binding agent, and 2 mL of natural inoculum per 20 g of coating material. Microbial inocula were obtained by collecting 100 g of forest litter from Quercus, Fagus, and Pinus stands and suspending it in 100 mL of water, followed by agitation for 30 min; subsequently, the solid fraction was removed by sieving, and the resulting liquid was used as the inoculum. Forest microbiomes from Fagus, Quercus, and Pinus litter were selected due to their widespread occurrence in Mediterranean ecosystems and well-documented microbial communities, known for their plant growth-promoting potential [29,32,42]. T. harzianum (T 22) strains from the collection of the Plant Pathology Section, Department of Agricultural Sciences, University of Naples Federico II (Portici), were used in this study. The inoculum was prepared by adding 2 mL of a conidial suspension at a concentration of 2 × 109 CFU per 100 g of seeds, according to [43]. The Fagus litter was collected from Mount Cervati, located in the municipality of Monte San Giacomo, at an altitude of 1200 m a.s.l., the Quercus litter was obtained from the municipality of Giffoni Valle Piana at an altitude of 450 m a.s.l., while the Pinus litter was obtained from the municipality of Eboli, at an altitude of 10 m a.s.l. The microbiome of Quercus and Fagus forest litter used in this study was previously characterized by [42], who reported distinct differences in the presence of beneficial microorganisms and phytopathogens between forest and agricultural soils. The preparation of the coating material began with the combination of powdered biochar and buffalo digestate and/or olive pomace in an 8:2 volume ratio, allowing biochar to adsorb the by-product compounds and enhance their availability to plants through cation exchange capacity. Once the organic materials were integrated, forest or industrial microbial inocula were added, followed by water to achieve a homogeneous mixture. The coating matrix was then left to air-dry until it reached a consistency like powdered biochar. The coating process was conducted using a rotary machine, the “Twirl coating machine” (Pastaline, Nerviano, Italy), operating at a speed of 60 rpm. Seeds were placed inside the rotating chamber, followed by the gradual addition of biochar (or enriched biochar) and Arabidòl® powder. After two minutes of mixing, water was sprayed into the rotating mixture to facilitate uniform adhesion. Coating was considered complete once all materials had adhered to the seeds and dried, making them ready for sowing. The amount of seed coating dried matrix applied was standardized at 10% of the seed weight (w/w). The required quantity of coating powder was measured accordingly and applied to the seeds to ensure the intended coating ratio. Coatings were applied by thoroughly mixing the seeds with the prepared solution, followed by controlled drying before use in the respective trials (Figure 1).
Figure 1.
Seeds without seed coating (right) and seeds coated with biochar and buffalo digestate (left). (A) D. tenuifolia, (B) L. sativa, (C) S. lycopersicum, (D) Z. mays. Images were acquired using a stereomicroscope at 10× magnification.
2.3. Germination Test In Vitro
Fifty seeds per treatment (15 treatments) of D. tenuifolia, L. sativa, S. lycopersicum, and Z. mays were coated and tested in a germination assay on Petri dishes (140 mm of diameter) under controlled growth chamber conditions. The soil used for the bioassay was mixed with 20% (v/v) vermiculite unsterilized. The Petri dishes were covered with lids before being kept in a growth climate chamber (Italy, model Angelantoni life-science EKOCH 700), set at a constant temperature of 25 ± 0.5 °C, 75% relative humidity, and in total darkness. Irrigation was performed every two days by spraying 30 mL of water per dish. Germination was monitored daily from the third to the tenth day, recording the germination rate, final germination percentage, and seedling length.
The statistical analyses were conducted using R (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria), SPSS (version 29.0.1.0; IBM Corp., Armonk, NY, USA), and Excel (version 2019, 17.0; Microsoft Corp., Redmond, WA, USA). Two parameters were evaluated: the seed germination rate, calculated relative to total seeds sown, and the seedling length ratio, obtained by comparing each individual treatment to the control. Germination percentage was analysed using a generalized linear model (GLM) with a binomial distribution, followed by a pairwise comparison test when necessary. The seedling length ratio was calculated as the ratio of seedling length in treated samples relative to the control and was analysed using one-way ANOVA, followed by Tukey’s post-hoc test, or Kruskal–Wallis with Dunn’s test if normality assumptions were not met. The results are presented as mean ± standard deviation (SD), and different letters indicate statistically significant differences among treatments at p < 0.05.
2.4. Open Field Trial
For the field trial, five seeds per treatment were sown for each of the four species (D. tenuifolia, L. sativa, S. lycopersicum, and Z. mays), resulting in 13 treatments per species. The experiment was conducted between March and July 2024 in an open-field greenhouse setting, with plants spaced 30 cm apart and arranged according to a randomized block design to minimize spatial variability. Unlike the previous germination test, in this case 13 treatments were tested, as those involving only olive pomace or buffalo digestate as seed coatings were excluded due to the extremely negative results obtained previously. A fertilization treatment was applied using the ternary fertilizer Nitrophoska® Special 12-12-17 at a rate of N (60 kg/ha), P (60 kg/ha), and K (85 kg/ha), produced by EuroChem Agro Spa, Cesano Moderno (MO), Italy. Irrigation was performed twice per week (0.5 L per plant) from March to May and three times per week (1 L per plant) in June and July. Growth performance was evaluated by measuring the fresh biomass of shoots and roots, as well as the total fruit yield at the end of the growth cycle. For each treatment, five biological replicates were measured using an OHAUS TS series precision balance (accuracy ±0.01 g). Statistical analyses were performed using R (version 4.2.2), SPSS (version 29.0.1.0), and Excel (version 2019, 17.0). For the field experiment, shoot biomass, root biomass, and total fruit biomass were measured and analysed. Data were tested following the same statistical approach as in the pot experiment, applying one-way ANOVA with Tukey’s post-hoc test or Kruskal-Wallis with Dunn’s test, depending on data normality. The results are presented as mean ± standard deviation (SD), and different letters indicate statistically significant differences among treatments at p < 0.05.
