Zingiberaceae in Roi Et Province, Thailand: Diversity, Ethnobotany, Horticultural Value, and Conservation Status
Abstract
1. Introduction
2. Materials and Methods
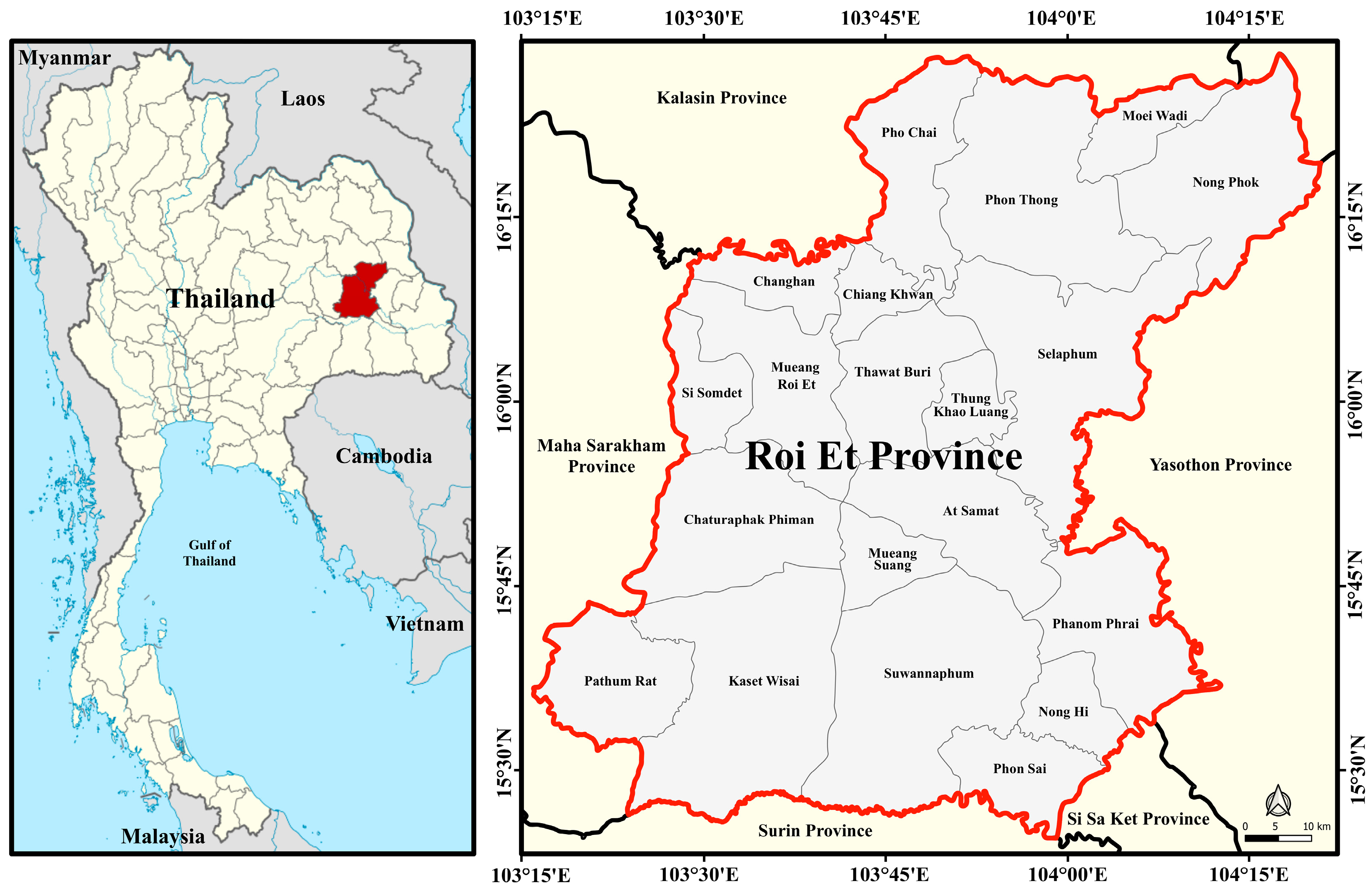
2.1. Study Area
2.2. Data Collection
2.3. Taxonomic Identification
2.4. Plant Specimen Preservation
2.5. Study of Habitats of Zingiberaceae in Roi Et Province
2.6. Study of Phenology of Zingiberaceae in Roi Et Province
2.7. Utilization
2.8. Quantitative Analysis
2.8.1. Jaccard’s Similarity Index (JI)
2.8.2. Species Use Value (SUV): For One Species Across All Informants
2.8.3. Genera Use Value (GUV)
2.8.4. Relative Frequency of Citation (RFC)
2.8.5. Informant Consensus Factor (Fic)
2.8.6. Fidelity Level (%FL)
2.8.7. Economic Value (EV)
2.9. Conservation Status
3. Results
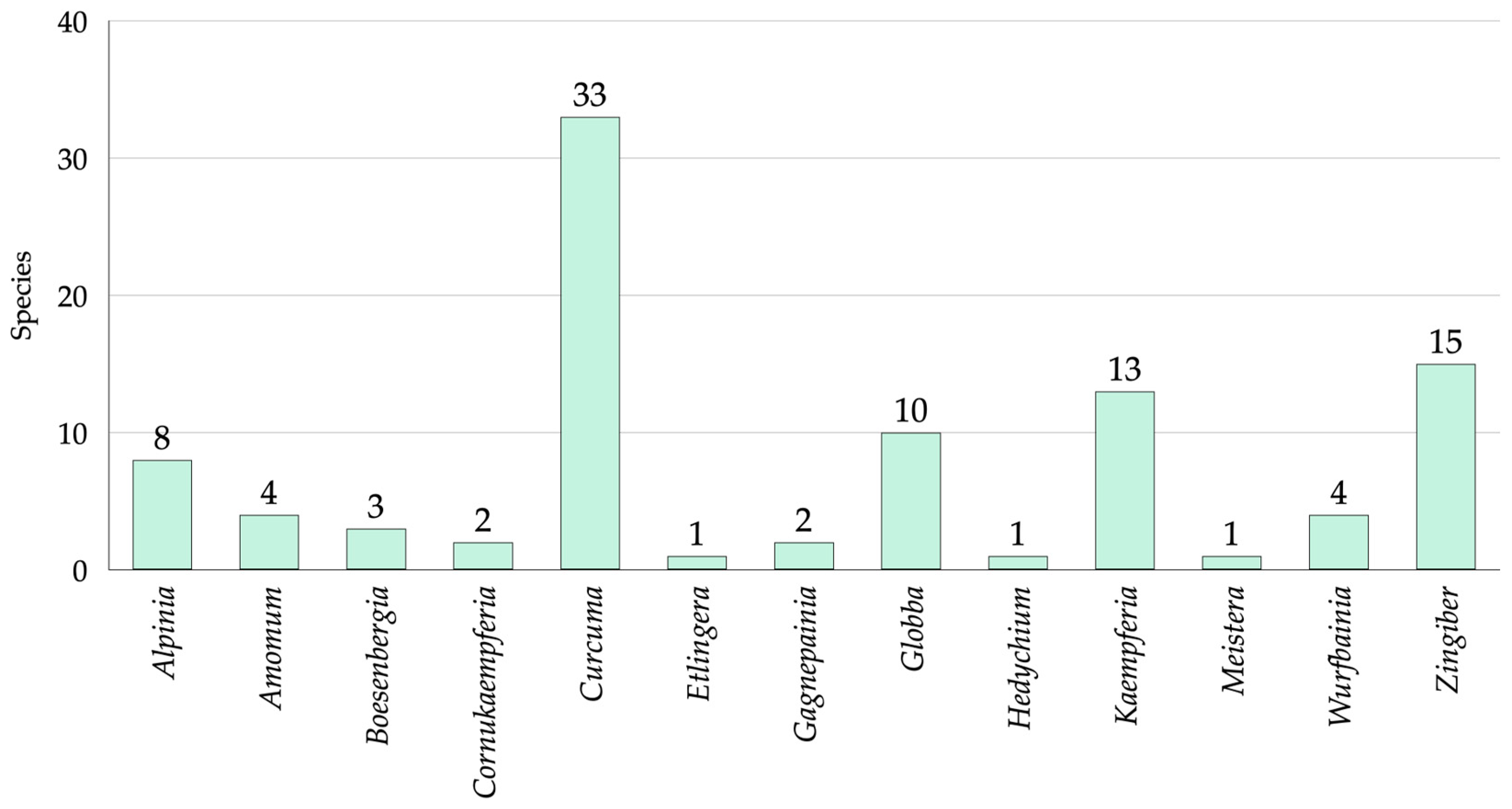
3.1. Species Diversity of Zingiberaceae in Roi Et Province
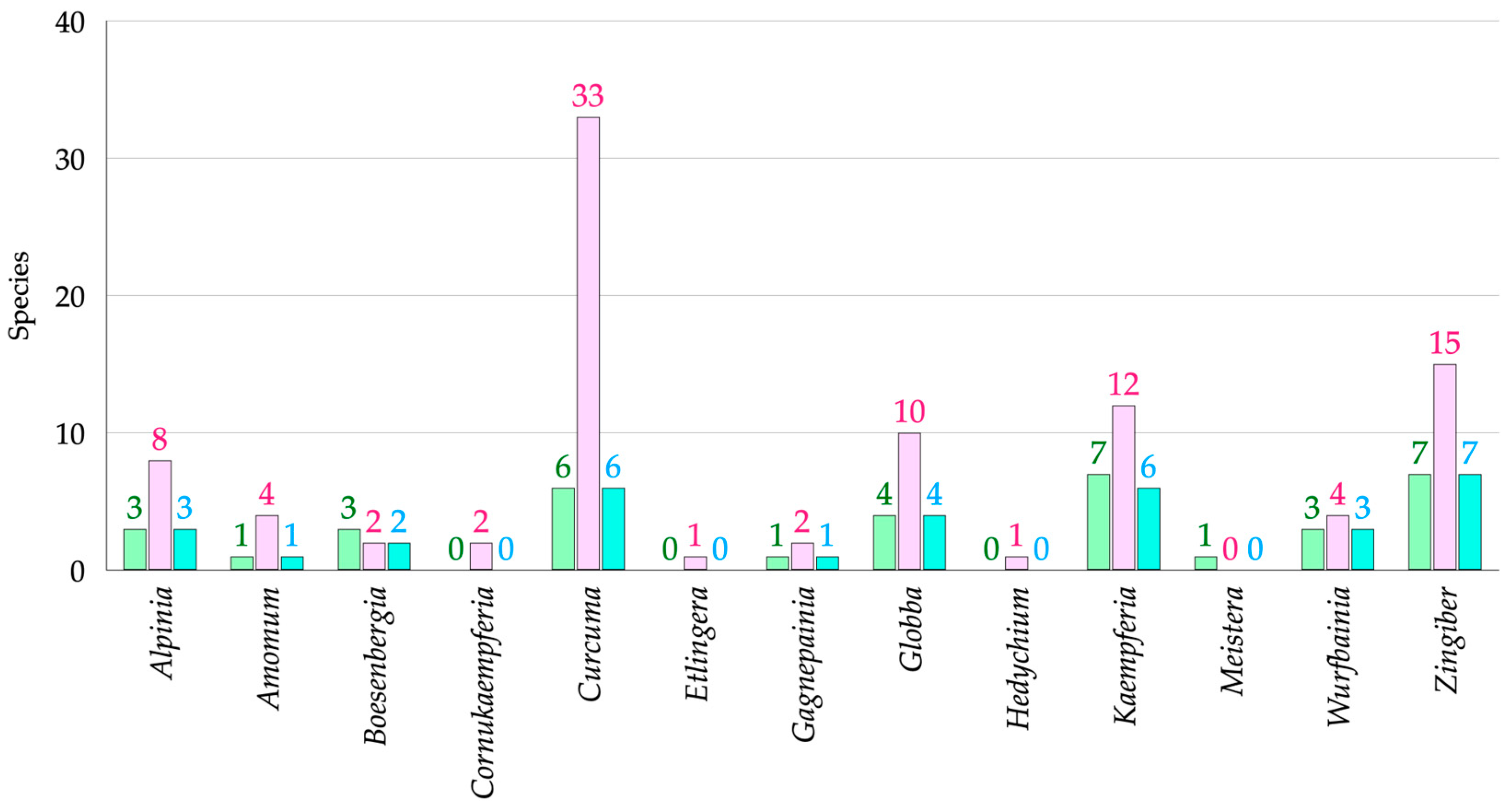
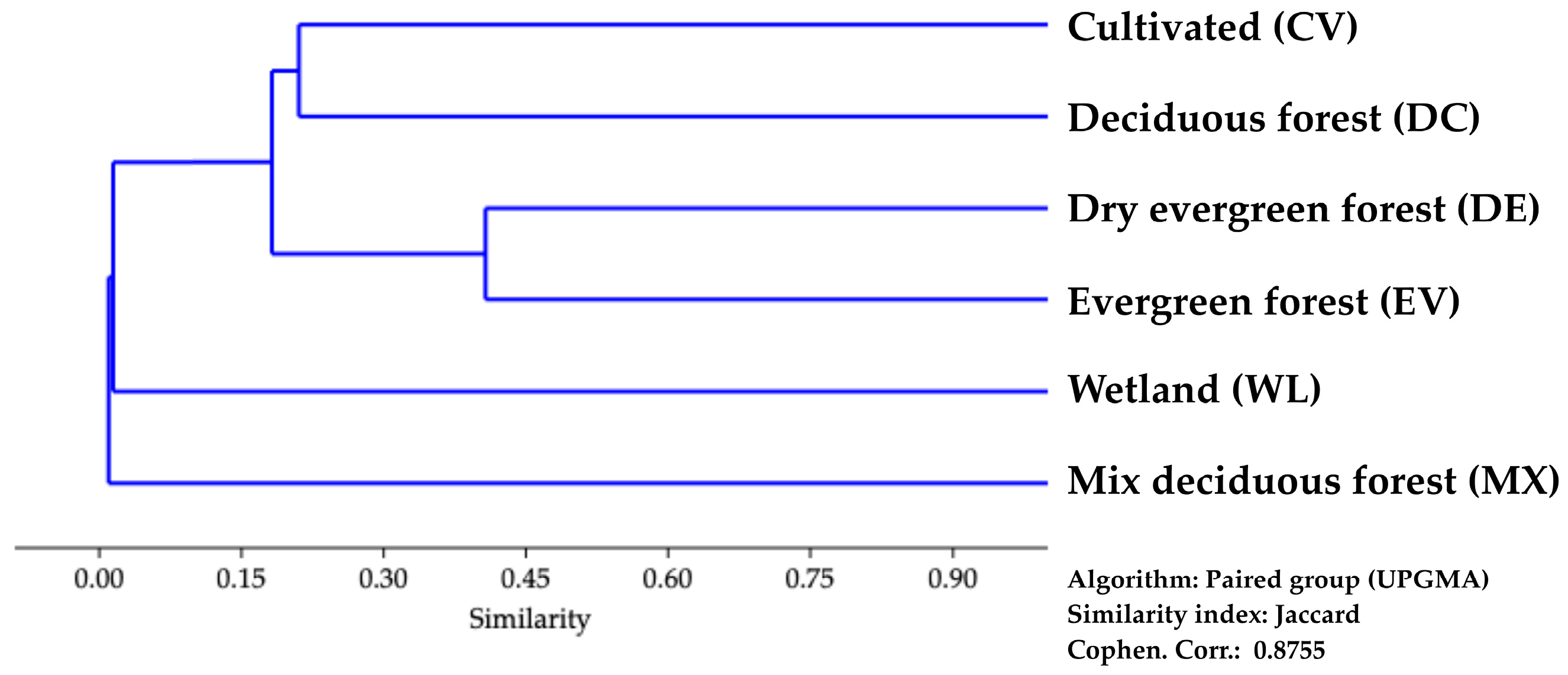
3.1.1. Habitats
3.1.2. Phenology
3.2. Ethnobotany: Utilization of Zingiberaceae in Roi Et Province
3.2.1. Zingiberaceae Species Used as Food
3.2.2. Zingiberaceae Species Used as Spices
3.2.3. Zingiberaceae Species Used for Ornamentals
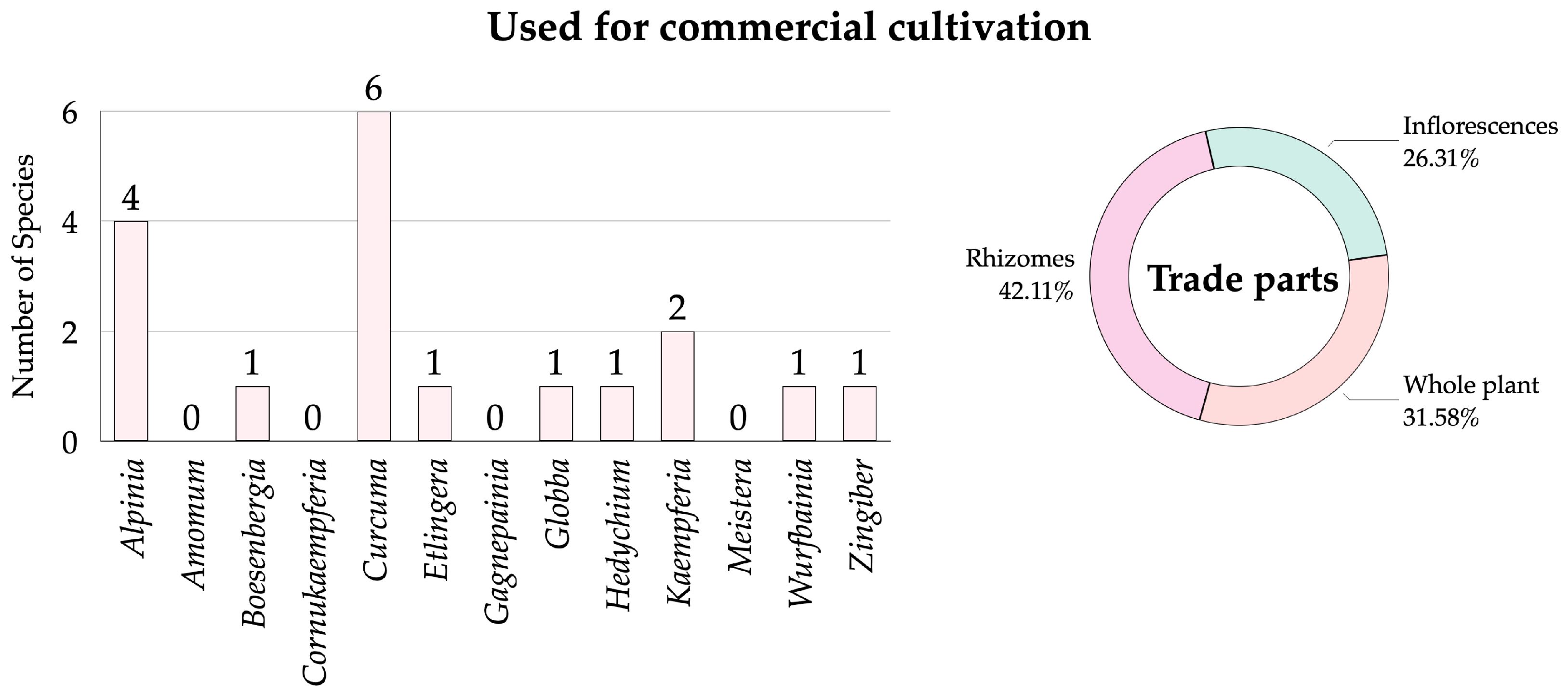
3.2.4. Zingiberaceae Species Used for Commercial Cultivation
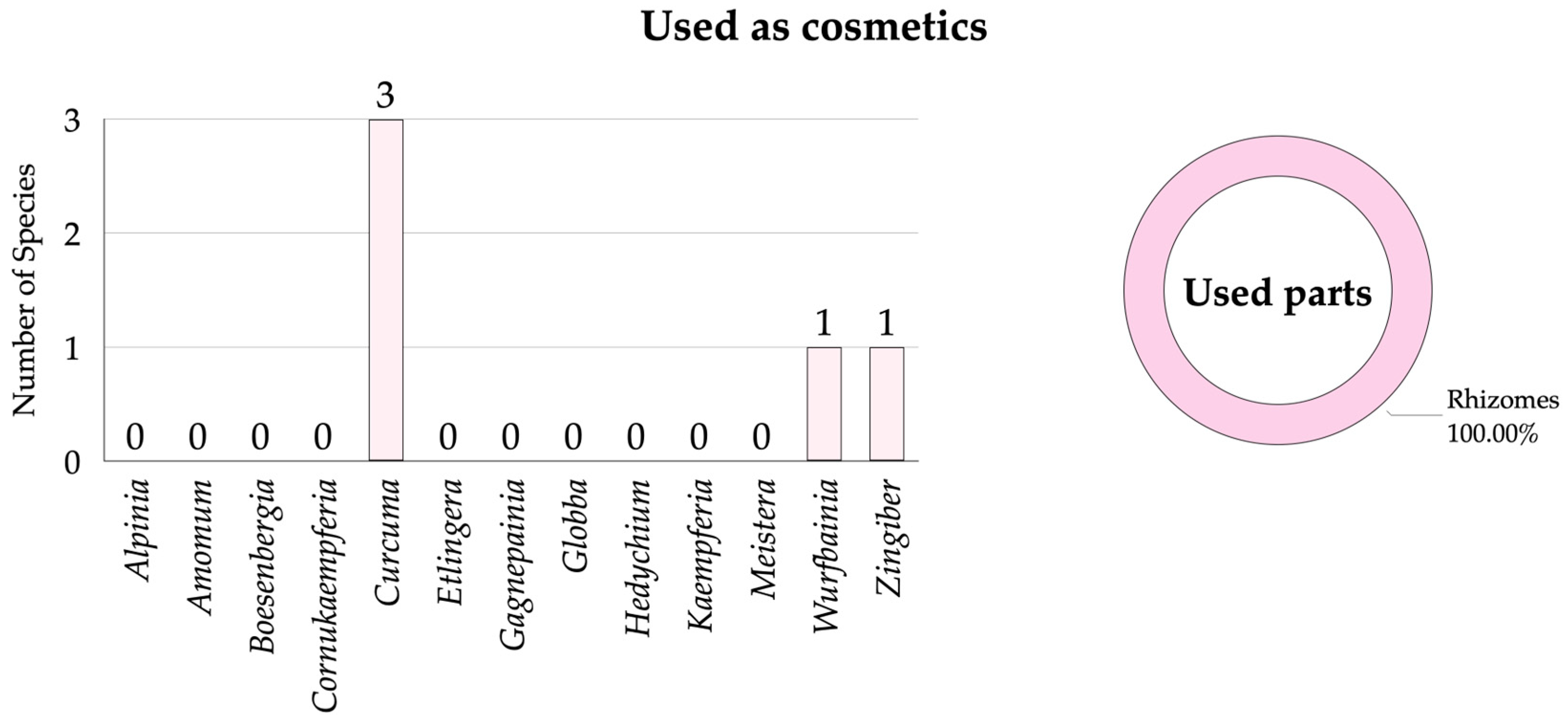
3.2.5. Zingiberaceae Species Used as Cosmetics
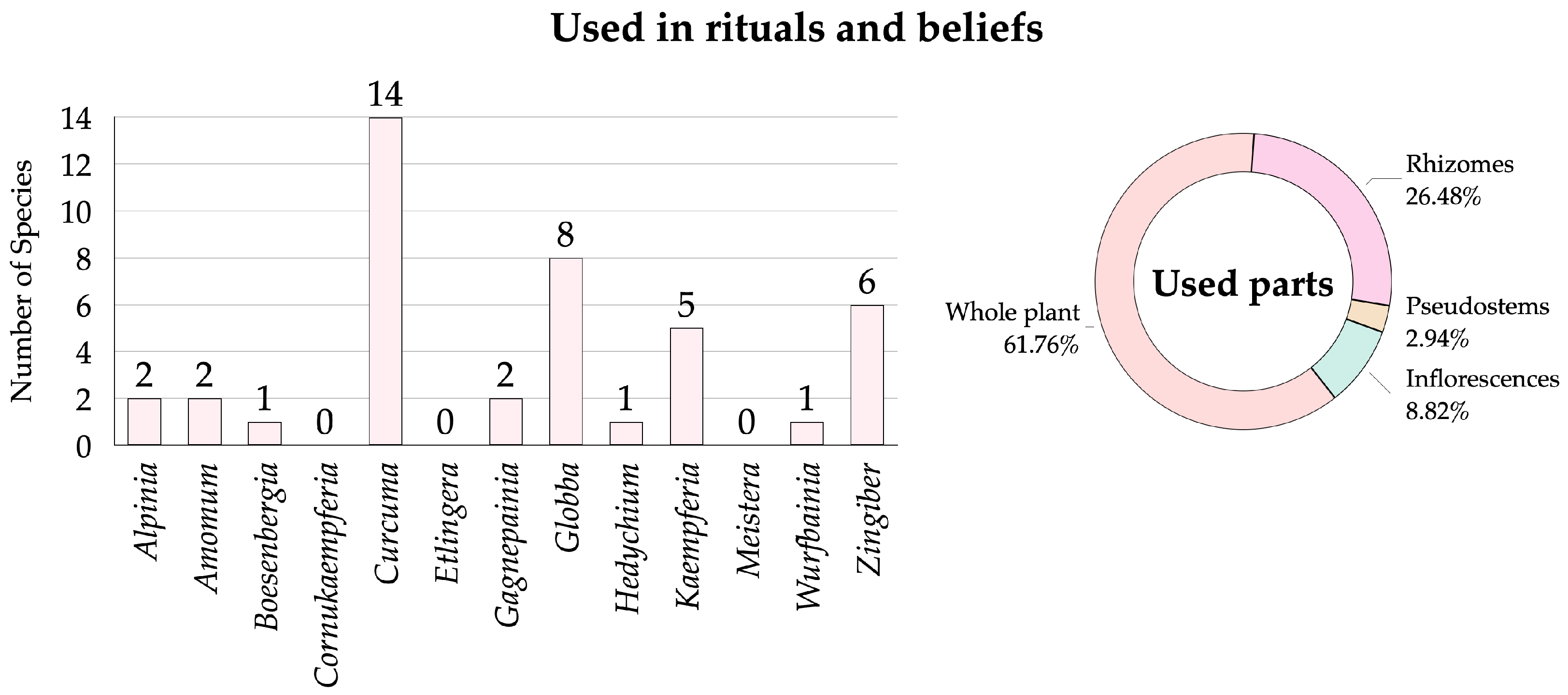
3.2.6. Zingiberaceae Species Used in Rituals and Beliefs
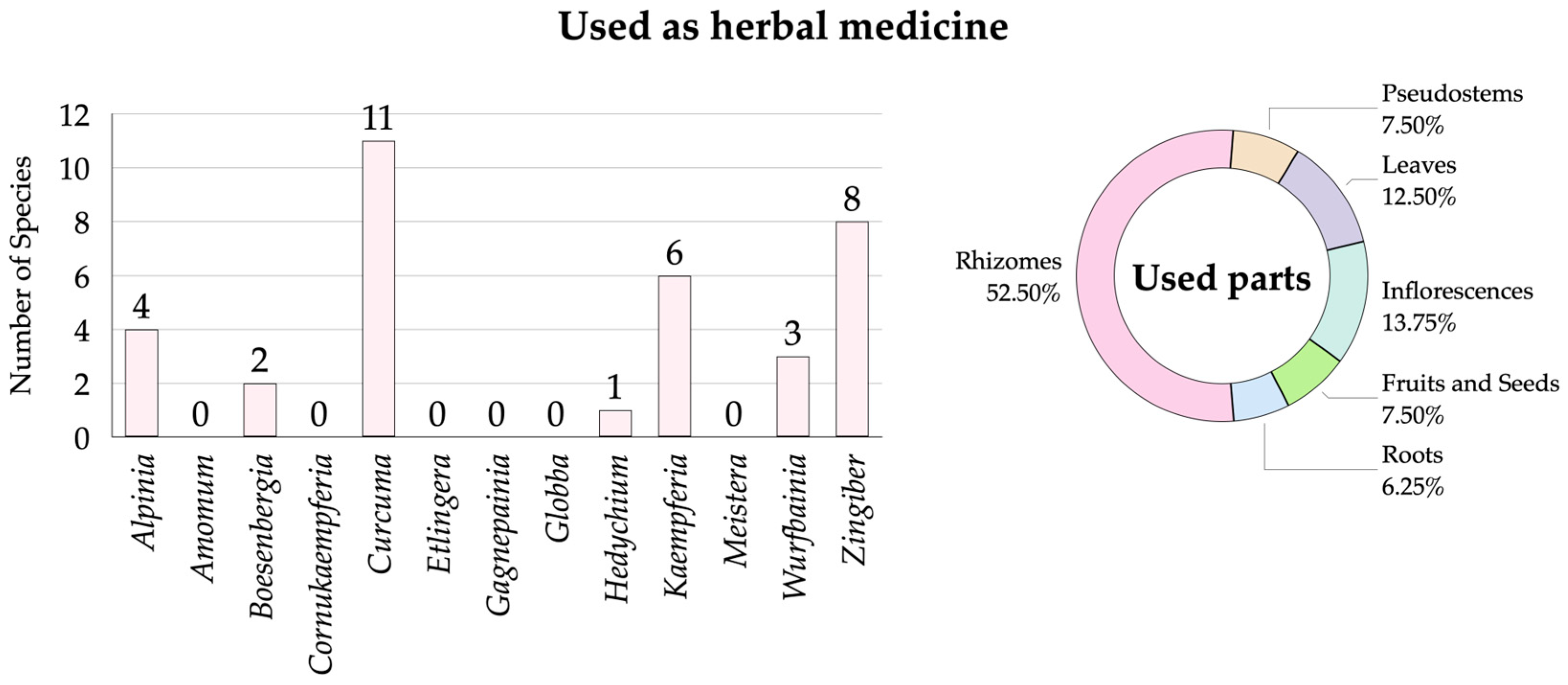
3.2.7. Zingiberaceae Species Used as Herbal Medicine
- Informant Consensus Factor of Zingiberaceae plants in Roi Et Province
- Fidelity Level of Zingiberaceae Plants in Roi Et Province
3.3. Species Use Value (SUV) of Zingiberaceae in Roi Et Province
3.4. Genera Use Value of Zingiberaceae in Roi Et Province
3.5. Relative Frequency of Citation of Zingiberaceae Plants in Roi Et Province
3.6. Economic Values of Zingiberaceae Plants in Roi Et Province
3.7. Conservation Status of Zingiberaceae in Roi Et Province
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plant of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org (accessed on 31 January 2025).
- Jitpromma, T.; Saensouk, S.; Saensouk, P.; Boonma, T. Diversity, Traditional Uses, Economic Values, and Conservation Status of Zingiberaceae in Kalasin Province, Northeastern Thailand. Horticulturae 2025, 11, 247. [Google Scholar] [CrossRef]
