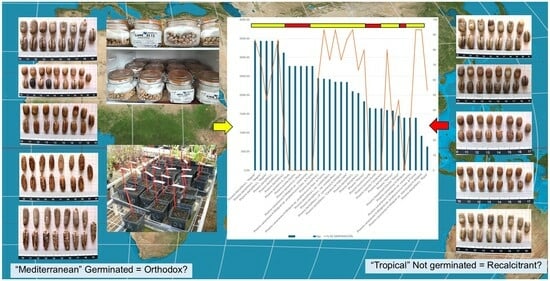
Orthodox vs. Recalcitrant? Germination and Early Growth of Phoenix Species (Arecaceae) Stored for up to Ten Years
Abstract
1. Introduction
- Orthodox seeds predominate in temperate regions, in arid environments, and among herbaceous plants.
- Within some families, such as Dipterocarpaceae, recalcitrance is the norm.
- Other families, like Fabaceae, exhibit a mix of both behaviors.
- Primitive seed plants often display recalcitrant characteristics.
- Some plant families show strong phylogenetic conservation of seed storage behavior.
- Cellular and biochemical adaptations:
- Orthodox seeds accumulate protective proteins (LEA proteins), sugars (especially oligosaccharides), and antioxidants during maturation and undergo cytoplasmic vitrification during drying.
- Recalcitrant seeds lack sufficient protective mechanisms against desiccation damage and maintain high metabolic activity [9].
- Water relations:
- Developmental pathways:
- Orthodox seeds typically undergo a programmed drying phase during maturation.
- Recalcitrant seeds are shed at high moisture content and remain metabolically active [16].
- Orthodox seeds are adapted to unpredictable or seasonal environments where dormancy increases survival probability and are often smaller with higher production numbers [17].
- Recalcitrant seeds are adapted to stable, humid environments where immediate germination is advantageous and are often larger with greater nutrient reserves.
- Dispersal mechanisms often align with storage behavior, with wind-dispersed seeds typically being orthodox.
- Pioneer species generally produce orthodox seeds, while climax forest species often produce recalcitrant seeds.
- Well suited for conventional seed banking (dried and stored at −18 °C).
- Predictable longevity under proper storage conditions.
- Cost-effective conservation of genetic diversity.
- Large sample sizes can be stored in limited space.
- Conventional seed banking is ineffective.
- Alternative approaches are required, such as cryopreservation of embryonic axes or embryos, in vitro culture of embryos or embryonic axes, field gene banks (living collections), pollen storage and artificial pollination, and synthetic seed technology.
- Identifying the molecular mechanisms underlying desiccation tolerance.
- Understanding the genetic basis of seed storage behavior to potentially engineer greater storage tolerance.
- Exploring the continuum of seed storage behaviors beyond the simple orthodox–recalcitrant dichotomy [10].
2. Materials and Methods
2.1. Plant Material
2.2. Planting Seeds
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Phoenix Seed Age and Germination
3.1.1. Seed Age and Germination Percentage: Pearson Correlation Coefficient
3.1.2. Seed Age and Germination Percentage: Spearman Correlation Coefficient
3.1.3. Seed Age and Germination Percentage: t-Test
3.2. Phoenix Seed Age, Species, and Germination
3.2.1. Overall Species Comparison
3.2.2. Bayesian Approach to Correlation Analysis
3.2.3. Relationship Between the Year of Collection and the Germination Percentage of the Species
3.3. Phoenix Seedling Development
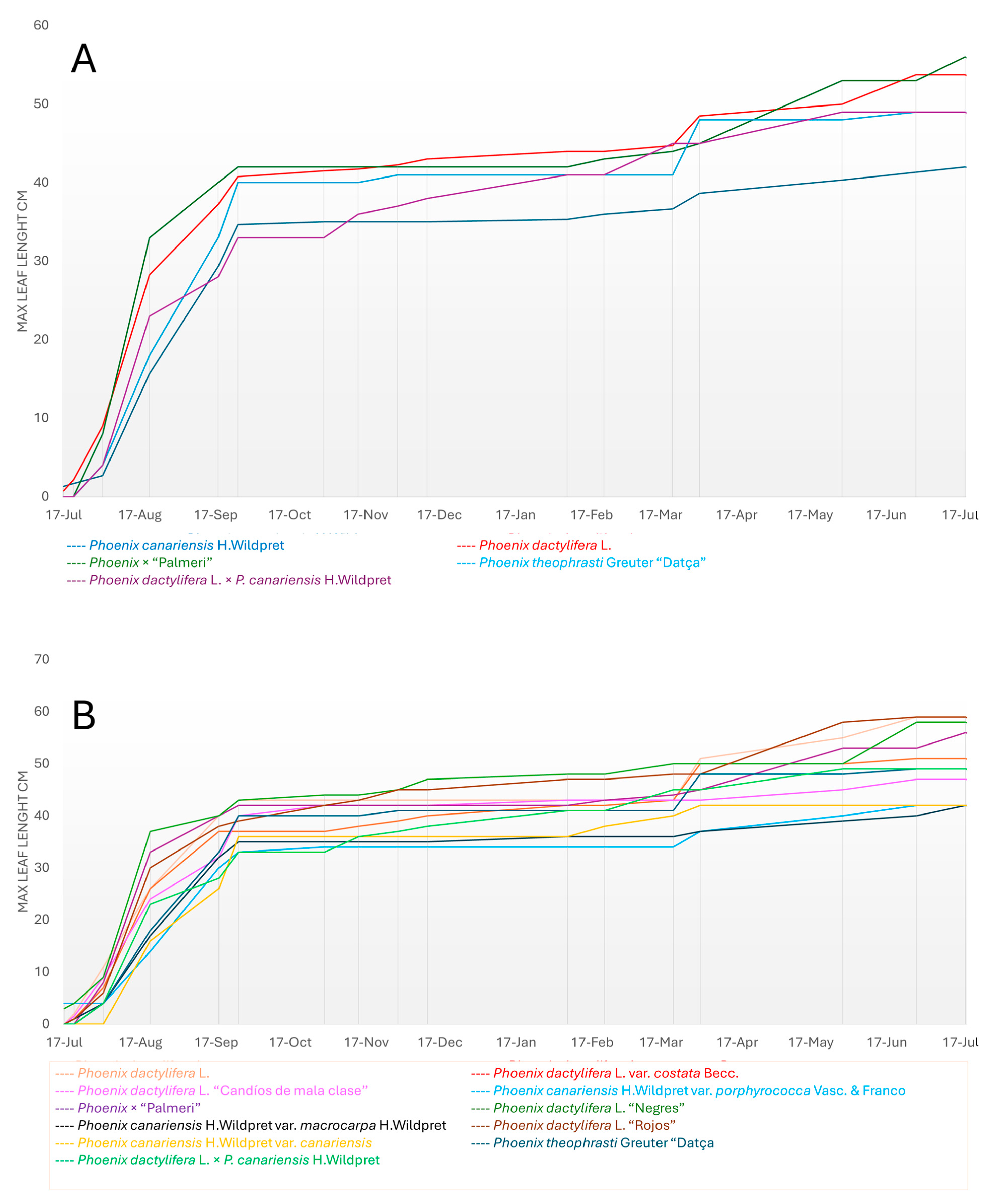
3.3.1. Leaf Number
3.3.2. Leaf Length
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beccari, O. Rivista monografica delle specie del genere Phoenix L. Malesia 1890, 3, 345–416. [Google Scholar]
- Barrow, S. A Monograph of Phoenix L. (Palmae: Coryphoidae). Kew Bull. 1998, 53, 513–575. [Google Scholar] [CrossRef]