A total of 14 coating treatments were tested, applied across four species in the germination test and three species in the field trial.
3. Results
3.1. D. tenuifolia, L. sativa, S. lycopersicum and Z. mays Germination
The germination percentage varied among treatments and species (Figure 2a), showing distinct responses to the applied coatings. Biochar alone consistently maintained or increased germination rates across all species, with the highest values observed in S. lycopersicum and Z. mays, where no significant reductions were recorded in any biochar-based treatment. In D. tenuifolia and L. sativa, biochar also supported high germination, but the addition of other components led to greater variability. Treatments containing digestate, either alone or combined with olive pomace, resulted in a significant reduction in germination across all species. This reduction was most pronounced in D. tenuifolia and L. sativa, where digestate alone showed the lowest germination percentages. The addition of olive pomace to digestate did not consistently mitigate this effect. The presence of T. harzianum did improve germination and, in some cases, maintained rates better than biochar alone. The effect of Fagus and Quercus microbiomes varied among species: in S. lycopersicum and Z. mays, germination remained high across all biochar-based treatments, including those with microbiomes, while in D. tenuifolia and L. sativa, the addition of Fagus and Quercus to biochar resulted in slight variations but did not cause significantly improve of biomass.
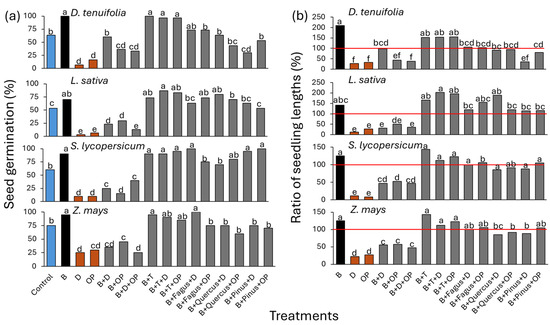
Figure 2.
Seed germination (%) (a) and the ratio of seedling length relative to control (%) (b) of treated seeds with seed coatings in four species (D. tenuifolia, L. sativa, S. lycopersicum, and Z. mays). Different letters indicate statistically significant differences according to Tukey’s HSD test following ANOVA (p < 0.05); the red line indicates the control level. D: digestate; B: biochar; OP: olive pomace; T: T. harzianum. Fagus, Quercus, and Pinus refer to the addition of forest-derived natural microbiomes.
Regarding seedling length, the ratio was expressed as a percentage relative to the control, with values varying across treatments and species (Figure 2b). Biochar alone consistently resulted in the highest values, particularly in S. lycopersicum and Z. mays, where all biochar-based treatments maintained or exceeded control levels. In D. tenuifolia and L. sativa, responses were more variable, though biochar still supported greater seedling length. Digestate-based treatments resulted in the lowest seedling length ratios across all species. In D. tenuifolia and L. sativa, digestate alone significantly reduced seedling length, with minimal recovery when combined with olive pomace. The presence of T. harzianum generally supported seedling growth, particularly in S. lycopersicum and Z. mays, while responses in D. tenuifolia and L. sativa were more variable. The Fagus-derived microbiome generally had a positive effect on root growth, particularly in L. sativa. A similar trend was observed with the Quercus microbiome, although in some cases its performance was comparable to that of the untreated control. In contrast, the Pinus microbiome produced inconsistent results, with modest effects in L. sativa and performance below the control in other species.
3.2. Response of L. sativa, S. lycopersicum and Z. mays to Coating in the Field
In the open field experimentation (Figure 3), biomass varied across species and treatments. Firstly, in the field trial, D. tenuifolia exhibited poor performance, as intense summer heat waves compromised its growth, leading to premature flowering and desiccation; For this reason, it was excluded from the sampling. The results indicate that biochar exerts a markedly positive effect, particularly when inoculated solely with T. harzianum, in S. lycopersicum and L. sativa, where an average biomass increase of 17.4% over the control was observed. In contrast, in Z. mays, the beneficial effect of biochar and T. harzianum appears to be limited or absent. Moreover, the addition of an agro-industrial by-product alongside T. harzianum was generally associated with a slight reduction in biomass production. Treatments containing digestate showed species-dependent effects. In L. sativa, they led to reduced biomass compared to biochar alone, while in S. lycopersicum and Z. mays, they maintained intermediate values. The addition of olive pomace and digestate did not enhance biomass and was associated with lower values in L. sativa. The presence of T. harzianum had a consistently positive effect, particularly in L. sativa and S. lycopersicum, further increasing aerial biomass. Root biomass showed less pronounced variations across treatments. Overall, biochar alone or in combination with T. harzianum led to the highest biomass, while digestate-containing treatments exhibited greater variability. The biomass results for S. lycopersicum and L. sativa showed significant differences for the Pinus-derived microbiome, which resulted in lower values compared to the control. No significant differences were observed for the Fagus and Quercus treatments. Finally, biomass data for Z. mays did not show significant differences among the three forest-derived inocula.