- Boonma, T.; Saensouk, S.; Saensouk, P. Biogeography, Conservation Status, and Traditional Uses of Zingiberaceae in Saraburi Province, Thailand, with Kaempferia chaveerachiae sp. nov. Horticulturae 2024, 10, 934. [Google Scholar] [CrossRef]
- Tangjitman, K.; Wongsawad, C.; Kamwong, K.; Sukkho, T.; Trisonthi, C. Ethnomedicinal plants used for digestive system disorders by the Karen of northern Thailand. J. Ethnobiol. Ethnomed. 2015, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Phumthum, M.; Balslev, H.; Kantasrila, R.; Kaewsangsai, S.; Inta, A. Ethnomedicinal Plant Knowledge of the Karen in Thailand. Plants 2020, 9, 813. [Google Scholar] [CrossRef]
- Phechphakdee, T.; Saensouk, S.; Saensouk, P. The diversity of Zingiberaceae in Pathumrat District, Roi-Et Province. Khon Kaen Agr. J. 2019, 47 (Suppl. S1), 1527–1532. [Google Scholar]
- Phechphakdee, T.; Saensouk, S.; Saensouk, P. Diversity, Conservation Status and Traditional uses of Family Zingiberaceae in Nong Phok District, Roi Et Province. Koch Cha Sarn J. Sci. 2020, 42, 70–82. Available online: https://li01.tci-thaijo.org/index.php/kochasarn/article/view/253190 (accessed on 31 March 2025).
- Newman, M.F.; Barfod, A.S.; Esser, H.J.; Simpson, D.; Parnell, J.A.N. Flora of Thailand Volume 16 Part 2; The Forest Herbarium, Department of National Parks, Wildlife and Plant Conservation: Bangkok, Thailand, 2023. [Google Scholar]
- Lu, Z.; Chen, H.; Lin, C.; Ou, G.; Li, J.; Xu, W. Ethnobotany of medicinal plants used by the Yao people in Gongcheng County, Guangxi, China. J. Ethnobiol. Ethnomed. 2022, 18, 1–37. [Google Scholar] [CrossRef]
- Yang, R.; Pei, S.; Xie, Y.; Yan, X.; Inta, A.; Yang, L. Ethnobotanical Research on Dye Plants Used by the Baiyi Indigenous Peoples’ from Heqing County, Dali, Yunnan, China. Diversity 2023, 15, 856. [Google Scholar] [CrossRef]
- Nahdi, M.S.; Martiwi, I.N.A.; Arsyah, D.C. The ethnobotany of medicinal plants in supporting the family health in Turgo, Yogyakarta, Indonesia. Biodiversitas 2016, 17, 900–906. [Google Scholar] [CrossRef]
- Retana-Cordero, M.; Fisher, P.R.; Gómez, C. Modeling the Effect of Temperature on Ginger and Turmeric Rhizome Sprouting. Agronomy 2021, 11, 1931. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria Version 16. 2024. Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf (accessed on 31 March 2025).