- Carreño, E. Diversidad Genética en Especies del Género Phoenix L. Ph.D. Thesis, Universidad Miguel Hernández de Elche, Orihuela, Spain, 2017. [Google Scholar]
- Johnson, D.V.; Al-Khayri, J.M.; Jain, S.M. Seedling date palms (Phoenix dactylifera L.) as genetic resources. Emir. J. Food Agric. 2013, 25, 809–830. [Google Scholar] [CrossRef]
- Hong, T.D.; Ellis, R.H. A Protocol to Determine Seed Storage Behaviour; IPGRI Technical Bulletin No. 1; International Plant Genetic Resources Institute: Rome, Italy, 1996; Available online: https://cropgenebank.sgrp.cgiar.org/images/file/learning_space/technicalbulletin1.pdf (accessed on 1 May 2025).
- Pammenter, N.W.; Berjak, P. Physiology of desiccation-sensitive (recalcitrant) seeds and the implications for cryopreservation. Int. J. Plant Sci. 2014, 175, 21–28. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. Implications of the lack of desiccation tolerance in recalcitrant seeds. Front. Plant Sci. 2013, 4, 478. [Google Scholar] [CrossRef]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Barbedo, C.J.; Centeno, D.C.; Ribeiro, R.C.F. Do recalcitrant seeds really exist? Hoehnea 2013, 40, 583–593. [Google Scholar] [CrossRef]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Matilla, A.J. The Orthodox Dry Seeds Are Alive: A Clear Example of Desiccation Tolerance. Plants 2022, 11, 20. [Google Scholar] [CrossRef]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2014; pp. 18–64. [Google Scholar]
- Tweddle, J.C.; Dickie, J.B.; Baskin, C.C.; Baskin, J.M. Ecological aspects of seed desiccation sensitivity. J. Ecol. 2003, 91, 294–304. [Google Scholar] [CrossRef]
- Wyse, S.V.; Dickie, J.B. Predicting the global incidence of seed desiccation sensitivity. J. Ecol. 2017, 105, 1082–1093. [Google Scholar] [CrossRef]
- Obroucheva, N.V.; Lityagina, S.V.; Novikova, G.V.; Sin’kevich, I.A. Vacuolar status and water relations in embryonic axes of recalcitrant Aesculus hippocastanum seeds during stratification and early germination. AoB Plants 2012, 2012, pls008. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, E. The ecology and physiology of viviparous and recalcitrant seeds. Annu. Rev. Ecol. Syst. 2000, 31, 107–138. [Google Scholar] [CrossRef]
- Hamilton, K.N.; Offord, C.A.; Cuneo, P.; Deseo, M.A. A comparative study of seed morphology in relation to desiccation tolerance and other physiological responses in 71 Eastern Australian rainforest species. Plant Species Biol. 2013, 28, 51–62. [Google Scholar] [CrossRef]
- Daws, M.I.; Garwood, N.C.; Pritchard, H.W. Prediction of desiccation sensitivity in seeds of woody species: A probabilistic model based on two seed traits and 104 species. Ann. Bot. 2006, 97, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1, cot030. [Google Scholar] [CrossRef]
- Sershen; Varghese, B.; Pammenter, N.W.; Berjak, P. Cryo-tolerance of zygotic embryos from recalcitrant seeds in relation to oxidative stress—A case study on two amaryllid species. J. Plant Physiol. 2012, 169, 999–1011. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W. The cryobiotechnology of oaks: An integration of approaches for the long-term ex situ conservation of Quercus species. Forests 2020, 11, 1281. [Google Scholar] [CrossRef]
- Hong, T.D.; Linington, S.; Ellis, R.H. Seed Storage Behaviour: A Compendium; Handbooks for Genebanks No. 4; International Plant Genetic Resources Institute: Rome, Italy, 1996; Available online: https://cgspace.cgiar.org/server/api/core/bitstreams/7e86d173-da38-406e-bd8f-7c971ce9d4b9/content (accessed on 1 May 2025).