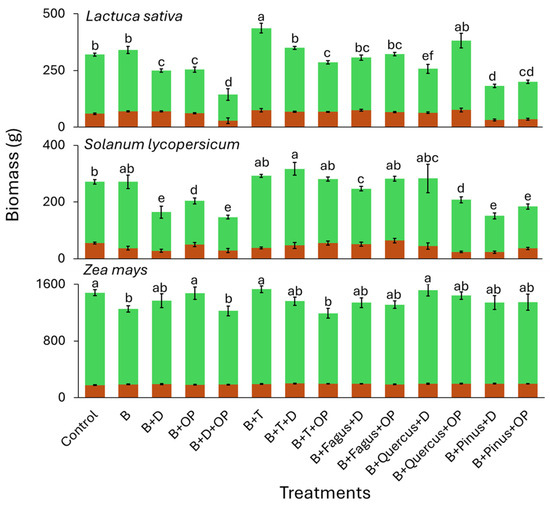
Figure 3.
Aerial (green) and root (brown) biomass from a field experiment on treated seeds with various seed coatings in L. sativa, S. lycopersicum, and Z. mays. Different letters indicate statistically significant differences according to Tukey’s HSD test following ANOVA (p < 0.05). D: digestate, B: biochar, OP: olive pomace, T: Trichoderma harzianum. Fagus, Quercus, and Pinus refer to the addition of forest-derived natural microbiomes. Error bars represent standard deviation.
4. Discussion
Industrial seed coating formulations typically contain fungicides, insecticides, nematicides, predator deterrents and herbicides [44]. However, excessive use of synthetic chemicals in agriculture has led to environmental concerns and pollution [45]. In the last decade different research has affirmed that some seed coatings can also incorporate nutrients, symbiotic microorganisms, and beneficial microbes, offering a more sustainable alternative than the chemical seed coating [46,47]. In this study, 15 different seed coatings were tested in germination experiments, along with 13 in field experiments. The coatings were composed of biochar, two nutrient-input by-products, and different associated microbiomes. The results of the seed germination test revealed a significant reduction in germination when seeds were treated with digestate and/or olive pomace without the addition of biochar or beneficial microbiomes. This effect was particularly pronounced in D. tenuifolia and L. sativa, where treatments containing only digestate or olive pomace exhibited markedly lower germination rates compared to the control. This reduction is likely attributable to the phytotoxic effects of ammonia, salts, and polyphenols present in these agro-industrial by-products, which may hinder seed water uptake and interfere with early metabolic processes [48,49,50,51,52]. In particular, the most severe effects were observed in leafy plants treated with digestate-based seed coatings, likely due to their heightened sensitivity to compounds present in digestate. This aligns with the findings of [53], who investigated seed germination and root elongation in D. tenuifolia, S. lycopersicum, Daucus carota, and Cucumis sativus using a different antibiotic commonly applied in agrofarms. Their results further confirmed that leafy plants are the most sensitive. However, the seed coating made up by only biochar significantly mitigated these negative effects, confirming its pivotal role in counteracting phytotoxicity [54]. Its high adsorption capacity reduces the concentration of inhibitory compounds, while its moisture retention properties create a more favourable environment for seed germination [55]. As reported by [56], treatments containing biochar alone resulted in the highest germination rates, further supporting its role in improving water availability and promoting early germination processes. The combination of biochar with low C:N, non-pyrogenic organic amendments allow the buffering of negative effects through the selective adsorption of several putative phytotoxic compounds [57]. This approach enables biochar to adsorb several organic molecules from the co-applied material, which not only leads to significant detoxification but also triggers a strong biostimulant effect on plant growth [57]. In addition, a key aspect of plant nutrition is the C:N ratio of biochar, which is closely related to both the feedstock type and the pyrolysis temperature [58]. High-quality biochar, such as the one used in this study, is typically produced at high pyrolysis temperatures from woody plant biomass and exhibits a C:N ratio above 100. As also demonstrated by [59], contrary to expectations, such biochars do not inhibit nitrogen mineralization. On the contrary, they may promote it, thereby enhancing the release of nitrogen in a bioavailable form. This may partly explain the overall increase in biomass observed following biochar application to seeds. Similar trends were observed in root elongation, emphasizing the impact of seed coatings not only on seed germination but also on early seedling development. Ref. [60] identified biochar’s water retention capacity as a key factor contributing to high germination rates and root elongation in corn seeds. This effect was further amplified by its ability to adsorb nutrients due to its physicochemical properties [61]. Ref. [62] tested a biochar-based seed coating on rice, confirming its positive effects on germination and seedling growth, and further stated that biochar is a suitable material for seed coating as it does not compromise germination or growth, degrading easily in water even when combined with an appropriate binding agent. Accordingly, Arabic gum (Arabidòl®) was used, as it forms a gel-like structure upon hydration, facilitating germination. Without biochar, digestate and olive pomace-based treatments led to a marked reduction in root length across all species, likely due to the same phytotoxic compounds affecting germination [50]. Ref. [25] conducted a germination and growth study in Morocco using different concentrations of olive mill wastewater, observing phytotoxic effects in both species, with S. lycopersicum being more sensitive and exhibiting severe toxicity at medium and high concentrations, while Z. mays showed greater tolerance, likely due to its higher nutrient demands. The impairment of root elongation suggests disruptions in nutrient uptake and cellular processes essential for early root development. In contrast, biochar significantly enhanced root growth by neutralizing inhibitory compounds. Also, ref. [63] found that seed pelleting with biochar improves cold resistance and promotes growth in Brassica napus plants.
