Abstract
Endophytes, as an integral part of plants, form unique relationships with their hosts that go beyond classical definitions of symbiosis and influence plant development, immunity, and stress responses. The pepper endophyte strain Pseudomonas putida A32 has several plant growth-promoting properties and increases the tolerance of pepper to drought, but its biocontrol potential is unknown. In this study, we investigated the protective role of P. putida A32 against infection with the pathogenic bacterium P. syringae pv. aptata P21 in two pepper genotypes in laboratory experiments. The percentage of lesion reduction in genotype 26 treated with P. putida A32 was 46.62%. The results showed a significant reduction in hydrogen peroxide and malondialdehyde levels by 29.45 and 20.22%, respectively, in infected genotype 26. The treated but uninfected controls showed a significant increase in superoxide dismutase activity in genotype 26 by 41.26% and ascorbate peroxidase activity in genotype 19 by 40.28% in the treated infected plants. The tolerant genotype 19 was much less dependent on the bacterial treatment under stress conditions than the susceptible genotype 26. Future research will investigate the role of P. putida A32 in the induced systemic resistance of different pepper genotypes to protect against pathogens.
1. Introduction
The demands placed on agricultural production are growing and are increasingly challenging to meet. The growth of the world’s population increases the need for food production. Agriculture currently still relies heavily on chemical fertilizers and pesticides, although there are numerous environmental concerns associated with their use [1]. The losses imposed by plant pathogens are very high, and finding a substitution for the intensive use of pesticides is a burning issue. The annual loss of food production due to infection with plant pathogens is in second place, immediately after the losses that could be caused by drought. Various strategies are being used to address this problem, from traditional breeding methods to transgenic technologies or priming methods [2]. However, an alternative that is increasingly being considered to overcome the effects of biotic and abiotic stressors is the use of microorganisms that promote plant growth and stress resilience as bioinoculants (biofertilizers) [3]. Sustainable agriculture is based on these complex, mostly unexplored relationships between plants and microbes that are crucial for plant growth, health, development, productivity, and disease suppression [4]. An effective method of improving soil microbial communities is reductive soil disinfestation (RSD) as an environmentally friendly method of controlling soil-borne diseases and ensuring crop productivity. This treatment can reduce bacterial and fungal diversity, but also alter the nutrient capacity of the soil, reduce pathogen abundance, and increase interactions within the microbial community of the plant rhizosphere [5]. It has been shown that there is a synergistic effect between RSD, biochar, and antagonistic microbes in the control of Fusarium wilt in cucumber by altering the structure of the microbial community in the rhizosphere [6]. The interaction between plants and soil microorganisms that colonize the rhizosphere and roots, and then the entire plant, is considered one of the key factors in the rapid adaptation of the plant to environmental stress [7]. Consequently, this interaction leads to the formation of the holobiont [8], a structure that influences the stability, adaptability, and evolution of the organisms it contains. This bacterial action, called plant growth promotion (PGP), is reflected in the synthesis of phytohormones, enzymes, siderophores, nitrogen fixation, and phosphate solubilization as a means of direct PGP mechanisms [9]. Plants also benefit from these bacteria through indirect PGP mechanisms that protect them from pathogens and induce systemic resistance to diseases. These beneficial microbes can act as biological control agents, suppressing pathogens either directly—through competition, antibiosis, or parasitism—or indirectly, by priming the plant’s immune system and enhancing its natural defenses [10].
Members of the Pseudomonas genus can grow under a variety of environmental conditions, which affects their wide distribution and the numerous roles they play in living organisms and the environment. They are well-known PGPs, widely used in sustainable agriculture to control disease and promote plant growth [11,12]. However, bacteria from the species Pseudomonas syringae are the most widespread plant pathogens on the planet due to their epiphytic and endophytic lifestyle [13]. Their pathogenicity is based on a number of virulence factors, including the translocation of effector proteins into plant cells via the type III secretion system (T3SS). This is their main weapon in plant infection and has been found in all pathogenic strains of this species, while other virulence factors may or may not be present [13]. The strain Pseudomonas syringae pv. aptata P21, which was originally isolated as a pathogen from sugar beet [14], showed its broad pathogenic potential for numerous plant species such as pepper, tomato, cucumber, zucchini, pea, watermelon, melon, cabbage, lettuce, parsley, onion, beetroot, and Swiss chard (unpublished data). Given its highly virulent potential, supported by the presence of the T3SS and the T6SS, as well as the fine-tuning of the secretion of the effector repertoire and the tuning of virulence [15], it is necessary to identify biological control agents for its control. Natural Bacillus spp. isolates and their lipopeptides were proposed as candidates for biological control of this strain [16]. However, the use of this type of secondary metabolites in the environment frequently could not be implemented due to their cytotoxicity and/or genotoxicity. The plant microbiome represents a rich source of potential biocontrol agents capable of protecting the host through both direct antagonistic activity and indirect mechanisms such as induced resistance. For example, sugar beet—identified as the primary site of isolation for P. syringae pv. aptata P21—also harbors beneficial Pseudomonas strains, which enhance resistance to infection with P. syringae pv. aptata P21 [17]. We have previously shown that the pepper-root endophyte bacterium P. putida A32, characterized as a strain with many PGP characteristics, is a good candidate for the protection of pepper against abiotic drought stress. The strain synthesizes hydrogen cyanide and has a broad spectrum of antimicrobial activity in vitro against various pepper phytopathogenic bacteria [18]. These properties make the strain P. putida A32 a promising agent for the biological control of plant pathogens by direct biocontrol mechanisms.