- Aishwarya, K.; Sabu, M. Boesenbergia pulcherrima and B. tiliifolia (Zingiberaceae) in India: Notes on the identity, variability and typification. Rheedea 2015, 25, 59–68. [Google Scholar]
- Aishwarya, K.; Sabu, M. On the IUCN status of Boesenbergia albolutea and B. rubrolutea (Zingiberaceae) and typification of B. rubrolutea. J. Threat. Taxa 2021, 13, 20133–20135. Available online: https://threatenedtaxa.org/index.php/JoTT/article/view/6707 (accessed on 31 March 2025). [CrossRef]
- Aishwarya, K.; Prabhu Kumar, K.M.; Sabu, M. Boesenbergia kingii (Zingiberaceae): A new record for South India. Webbia 2015, 70, 319–322. [Google Scholar] [CrossRef]
- Aishwarya, K.; Vinitha, M.R.; Thomas, G.; Sabu, M. A new species of Boesenbergia and rediscovery of B. rotunda (Zingiberaceae) from India. Phytotaxa 2015, 197, 186–196. [Google Scholar] [CrossRef]
- Chen, J.; Xia, N.H. A taxonomic revision of Chinese Boesenbergia (Zingiberaceae), with a new record. Phytotaxa 2019, 424, 217–231. [Google Scholar] [CrossRef]
- Saensouk, P.; Boonma, T.; Saensouk, S. Curcuma pulcherrima (Zingiberaceae), a new rare species of Curcuma subgen. Ecomata from eastern Thailand. Biodiversitas 2022, 23, 6635–6644. [Google Scholar] [CrossRef]
- Lam, N.F.; Ibrahim, H.; Sam, Y.Y.; Zakaria, R.M.; Poulsen, A.D. Two new species of Boesenbergia (Zingiberaceae) from Sabah, Malaysia. Phytokeys 2022, 211, 81–92. [Google Scholar] [CrossRef]
- Lam, N.F.; Ibrahim, H.; Sam, Y.Y.; Zakaria, R.M.; Poulsen, A.D. Three new species of Boesenbergia (Zingiberaceae) from Sabah, Malaysia. Phytokeys 2024, 247, 39–53. [Google Scholar] [CrossRef]
- Li, W.J.; Yang, H.J.; Ye, Y.S.; Kang, Y.; Zhang, W.; Jiang, X.L.; Pan, Y.X.; Zheng, X.L. Boesenbergia quangngaiensis N.S.Lý, a newly recorded species of Zingiberaceae from China. J. Trop. Subtrop. Bot. 2020, 28, 241–244. [Google Scholar] [CrossRef]
- Saravanan, T.S.; Kaliamoorthy, S. Boesenbergia kalakadensis (Zingiberaceae), a new species from southern Western Ghats, India. Nord. J. Bot. 2024, 2024, e04445. [Google Scholar]
- Saensouk, S.; Boonma, T.; Saensouk, P. Curcuma achrae (Zingiberaceae), a new species from Central Thailand. Rheedea 2022, 32, 30–45. Available online: http://rheedea.in/storages/submission/file/492773023.pdf (accessed on 31 January 2025).