Beneficial microbes, such as arbuscular mycorrhizal fungi and plant growth-promoting bacteria, have been extensively used to enhance crop performance [64]. Seed coating is recognized as an effective and practical method for delivering these beneficial microbes directly to the soil [65], facilitating their establishment in the rhizosphere or plant tissues. Unlike other application methods, seed coating ensures that microorganisms reach not only the soil but also the plant roots, supporting seedlings during the critical early stages of development [66,67]. This approach enables microorganisms to adhere to the seed surface and establish an early relationship with the radicle, forming the first line of defence against external pathogens [68]. Additionally, interactions with certain rhizosphere microorganisms and symbionts can stimulate plant immunity through the secretion of defence-related molecules by the plant [68]. Although microbial seed coating is a precise technique that reduces chemical inputs in seed treatments, one of the main challenges in its application is ensuring the survival of the microbial community on the seed surface [69,70]. The combined use of biochar and microbial inoculants increases the likelihood of microbial survival due to the protective role provided by biochar’s porous structure, which serves as a refuge for microorganisms [71,72]. Moreover, seed coating and encrustment enhance the efficiency and precision of mechanical sowing [42]. A further improvement in both germination and root elongation was observed with the addition of T. harzianum, which enhanced seedling establishment by protecting against pathogens [73], stimulating root development, and improving nutrient availability [74]. The synergistic effect of biochar and T. harzianum was particularly evident, resulting in the highest germination rates and the longest root development, further confirming its role in promoting root elongation and nutrient assimilation. Several studies have reported that the co-application of Trichoderma and biochar improves plant performance and suppresses major soil-borne pathogens. For example, ref. [75] demonstrated effective control of Ralstonia solanacearum in eggplant (Solanum melongena) through combined biochar and Trichoderma spp. treatment. Ref. [39] showed that the integration of Trichoderma with biochar and Bacillus spp. enhanced resistance against Fusarium spp. in cucumber. Similarly, ref. [76] reported that biochar combined with T. harzianum effectively suppressed Macrophomina phaseolina in bean (Phaseolus vulgaris). The synergistic use of biochar and Trichoderma has also been shown to improve strawberry resistance to Fusarium [77] and to enhance productivity in tomato (S. lycopersicum) [78]. Ref. [79] demonstrated that inoculation with T. asperellum, T. harzianum, and T. pseudokoningii in combination with biochar suppressed Fusarium oxysporum and stimulated metabolic activity in cucumber plants. In cassava, the co-application of Trichoderma-inoculated biochar reduced root rot incidence and improved soil quality [80]. Furthermore, T. viride combined with wood-derived biochar enhanced the growth of rye (Secale cereale L.) [81]. Several studies in the literature highlight the application of Trichoderma spp. in seed coating. Ref. [82] found that biochar combined with organic feedstocks and T. harzianum produced variable effects, from inhibition to stimulation. Positive outcomes occurred with biochar and non-pyrogenic amendments, while negative effects were linked to nitrogen-rich, non-stabilized inputs. In this case, the organic matter appears to modulate the action of Trichoderma, with phytotoxic amendments exacerbating its negative effects. Ref. [33] reported that coating Brassica napus seeds with Trichoderma viride spores significantly enhanced plant growth and resistance to fungal pathogens. Similarly, ref. [75] demonstrated that Trichoderma koningiopsis applied as a seed coating effectively protects Sorghum bicolor seeds from fungal contamination during storage. The combination of biochar and T. harzianum offers multiple agronomic benefits. Ref. [83] found that the synergistic effect of biochar and T. harzianum resulted in greater suppression of wilt disease and improved physiological parameters in Solanum melongena plants. Several examples in the literature illustrate the use of seed coating with beneficial microorganisms. Ref. [84] successfully utilized the biocontrol bacterium Bacillus subtilis ZF71 as a seed coating agent to control Fusarium root rot in Cucumis sativus. Ref. [85], in a comprehensive review, examined the combined use of Trichoderma spp. and beneficial bacteria for crop development and disease suppression. Based on this evidence, a natural forest microbiome was incorporated into the seed coatings. Litter forest-derived microbial consortia (Fagus, Quercus, and Pinus) positively influenced germination and root development, albeit to a lesser extent than T. harzianum. Their primary mode of action appears to be disease suppression and overall substrate quality improvement rather than direct stimulation of root elongation. Nevertheless, their presence in seed coatings provided an advantage over treatments containing digestate or olive pomace alone, suggesting that they contribute to the long-term balance of soil microbiota, which may support plant development in later growth stages. Ref. [42] reported that Quercus and Fagus forest soils host more diverse microbial communities than agricultural soils, where pathogenic taxa tend to prevail over beneficial ones. Quercus soils were dominated by Actinomycetota, and Fagus by Pseudomonadota and Acidobacteriota—phyla that include several plant growth-promoting (PGP) bacteria. This may partly explain the growth-promoting effects observed following the application of Fagus-derived microbiomes. These microbial groups are known to enhance root development, germination, and nitrogen fixation. Fungal communities also differed, with Basidiomycota dominating Quercus and Ascomycota in Fagus, both of which can degrade phytotoxic compounds. Overall, forest-derived microbiomes, particularly from Fagus and Quercus, appear enriched in beneficial taxa and may help suppress disease through associations with ectomycorrhizal fungi. The dominance of fungal taxa such as Ascomycota and Basidiomycota within these communities highlights the key role of fungal-mediated processes in maintaining soil health and fertility [32].