- Nagel, M.; Rehman Arif, M.; Rosenhauer, M.; Börner, A. Longevity of seeds—Intraspecific differences in the Gatersleben genebank collections. In 60. Tagung der Vereinigung der Pfl anzenzüchter und Saatgutkaufleute Österreichs Lehr- und Forschungszentrum für Landwirtschaft, Raumberg-Gumpenstein 2009; Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs: St. Pölten, Austria, 2010; pp. 179–182. [Google Scholar]
- Solberg, S.; Yndgaard, F.; Andreasen, C.; von Bot, R.; Loskutov, I.G.; Asdal, Å. Long-Term Storage and Longevity of Orthodox Seeds: A Systematic Review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Nadarajan, J.; Walters, C.; Pritchard, H.W.; Ballesteros, D.; Colville, L. Seed Longevity—The Evolution of Knowledge and a Conceptual Framework. Plants 2023, 12, 471. [Google Scholar] [CrossRef]
- Yashina, S.; Gubin, S.; Maksimovich, S.; Yashina, A.; Gakhova, E.; Gilichinsky, D. Regeneration of whole fertile plants from 30,000-y-old fruit tissue buried in Siberian permafrost. Proc. Natl. Acad. Sci. USA 2012, 109, 4008–4013. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Zhang, X.; Zafar, M.M.; Ma, W.; Zhao, J. Roles of reactive oxygen species and mitochondria in seed germination. Front. Plant Sci. 2021, 12, 781734. [Google Scholar] [CrossRef]
- Crowe, J.H. Anhydrobiosis: An unsolved problem. Am. Nat. 1971, 105, 563–573. [Google Scholar] [CrossRef]
- Lloyd, F.E. Development and Nutrition of the Embryo, Seed and Carpel in the Date, Phoenix dactylifera L. Mo. Bot. Gard. Annu. Rep. 1910, 1910, 103–164. [Google Scholar] [CrossRef]
- von Fintel, G.T.; Berjak, P.; Pammenter, N.W. Seed behaviour in Phoenix reclinata Jacquin, the wild date palm. Seed Sci. Res. 2004, 14, 197–204. [Google Scholar] [CrossRef]
- Muhammad, M.; Ringim, A.S.; Dangora, I.I. Effects of different methods of breaking dormancy and seed germination rate in date palm (Phoenix dactylifera L.). J. Res. For. Wildl. Environ. 2017, 9, 28–35. [Google Scholar]
- Mohammed, N.M.I.; Said, A.G.E. Date palm (Phoenix dactylifera) seeds germination. Cell Biol. Dev. 2018, 2, 63–68. [Google Scholar] [CrossRef]
- Sallon, S.; Solowey, E.; Cohen, Y.; Korchinsky, R.; Egli, M.; Woodhatch, I.; Simchoni, O.; Kislev, M. Germination, genetics, and growth of an ancient date seed. Science 2008, 320, 1464. [Google Scholar] [CrossRef]
- Sallon, S.; Cherif, E.; Chabrillange, N.; Solowey, E.; Gros-Balthazard, M.; Ivorra, S.; Terral, J.F.; Egli, M.; Aberlenc, F. Origins and insights into the historic Judean date palm based on genetic analysis of germinated ancient seeds and morphometric studies. Sci. Adv. 2020, 6, eaax0384. [Google Scholar] [CrossRef]
- Obón, C.; Rivera, D.; Amorós, A.; Alcaraz, F.; Díaz, G.; Carreño, E.; Martínez-Rico, M.; Larrosa, E.; Laguna, E. El Banco de Germoplasma Español de Palmera Datilera y Especies Próximas. In Huellas inéditas del VI Congreso Internacional de Etnobotánica/Recovered Tracks from the VIth International Congress of Ethnobotany, ICEB 2014; Herrera, F., Hurrel, J., Tarifa, F., Hernández-Bermejo, J., Eds.; UCO Press: Córdoba, Spain, 2017; pp. 235–254. [Google Scholar]
- AEMET. Agencia Estatal de Meteorología. 2025. Available online: https://www.aemet.es/es/portada (accessed on 20 July 2018).