- Saensouk, P.; Boonma, T.; Rakarcha, S.; Maknoi, C.; Wongnak, M.; Saensouk, S. Two new species of Curcuma subgenus Ecomata (Zingiberaceae: Zingibereae), from Central and Southwestern Thailand. Biodiversitas 2022, 23, 4578–4588. Available online: https://smujo.id/biodiv/article/view/11872 (accessed on 31 January 2025). [CrossRef]
- Nguyen, D.D.; Le, T.A.; Hoang, Q.H.; Le, Q.T.; Nguyen, E. Two new taxa of Curcuma subgen. Ecomata (Zingiberaceae: Zingibereae), from coastal Central Vietnam. Biodiversitas 2022, 23, 2512–2519. [Google Scholar] [CrossRef]
- Saensouk, P.; Boonma, T.; Maknoi, C.; Saensouk, S. Curcuma ubonensis (Zingiberaceae), a new species of Curcuma subgen. Hitcheniopsis from Eastern Thailand. Not. Bot. Horti. Agrobo. 2023, 51, 13374. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, N.A.; Averyanov, L.; Nguyen, D.D.; Le, C.T. Curcuma tuanii (Zingiberaceae) a new species of subgenus Ecomata from Vietnam based on morphological and molecular evidence. Acta Bot. Bras. 2023, 37, e20230028. [Google Scholar] [CrossRef]
- Mood, J.D.; Veldkamp, J.F.; Dey, S.; Prince, L.M. Nomenclatural changes in Zingiberaceae: Caulokaempferia is a superfluous name for Monolophus and Jirawongsea is reduced to Boesenbergia. Gard. Bull. Sing. 2014, 66, 215–231. [Google Scholar]
- Mood, J.D.; Tanaka, N.; Aung, M.M.; Murata, J. The genus Boesenbergia (Zingiberaceae) in Myanmar with two new records. Gard. Bull. Sing. 2016, 68, 299–318. [Google Scholar] [CrossRef]
- Mood, J.D.; Trần, H.Đ.; Prince, L.M.; Veldkamp, J.F. Boesenbergia siphonantha (Zingiberaceae)—A new record for Thailand and Vietnam. Gard. Bull. Sing. 2016, 68, 125–137. [Google Scholar] [CrossRef]
- Lý, N.S. Boesenbergia quangngaiensis (Zingiberaceae), a new species from Central Vietnam. Phytotaxa 2017, 324, 83–88. [Google Scholar] [CrossRef]
- Tanaka, N.; Tagane, S.; Naiki, A.; Aung, M.M.; Tanaka, N.; Dey, S.; Mood, J.; Murata, J. Contributions to the Flora of Myanmar I: Nine taxa of monocots newly recorded from Myanmar. Bull. Natl. Mus. Nat. Sci. Ser. B 2018, 44, 31–39. [Google Scholar]
- Mood, J.D.; Trần, H.Đ.; Veldkamp, J.F.; Prince, L.M. Taxonomy of Boesenbergia parvula (Zingiberaceae) with new synonymy. Thai. For. Bull. Bot. 2018, 46, 10–24. [Google Scholar] [CrossRef]
- Mood, J.D.; Prince, L.M.; Veldkamp, J.F.; Dey, S. The history and identity of Boesenbergia longiflora (Zingiberaceae) and descriptions of five related new taxa. Gard. Bull. Sing. 2013, 65, 47–95. [Google Scholar]
- Mood, J.D.; Ardiyani, M.; Veldkamp, J.F.; Mandáková, T.; Prince, L.M.; de Boer, H.J. Nomenclatural changes in Zingiberaceae: Haplochorema is reduced to Boesenbergia. Gard. Bull. Sing. 2020, 72, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Saensouk, P.; Saensouk, S.; Maknoi, C.; Setyawan, A.D.; Boonma, T. A horticultural Gem Unveiled: Curcuma peninsularis sp. nov. (Zingiberaceae), a new species from Peninsular Thailand, previously misidentified as Curcuma aurantiaca Zijp. Horticulturae 2024, 10, 950. [Google Scholar] [CrossRef]
- Binh, N.Q.; Tuan, H.A.; Dat, N.V.; Thanh, T.T.V.; Hanh, N.P.; Cuong, N.M.; Thanh, N.T. A new record species for Flora of Vietnam—Curcuma singularis Gagnep. (Zingiberaceae). VNU J. Sci. Nat. Sci. Technol. 2017, 33, 25–29. [Google Scholar]
- Chaveerach, A.; Mokkamul, P.; Sudmoon, R.; Tanee, T.; Garcia, V.F. A new species of Stahlianthus (Zingiberaceae) from Northeastern Thailand. Taiwania 2007, 52, 315–319. [Google Scholar]
- Chaveerach, A.; Mokkamul, P.; Sudmoon, R.; Tanee, T. A new species of Alpinia Roxb. (Zingiberaceae) from Northeastern Thailand. Taiwania 2008, 53, 1–5. [Google Scholar]
- Boonma, T.; Saensouk, S.; Saensouk, P. Kaempferia sipraiana (Zingiberaceae), a new species from Thailand and a new record of Kaempferia pseudoparviflora for Myanmar. Biodiversitas 2022, 23, 2203–2211. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Boonma, T.; Song, D.; Maknoi, C.; Setyawan, A.D. Boesenbergia Kuntze (Zingiberaceae) in Cambodia: Four New Records with Notes on Their Potential Horticultural Significance, Cultivation Guidelines, and Lectotypification of B. xiphostachya (Gagnep.) Loes. Horticulturae 2025, 11, 178. [Google Scholar] [CrossRef]
- Saensouk, P.; Boonma, T.; Saensouk, S. Revision of the genus Cornukaempferia Mood & K.Larsen (Zingiberaceae), and a new species from Thailand. Biodiversitas 2022, 23, 5718–5729. [Google Scholar] [CrossRef]
- Ye, X.B.; Chen, J.; Liu, N. Curcuma nankunshanensis (Zingiberaceae), a New Species from China. J. Trop. Subtrop. Bot. 2008, 16, 472–476. [Google Scholar]
- Wongsuwan, P. A New Species of Hedychium (Zingiberaceae) from Southern Laos. Taiwania 2008, 53, 401–405. [Google Scholar]
- Wongsuwan, P.; Meechonkit, P.; Phokham, B.; Sangnark, S.; Yupparach, P.; Picheansoonthon, C. A New Species of Kaempferia (Zingiberaceae) from Northern Thailand. J. Jpn. Bot. 2020, 95, 34–38. [Google Scholar] [CrossRef]
- Wongsuwan, P.; Picheansoonthon, C. Taxonomic Revision of the Genus Hedychium J.Koenig (Zingiberaceae) in Thailand (Part 1). J. R. Inst. Thail. 2011, 3, 126–149. [Google Scholar]
- Wongsuwan, P.; Picheansoonthon, C. Taxonomic Revision of the Genus Hedychium J.Koenig (Zingiberaceae) in Thailand (Part 2). J. R. Inst. Thail. 2012, 4, 250–267. [Google Scholar]
- Wongsuwan, P.; Phokham, B.; Yupparach, P.; Picheansoonthon, C. A New Caulokaempferia (Zingiberaceae) from Northeast Thailand. J. Jpn. Bot. 2020, 95, 321–326. [Google Scholar]
- Wongsuwan, P.; Prasarn, S.; Picheansoonthon, C. Kaempferia koontermii (Zingiberaceae)—A New Species from Thailand. J. Jpn. Bot. 2015, 90, 29–33. [Google Scholar] [CrossRef]
- Wong, S.Y.; Ooi, I.H.; Boyce, P.C. A New Haniffia Species (Zingiberaceae) and a New Generic Record from Sarawak, Malaysian Borneo. Bot. Stud. 2014, 55, 51. [Google Scholar] [CrossRef]
- Veldkamp, J.F. The Valid Publication of Monolophus (Zingiberaceae) Revisited. Gard. Bull. Singap. 2016, 68, 173–174. [Google Scholar] [CrossRef]
- Triboun, P. Biogeography and Biodiversity of the Genus Zingiber in Thailand. Ph.D. Thesis, Khon Kaen University, Khon Kaen, Thailand, 2006. [Google Scholar]
- Triboun, P.; Chantaranothai, P.; Larsen, K. Taxonomic changes regarding three species of Zingiber (Zingiberaceae) from Thailand. Acta Phytotax. Sin. 2007, 45, 403–404. [Google Scholar] [CrossRef]
- Triboun, P.; Larsen, K.; Chantaranothai, P. A key to the genus Zingiber (Zingiberaceae) in Thailand with descriptions of 10 new taxa. Thai J. Bot. 2014, 6, 53–77. [Google Scholar]
- Theerakulpisut, P.; Triboun, P.; Mahakham, W.; Maensiri, D.; Khampila, J.; Chantaranothai, P. Phylogeny of the genus Zingiber (Zingiberaceae) based on nuclear ITS sequence data. Kew Bull. 2012, 67, 389–395. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Tangjitman, K. Ethnobotany of the Karen at Huay Nam Nak village, Tanaosri subdistrict, Suanphueng district, Ratchaburi province. Thai J. Bot. 2017, 9, 253–272. [Google Scholar]
- Tan, S.; Hollands, R.; Pavlíková, M.; Fér, T.; Newman, M. A revision of Gagnepainia and Hemiorchis (Globbeae: Zingiberaceae). Edinb. J. Bot. 2020, 77, 455–490. [Google Scholar] [CrossRef]
- Sakhanokho, H.F.; Rajasekaran, K. Pollen biology of ornamental ginger (Hedychium spp. J. Koenig). Sci. Hortic. 2010, 125, 129–135. [Google Scholar] [CrossRef]
- Salasiah, M.; Meekiong, K. Plagiostachys strobilifera var. conica, a new variety from Sarawak, Borneo. Reinwardtia 2020, 19, 109–116. [Google Scholar] [CrossRef]
- Sam, Y.Y.; Ibrahim, H.; Saw, L.G. Four new species of Scaphochlamys (Zingiberaceae) from Peninsular Malaysia. Phytotaxa 2015, 221, 21–34. [Google Scholar] [CrossRef][Green Version]
- Sam, Y.Y.; Julius, A.; Chew, M.Y. Haniffia flavescens (Zingiberaceae): A new species from Peninsular Malaysia. Bot. Stud. 2009, 50, 359–364. [Google Scholar]
- Sakai, S.; Nagamasu, H. Notes on inflorescence structure of Boesenbergia (Zingiberaceae). Acta Phytotax. Geobot. 2006, 57, 107–111. [Google Scholar]
- Phokham, B.; Wongsuwan, P.; Picheansoonthon, C. Three new species of Kaempferia (Zingiberaceae) from Thailand and Laos. J. Jpn. Bot. 2013, 88, 297–308. [Google Scholar] [CrossRef]
- Muangyen, N.; Pongamornkul, W.; Maknoi, C. Rhynchanthus bluthianus (Zingiberaceae), a new record for Thailand. Thai J. Bot. 2015, 7, 79–81. [Google Scholar]
- Mohamad, S.; Kalu, M. Assessment of Zingiberaceae (Tribe Alpinieae) from North East Sarawak, Malaysia. IOP Conf. Ser. Earth Environ. Sci. 2019, 269, 012032. [Google Scholar] [CrossRef]
- Maknoi, C.; Sirirugsa, P. New records of Zingiberaceae from Southern Thailand. Nat. Hist. Bull. Siam Soc. 2002, 50, 225–237. [Google Scholar]
- Maknoi, C.; Sirirugsa, P. The Genus Curcuma L. (Zingiberaceae) in Thailand; The Botanical Garden Organization: Chiang Mai, Thailand, 2012. [Google Scholar]
- Maknoi, C.; Sirirugsa, P.; Larsen, K. New records of Curcuma L. (Zingiberaceae) in Thailand. Thai For. Bull. (Bot.) 2005, 33, 71–74. [Google Scholar]
- Maknoi, C.; Sirirugsa, P.; Larsen, K. Curcuma bella (Zingiberaceae), a new species from Thailand. Thai J. Bot. 2011, 3, 121–124. [Google Scholar]
- Latiff, A.; Roslan, I. A revision on Hornstedtia Retz. (Zingiberaceae) in Peninsular Malaysia. Malay. Nat. J. 2015, 67, 328–341. [Google Scholar]
- Lau, K.H. The conservation of Peninsular Malaysian Geostachys (Zingiberaceae). Gard. Bull. Singap. 2014, 66, 3–14. [Google Scholar]
- Lau, K.H.; Lim, C.K. Revision of the genus Geostachys (Zingiberaceae) in Peninsular Malaysia. Folia Malays. 2012, 13, 7–42. [Google Scholar]
- Lau, K.H.; Lim, C.K.; Mat-Salleh, K. Materials for a taxonomic revision of Geostachys (Baker) Ridl. (Zingiberaceae) in Peninsular Malaysia. Gard. Bull. Singap. 2007, 59, 129–138. [Google Scholar]
- Kaewsri, W. Amomum tomrey Gagnep. (Zingiberaceae), a new record for Thailand. Thai For. Bull. (Bot.) 2012, 40, 17–19. [Google Scholar]
- Kaewsri, W.; Kanjanawattanawong, S. Amomum spathilabium (Zingiberaceae: Alpinieae), a new species from Northern Thailand. Thai For. Bull. (Bot.) 2019, 47, 193–195. [Google Scholar] [CrossRef]
- Kaewsri, W.; Phankamolsil, Y. A new synonym for Amomum molle Ridl. (Zingiberaceae). Nat. Hist. Bull. Siam Soc. 2012, 58, 53–57. [Google Scholar]
- Kaewsri, W.; Paisooksantivatana, Y. Morphology and Palynology of Amomum Roxb. in Thailand. Gard. Bull. Singap. 2007, 59, 105–112. [Google Scholar]
- Kaewsri, W.; Paisooksantivatana, Y.; Veesommai, U. A new record and a new synonym in Amomum Roxb. (Zingiberaceae) in Thailand. Thai For. Bull. (Bot.) 2009, 37, 32–35. [Google Scholar]
- Chen, J.; Xia, N.H. Curcuma gulinqingensis sp. nov. (Zingiberaceae) from Yunnan, China. Nord. J. Bot. 2013, 31, 711–716. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Y.S.; Xia, N.H. Curcuma ruiliensis (Zingiberaceae), a new species from Yunnan, China. Nord. J. Bot. 2021, 39, e02910. [Google Scholar] [CrossRef]
- Jaccard, P. Lois de distribution florale dans la zone alpine. Bull. Soc. Vaud. Sci. Nat. 1902, 38, 69–130. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 31 March 2025).