The trends in aerial and root biomass under field conditions aligned with germination and root length results, highlighting the impact of seed coating on plant development. In L. sativa, the highest biomass was recorded with biochar and T. harzianum, followed by biochar with digestate, confirming biochar’s role in improving soil structure, water retention, and mitigating phytotoxicity. T. harzianum further enhanced nutrient uptake and pathogen resistance. Conversely, digestate or olive pomace without biochar led to significantly lower biomass, reinforcing their phytotoxicity when destabilised. Similar trend was observed in S. lycopersicum, where biochar-based treatments, especially with T. harzianum and digestate, increased biomass. Forest microbial consortia contributed to microbial balance and disease suppression but had a weaker effect than T. harzianum. Long-term microbial viability may be lower in intensively managed soils, reducing their lasting impact. In Z. mays, biomass response was more stable across treatments, with the highest values in biochar-containing formulations, especially those with T. harzianum and digestate. Z. mays’s higher nutrient demand likely enhanced its tolerance to digestate and olive pomace, though treatments without biochar still resulted in lower biomass due to early phytotoxicity. Overall, biochar-enriched seed coatings, particularly with T. harzianum, proved the most effective for biomass production. Their synergy improved nutrient availability, root development, and resistance to soil stressors. While forest microbial consortia offered some benefits, their influence was more related to soil health than direct plant growth stimulation. In the field experiment, as in the germination trial, the strong phytotoxic effect of olive pomace and buffalo digestate was evident when applied individually with biochar [25,27]. However, contrary to previous observations, no significant differences were detected among the other treatments, except for those containing T. harzianum. This is likely due to the temporary protective and soil-conditioning effects of other treatments, which were particularly evident during the first two weeks of seedling development. In the later stages of the experiment, only the T. harzianum-enriched treatments resulted in significantly higher biomass accumulation. This may be attributed to the successful root colonization by T. harzianum spores, leading to a beneficial plant-fungal interaction comparable to that observed in root-dipping applications [85]. Although a direct comparison with existing microbial seed coatings was not performed in this study, our results suggest that the integration of biochar with microbial inoculants—particularly T. harzianum—and agro-industrial by-products offers several potential advantages. Biochar not only acts as a carrier that enhances microbial stability during storage, adhesion to seeds, and soil quality, but also plays a key role in mitigating the phytotoxic effects of raw materials such as olive pomace and buffalo digestate. This neutralization allows these by-products to be repurposed as nutrient sources within the coating, contributing to plant growth while reducing environmental impact. Compared to conventional microbial coatings, this approach introduces a multifunctional system that combines physical, biological, and nutritional benefits, potentially enhancing seedling establishment and field performance. Further comparative studies with existing commercial products are recommended to validate these advantages under diverse conditions.
5. Conclusions
This study demonstrates that seed coatings with biochar and T. harzianum significantly enhance germination, root elongation, and plant biomass. In contrast, treatments with olive pomace or digestate alone exhibited phytotoxic effects, while the addition of biochar mitigated these, likely through adsorption of inhibitory compounds and improved moisture retention. T. harzianum further promoted seedling vigour, possibly by enhancing nutrient uptake and offering pathogen protection. Microbial consortia from forest litter had milder effects, suggesting a contribution to long-term soil health rather than immediate growth stimulation. Field trials confirmed the effectiveness of biochar + T. harzianum + digestate combinations, particularly in L. sativa and S. lycopersicum, while Z. mays showed greater tolerance to raw byproducts, though still benefiting from biochar. These findings support the use of biochar-based seed coatings with beneficial microbes as sustainable alternatives to synthetic treatments. Future research should focus on optimizing biochar-based coatings to enhance plant resilience across different crop systems and environmental conditions, as well as on elucidating the underlying mechanisms responsible for their beneficial effects.
Author Contributions
Conceptualization, G.B. and G.A.; methodology, G.A. and G.B.; software, G.A. and A.C.; validation, M.I., R.M. and G.B.; formal analysis, G.A. and A.C.; investigation, G.A. and A.G.; resources, G.A.; data curation, G.A and A.G.; writing—original draft preparation, G.A.; writing—review and editing, G.A., R.M., M.I., A.C. and G.B.; visualization, G.B.; supervision, R.M., M.I. and G.B.; project administration, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lichtfouse, E.; Navarrete, M.; Debaeke, P.; Souchère, V.; Alberola, C.; Ménassieu, J. Agronomy for sustainable agriculture: A review. Sustain. Agric. 2009, 1, 1–7. [Google Scholar]
- Pacini, C.; Wossink, A.; Giesen, G.; Vazzana, C.; Huirne, R. Evaluation of sustainability of organic, integrated and conventional farming systems: A farm and field-scale analysis. Agric. Ecosyst. Environ. 2003, 95, 273–288. [Google Scholar] [CrossRef]