The primary objective of the study was to investigate the protective role of P. putida A32 against infection with the pathogenic bacterium P. syringae pv. aptata P21 in two pepper genotypes, 26 and 19. We also aimed to test the hypothesis that genotype 19, which exhibits tolerance to Xanthomonas euvesicatoria and greater drought resilience compared to genotype 26, would also display an enhanced response to infection with Pseudomonas syringae pv. aptata P21. Moreover, we hypothesize that the enhanced baseline tolerance of genotype 19 to both biotic and abiotic stressors will impact its reliance on microbial assistance, resulting in a comparatively weaker response to beneficial treatments such as P. putida A32. In contrast, stress-sensitive genotypes like 26 may exhibit a more pronounced response. Therefore, we aimed to compare these two genotypes but also to look into the mechanisms underlying the induction of infection resistance. This study provides valuable insights into genotype-dependent responses to both pathogenic challenge and beneficial bacterial treatment, highlighting the complex interplay between inherent stress tolerance and microbe-mediated protection in pepper.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
The strain Pseudomonas putida A32 used in this study is an endophyte isolated from the roots of pepper genotype 26, as described previously [18]. Plant pathogen P. syringae pv. aptata P21, previously isolated from sugar beet [14], was used for the infection tests. Both bacterial strains were routinely cultured on Luria–Bertani agar (LA) (all components from Torlak, Belgrade, Serbia) and King’s B agar (KB) (TM Media, Titan Biotech LTD, Rajasthan, India) plates at 30 °C. Prior to experimental use, the strains were grown in Luria–Bertani (LB) broth for 16 h at 30 °C with shaking (IKA KS 3000 i control, IKA-Werke GmbH & Co. KG, Staufen, Germany) to obtain fresh bacterial cultures.
2.2. Antibacterial Activity In Vitro Assay
The antibacterial activity of P. putida A32 against P. syringae pv. aptata P21 was tested using the well-diffusion assay described by Krstić Tomić et al. [19]. To evaluate the antibacterial activity of previously detected volatile antimicrobial compounds produced by P. putida A32 [18], split Petri dishes were used. One half of this Petri dish was filled with the LA medium and the other with the KB medium. An overnight culture of P. syringae pv. aptata P21 adjusted to an OD600 of 1 was spotted onto the LA medium as 10 μL drops of 10−1 to 10−4 serial dilutions, while P. putida A32 was inoculated onto the KB agar medium. As a control, plates were prepared in which only the pathogen was inoculated on the LA side, while the opposite side of the plate with KB medium remained uninoculated. The Petri dish was sealed with parafilm and incubated at 30 °C. After 24 h of growth and exposure, colonies growing on serial dilutions spotted on plates were quantified. Each spot was carefully punched out from the agar plate, transferred to a tube containing 1 mL of sterile distilled water, and vortexed for 1 min. The resulting suspension was then transferred to a cuvette, and the optical density at 600 nm was measured (Orion Aquamate 8000, UV-VIS Spectrophotometer, Thermo Scientific, Waltham, MA, USA) [20].
2.3. Bacterial Treatment and Infection Susceptibility Assay
This study included two different genotypes of pepper plants (Capsicum annuum L.), the genotype 26 (obtained by self-pollination of a plant of the variety Amfora), which is susceptible to phytopathogenic bacteria Xanthomonas euvesicatoria, and the genotype 19, crossed between the Amfora variety and the pepper line with tolerance to X. euvesicatoria [21]. Pepper seeds were sterilized with sodium hypochlorite and ethanol and treated overnight with a culture of P. putida A32 strain adjusted to a concentration of 108 CFU/mL, as previously established by Hyder et al. (2021) [22]. The seeds of the control groups were treated with sterile water. Seeds were then sown in plastic pots (10 cm × 10 cm × 10 cm) filled with commercial soilless substrate (Plagron, Weert, The Netherlands), placed in a growth box, and watered regularly. The temperature was maintained at 20 ± 2 °C, the relative humidity was 20%, and a 16/8 h photoperiod was applied. The experiment was set up in a completely randomized design and the pots were rotated weakly across the bench to minimize the effects of position on the variability of temperature and light conditions. After six weeks, the plants were infected with P. syringae pv. aptata P21. The bacterial suspension was adjusted to a concentration of 105 CFU/mL and 100 µL of the suspension was injected into the abaxial side of the first true leaf using a sterile syringe. Leaves in the control group were injected with sterile distilled water. Five days after the infection, the leaves were harvested and scanned with a computer scanner. Necrotic lesions were quantified using ImageJ software, version 1.54m (December 2024). [23]. Leaf samples were ground in liquid nitrogen and stored at −80 °C until further use.