- Phillips, O.; Gentry, A.H. The useful plants of Tambopata, Peru: I. Statistical hypotheses tests with a new quantitative technique. Econ. Bot. 1993, 47, 15–32. [Google Scholar] [CrossRef]
- Hoffman, B.; Gallaher, T. Importance indices in ethnobotany. Ethnobot. Res. Appl. 2007, 5, 201. Available online: https://ethnobotanyjournal.org/index.php/era/article/view/130/115 (accessed on 31 March 2025). [CrossRef]
- Vitalini, S.; Iriti, M.; Puricelli, C.; Ciuchi, D.; Segale, A.; Fico, G. Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—An alpine ethnobotanical study. J. Ethnopharmacol. 2013, 145, 517–529. [Google Scholar] [CrossRef]
- Trotter, R.T.; Logan, M.H. Informant consensus: A new approach for identifying potentially effective medicinal plants. In Plants in Indigenous Medicine and Diet: Biobehavioral Approaches; Etkin, N.L., Ed.; Redgrave Publishers: Bedford Hills, NY, USA, 1986; pp. 91–112. [Google Scholar]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. Medicinal plants in Mexico: Healers’ consensus and cultural importance. Soc. Sci. Med. 1998, 47, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Yaniv, Z.; Dafni, A.; Palewitch, D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Saensouk, S.; Saensouk, P. Kaempferia mahasarakhamensis, a new species from Thailand. Taiwania 2019, 64, 39–42. [Google Scholar] [CrossRef]
- Dolase, P.; Chaudhari, V. Review on cultivation practices of Haridra (Curcuma longa Linn.). Int. J. Ayurveda Pharm. Res. 2024, 12, 56–61. [Google Scholar] [CrossRef]
- Giang, L.D.; Tran-Trung, H.; Thuy, P.T.; An, N.T.G.; Nguyen-Ngoc, H.; Nguyen, T.H.D.; Nguyen, D.K.; Nguyen, A.V.; Chen, T.V.; Ha, N.X.; et al. Chemical Constituents, Antioxidant, and Antimicrobial Activities of Ethyl Acetate Fractionated Extract from Rhizomes of Zingiber monophyllum Gagnep.: In vitro and in silico Screenings. Nat. Prod. Commun. 2024, 19, 3443. [Google Scholar] [CrossRef]
- Tran-Trung, H.; Le, D.G.; Hoang, V.T.; Vu, D.C.; Thang, T.D.; Nguyen-Ngoc, H.; Tran Van, C.; Nguyen, T.T.; Tuan, N.H.; Nguyen, T.H.D. Essential Oils of Two Species of the Zingiberaceae Family from Vietnam: Chemical Compositions and α-Glucosidase, α-Amylase Inhibitory Effects. Nat. Prod. Commun. 2024, 19, 2281. [Google Scholar] [CrossRef]
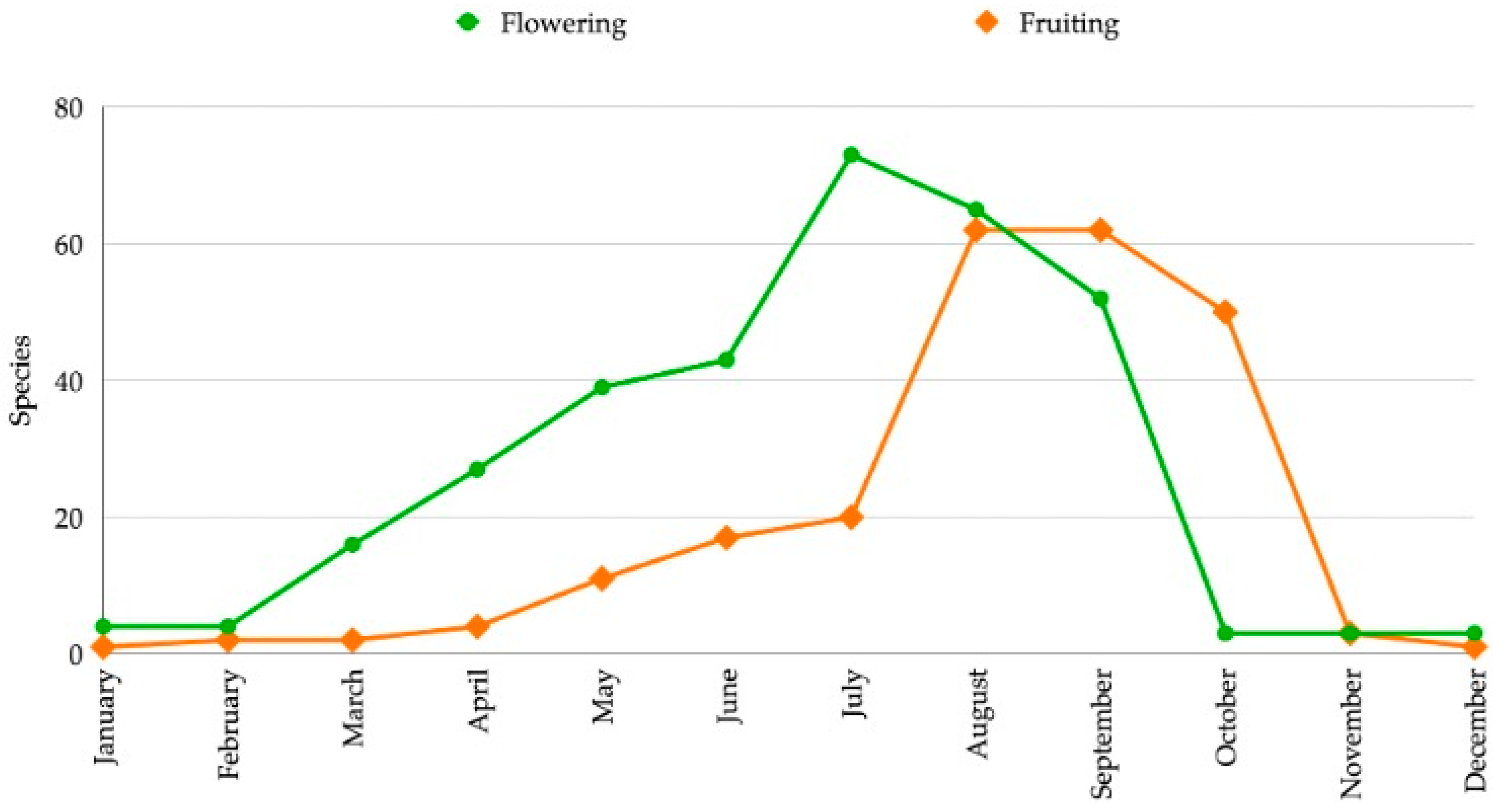
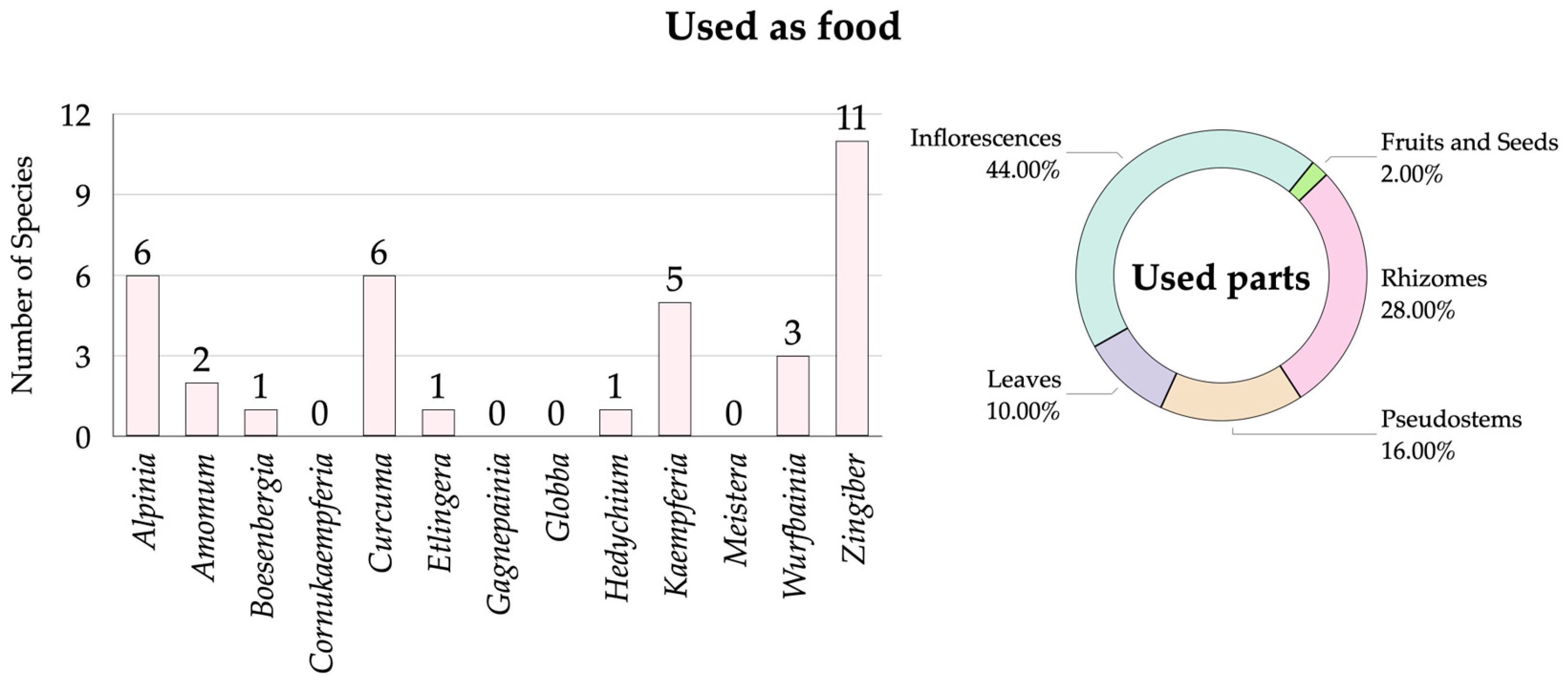
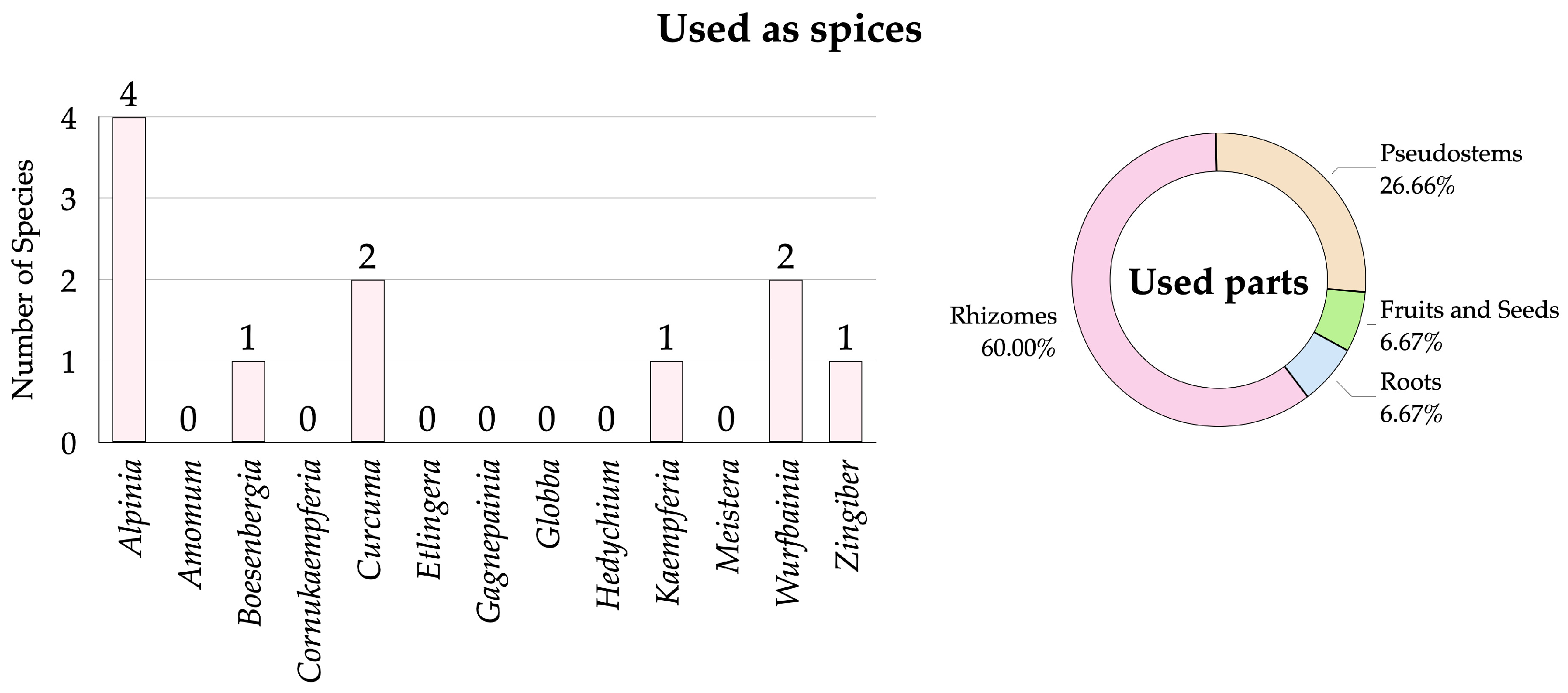
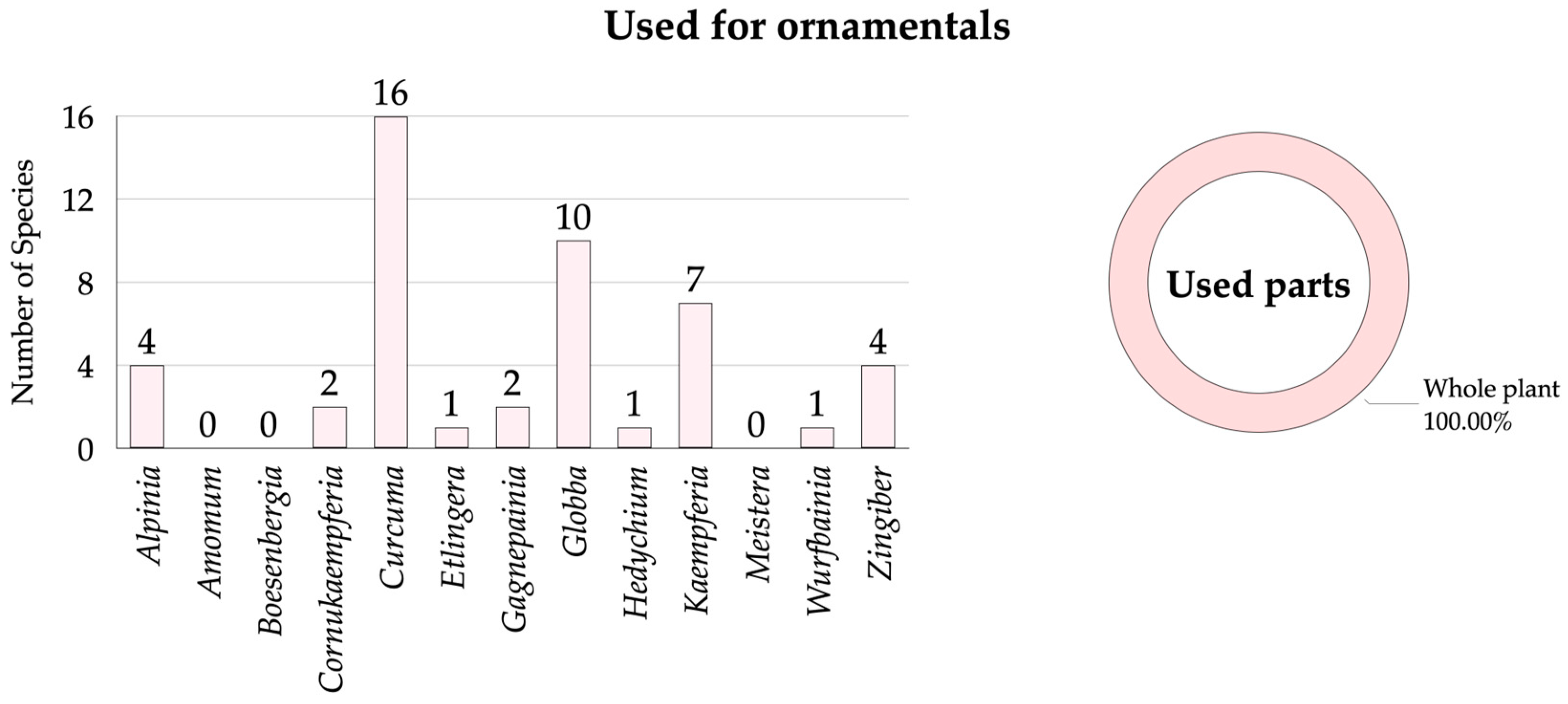
| No. | Scientific Name | Vernacular Name | Distribution for Thailand | Occurrence 1 | Habitats 2 | Phenology 3 | Utilization 4 | SUV | RFC | Conservation Status 5 | Voucher Specimens | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flowering | Fruiting | Purpose | Used Parts | IUCN | Author Proposed | |||||||||
| 1. | Alpinia conchigera Griff. | Kha Ling | Native | C | CV | 6–9 | 8–9 | FD, MP, SP | Rz, In | 0.068 | 0.038 | LC | ZR001 | |
| 2. | Alpinia galanga (L.) Willd. | Kha | Native | B | DE, CV | 5–9 | 7–10 | CC, FD, MP, SP | Rz, Ps, In, Lv, Wp | 2.043 | 0.920 | NE | LC | ZR002 |
| 3. | Alpinia laosensis Gagnep. | Kha Ling Dok Daeng | Native | B | DE, EV, CV | 6–9 | 7–10 | FD | Ps, In | 0.013 | 0.013 | NE | LC | ZR003 |
| 4. | Alpinia latilabris Ridl. | Kha Pa | Native | B | DE, EV, CV | 11–2 | 2–4 | FD, ON, SP | Rz, Ps, In, Wp | 0.025 | 0.010 | LC | LC | ZR004 |
| 5. | Alpinia mutica Roxb. | Wan Saneh Haa | Native | C | CV | 3–8 | 5–10 | FD, MP, ON, RB | Rz, Ps, In, Wp | 0.213 | 0.128 | LC | ZR005 | |
| 6. | Alpinia purpurata (Vieill.) K.Schum. | Kha Daeng | Introduced | C | CV | 1–12 | Not seen | CC, ON | In, Wp | 0.210 | 0.165 | NE | ZR006 | |
| 7. | Alpinia siamensis K.Schum. | Kha Ta Daeng | Native | C | CV | 5–8 | 7–11 | CC, FD, MP, SP | Rz, Ps, In | 2.505 | 0.988 | NE | ZR007 | |
| 8. | Alpinia vittata W.Bull | Kha Daang | Introduced | C | CV | 6–8 | 7–9 | CC, ON, RB | Wp | 0.135 | 0.058 | NE | ZR008 | |
| 9. | Amomum foetidum Boonma and Saensouk | Ton Maeng Khaeng | Endemic | C | CV | 1–3 | Not seen | FD | Wp | 0.068 | 0.068 | NE | ZR009 | |
| 10. | Amomum repoeense Pierre ex Gagnep. | Raiw Pa | Native | B | DE, EV, CV | 4–7 | 6–9 | FD | Ps | 0.008 | 0.008 | LC | LC | ZR010 |
| 11. | Amomum trilobum Gagnep. | Pud Nhoo | Native | C | CV | 5–6 | 6–9 | RB | Wp | 0.083 | 0.083 | NE | ZR011 | |
| 12. | Amomum wandokthong (Picheans. and Yupparach) Škorničk. and Hlavatá | Wan Dok Thong | Endemic | C | CV | 4–10 | Not seen | RB | Wp | 0.135 | 0.135 | NE | ZR012 | |
| 13. | Boesenbergia parvula (Wall. ex Baker) Kuntze | Ka Tue Ling | Native | W | EV | 7–9 | 9–10 | NA | – | 0.000 | 0.000 | LC | EN | ZR013 |
| 14. | Boesenbergia rotunda (L.) Mansf. | Kha Chai | Native | B | MX, DE, CV | 7–9 | 9–10 | CC, FD, MP, SP | Rt, Rz, Ps | 1.933 | 0.980 | LC | VU | ZR014 |
| 15. | Boesenbergia xiphostachya (Gagnep.) Loes. | Ngon Nark | Native | B | DC, DE, CV | 5–8 | 8–10 | MP, RB | Rz, In, Wp | 0.058 | 0.030 | LC | VU | ZR015 |
| 16. | Cornukaempferia argentifolia Boonma and Saensouk | Proh Thong Bai Ngern | Endemic | C | CV | 7–9 | 8–10 | ON | Wp | 0.008 | 0.008 | NE | ZR016 | |
| 17. | Cornukaempferia srisumoniae P.Saensouk, Saensouk, and Boonma | Proh Thong Srisumon | Endemic | C | CV | 7–9 | 8–10 | ON | Wp | 0.005 | 0.005 | NE | ZR017 | |
| 18. | Curcuma aeruginosa Roxb. | Wan Maha Mek | Native | C | CV | 3–6 | Not seen | FD, MP, ON | Rz, In, Wp | 0.133 | 0.090 | LC | ZR018 | |