- Python. Python [software]. Version 3.11.5. Python Software Foundation. Available online: https://www.python.org/ (accessed on 8 March 2025).
- Anthropic. Claude Analysis [Internet]. Anthropic—Cited 10 March 2025. Available online: https://claude.ai (accessed on 8 March 2025).
- Baskin, J.M.; Baskin, C.C. What Kind of Seed Dormancy Might Palms Have? Seed Sci. Res. 2013, 24, 17–22. [Google Scholar] [CrossRef]
- Biradar, N.; Mahabale, T. Studies on palms: Fruits, seeds and seed germination in the genus Phoenix L. Proc. Indian Acad. Sci. Sect. B 1969, 70, 55–65. [Google Scholar] [CrossRef]
- Iossi, E.; Moro, F.V.; Sader, R. Seed anatomy and germination of Phoenix roebelenii O’Brien (Arecaceae). Rev. Bras. De Sementes 2006, 28, 121–128. [Google Scholar] [CrossRef]
- Corbineau, F. The effects of storage conditions on seed deterioration and ageing: How to improve seed longevity. Seeds 2024, 3, 56–75. [Google Scholar] [CrossRef]
- Duncan, C.; Schultz, N.; Lewandrowski, W.; Good, M.K.; Cook, S. Lower dormancy with rapid germination is an important strategy for seeds in an arid zone with unpredictable rainfall. PLoS ONE 2019, 14, e0218421. [Google Scholar] [CrossRef]
- Khurana, E.; Singh, J.S. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: A review. Environ. Conserv. 2001, 28, 39–52. [Google Scholar] [CrossRef]
- Ellis, R.H. The longevity of seeds. HortScience 1991, 26, 1119–1125. [Google Scholar] [CrossRef]
- Pimenta, R.S.; Luz, P.B.; Pivetta, K.F.; Castro, A.D.; Pizetta, P.U. Efeito da maturação e temperatura na germinação de sementes de Phoenix canariensis hort. ex Chabaud-Arecaceae. Rev. Árvore 2010, 34, 31–38. [Google Scholar] [CrossRef]
- Azad, M.S.; Rahman, M.T.; Matin, M.A. Seed germination techniques of Phoenix dactylifera: A new experience from Bangladesh. Front. Agric. China 2011, 5, 241–246. [Google Scholar] [CrossRef]
- Porteous, G.; Nesbitt, M.; Kendon, J.P.; Prychid, C.J.; Stuppy, W.; Conejero, M.; Ballesteros, D. Assessing extreme seed longevity: The value of historic botanical collections to modern research. Front. Plant Sci. 2019, 10, 1181. [Google Scholar] [CrossRef]
- Godwin, H. Evidence for longevity of seeds. Nature 1968, 220, 708–709. [Google Scholar] [CrossRef]