2.4. Quantification of Lipid Peroxidation and Hydrogen Peroxide in Pepper Leaves
The determination of the level of lipid peroxidation and H2O2 content as a parameter of the stress response was determined using previously established methods [24]. The degree of lipid peroxidation was determined indirectly by measuring the malondialdehyde (MDA) content, using the extinction coefficient of 155 mM−1 cm−1 and expressed as nmol MDA/g dry weight (DW). The hydrogen peroxide content was determined using a spectrophotometric assay based on the oxidation of iodide by H2O2 to iodine in an acidic medium. The amount of H2O2 was determined using the standard curve and expressed as nmol/g DW.
2.5. Antioxidant Enzyme Activity
The activity of antioxidant enzymes, total soluble peroxidases (POD), ascorbate peroxidase (APX) and superoxide dismutase (SOD) was determined as previously described [24]. In brief, previously prepared protein extracts were used for all spectrophotometric measurements of enzyme activity. For the activity of POD, absorbance was measured at 470 nm for colored products formed in the reaction with guaiacol. The activity of APX was measured at 290 nm, and ascorbate was used as a reactive reagent. SOD activity was measured at 560 nm as an inhibition of the photochemical reduction in nitro blue tetrazolium.
2.6. Determination of Phenylalanine Ammonia Lyase Activity
Phenylalanine ammonia lyase (PAL) activity was assessed by quantifying the production of trans-cinnamic acid, as previously described [25]. The reaction mixture consisted of 100 µL of enzyme extract, 0.5 mL of 50 mM Tris-Cl buffer (pH 8.8), and 0.6 mL of 1 mM L-phenylalanine. The mixture was incubated in water bath at 40 °C for 60 min, and the reaction was stopped by adding 1 mL of 2 N HCl. Absorbance was measured at 290 nm, and enzyme activity was expressed as µg of trans-cinnamic acid per gram of leaf tissue.
2.7. Statistical Analysis
All experiments were performed with three biological replicates. All data were analyzed with an unpaired t-test with Welch’s correction at the 0.05 probability level using GraphPad Prism version 9.0.0 for Windows (GraphPad Software, San Diego, CA, USA).
3. Results
In Vitro and In Planta Antagonistic Activity of Pseudomonas Putida A32 Against Phytopathogen Bacteria Pseudomonas syringae pv. aptata P21
In a well-diffusion essay, the strain P. putida A32 showed no inhibitory effect on P. syringae pv. aptata P21. To confirm the result obtained, we tested different concentrations of the indicator strain P21 and the growth time for the A32 overnight culture. None of these approaches gave a positive result. To determine the antagonistic activity of volatile metabolites produced by this endophyte strain against P. syringae pv. aptata P21, split Petri dishes were used (Figure 1). The results showed that in the presence of the antagonistic strain, although there was no contact between them, the number of pathogenic bacteria decreased significantly, making this strain a potential candidate as a biological control agent.
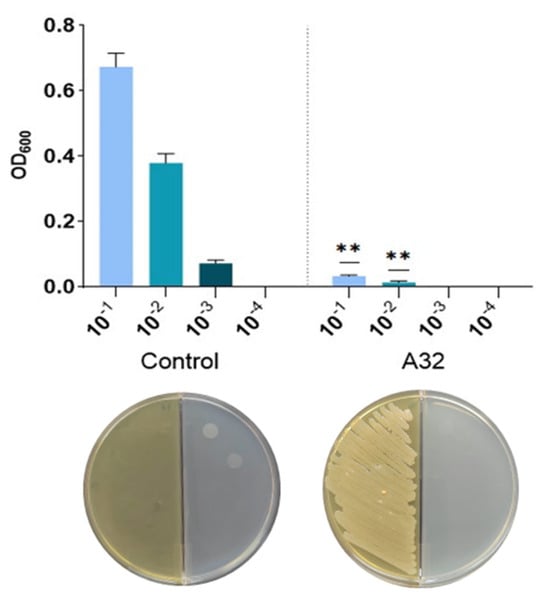
Figure 1.
The quantification of the effect of exposure to volatile metabolites of P. putida on the growth of P. syringae pv. aptata P21 on LB agar medium in split Petri dishes. Pathogenic bacteria were grown on 10−1 to 10−4 dilution spots and exposed to P. putida volatiles (A32) or not (control). Images were taken after 24 h of incubation at 30 °C. Each spot was punched out of the LB agar plate and resuspended in water, and the corresponding OD600 was determined. Statistics correspond to a two-tailed, unpaired t-test with Welch correction. ** p ≤ 0.01.