| 19. | Curcuma alismatifolia Gagnep. | Krachiao, Pathumma | Native | C | CV | 7–9 | 8–10 | FD, ON | In, Wp | 0.178 | 0.120 | NT | ZR019 | |
| 20. | Curcuma amada Roxb. | Kha Min Khaow Pa | Native | C | CV | 7–9 | Not seen | MP | Rz | 0.028 | 0.028 | NE | ZR020 | |
| 21. | Curcuma amarissima Roscoe | Kha Min Dum | Native | C | CV | 3–5 | Not seen | MP | Rz | 0.063 | 0.063 | NE | ZR021 | |
| 22. | Curcuma angustifolia Roxb. | Krachiao Daeng | Native | B | DC, CV | 3–9 | 5–10 | CC, FD, MP | Rz, In, Wp | 1.203 | 0.805 | NE | LC | ZR022 |
| 23. | Curcuma aromatica Salisb. | Wan Nang Kham | Native | C | CV | 3–5 | 4–10 | CM, MP | Rz | 0.173 | 0.153 | NE | ZR023 | |
| 24. | Curcuma borealis Saensouk, P.Saensouk, and Boonma | Krachiao Thep Apsorn | Endemic | C | CV | 4–7 | 7–9 | ON | Wp | 0.078 | 0.078 | NE | ZR024 | |
| 25. | Curcuma caesia Roxb. | Wan Mek Sit | Introduced | C | CV | 4–5 | Not seen | MP, RB | Rz, Wp | 0.030 | 0.020 | NE | ZR025 | |
| 26. | Curcuma campanulata (Kuntze) Škorničk. | Wan Duk Dae | Native | B | DC, CV | 3–5 | Not seen | RB | Wp | 0.005 | 0.005 | NE | VU | ZR026 |
| 27. | Curcuma clovisii Škorničk. | Jok Daeng | Native | C | CV | 5–6 | 6–9 | ON | Wp | 0.020 | 0.020 | NE | ZR027 | |
| 28. | Curcuma comosa Roxb. | Wan Chuck Mod Look | Native | C | CV | 3–5 | Not seen | CC, CM, MP | Rz, Wp | 0.173 | 0.135 | NE | ZR028 | |
| 29. | Curcuma gracillima Gagnep. | Krachiao Jew | Native | C | CV | 6–8 | 8–10 | ON | Wp | 0.010 | 0.010 | LC | ZR029 | |
| 30. | Curcuma harmandii Gagnep. | Chor Morrakot | Native | C | CV | 7–9 | 8–10 | ON, RB | Wp | 0.050 | 0.028 | LC | ZR030 | |
| 31. | Curcuma involucrata (King ex Baker) Škorničk. | Wan Phet Noi | Native | B | DC, CV | 5–6 | 6–8 | ON | Wp | 0.013 | 0.013 | NE | VU | ZR031 |
| 32. | Curcuma longa L. | Kha Min, Kha Min Chan | Introduced | C | CV | 7–9 | 8–10 | CC, CM, FD, MP, RB, SP | Rz, In, Wp | 1.863 | 0.970 | DD | ZR032 | |
| 33. | Curcuma mangga Valeton and Zijp | Kha Min Khaow | Introduced | C | CV | 3–5 | 5–6 | CC, FD, SP | Rz, In | 0.935 | 0.780 | DD | ZR033 | |
| 34. | Curcuma parviflora Wall. | Wan Thep Ram Luek | Native | B | DC, DE, CV | 7–9 | 8–10 | ON | Wp | 0.280 | 0.280 | NE | VU | ZR034 |
| 35. | Curcuma pedicellata (Chaveer. and Mokkamul) Škorničk. | Wan Phet Phraiwan | Endemic | C | CV | 6–8 | Not seen | ON, RB | Wp | 0.050 | 0.030 | NE | ZR035 | |
| 36. | Curcuma peninsularis Saensouk, P.Saensouk, Maknoi, and Boonma | Krachiao Ploy Andaman | Endemic | C | CV | 6–9 | Not seen | ON | Wp | 0.058 | 0.058 | NE | ZR036 | |
| 37. | Curcuma peramoena Souvann. and Maknoi | Wan Houa Noi | Native | C | CV | 6–8 | Not seen | RB | Wp | 0.015 | 0.015 | NE | ZR037 | |
| 38. | Curcuma petiolata Roxb. | Bua Chan | Native | C | CV | 7–9 | 8–10 | ON, RB | Wp | 0.228 | 0.170 | DD | ZR038 | |
| 39. | Curcuma phrayawan Boonma and Saensouk | Phraya Wan | Endemic | C | CV | 7–9 | 8–10 | MP, RB | Rz, Wp | 0.103 | 0.053 | NE | ZR039 | |
| 40. | Curcuma rangjued Saensouk and Boonma | Rang Jued | Native | C | CV | 7–9 | 8–10 | MP | Rz | 0.118 | 0.118 | NE | ZR040 | |
| 41. | Curcuma rosea P.Saensouk, Saensouk, and Boonma | Maha Udom Umawadee | Endemic | C | CV | 7–9 | 8–10 | RB | Wp | 0.018 | 0.018 | NE | ZR041 | |
| 42. | Curcuma rubescens Roxb. | Wan Mahaprab | Introduced | C | CV | 3–5 | 4–7 | ON, RB | Wp | 0.078 | 0.055 | NE | ZR042 | |
| 43. | Curcuma saraburiensis Boonma and Saensouk | Wan Klom Nang None | Endemic | C | CV | 7–9 | 8–10 | RB | Wp | 0.005 | 0.005 | NE | ZR043 | |
| 44. | Curcuma siamensis Saensouk and Boonma | Kha Min Siam | Endemic | C | CV | 7–9 | 8–10 | RB | Wp | 0.008 | 0.008 | NE | ZR044 | |
| 45. | Curcuma singularis Gagnep. | Krachiao Khaow, Dok Din | Native | B | DC, CV | 3–6 | 5–7 | CC, FD | Rz, In | 1.325 | 0.988 | NE | VU | ZR045 |
| 46. | Curcuma suraponii Boonma | Wan Krabi Thong | Endemic | C | CV | 7–9 | Not seen | ON, RB | Wp | 0.125 | 0.108 | NE | ZR046 | |
| 47. | Curcuma thorelii Gagnep. | Krachiao Khaow | Native | B | DC, DE, CV | 7–9 | 8–10 | ON, RB | Wp | 0.163 | 0.128 | NE | VU | ZR047 |
| 48. | Curcuma ubonensis Boonma, Saensouk, Maknoi, and P.Saensouk | Krachiao Ubon | Endemic | C | CV | 7–9 | 8–10 | ON | Wp | 0.005 | 0.005 | NE | ZR048 | |
| 49. | Curcuma wanchaii Saensouk, P.Saensouk, Maknoi, and Boonma | Krachiao Wanchaii | Endemic | C | CV | 7–9 | 8–10 | ON | Wp | 0.005 | 0.005 | NE | ZR049 | |
| 50. | Curcuma wanenlueanga Saensouk, Thomudtha, and Boonma | Wan Enlueang | Endemic | C | CV | 7–9 | 8–10 | CC, MP | Rz | 0.103 | 0.080 | NE | ZR050 | |
| 51. | Etlingera elatior (Jack) R.M.Sm. | Da La | Native | C | CV | 1–12 | 1–12 | CC, FD, ON | Wp | 0.313 | 0.273 | DD | ZR051 | |
| 52. | Gagnepainia godefroyi (Baill.) K.Schum. | Wan Phet Na Tang | Native | C | CV | 4–6 | Not seen | ON, RB | Wp | 0.043 | 0.030 | LC | ZR052 | |
| 53. | Gagnepainia harmandii (Baill.) K.Schum. | Wan Morrakot | Native | B | DE, CV | 4–6 | Not seen | ON, RB | Wp | 0.035 | 0.028 | NE | VU | ZR053 |
| 54. | Globba cambodgensis Gagnep. | Khaophansa Hu Kra Tai | Native | C | CV | 6–8 | 8–10 | ON, RB | Wp | 0.025 | 0.013 | NE | ZR054 | |
| 55. | Globba cernua Baker | Pud Hin | Native | C | CV | 6–8 | 8–10 | ON, RB | Wp | 0.068 | 0.053 | LC | ZR055 | |
| 56. | Globba conferta M.F.Newman | Khaophansa Chor Tab Tim | Endemic | B | DC, DE, CV | 6–8 | 8–10 | ON, RB | Rz, Wp | 0.165 | 0.085 | LC | VU | ZR056 |