- Toole, V.K. Ancient seeds; seed longevity. J. Seed Technol. 1986, 10, 1–23. [Google Scholar]
- Zazula, G.; Harington, R.; Telka, A.; Brock, F. Radiocarbon dates reveal that Lupinus arcticus plants were grown from modern not Pleistocene seed. New Phytol. 2009, 182, 788–792. [Google Scholar] [CrossRef]
- Leino, M.; Edqvis, J. Germination of 151-year old Acacia spp. seeds. Genet. Resour. Crop Evol. 2010, 57, 741–746. [Google Scholar] [CrossRef]
- Colvill, W.H. Some observations on the vegetable productions and the rural economy of the province of Baghdad. Bot. J. Linn. Soc. 1875, 14, 399–410. [Google Scholar] [CrossRef]
- Perez, M.A.G.; Cabrera-García, N.; Curbelo, L.; Sosa, P.A. Is the endemic Phoenix canariensis H. Wildpret an orthodox species? Implications for its conservation. N. Z. J. Bot. 2025, 1–9. [Google Scholar] [CrossRef]
- Beltrame, R.A.; Mendes Jasmim, J.; Duarte Vieira, H.; Acha, A.J. Desecación, almacenamiento y calidad fisiológica de las semillas de Phoenix roebelenii O’Brien (Arecaceae). Rev. Fac. Cienc. Agrarias. Univ. Nac. Cuyo 2022, 54, 25–34. [Google Scholar]
- Davies, R.; Pritchard, H. Seed Conservation of Dryland Palms of Africa and Madagascar: Needs and Prospects. For. Genet. Resour. 1998, 26, 36–44. [Google Scholar]
- Albani-Rocchetti, G.; Carta, A.; Mondoni, A.; Godefroid, S.; Davis, C.C.; Caneva, G.; Albrecht, M.A.; Alvarado, K.; Bijmoer, R.; Borosova, R.; et al. Selecting the best candidates for resurrecting extinct-in-the-wild plants from herbaria. Nat. Plants 2022, 8, 1385–1393. [Google Scholar] [CrossRef]

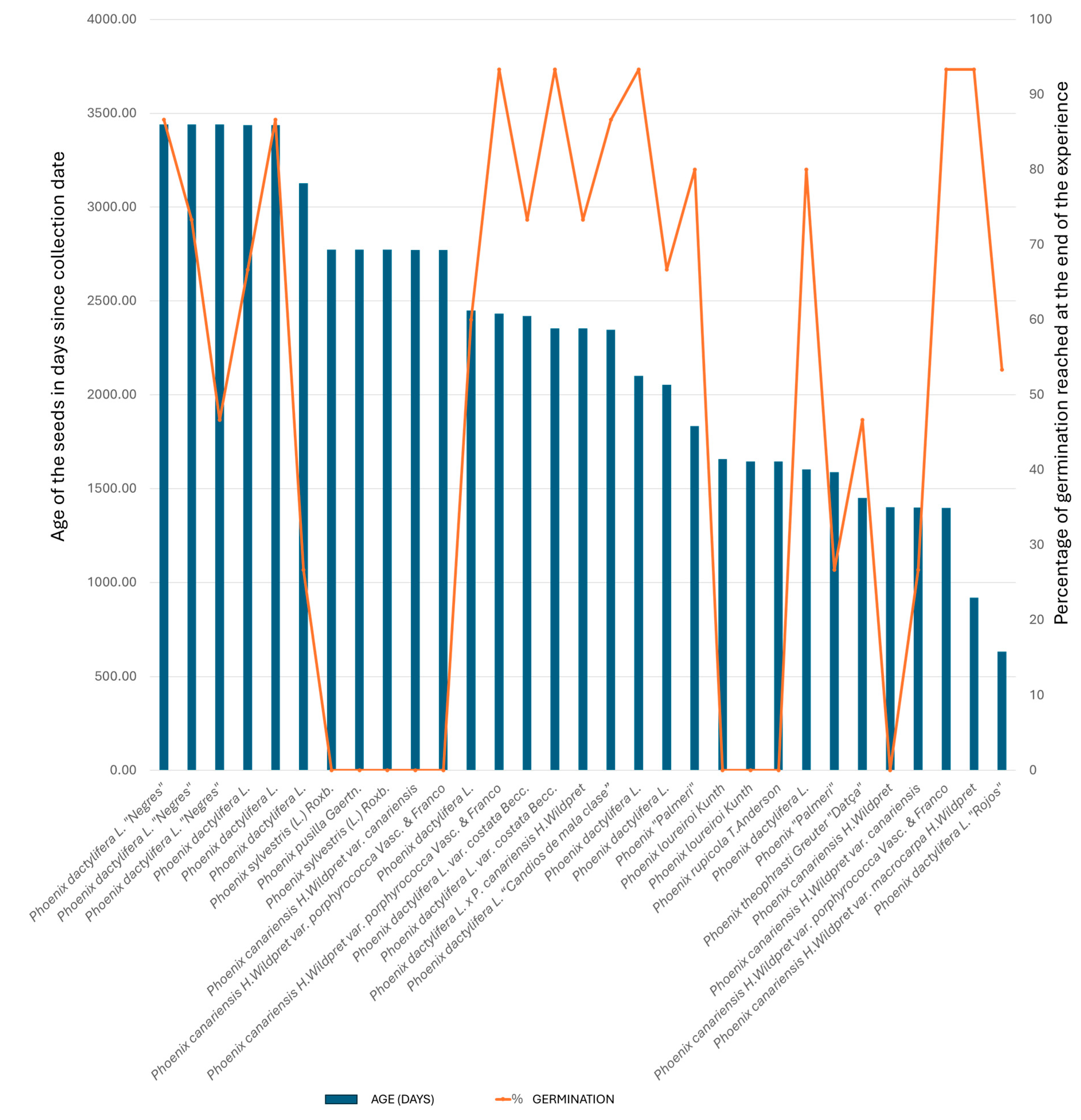

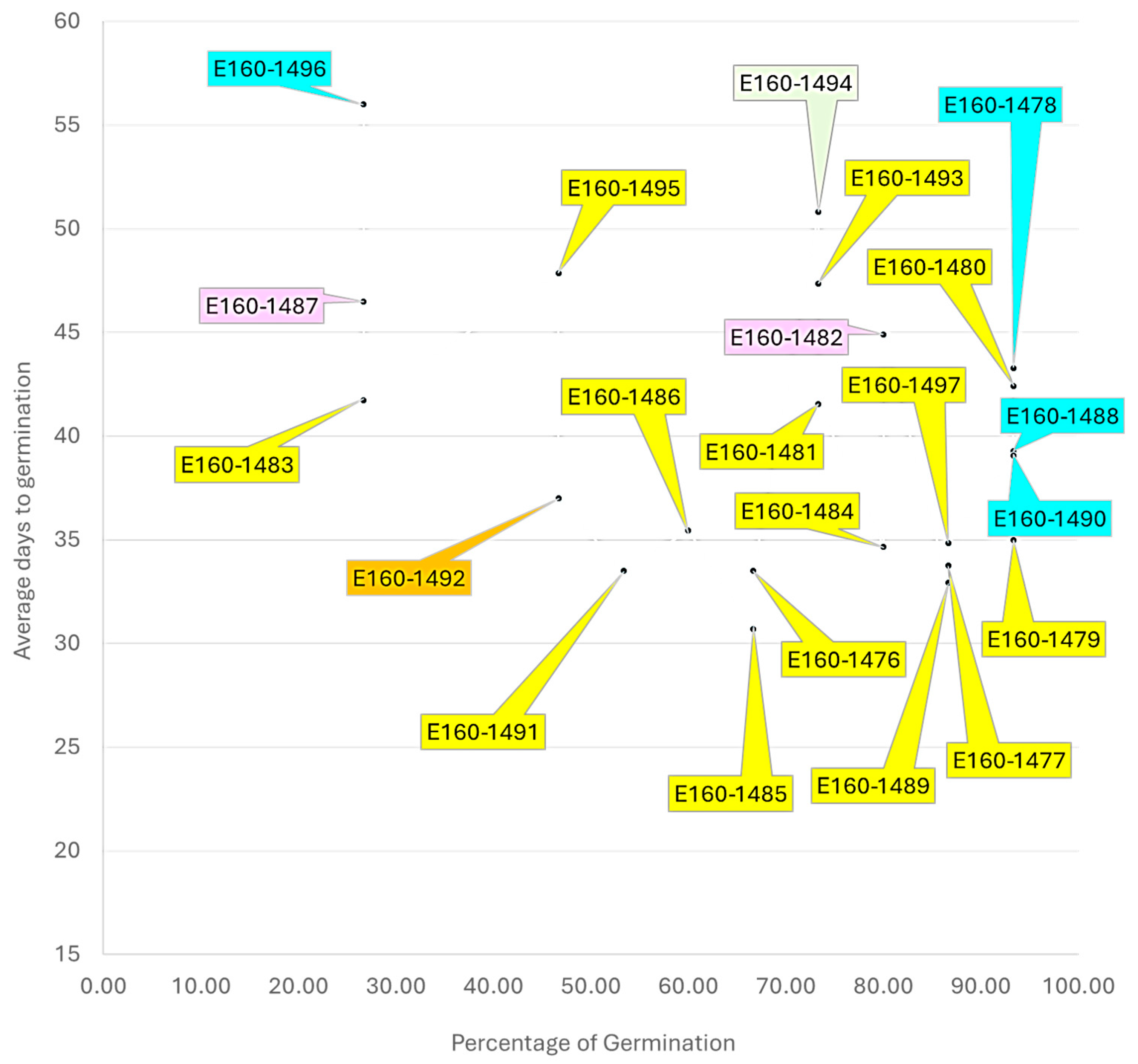

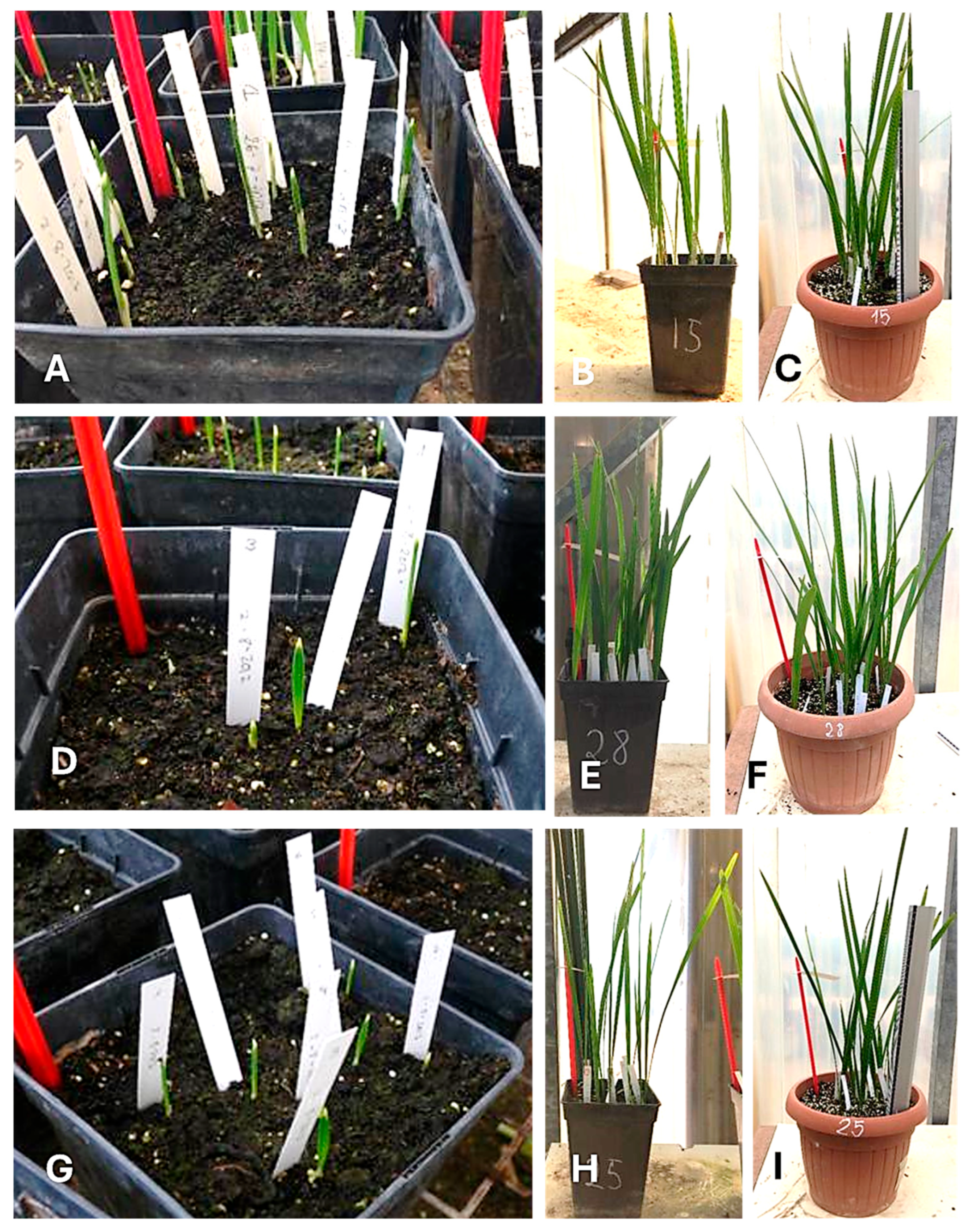
| Repository Code | Species and Variety | Accessions Data (Date and Locality of Seed Collection) | Storage (Days) ** | Previous Germination Results |
|---|---|---|---|---|
| Phoenix canariensis H. Wildpret | 1870 * | |||
| E160-1677 | P. canariensis var. porphyrococca Vasc. & Franco | 24-11-2009; Espinardo (Murcia, Spain) | 2771 | 30-11-2009, − |
| E160-1036 | P. canariensis var. canariensis | 26-08-2013; Colombres (Asturias, Spain) | 1400 | 17-09-2013, − |
| E160-1478 | P. canariensis var. porphyrococca | 28-08-2013; Cambados (Pontevedra, Spain) | 1398 | 20-06-2014, + |