To verify the potential biocontrol activity of P. putida A32, we performed experiments in plants. The protective effect of seed pretreatment with the strain P. putida A32 was determined by the reduction in the development of lesions due to infection with the phytopathogenic bacterium P. syringae pv. aptata P21 in both pepper genotypes (Figure 2). This reduction was significant in genotype 26, where lesions were 46.62% smaller than in the control group, and 45% smaller than in genotype 19 plants (Figure 2A,B).
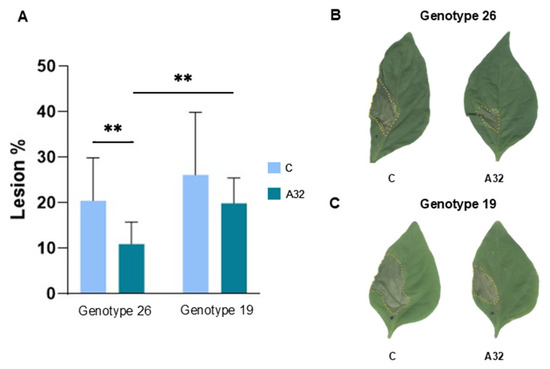
Figure 2.
The effect of treatment with P. putida A32 on the susceptibility of two pepper genotypes to infection with P. syringae pv. aptata P21. Pepper seeds were pretreated with P. putida A32, and the control group was not exposed to the bacteria. The leaves were infected with P. syringae pv. aptata P21, and the susceptibility was evaluated by the size of the necrotic lesions at the infection sites. (A) Percentage of lesions due to P. syringae pv. aptata P21 infection in genotypes 26 and 19 treated with A32 compared to the untreated control group (C). The statistics correspond to a two-tailed unpaired t-test with Welch correction. ** p ≤ 0.01. (B,C) Representative necrotic lesions in genotype 26 (B) and genotype 19 (C) in the control and the A32-treated group.
An increased concentration of reactive oxygen species (ROS) is the first stress response, followed by lipid peroxidation among other changes in plant tissue. One of the most important types of ROS is hydrogen peroxide (H2O2). After infection, the H2O2 concentration decreased significantly in genotype 26, whose seeds had been pretreated with P. putida A32. This genotype was more sensitive to the bacterial treatment, as the H2O2 concentration increased significantly by 82.16% in uninfected but P. putida A32-treated plants compared to the control (Figure 3A). Both genotypes showed a reduction in the concentration of malondialdehyde (MDA), a marker for lipid peroxidation, in the uninfected plants after treatment with P. putida A32 (Figure 3B). It is important to point out that the MDA concentration in the infected plants was also significantly reduced in genotype 26 treated with P. putida A32.
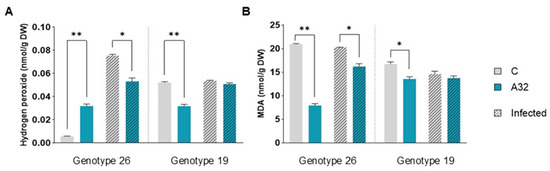
Figure 3.
Changes in H2O2 and MDA content in pepper plants uninfected and infected with P. syringae pv. aptata P21. Two pepper genotypes treated with P. putida A32 (A32) were compared with the control group (C), which was not exposed to the bacteria. The leaves were harvested 5 days after infection, and the biochemical parameters were analyzed in comparison to the uninfected leaves. (A) Changes in H2O2 content. (B) Changes in MDA content. The statistics correspond to a two-tailed unpaired t-test with Welch correction. Significant differences are indicated as follows: p < 0.05 (*), p < 0.01 (**).
The activation of antioxidant enzymes in plants exposed to biotic and abiotic stress is one of the first lines of defense. In genotype 26, uninfected plants treated with P. putida A32 exhibited increased superoxide dismutase (SOD) activity (Figure 4A). In contrast, P. putida A32-treated plants of genotype 19 showed a significant activation of ascorbate peroxidase (APX) in the infected plants (Figure 4B), while a significant decrease in APX activity was observed in the uninfected plants. Both genotypes showed a significant decrease in the activity of total soluble peroxidases in the infected plants after treatment with P. putida A32 (Figure 4C). The activity of phenylalanine ammonia lyase (PAL), an enzyme of phenylpropanoid metabolism related to the biosynthesis of phytoalexins and other compounds that contribute to pathogen resistance, was determined. However, the activity of PAL was reduced in both uninfected and infected plants after treatment with P. putida A32 (Figure 4D).