- Bonanomi, G.; D’Ascoli, R.; Antignani, V.; Capodilupo, M.; Cozzolino, L.; Marzaioli, R.; Zoina, A. Assessing soil quality under intensive cultivation and tree orchards in Southern Italy. Appl. Soil Ecol. 2011, 47, 184–194. [Google Scholar] [CrossRef]
- Brunelle, T.; Chakir, R.; Carpentier, A.; Dorin, B.; Goll, D.; Guilpart, N.; Tang, F.H. Reducing chemical inputs in agriculture requires a system change. Commun. Earth Environ. 2024, 5, 369. [Google Scholar] [CrossRef]
- Javed, T.; Afzal, I.; Shabbir, R.; Ikram, K.; Zaheer, M.S.; Faheem, M.; Iqbal, J. Seed coating technology: An innovative and sustainable approach for improving seed quality and crop performance. J. Saudi Soc. Agric. Sci. 2022, 21, 536–545. [Google Scholar] [CrossRef]
- Miyamoto, H.; Shigeta, K.; Suda, W.; Ichihashi, Y.; Nihei, N.; Matsuura, M.; Hirai, Y. Agricultural quality matrix-based multiomics structural analysis of carrots in soils fertilized with thermophile-fermented compost. arXiv 2022, arXiv:2202.03132. [Google Scholar]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications—Sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Iacomino, G.; Sarker, T.C.; Ippolito, F.; Bonanomi, G.; Vinale, F.; Staropoli, A.; Idbella, M. Biochar and compost application either alone or in combination affects vegetable yield in a volcanic Mediterranean soil. Agronomy 2022, 12, 1996. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Bonanomi, G.; Alioto, D.; Minutolo, M.; Marra, R.; Cesarano, G.; Vinale, F. Organic amendments modulate soil microbiota and reduce virus disease incidence in the TSWV-tomato pathosystem. Pathogens 2020, 9, 379. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.L.; Abd-ElGawad, A.M. Mixtures of organic amendments and biochar promote beneficial soil microbiota and affect Fusarium oxysporum f. sp. lactucae, Rhizoctonia solani and Sclerotinia minor disease suppression. Plant Pathol. 2022, 71, 818–829. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K.; Page-Dumroese, D.S.; Hardegree, S.P. Can biochar be used as a seed coating to improve native plant germination and growth in arid conditions? J. Arid. Environ. 2016, 125, 8–15. [Google Scholar] [CrossRef]
- Debode, J.; Viaene, J.; Maenhout, K.; Joos, L.; França, S.C.; Cuypers, A.; Vandecasteele, B. Wood-based biochars produced at low pyrolysis temperatures are good carriers for a Trichoderma-based biopesticide. Biochar 2024, 6, 1–8. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Bolan, N. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; El-Bassi, L.; Charabi, Y.; Usman, M.; Khiari, B.; Al-Wardy, M.; Jeguirim, M. Recent advancements on biochars enrichment with ammonium and nitrates from wastewaters: A critical review on benefits for environment and agriculture. J. Environ. Manag. 2022, 305, 114368. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Sudoma, M.; Kintl, A.; Vopravil, J.; Ryant, P.; Brtnicky, M. Biochar and sulphur enriched digestate: Utilization of agriculture-associated waste products for improved soil carbon and nitrogen content, microbial activity, and plant growth. Agronomy 2021, 11, 2041. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Liaquat, F.; Rehman, R.A.; Gul, M.; ul Hye, M.Z.; Rizwan, M.; Rehaman, M.Z.U. Effects of co-composting of farm manure and biochar on plant growth and carbon mineralization in an alkaline soil. Environ. Sci. Pollut. Res. 2017, 24, 26060–26068. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods: A review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Scotto Di Petra, E.; Cervelli, E.; Caro, S.; Faugno, S.; Pindozzi, S. Monitoring of ammonia emissions from stored buffalo digestate covered with biochar. In Proceedings of the 28th European Biomass Conference and Exhibition, Virtual, 6–9 July 2020. ETA-Florence Renewable Energies. [Google Scholar]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnürer, A. Comparative characterization of digestate versus pig slurry and cow manure: Chemical composition and effects on soil microbial activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Medouni-Haroune, L.A.M.; Zaidi, F.; Medouni-Adrar, S.O.N.; Kecha, M. Olive pomace: From an olive mill waste to a resource, an overview of the new treatments. J. Crit. Rev. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Alaoui, I.; El Ghadraoui, O.; Tanji, K.; Harrach, A.; Farah, A. The olive mill pomace: A sustainable biofertilizer to improve soil proprieties and plant nutrient uptake. Waste Biomass Valorization 2024, 15, 2575–2590. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Belaqziz, M.; Wichern, M.; Lübken, M. Phytotoxicity assessment of olive mill wastewater treated by different technologies: Effect on seed germination of maize and tomato. Environ. Sci. Pollut. Res. 2020, 27, 8034–8045. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A new nutrient source—Review. Biogas 2012, 14, 295–312. [Google Scholar]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, G.; Cozzolino, A.; Idbella, M.; Iacomino, G.; Motti, R.; Bonanomi, G. The decomposition dynamics and substrate component potential of biomass from the seagrass Posidonia oceanica (L.) Delile. Horticulturae 2024, 10, 58. [Google Scholar] [CrossRef]
- Alori, E.T.; Babalola, O.O. Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Idbella, M.; Iacomino, G.; Abd-ElGawad, A.M.; Bonanomi, G. Soil microbial co-occurrence networks across climate and land use gradient in Southern Italy. Environ. Microbiol. Rep. 2025, 17, e70093. [Google Scholar] [CrossRef] [PubMed]