| E160-1488 | P. canariensis var. porphyrococca | 29-10-2010; Valencia (Spain) | 2432 | 20-06-2011, + |
| E160-1490 | P. canariensis var. macrocarpa H. Wildpret | 20-12-2014; Granada (Spain) | 919 | 30-06-2015, + |
| E160-1496 | P. canariensis var. canariensis | 27-08-2013; Cudillero (Asturias, Spain) | 1399 | 10-07-2014, + |
| E160-1676 | P. canariensis var. canariensis | 24-11-2009; Espinardo (Murcia, Spain) | 2771 | 30-11-2009, − |
| Phoenix dactylifera L. | 2591 * | |||
| E160-1476 | P. dactylifera var. dactylifera | 28-01-2008; Fortuna (Murcia, Spain) | 3437 | none |
| E160-1477 | P. dactylifera var. dactylifera | 28-01-2008; Fortuna (Murcia, Spain) | 3437 | none |
| E160-1479 | P. dactylifera var. dactylifera | 25-09-2011; Elche (Alicante, Spain) | 2101 | 30-06-2012, + |
| E160-1480 | P. dactylifera var. costata Becc. | 16-01-2011; Santomera (Murcia, Spain) | 2353 | 30-6-2011, + |
| E160-1481 | P. dactylifera var. costata | 10-11-2010; Santomera (Murcia, Spain) | 2420 | 30-6-2011, + |
| E160-1483 | P. dactylifera var. dactylifera | 03-12-2008; Ojós (Murcia, Spain) | 3127 | 20-07-2009, + |
| E160-1484 | P. dactylifera var. dactylifera | 04-02-2013; Elche (Alicante, Spain) | 1603 | 04-02-2013, − |
| E160-1485 | P. dactylifera var. dactylifera | 11-11-2011; Orihuela (Alicante, Spain) | 2054 | 29-12-2011, − |
| E160-1486 | P. dactylifera var. dactylifera | 12-10-2010; Albatera (Alicante, Spain) | 2449 | 30-06-2011, + |
| E160-1489 | P. dactylifera var. dactylifera “Negres” | 25-01-2008; Elche (Alicante, Spain) | 3440 | 30-06-2009, − |
| E160-1491 | P. dactylifera var. dactylifera “Rojos” | 02-10-2015; Elche (Alicante, Spain) | 633 | 20-06-2016, + |
| E160-1493 | P. dactylifera var. dactylifera “Negres” | 25-01-2008; Elche (Alicante, Spain) | 3440 | 10-07-2016, + |
| E160-1495 | P. dactylifera var. dactylifera “Negres” | 25-01-2008; Elche (Alicante, Spain) | 3440 | 10-07-2016, + |
| E160-1497 | P. dactylifera var. dactylifera “Candios de mala clase” | 23-01-2011; Elche (Alicante, Spain) | 2346 | 20-06-2011, + |
| Phoenix × “Palmeri” | 1710 * | |||
| E160-1482 | Phoenix × “Palmeri” | 18-06-2012; B & T World Seeds 1 | 1834 | 10-07-2014, + |