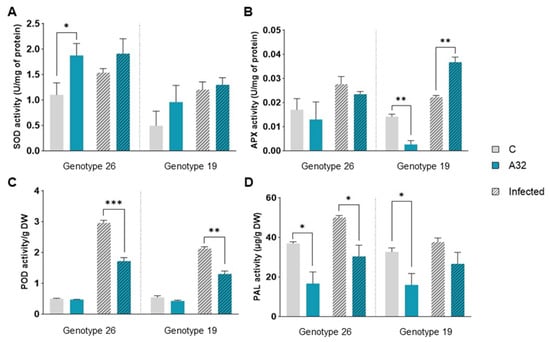
Figure 4.
Changes in protective enzyme activity in pepper plants not infected and infected with P. syringae pv. aptata P21. Two pepper genotypes treated with P. putida A32 (A32) were compared with the control group (C), which was not exposed to bacteria. The leaves were harvested 5 days after infection, and the biochemical parameters were analyzed in comparison to the uninfected leaves. (A) Superoxide dismutase (SOD) activity. (B) Ascorbate peroxidase (APX) activity. (C) Peroxidase (POD) activity. (D) Phenylalanine ammonia lyase (PAL) activity. The statistics correspond to a two-tailed unpaired t-test with Welch correction. Significant differences are indicated as follows: p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
4. Discussion
Plants, like all other organisms, have a microbiome that serves as a partner in their growth, development, protection, and maintenance of plant health [26,27]. The coevolution of the microbiome with its host plant leads to the development of an even more complex relationship [28]. A unique and very specific part of this microbiome consists of endophytes, microorganisms that inhabit the inner plant tissue and form very close and specific relationships with the plant itself [29]. In this study, we investigated the biocontrol potential of the endophytic pepper-root bacterium P. putida A32 against the highly virulent phytopathogenic bacterium P. syringae pv. aptata P21. Although the strain did not inhibit the growth of the pathogen in a well-diffusion assay, a split Petri dish test showed an antagonistic effect of the volatile compounds produced, most likely due to HCN synthesis, which has been previously demonstrated [18]. The antagonistic effect of strains synthesizing HCN against various bacteria, fungi, and nematodes, especially those belonging to the group of pathogenic microorganisms, has been previously demonstrated [30]. The results of this study point to previous research that has shown that HCN plays more than just an antagonistic role, as it is involved in the regulation of gene expression in the producer and the host [31]. We were able to demonstrate a protective effect in plants treated with P. putida A32 by reducing the size of lesions caused by P. syringae pv. aptata P21 infection compared to untreated plants. Since direct inhibition did not yield results, we hypothesize that secondary metabolites are involved in modulating the plant response to pathogen infection. Recent research highlights the role of HCN as a signaling molecule that modulates plant immunity by inducing the expression of defense-related genes, promoting the production of reactive oxygen species (ROS), and mediating the function of proteins through S-cyanylation, thereby increasing plant resistance not by directly inhibiting the pathogen but by activating its defense mechanisms [31]. Our results emphasize this finding by indicating that the protective effect of the strain is not due to a direct effect on the pathogen, but most likely to a modulation of the response of the plant itself, making it more resistant to the pathogen’s action. The seed treatment led to changes in the plant that were most likely caused by the colonization of the plant by P. putida A32 that we assume had a greater potential for this due to its endophytic origin, which has already been shown previously [32]. The ability of Pseudomonas species to efficiently colonize plants has been demonstrated for Pseudomonas aurantiaca JD37, which efficiently colonizes maize roots and protects the plants by inducing resistance to Bipolaris maydis [33]. The endophytic nature of the strain Pseudomonas aeruginosa FG106 enables it to effectively colonize not only the tomatoes from which it was isolated, but also potatoes, taro, and strawberries, providing effective protection against various pathogenic bacteria and fungi [34].
The genotype of the plant is an important determinant for the development of a positive plant–endophyte association. Several techniques must be used to minimize the effects of the host genotype in plant–bacteria interactions, like micropropagation and vegetative growth [35]. In our study, both pepper genotypes had in common that the plants treated with P. putida A32 showed a lower concentration of H2O2 and malondialdehyde (MDA) after infection. The measured parameters are interdependent, as the accumulation of reactive oxygen species (ROS) causes lipid peroxidation. This effect of bacterial treatment can be explained by their ability to neutralize the effects of the ROS produced and possibly reduce their production [36]. Our study showed that the genotype 26 plants treated with P. putida A32 had significantly increased levels of H2O2 under uninfected conditions, which can be explained by the fact that H2O2 is not only a reactive oxygen species but also a signaling molecule [37]. This increase could reflect the interactions between this strain and the plant under stress conditions, especially the colonization of the host plant by bacteria. The endophytic nature of P. putida A32 allows it to interact with and colonize plants more rapidly, which has already been confirmed for endophytic bacteria [32]. Contrasting results were obtained when duckweed was co-cultivated with P. gessardii C31-106/3, with a decrease in H2O2 concentration, while the same treatment under salt-stress conditions led to an increase [38,39]. The treatment of duckweed with P. oryzihabitans D1-104/3 decreased the H2O2 content under salt stress [39]. However, duckweed rhizobacteria P. oryzihabitans D1-104/3 and P. gessardii C31-106/3 decreased MDA in treated duckweed exposed to salt stress [39]. The treatment of rapeseed growing under water stress with the strain P. protegens ML15 also had a similar effect [40]. In addition to abiotic stress, the reduction in MDA after treatment with PGP strains was also demonstrated under biotic stress conditions. The strain Bacillus subtilis BS-2301 with biocontrol potential against Sclerotinia sclerotiorum reduced the MDA concentration in infected soybean plants [41].