| 57. | Globba laeta K.Larsen | Khaophansa Khaow | Native | C | CV | 6–9 | 8–9 | ON, RB | Wp | 0.100 | 0.085 | EN | ZR057 | |
| 58. | Globba marantina L. | Wan E Moob | Native | B | DC, DE, CV | 7–9 | 8–10 | ON | Wp | 0.020 | 0.020 | LC | LC | ZR058 |
| 59. | Globba pendula Roxb. | Pud Nok Yoong | Native | C | CV | 6–7 | Not seen | ON, RB | Wp | 0.030 | 0.023 | LC | ZR059 | |
| 60. | Globba rosea Gagnep. | Khaophansa Chor Chomphoo | Native | B | DC, DE, CV | 7–9 | 8–10 | CC, ON, RB | Rz, Wp | 0.113 | 0.045 | NE | VU | ZR060 |
| 61. | Globba sherwoodiana W.J.Kress and V.Gowda | Khaophansa Khaow Phama | Native | C | CV | 7–9 | 8–10 | ON, RB | Wp | 0.140 | 0.140 | NE | ZR061 | |
| 62. | Globba schomburgkii Hook.f. | Khaophansa | Native | B | DC, DE, CV | 7–9 | 8–10 | ON | Wp | 0.088 | 0.053 | NE | LC | ZR062 |
| 63. | Globba williamsiana M.F.Newman and Sangvir. | Hong Hern | Endemic | C | CV | 7–9 | 8–10 | ON, RB | Wp | 0.365 | 0.190 | NE | ZR063 | |
| 64. | Hedychium coronarium J.Koenig | Sa Le Te | Native | C | CV | 7–9 | 8–10 | CC, FD, MP, ON, RB | Rz, Wp | 0.223 | 0.120 | DD | ZR064 | |
| 65. | Kaempferia angustifolia Roscoe | Wan Prab Samut | Native | C | CV | 7–9 | 8–10 | CC, MP, RB | Rz, Wp | 0.158 | 0.060 | LC | ZR065 | |
| 66. | Kaempferia elegans Wall. | Wan Nok Khoom | Native | C | CV | 7–9 | 8–10 | CC, MP, ON, RB | Rz, Wp | 0.268 | 0.135 | NE | ZR066 | |
| 67. | Kaempferia galanga L. | Wan Proh Hom | Native | B | DC, CV | 7–9 | 8–10 | FD, MP, RB | Rz, Wp | 0.175 | 0.085 | DD | VU | ZR067 |
| 68. | Kaempferia gilbertii W.Bull | Wan Maha Niyom | Introduced | C | CV | 7–9 | 8–10 | ON, RB | Wp | 0.090 | 0.053 | NE | ZR068 | |
| 69. | Kaempferia isanensis Saensouk and P.Saensouk | Toob Moob Isan | Endemic | B | DC, CV | 5–6 | 8–9 | FD, SP | Rz, Lv | 0.055 | 0.040 | NE | VU | ZR069 |
| 70. | Kaempferia mahasarakhamensis Saensouk and P.Saensouk | Toob Moob Sarakham | Endemic | B | DC, WL, CV | 6–8 | 8–9 | FD | In, Ps | 0.043 | 0.043 | NE | EN | ZR070 |
| 71. | Kaempferia marginata Carey ex Roscoe | Toob Moob | Native | B | DC, CV | 7–9 | 8–10 | FD, MP | Rz, Lv | 0.235 | 0.213 | NE | LC | ZR071 |
| 72. | Kaempferia parviflora Wall. ex Baker | Kra Chai Dam | Native | C | CV | 7–9 | 8–10 | MP | Rz | 0.215 | 0.215 | DD | ZR072 | |
| 73. | Kaempferia pulchra Ridl. | Proh Pa | Native | C | CV | 6–9 | Not seen | ON | Wp | 0.030 | 0.030 | NE | ZR073 | |
| 74. | Kaempferia rotunda L. | Wan Haow None | Native | B | DC, CV | 3–5 | 5–6 | FD, MP, ON, RB | Rz, Wp | 0.445 | 0.230 | NE | VU | ZR074 |
| 75. | Kaempferia sakolchaii P.Saensouk, Saensouk, and Boonma | Proh Bai Lai Taeng Mo | Endemic | B | DC, DE, CV | 5–7 | Not seen | ON | Wp | 0.010 | 0.010 | NE | VU | ZR075 |
| 76. | Kaempferia sakonensis Saensouk, P.Saensouk, and Boonma | Proh Sakon | Endemic | W | DC, DE | 6–9 | Not seen | ON | Wp | 0.030 | 0.030 | NE | VU | ZR076 |
| 77. | Kaempferia sipraiana Boonma and Saensouk | Proh Siprai | Endemic | C | CV | 4–5 | Not seen | ON | Wp | 0.005 | 0.005 | NE | ZR077 | |
| 78. | Meistera chinensis (Chun ex T.L.Wu) Škorničk. and M.F.Newman | Raew Pa | Native | W | DE, EV | 4–5 | Not seen | NA | - | 0.000 | 0.000 | LC | LC | ZR078 |
| 79. | Wurfbainia schmidtii (K.Schum.) Škorničk. and A.D.Poulsen | Wan Saow Long | Native | B | DE, EV, CV | 5–6 | 6–9 | FD, ON, RB, CM | Rz, Wp | 0.168 | 0.108 | LC | LC | ZR079 |
| 80. | Wurfbainia uliginosa (J.Koenig) Giseke | Raew | Native | B | DE, EV, CV | 4–7 | 5–8 | FD, MP, SP | Rz, In | 0.083 | 0.033 | LC | LC | ZR080 |
| 81. | Wurfbainia vera (Blackw.) Škorničk. and A.D.Poulsen | Krawan | Native | C | CV | 4–7 | 5–8 | MP, SP | Rz, Fr | 0.063 | 0.048 | DD | ZR081 | |
| 82. | Wurfbainia villosa (Lour.) Škorničk. and A.D.Poulsen | Mak Naeng | Native | B | DE, EV, CV | 5–9 | 8–11 | FD, MP | Rz, Fr | 0.028 | 0.020 | DD | LC | ZR082 |
| 83. | Zingiber chrysostachys Ridl. | Khing Pa | Native | C | CV | 7–9 | 9–10 | ON | In, Wp | 0.003 | 0.003 | EN | ZR083 | |
| 84. | Zingiber citriodorum Theilade and Mood | Ta Krai Pran | Endemic | C | CV | 4–5 | 5–7 | FD, MP, RB | Rz, Wp | 0.043 | 0.035 | NE | ZR084 | |
| 85. | Zingiber isanense Triboun and K.Larsen | Khing Isan | Native | B | DC, DE, CV | 5–7 | 6–8 | FD, MP | Rz, Ps, In | 0.070 | 0.070 | LC | VU | ZR085 |
| 86. | Zingiber junceum Gagnep. | Khing Kra Tai | Native | B | DC, DE, CV | 7–9 | Not seen | FD, ON | In, Wp | 0.278 | 0.260 | LC | VU | ZR086 |
| 87. | Zingiber ligulatum Roxb. | Khing Haeng | Introduced | C | CV | 6–8 | 8–10 | MP | Rz | 0.008 | 0.008 | DD | ZR087 | |
| 88. | Zingiber mekongense Gagnep. | Khing Mae Kong | Native | B | DE, CV | 4–7 | Not seen | MP | Rz | 0.033 | 0.033 | NE | VU | ZR088 |
| 89. | Zingiber montanum (J.Koenig) Link ex A.Dietr. | Wan Fai | Native | C | CV | 3–5 | Not seen | FD, MP, RB, CM | Rz, Wp | 0.303 | 0.203 | NE | ZR089 | |
| 90. | Zingiber officinale Roscoe | Khing | Introduced | C | CV | 7–9 | 8–10 | CC, FD, MP, SP | Rz, Ps, Lv, In | 2.105 | 0.980 | DD | ZR090 | |
| 91. | Zingiber ottensii Valeton | Plai Dam | Introduced | C | CV | 7–9 | 8–10 | FD, MP, RB | Rz, In, Ps, Wp | 0.138 | 0.053 | DD | ZR091 | |
| 92. | Zingiber purpureum Roscoe | Plai Lueang | Introduced | C | CV | 3–6 | Not seen | MP, RB | Rz, Wp | 0.265 | 0.220 | DD | ZR092 | |