| E160-1487 | Phoenix × “Palmeri” | 20-02-2013; KPR 2 | 1587 | 10-07-2014, + |
| Phoenix theophrasti Greuter | ||||
| E160-1492 | P. theophrasti “Datça” | 06-07-2013; Palmiye Merkezi 3 | 1451 | 30-9-2013, + |
| Phoenix × hybrida André (Phoenix dactylifera L. × Phoenix canariensis H. Wildpret) | ||||
| E160-1494 | P. × hybrida | 16-01-2011; El Siscar (Murcia, Spain) | 2353 | 10-07-2011, + |
| Phoenix pusilla Gaertn. | ||||
| E160-327 | P. pusilla | 22-11-2009; Kenibreed 4 | 2773 | 24-12-2011, − |
| Phoenix sylvestris (L.) Roxb. | 2773 * | |||
| E160-328 | P. sylvestris | 22-11-2009; Kenibreed 4 | 2773 | 30-06-2010, + |
| E160-329 | P. sylvestris | 22-11-2009; Kenibreed 4 | 2773 | 30-06-2010, + |
| Phoenix loureiroi Kunth | 1650 * | |||
| E160-712 | P. loureiroi | 25-12-2012; Kenibreed 4 | 1644 | 30-06-2013, + |
| E160-714 | P. loureiroi | 12-12-2012; Kenibreed 4 | 1657 | 30-06-2013, + |
| Phoenix rupicola T. Anderson | ||||
| E160-713 | P. rupicola | 25-12-2012; Kenibreed 4 | 1644 | 30-06-2013, + |
| Species | Varieties | Storage Conditions | Germination Percentage | Age of the Seeds (Years) | Refs |
|---|---|---|---|---|---|
| Phoenix dactylifera | “Methuselah” and “Hannah” | Dry archaeological, Masada | c. 19% | c. 2200 | 1,2 |
| Phoenix dactylifera | “Adam” | Dry archaeological, Wadi Makukh | c. 19% | c. 2200 | 1 |
| Phoenix dactylifera | “Judith” and “Boaz” | Dry archaeological, Qumran | c. 19% | c. 2000 | 1 |
| Phoenix dactylifera | “Uriel” and “Jonah” | Dry archaeological, Qumran | c. 19% | c. 1900 | 1 |
| Phoenix dactylifera | Baghdad, 1873, 35,988 (“Drah Subbah”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 35,990 (“Zadie”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 35,991 (“Brem”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 35,992 (“Makawieh Eshgar”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 35,993 (“Etchrisieh”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 36,006 (“Tiburzel”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 36,007 (“Sin Mufta”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 36,008 (“Khaderawieh”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 36,011 (“Makawieh Ahmur”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 36,012 (“El Washa”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 36,021 (“Sukri”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 36,024 (“Bedraieh”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 36,025 (“Khustawieh”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C, at Kew | 0% | 144 | 4,5 |