An increase in the activity of antioxidant enzymes and, thereby, an increase in the resistance of plants to stress as a consequence of treatment with PGP bacteria, was reported previously [42]. Our study confirmed a plant genotype-specific response in the activity of enzymes of antioxidant protection. Genotype 19, which is tolerant to Xanthomonas euvesicatoria, a biotic stressor, activates ascorbate peroxidase (APX), which is directly involved in the elimination of H2O2 as a first line of defense, as we have previously shown for drought as an abiotic stress [18]. Moreover, a comparison of these two genotypes under different growth conditions has shown that both increase APX activity in the field when multiple stressors are present [21]. One explanation for this is the fact that plant peroxidases not only serve to remove excess hydrogen peroxide but are also the first line of defense against pathogens and toxic compounds in many plants [43]. Thus, APX in genotype 19 is the first antioxidant enzyme to be activated during the defense response. Although we observed a significant decrease in lipid peroxidation in P. putida A32-treated and infected plants, indicating a possible increase in superoxide dismutase (SOD) activity, SOD activity was significantly increased only in uninfected plants of genotype 26. This suggests that superoxide anions were removed with bacterial SOD or that P. putida A32 produces protective substances that protect the electron transport chains in the mitochondria and chloroplasts, where most superoxide anions are produced [36]. Our previous study has also shown that SOD was one of the enzymes activated in genotype 26 under certain growth conditions in which the plant was isolated with a plastic cover [21]. This renewed response to the bacterial treatment explains the high H2O2 concentration even in uninfected plants.
The genotype-specific response was expected, but from all the studies we conducted on these two pepper genotypes, we were able to draw conclusions and propose some hypotheses for future research. It is interesting to note that the tolerant genotype 19 showed a weaker response to treatment with strain P. putida A32 in our earlier study [18] and this study. We hypothesize that the reason for this could be that since less decay occurred under the given conditions, the plant was able to conserve energy to initiate various biochemical processes and respond to stress and treatment. On the other hand, the more sensitive genotype 26 responded much better to treatment with the P. putida A32 strain. This not only highlights the protective effect of the strain, which is evident in both genotypes, but also demonstrates how the plant responds under the given conditions. Drought and high temperatures lead to changes in the plant that make it more susceptible to biotic factors and eventually lead to its complete collapse. Formulating bioinoculants with strains that can perform more than one protective function makes them more effective and cheaper to produce, and therefore more accessible to the end user in food production. This ability of the proposed candidate strains is rarely analyzed, but its importance, particularly concerning abrupt climate change, is highlighted in a review paper by Anuar and coauthors. [44]. Strain P. putida A32 is an excellent candidate that has been shown to be effective in protecting peppers against drought [18] and infection with phytopathogenic bacteria, as demonstrated in this study. In addition, this isolate is an endophyte, which better prepares it for close interaction with the plant. Furthermore, we believe that this classifies it as a strain that is safe for use in biological preparations for sustainable agriculture, as it was already an integral part of the plant tissue. In the future, it will be necessary to validate its effect on induced systemic resistance in plants and protection against disease, as well as the possible role of HCN in this activity. Another future research direction will be to test the efficacy of P. putida A32 in the simultaneous application of both stressors, not only in pepper as a model organism, but also in other crops.