| 93. | Zingiber pyroglossum Triboun and K.Larsen | Plai Dok Lueang | Native | B | DE, EV, CV | 7–9 | 8–10 | FD, MP, ON | Rz, In, Wp | 0.083 | 0.035 | LC | VU | ZR093 |
| 94 | Zingiber rubens Roxb. | Khing Paa | Native | B | DE, EV, CV | 7–9 | 8–9 | FD, RB | In, Wp | 0.060 | 0.055 | NE | VU | ZR094 |
| 95 | Zingiber spectabile Griff. | Katue Pilat | Native | C | CV | 7–9 | 8–9 | FD, MP, ON, RB | Rz, In, Wp | 0.018 | 0.008 | DD | ZR095 | |
| 96 | Zingiber thorelii Gagnep. | Dok Din | Native | B | DE, EV, CV | 7–9 | 9–10 | FD | In | 0.080 | 0.080 | LC | VU | ZR096 |
| 97 | Zingiber zerumbet (L.) Roscoe ex Sm. | E-Tue | Native | B | DE, DC, EV, CV | 5–8 | 8–10 | FD, MP | In, Ps | 0.163 | 0.133 | DD | LC | ZR097 |
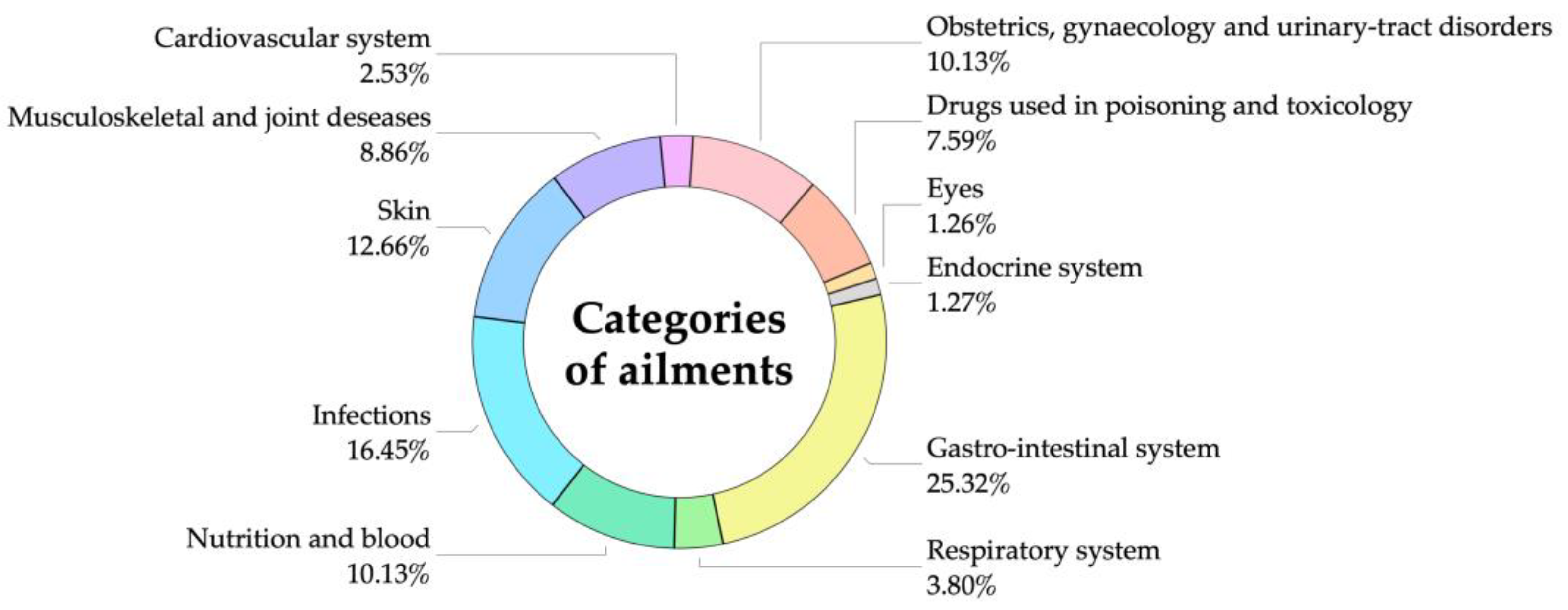
| Medical Categories | Number of Use Report (nur) | Number of Species (nt) | Fic |
|---|---|---|---|
| Cardiovascular system | 48 | 2 | 0.98 |
| Drugs used in poisoning and toxicology | 294 | 5 | 0.99 |
| Endocrine system | 18 | 1 | 1.00 |
| Eyes | 36 | 1 | 1.00 |
| Gastrointestinal system | 714 | 19 | 0.97 |
| Infections | 510 | 12 | 0.98 |
| Musculoskeletal and joint diseases | 160 | 6 | 0.97 |
| Nutrition and blood | 258 | 6 | 0.98 |
| Obstetrics, gynecology, and urinary tract disorders | 312 | 8 | 0.98 |
| Respiratory system | 108 | 3 | 0.98 |
| Skin | 330 | 8 | 0.98 |
| No. | Genera | Number of Species | Genera Use Value (GUV) |
|---|---|---|---|
| 1. | Alpinia | 8 | 0.651 |
| 2. | Amomum | 4 | 0.073 |
| 3. | Boesenbergia | 3 | 0.663 |
| 4. | Cornukaempferia | 2 | 0.006 |
| 5. | Curcuma | 33 | 0.231 |
| 6. | Etlingera | 1 | 0.313 |
| 7. | Gagnepainia | 2 | 0.039 |
| 8. | Globba | 10 | 0.111 |
| 9. | Hedychium | 1 | 0.223 |
| 10. | Kaempferia | 13 | 0.135 |
| 11. | Meistera | 1 | 0.00 |
| 12. | Wurfbainia | 4 | 0.085 |
| 13. | Zingiber | 15 | 0.243 |
| Scientific Name | RFC | SUV |
|---|---|---|
| Alpinia siamensis K. Schum. | 0.988 | 2.505 |
| Zingiber officinale Roscoe | 0.980 | 2.105 |
| Alpinia galanga (L.) Willd. | 0.920 | 2.043 |
| Boesenbergia rotunda (L.) Mansf. | 0.980 | 1.933 |
| Curcuma longa L. | 0.970 | 1.863 |
| Curcuma singularis Gagnep. | 0.988 | 1.325 |
| Curcuma angustifolia Roxb. | 0.805 | 1.203 |
| Curcuma mangga Valeton and Zijp | 0.780 | 0.935 |
| Kaempferia rotunda L. | 0.230 | 0.445 |
| Globba williamsiana M. F. Newman and Sangvir. | 0.190 | 0.365 |
| Alpinia siamensis K. Schum. | 0.988 | 2.505 |
| No. | Scientific Name | Part of Trades | Price (THB/kg) | Trading Periods (Months) 1 | Monthly Sales Volume (kg) | Average Yearly Income (THB/Trader) | |
|---|---|---|---|---|---|---|---|
| Min | Max | ||||||
| 1. | Alpinia galanga (L.) Willd. | Inflorescences, Rhizome | 35 | 40 | 1–12 | 85 | 38,250 |
| 2. | Alpinia purpurata (Vieill.) K. Schum. | Whole plants | 150 * | 180 * | 1–12 | 60 | 118,800 |
| 3. | Alpinia siamensis K. Schum. | Inflorescences, Rhizome | 35 | 40 | 1–12 | 90 | 40,500 |
| 4. | Alpinia vittata W. Bull | Whole plants | 80 * | 100 * | 1–12 | 70 | 75,600 |
| 5. | Boesenbergia rotunda (L.) Mansf. | Rhizome | 150 | 100 | 1–12 | 100 | 151,200 |
| 6. | Curcuma angustifolia Roxb. | Inflorescences | 60 | 100 | 6–10 | 50 | 20,000 |
| 7. | Curcuma comosa Roxb. | Rhizome | 180 | 190 | 1–12 | 45 | 99,900 |
| 8. | Curcuma longa L. | Rhizome | 50 | 60 | 1–12 | 80 | 52,800 |
| 9. | Curcuma mangga Valeton and Zijp | Rhizome | 50 | 70 | 1–12 | 74 | 53,280 |
| 10. | Curcuma singularis Gagnep. | Inflorescences | 100 | 200 | 4–6 | 67 | 30,150 |
| 11. | Curcuma wanenlueanga Saensouk, Thomudtha, and Boonma | Rhizome | 200 | 230 | 1–12 | 43 | 110,940 |
| 12. | Etlingera elatior (Jack) R. M. Sm. | Whole plants | 150 * | 280 * | 1–12 | 86 | 221,880 |
| 13. | Globba rosea Gagnep. | Whole plants | 80 * | 100 * | 1–12 | 40 | 43,200 |
| 14. | Hedychium coronarium J.Koenig | Inflorescences | 50 | 70 | 6–12 | 75 | 27,000 |
| 15. | Kaempferia angustifolia Roscoe | Whole plants | 50 * | 80 * | 1–12 | 40 | 31,200 |
| 16. | Kaempferia elegans Wall. | Whole plants | 50 * | 100 * | 1–12 | 43 | 38,700 |
| 17. | Zingiber officinale Roscoe | Rhizome | 50 | 70 | 1–12 | 85 | 61,200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saensouk, P.; Saensouk, S.; Boonma, T.; Junsongduang, A.; Rakarcha, S.; Chanthavongsa, K.; Jitpromma, T. Zingiberaceae in Roi Et Province, Thailand: Diversity, Ethnobotany, Horticultural Value, and Conservation Status. Horticulturae 2025, 11, 527. https://doi.org/10.3390/horticulturae11050527
Saensouk P, Saensouk S, Boonma T, Junsongduang A, Rakarcha S, Chanthavongsa K, Jitpromma T. Zingiberaceae in Roi Et Province, Thailand: Diversity, Ethnobotany, Horticultural Value, and Conservation Status. Horticulturae. 2025; 11(5):527. https://doi.org/10.3390/horticulturae11050527
Chicago/Turabian StyleSaensouk, Piyaporn, Surapon Saensouk, Thawatphong Boonma, Auemporn Junsongduang, Sarayut Rakarcha, Khamfa Chanthavongsa, and Tammanoon Jitpromma. 2025. "Zingiberaceae in Roi Et Province, Thailand: Diversity, Ethnobotany, Horticultural Value, and Conservation Status" Horticulturae 11, no. 5: 527. https://doi.org/10.3390/horticulturae11050527
APA StyleSaensouk, P., Saensouk, S., Boonma, T., Junsongduang, A., Rakarcha, S., Chanthavongsa, K., & Jitpromma, T. (2025). Zingiberaceae in Roi Et Province, Thailand: Diversity, Ethnobotany, Horticultural Value, and Conservation Status. Horticulturae, 11(5), 527. https://doi.org/10.3390/horticulturae11050527