| Phoenix dactylifera | Baghdad, 1873, 35,988 (“Drah Subbah”) | Sealed glass jars and later acid-free card boxes, at c. 16 °C | 0% | 144 | 4,5 |
| Phoenix dactylifera | Balqa, Jordan, 2003 | Dried at 15% [RH], stored at −20 °C | 88% | 11 | 4 |
| Phoenix dactylifera | “Negres” | Desiccated, stored at 5 °C | 47–87% | 9 | 3 |
| Phoenix canariensis | var. porphyrococca Vasc. & Franco | Desiccated, stored at 5 °C | 93% | 7 | 3 |
| Phoenix dactylifera | var. costata Becc. | Desiccated, stored at 5 °C | 93% | 6 | 3 |
| Phoenix canariensis | var. porphyrococca Vasc. & Franco | Desiccated, stored at 5 °C | 93% | 4 | 3 |
| Phoenix. canariensis | Dried at 15% [RH], stored at −20 °C | 72% | 17 | 6 | |
| Phoenix. canariensis | Dried at 15% [RH], stored at −20 °C | 52% | 6 | 6 | |
| Phoenix. canariensis | Dried at 15% [RH], stored at −20 °C | 72% | 2 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obón, C.; Pardo-Pina, S.; Johnson, D.; Rivera, D. Orthodox vs. Recalcitrant? Germination and Early Growth of Phoenix Species (Arecaceae) Stored for up to Ten Years. Horticulturae 2025, 11, 537. https://doi.org/10.3390/horticulturae11050537
Obón C, Pardo-Pina S, Johnson D, Rivera D. Orthodox vs. Recalcitrant? Germination and Early Growth of Phoenix Species (Arecaceae) Stored for up to Ten Years. Horticulturae. 2025; 11(5):537. https://doi.org/10.3390/horticulturae11050537
Chicago/Turabian StyleObón, Concepción, Sofía Pardo-Pina, Dennis Johnson, and Diego Rivera. 2025. "Orthodox vs. Recalcitrant? Germination and Early Growth of Phoenix Species (Arecaceae) Stored for up to Ten Years" Horticulturae 11, no. 5: 537. https://doi.org/10.3390/horticulturae11050537
APA StyleObón, C., Pardo-Pina, S., Johnson, D., & Rivera, D. (2025). Orthodox vs. Recalcitrant? Germination and Early Growth of Phoenix Species (Arecaceae) Stored for up to Ten Years. Horticulturae, 11(5), 537. https://doi.org/10.3390/horticulturae11050537