Author Contributions
Conceptualization, J.L.; methodology, A.M.; formal analysis, A.M., M.N. and M.A.; resources, D.D.; data curation, I.A.; writing—original draft preparation, J.L.; writing—review and editing, S.S. and I.A.; supervision, J.L.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant numbers 451-03-137/2025-03/200178, 451-03-136/2025-03/200178, and 451-03-136/2025-03/200032 and the International Centre for Genetic Engineering and Biotechnology grant CRP/SRB23-04_EC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Niranjana, M.; Jha, S.; Mallick, N.; Agarwal, P. QTL detection and putative candidate gene prediction for leaf rolling under moisture stress condition in wheat. Sci. Rep. 2020, 10, 18696. [Google Scholar] [CrossRef]
- Laishram, B.; Devi, O.R.; Dutta, R.; Senthilkumar, T.; Goyal, G.; Paliwal, D.K.; Panotra, N.; Rasool, A. Plant-microbe interactions: PGPM as microbial inoculants/biofertilizers for sustaining crop productivity and soil fertility. Curr. Res. Microb. Sci. 2024, 18, 100333. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Zaib, S. Mighty Microbes: Plant Growth Promoting Microbes in Soil Health and Sustainable Agriculture. In Soil Health. Soil Biology; Giri, B., Varma, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 59. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, H.; Ran, T.; Yang, H. Changes in rhizosphere soil bacterial, fungal, and protistan communities during tomato (Solanum lycopersicum) growth after reductive soil disinfestation. Plant Soil. 2025, 506, 573–589. [Google Scholar] [CrossRef]
- Ali, A.; Elrys, A.S.; Liu, L.; Xia, Q.; Wang, B.; Li, Y.; Dan, X.; Iqbal, M.; Zhao, J.; Huang, X.; et al. Deciphering the synergies of reductive soil disinfestation combined with biochar and antagonistic microbial inoculation in cucumber Fusarium wilt suppression through rhizosphere microbiota structure. Microb. Ecol. 2022, 85, 980–997. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Proietti, S.I.; Pantelides, S.; Stringlis, I.A. Modulation of the root microbiome by plant molecules: The basis for targeted disease suppression and plant growth promotion. Front. Plant Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Simon, J.C.; Marchesi, J.R.; Mougel, C.; Selosse, M.A. Host-Microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef]
- Scagliola, M.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Crecchio, C.; Pii, Y. Bioinoculants as promising complement of chemical fertilizers for a more sustainable agricultural practice. Front. Sustain. Food Syst. 2021, 4, 622169. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as biological control agents of plant diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Sahgal, M.; Saini, N.; Jaggi, V.; Brindhaa, N.T.; Kabdwal, M.; Singh, R.P.; Prakash, A. Antagonistic potential and biological control mechanisms of Pseudomonas strains against banded leaf and sheath blight disease of maize. Sci. Rep. 2024, 14, 13580. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S. Evaluation of Pseudomonas sp. for its multifarious plant growth promoting potential and its ability to alleviate biotic and abiotic stress in tomato (Solanum lycopersicum) plants. Sci. Rep. 2020, 10, 20951. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Nikolić, I.; Stanković, S.; Dimkić, I.; Berić, T.; Stojšin, V.; Janse, J.; Popović, T. Genetic diversity and pathogenicity of Pseudomonas syringae pv aptata isolated from sugar beet. Plant Pathol. 2018, 67, 1194–1207. [Google Scholar] [CrossRef]
- Nikolić, I.; Glatter, T.; Ranković, T.; Berić, T.; Stanković, S.; Diepold, A. Repertoire and abundance of secreted virulence factors shape the pathogenic capacity of Pseudomonas syringae pv aptata. Front. Microbiol. 2023, 14, 1205257. [Google Scholar] [CrossRef]
- Nikolić, I.; Berić, T.; Dimkić, I.; Popović, T.; Lozo, J.; Fira, D.; Stanković, S. Biological control of Pseudomonas syringae pv. aptata on sugar beet with Bacillus pumilus SS-10.7 and Bacillus amyloliquefaciens (SS-12.6 and SS-38.4) strains. J. Appl. Microbiol. 2019, 126, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Mesaroš, A.; Rašić, V.; Nikolić, I.; Stanković, S.; Lozo, J.; Atanasković, I. Effects of T3SS-positive Pseudomonas isolates on sugar beet growth stimulation and pathogen resistance. Plant Soil. 2024. [Google Scholar] [CrossRef]
- Mesaroš, A.; Atanasković, I.; Nedeljković, M.; Stanković, S.; Lozo, J. Differential responses of bell pepper genotypes to indigenous Pseudomonas putida A32 treatment: Implications for drought resilience. J. Appl. Microbiol. 2024, 135, lxae190. [Google Scholar] [CrossRef]
- Krstić Tomić, T.; Atanasković, I.; Nikolić, I.; Joković, N.; Stević, T.; Stanković, S.; Berić, T.; Lozo, J. Culture-dependent and metabarcoding characterization of the sugar beet (Beta vulgaris L.) microbiome for high-yield isolation of bacteria with plant growth-promoting traits. Microorganisms 2023, 11, 1538. [Google Scholar] [CrossRef]