- Hoitink, H.A.J.; Madden, L.V.; Dorrance, A.E. Systemic resistance induced by Trichoderma spp.: Interactions between the host, the pathogen, the biocontrol agent, and soil organic matter quality. Phytopathology 2006, 96, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Turkan, S.; Mierek-Adamska, A.; Kulasek, M.; Konieczna, W.B.; Dąbrowska, G.B. New seed coating containing Trichoderma viride with anti-pathogenic properties. PeerJ 2023, 11, e15392. [Google Scholar] [CrossRef]
- Juntahum, S.; Klinsukon, C.; Senawong, K.; Katekaew, S.; Sokudlor, N.; Laloon, K. Assessing the Potential for Producing Compost Pellets with Binders from Cassava Industry By-products and Supplemented with Trichoderma. In Case Studies in Chemical and Environmental Engineering; Elsevier: Amsterdam, The Netherlands, 2025; p. 101221. [Google Scholar] [CrossRef]
- Munnysha, S.; Bunker, R.N.; Abhi, R.; Shree, D.; Mondal, K.; Akodiya, S.; Beniwal, M. Trichoderma: A Multifaceted Ally in Plant Growth Promotion and Disease Resistance. Plant Arch. 2025, 25, 1472–1478. [Google Scholar]
- Rodríguez-Martínez, E.S.; Torres-Torres, E.; Guigón-López, C.; Alvarado-González, M. Trichoderma Roles in Sustainable Agriculture. In Sustainable Engineering and Agro-Food Processing; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 147–187. [Google Scholar]
- Ali, A.; Elrys, A.S.; Liu, L.; Xia, Q.; Wang, B.; Li, Y.; Cai, Z. Deciphering the synergies of reductive soil disinfestation combined with biochar and antagonistic microbial inoculation in cucumber fusarium wilt suppression through rhizosphere microbiota structure. Microb. Ecol. 2023, 85, 980–997. [Google Scholar] [CrossRef]
- de Medeiros, E.V.; da Costa, D.P.; Silva, E.L.D.; de França, A.F.; de Sousa Lima, J.R.; Hammecker, C.; Araujo, A.S.F. Biochar and Trichoderma as an eco-friendly and low-cost alternative to improve soil chemical and biological properties. Waste Biomass Valorization 2024, 15, 1439–1450. [Google Scholar] [CrossRef]
- Jatuwong, K.; Aiduang, W.; Kiatsiriroat, T.; Kamopas, W.; Lumyong, S. A Review of Biochar from Biomass and Its Interaction with Microbes: Enhancing Soil Quality and Crop Yield in Brassica Cultivation. Life 2025, 15, 284. [Google Scholar] [CrossRef]
- Idbella, M.; Bonanomi, G. Uncovering the dark side of agriculture: How land use intensity shapes soil microbiome and increases potential plant pathogens. Appl. Soil Ecol. 2023, 192, 105090. [Google Scholar] [CrossRef]
- Couto, A.P.S.; Pereira, A.E.; Abati, J.; Fontanela, M.L.C.; Dias-Arieira, C.R.; Krohn, N.G. Seed treatment with Trichoderma and chemicals to improve physiological and sanitary quality of wheat cultivars. Rev. Caatinga 2021, 34, 813–823. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: Science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Pirzada, T.; Opperman, C.H.; Khan, S.A. Recent advances in seed coating technologies: Transitioning toward sustainable agriculture. Green Chem. 2022, 24, 6052–6085. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern seed technology: Seed coating delivery systems for enhancing seed and crop performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Jarecki, W.; Wietecha, J. Effect of seed coating on the yield of soybean Glycine max (L.) Merr. Plant Soil Environ. 2021, 67, 468–473. [Google Scholar] [CrossRef]
- Vitti, A.; Elshafie, H.S.; Logozzo, G.; Marzario, S.; Scopa, A.; Camele, I.; Nuzzaci, M. Physico-chemical characterization and biological activities of a digestate and a more stabilized digestate-derived compost from agro-waste. Plants 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Vinale, F.; Cesarano, G.; Lombardi, N.; d’Errico, G.; Crasto, A.; Bonanomi, G. Biochars from olive mill waste have contrasting effects on plants, fungi and phytoparasitic nematodes. PLoS ONE 2018, 13, e0198728. [Google Scholar] [CrossRef]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef]
- Mekki, A.; Arous, F.; Aloui, F.; Sayadi, S. Disposal of agro-industrial wastes as soil amendments. Am. J. Environ. Sci. 2013, 9, 458. [Google Scholar] [CrossRef][Green Version]
- Bonanomi, G.; Giorgi, V.; Neri, D.; Scala, F. Olive mill residues affect saprophytic growth and disease incidence of foliar and soilborne plant fungal pathogens. Agric. Ecosyst. Environ. 2006, 115, 194–200. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Phytotoxicity of veterinary antibiotics to seed germination and root elongation of crops. Ecotoxicol. Environ. Saf. 2016, 126, 228–237. [Google Scholar] [CrossRef]
- Abou Jaoude, L.; Nassif, N.; Garau, G.; Darwish, T.; Castaldi, P. Biochar addition decreases the mobility, bioavailability, and phytotoxicity of potentially toxic elements in an agricultural contaminated soil. Commun. Soil Sci. Plant Anal. 2022, 53, 1655–1671. [Google Scholar] [CrossRef]
- Bu, X.; Xue, J.; Wu, Y.; Ma, W. Effect of biochar on seed germination and seedling growth of Robinia pseudoacacia L. in karst calcareous soils. Commun. Soil Sci. Plant Anal. 2020, 51, 352–363. [Google Scholar] [CrossRef]
- Zhang, K.; Khan, Z.; Yu, Q.; Qu, Z.; Liu, J.; Luo, T.; Luo, L. Biochar coating is a sustainable and economical approach to promote seed coating technology, seed germination, plant performance, and soil health. Plants 2022, 11, 2864. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Abd-ElGawad, A.M.; Iacomino, G.; Nappi, A.; Grauso, L.; Idbella, M. Plant-growth promotion by biochar–organic amendments mixtures explained by selective chemicals adsorption of inhibitory compounds. J. Environ. Chem. Eng. 2023, 11, 109009. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Phillips, C.L.; Meyer, K.M.; Garcia-Jaramillo, M.; Weidman, C.S.; Stewart, C.E.; Wanzek, T.; Grusak, M.A.; Watts, D.W.; Novak, J. Towards predicting biochar impacts on plant-available soil nitrogen content. Biochar 2022, 4, 9. [Google Scholar] [CrossRef]