- Létoffé, S.; Wu, Y.; Darch, S.E.; Beloin, C.; Whiteley, M.; Touqui, L.; Ghigo, J.M. Pseudomonas aeruginosa production of hydrogen cyanide leads to airborne control of Staphylococcus aureus growth in biofilm and in vivo lung environments. mBio 2022, 13, e0215422. [Google Scholar] [CrossRef]
- Mesaroš, A.; Nedeljković, M.; Danojević, D.; Medić-Pap, S.; Stanković, S.; Radović, S.; Lozo, J. Influence of growth conditions on an antioxidative system in two bell pepper genotypes differing in susceptibility to phytopathogen bacteria Xanthomonas euvesicatoria. Plant Growth Regul. 2023, 100, 609–617. [Google Scholar] [CrossRef]
- Hyder, S.; Gondal, A.S.; Rizvi, Z.F.; Atiq, R.; Haider, M.I.S.; Fatima, N.; Inam-ul-Haq, M. Biological control of chili damping-off disease, caused by Pythium myriotylum. Front. Microbiol. 2021, 12, 587431. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lozo, J.; Danojević, D.; Jovanović, Ž.; Nenadović, Ž.; Fira, D.; Stanković, S.; Radović, S. Genotype-dependent antioxidative response of four sweet pepper cultivars to water deficiency as affected by drought-tolerant Bacillus safensis SS-2.7 and Bacillus thuringien sis SS-29.2 strains. Horticulturae 2022, 8, 236. [Google Scholar] [CrossRef]
- Kleinhofs, A.; Haskins, F.A.; Gorz, H.J. Relationship of Phenylalanine ammonia-lyase activity to o-hydroxycinnamic acid content in Melilotus alba. Plant Physiol. 1966, 41, 1276–1279. [Google Scholar] [CrossRef]
- Soth, S.; Hampton, J.G.; Alizadeh, H.; Wakelin, S.A.; Mendoza-Mendoza, A. Microbiomes in action: Multifaceted benefits and challenges across academic disciplines. Front. Microbiol. 2025, 16, 1550749. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Tack, A.J.; Wasserman, B.; Liu, J.; Berg, G.; Norelli, J.; Droby, S.; Wisniewski, M. Evidence for host–microbiome co-evolution in apple. New Phytol. 2022, 234, 2088–2100. [Google Scholar] [CrossRef]
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Sahu, P.K.; Pandey, A.; White, J.F.; Verma, S.K. Endophytic Burkholderia: Multifunctional roles in plant growth promotion and stress tolerance. Microbiol. Res. 2022, 265, 127201. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Díaz-Rueda, P.; Morales de los Ríos, L.; Romero, C.; García, I. Old poisons, new signaling molecules: The case of hydrogen cyanide. J. Exp. Bot. 2023, 74, 6040–6051. [Google Scholar] [CrossRef]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef]
- Fang, R.; Lin, J.; Yao, S.; Wang, Y.; Wang, J.; Zhou, C.; Wang, H.; Xiao, M. Promotion of plant growth, biological control and induced systemic resistance in maize by Pseudomonas aurantiaca JD37. Ann. Microbiol. 2013, 63, 1177–1185. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant growth-promoting activity of Pseudomonas aeruginosa FG106 and its ability to act as a biocontrol agent against potato, tomato and taro pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Karpinska, B.; Foyer, C.H. Superoxide signalling and antioxidant processing in the plant nucleus. J. Exp. Bot. 2024, 75, 4599–4610. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 6. [Google Scholar] [CrossRef]
- Popržen, T.; Jevremović, S.; Milošević, S.; Ðurić, M.; Uzelac, B.; Stanković, S.; Radulović, O. Antioxidative response of duckweed (Lemna minor L.) to rhizosphere- associated Pseudomonas strains and exogenous indole-3-acetic acid. Horticulturae 2024, 10, 562. [Google Scholar] [CrossRef]
- Popržen, T.; Antonić Reljin, D.; Uzelac, B.; Milovančević, M.; Paunović, D.; Trifunović-Momčilov, M.; Marković, M.; Raspor, M.; Nikolić, I.; Stanković, S.; et al. Pseudomonas oryzihabitans D1-104/3 and P. gessardii C31-106/3 differentially modulate the antioxidative response of duckweed (Lemna minor L.) to salt stress. Front. Microbiol. 2024, 15, 1481437. [Google Scholar] [CrossRef] [PubMed]
- Ajijah, N.; Fiodor, A.; Dziewit, L.; Pranaw, K. Biological amelioration of water stress in rapeseed (Brassica napus L.) by exopolysaccharides-producing Pseudomonas protegens ML15. Physiol. Plant. 2024, 176, e70012. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Ali, F.; Rashid, K.A.; Cao, S.; He, Y.-q.; Bukero, A.A.; Huang, W.-K.; et al. Exploring plant growth promoting traits and biocontrol potential of new isolated Bacillus subtilis BS-2301 strain in suppressing Sclerotinia sclerotiorum through various mechanisms. Front. Plant Sci. 2024, 15, 1444328. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent advances in bacterial amelioration of plant drought and salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.D.; Franco, O.L. Pathogenesis-related proteins (PRs) with enzyme activity activating plant defense responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef] [PubMed]
- Anuar, M.S.K.; Hashim, A.M.; Ho, C.L.; Wong, M.Y.; Sundram, S.; Saidi, N.B.; Yusof, M.T. Synergism: Biocontrol agents and biostimulants in reducing abiotic and biotic stresses in crop. World J. Microbiol. Biotechnol. 2023, 39, 123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).