- Głodowska, M.; Husk, B.; Schwinghamer, T.; Smith, D. Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agron. Sustain. Dev. 2016, 36, 1–10. [Google Scholar] [CrossRef]
- Khan, Z.; Zhang, K.; Khan, M.N.; Bi, J.; Zhu, K.; Luo, L.; Hu, L. How biochar affects nitrogen assimilation and dynamics by interacting soil and plant enzymatic activities: Quantitative assessment of 2 years potted study in a rapeseed-soil system. Front. Plant Sci. 2022, 13, 853449. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Fu, Y.; Zhou, Y.; Khan, Z.; Bi, J.; Luo, L. Biochar coating as a cost-effective delivery approach to promoting seed quality, rice germination, and seedling establishment. Plants 2023, 12, 3896. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Z.; Zhang, Y.; Wang, X.; Shao, D.; Wang, C.; Zhou, G. Biochar-based pelletized seed enhances the yield of late-sown rapeseed by improving the relative growth rate and cold resistance of seedlings. Ind. Crops Prod. 2025, 223, 119993. [Google Scholar] [CrossRef]
- Chaudhary, T.; Dixit, M.; Gera, R.; Shukla, A.K.; Prakash, A.; Gupta, G.; Shukla, P. Techniques for improving formulations of bioinoculants. Biotech 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.J.; Mead, A.; Whipps, J.M. Performance of carrot and onion seed primed with beneficial microorganisms in glasshouse and field trials. Biol. Control. 2009, 51, 417–426. [Google Scholar] [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed treatments with microorganisms can have a biostimulant effect by influencing germination and seedling growth of crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef]
- Naz, R.; Asif, T.; Mubeen, S.; Khushhal, S. Seed application with microbial inoculants for enhanced plant growth. In Sustainable Horticulture; Academic Press: London, UK, 2022; pp. 333–368. [Google Scholar]
- Kumar, S.; Arutselvan, R.; Greeshma, K.; Bodhankar, S.; Akash, A.U.; Prasad, V.S.S.K.; Keswani, C. Unraveling the seed bio-priming contours for managing plant health. J. Plant Growth Regul. 2024, in press. [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Iacomino, G.; Idbella, M.; Laudonia, S.; Vinale, F.; Bonanomi, G. The suppressive effects of biochar on above—And belowground plant pathogens and pests: A review. Plants 2022, 11, 3144. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Routledge: London, UK, 2012; pp. 117–138. [Google Scholar]
- Malik, M.A.; Ahmad, N.; Bhat, M.Y. The green shield: Trichoderma’s role in sustainable agriculture against soil-borne fungal threats. Curr. Res. Microb. Sci. 2024, 7, 100313. [Google Scholar] [CrossRef]
- Gutiérrez-Chávez, A.; Robles-Hernández, L.; Guerrero, B.I.; González-Franco, A.C.; Medina-Pérez, G.; Acevedo-Barrera, A.A.; Hernández-Huerta, J. Potential of Trichoderma asperellum as a growth promoter in hydroponic lettuce cultivated in a floating-root system. Plants 2025, 14, 382. [Google Scholar] [CrossRef]
- Ahmad, C.A.; Akhter, A.; Haider, M.S.; Abbas, M.T.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F. Demonstration of the synergistic effect of biochar and Trichoderma harzianum on the development of Ralstonia solanacearum in eggplant. Front. Microbiol. 2024, 15, 1360703. [Google Scholar] [CrossRef]
- Almasrahi, A.; Alamin, M.Y.; Molan, Y.Y.; Alhashel, A.F.; Widyawan, A.; Ibrahim, Y.E.; El-Komy, M.H. Synergistic effects of Trichoderma asperellum mixture strains and biochar-amended soil on Fusarium wilt of strawberry. Plant Pathol. 2025, 1–16. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Hasan, M.; Uddain, J.; Subramaniam, S. Impact of application of Trichoderma and biochar on growth, productivity and nutritional quality of tomato under reduced NPK fertilization. Ann. Agric. Sci. 2020, 65, 107–115. [Google Scholar] [CrossRef]
- Mei, L.I.; Hua, L.I.A.N.; Su, X.L.; Ying, T.I.A.N.; Huang, W.K.; Jie, M.E.I.; Jiang, X.L. The effects of Trichoderma on preventing cucumber fusarium wilt and regulating cucumber physiology. J. Integr. Agric. 2019, 18, 607–617. [Google Scholar] [CrossRef]
- Da Silva, J.S.A.; de Medeiros, E.V.; Da Costa, D.P.; de Souza, C.A.F.; De Oliveira, J.B.; da França, R.F.; Hammecker, C. Biochar and Trichoderma aureoviride URM 5158 as alternatives for the management of cassava root rot. Appl. Soil Ecol. 2022, 172, 104353. [Google Scholar] [CrossRef]
- Vecstaudza, D.; Grantina-Ievina, L.; Makarenkova, G.; Kasparinskis, R.; Selga, T.; Steinberga, V.; Muter, O. The impact of wood-derived biochar on the survival of Trichoderma spp. and growth of Secale cereale L. in sandy soil. Biocontrol. Sci. Technol. 2018, 28, 341–358. [Google Scholar] [CrossRef]
- Iacomino, G.; Bonanomi, G.; Motti, R.; Idbella, M. Trick of the trade: Unveiling the importance of feedstock chemistry in Trichoderma-organic amendments-based bio-stimulants. Horticulturae 2023, 9, 957. [Google Scholar] [CrossRef]
- Osorio-Guerrero, K.V.; Patiño-Moscoso, M.A.; Flórez-Gómez, D.L.; Cortés-Rojas, D.F. Trichoderma koningiopsis applied as seed coating protects sweet sorghum (Sorghum bicolor (L.) Moench) from fungal contaminants during storage. Eur. J. Plant Pathol. 2024, 169, 581–591. [Google Scholar] [CrossRef]
- Abdukerim, R.; Li, L.; Li, J.H.; Xiang, S.; Shi, Y.X.; Xie, X.W.; Li, B.J. Coating seeds with biocontrol bacteria-loaded sodium alginate/pectin hydrogel enhances the survival of bacteria and control efficacy against soil-borne vegetable diseases. Int. J. Biol. Macromol. 2024, 279, 135317. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control 2022, 176, 105100. [Google Scholar] [CrossRef]
- Herrera-Estrella, A.; Chet, I. The biological control agent Trichoderma: From fundamentals to applications. Mycol. Ser. 2004, 21, 147–156. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).