Investigating the Effect of Two Interstocks, Changshanhuyou and Ponkan, on the Fruit Quality and Volatile Flavor of Cocktail Grapefruit (Citrus paradisi Macf. cv. Cocktail)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Collection
2.2. Sample Preparation and Treatment
2.3. GC-MS Conditions
2.4. Metabolomic Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Biological Characteristics of Cocktail Grapefruit Grafted on Different Stocks
3.1.1. Basic Characteristics of Fruits
3.1.2. Differences in Fruit Color Grafted on Different Stocks
3.1.3. Soluble Sugars, Vitamin C, and Organic Acid in Fruits on Different Stocks
3.2. Classification of Volatile Organic Compounds in the Fruit Peel and Pulp of Cocktail Grapefruit
3.3. Differential Analysis of Cocktail Grapefruit Fruit VOCs Grafted on Different Stocks
3.4. Differential Accumulation of Volatile Flavor Substances of Fruit on Different Interstocks
4. Discussion
4.1. Effects of the Interstocks on the Fruit Quality of Cocktail Grapefruit
4.2. Effect of Interstocks on Fruit Flavor Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [PubMed]
- Penjor, T.; Mimura, T.; Matsumoto, R.; Yamamoto, M.; Nagano, Y. Characterization of limes (Citrus aurantifolia) grown in Bhutan and Indonesia using high-throughput sequencing. Sci. Rep. 2014, 4, 4853. [Google Scholar]
- Agustí, M.; Reig, C.; Martínez-Fuentes, A.; Mesejo, C. Advances in citrus flowering: A review. Front. Plant Sci. 2022, 13, 868831. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar]
- Xie, R.; He, W.; Chai, J.; Luo, L.; Wang, Y.; Chen, Q.; Tang, H.; Wang, X. A study of scion phenotypes in pummelo grafted onto a new citrus rootstock Citrus junos ‘Pujiang Xiangcheng’. Horticulturae 2022, 8, 1039. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, Z.; Deng, L.; Li, W.; Yuan, Y.; Zhang, M.; Sun, G.; He, S.; Wang, J.; Wang, Z.; et al. Effect of natural variation and rootstock on fruit quality and volatile organic compounds of ‘Kiyomi tangor’ (Citrus reticulata Blanco) citrus. Int. J. Mol. Sci. 2023, 24, 16810. [Google Scholar] [CrossRef] [PubMed]
- Morales Alfaro, J.; Bermejo, A.; Navarro, P.; Quiñones, A.; Salvador, A. Effect of rootstock on citrus fruit quality: A review. Food Rev. Int. 2021, 39, 2835–2853. [Google Scholar]
- Domingues, A.R.; Marcolini, C.D.; Gonçalves, C.H.; Gonçalves, L.S.; Roberto, S.R.; Carlos, E.F. Fruit ripening development of ‘Valencia’ orange trees grafted on different ‘Trifoliata’ hybrid rootstocks. Horticulturae 2021, 7, 3. [Google Scholar]
- Benjamin, G.; Tietel, Z.; Porat, R. Effects of rootstock/scion combinations on the flavor of citrus fruit. J. Agric. Food. Chem. 2013, 61, 11286–11294. [Google Scholar] [CrossRef]
- Cardeñosa, V.; Barros, L.; Barreira, J.C.; Arenas, F.; Moreno-Rojas, J.M.; Ferreira, I.C. Different citrus rootstocks present high dissimilarities in their antioxidant activity and vitamins content according to the ripening stage. J. Plant Physiol. 2015, 174, 124–130. [Google Scholar] [CrossRef]
- Martins, R.C.; Leonel, S.; Souza, J.M.A.; Leonel, M.; Putti, F.F.; ZÜGe, P.G.U.; Ferreira, R.B.; Silva, M.S.; Duarte Filho, J. Citrus crop performance and fruit quality in response to different scion-rootstock combinations. Span. J. Agric. Res. 2023, 21, e0903. [Google Scholar]
- Dutt, M.; Mahmoud, L.M.; Grosser, J.W. Field Performance of ‘Valencia’ sweet orange trees grafted onto pummelo interstocks and swingle citrumelo rootstocks under Huanglongbing (HLB) endemic conditions. Horticulturae 2023, 9, 719. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, B.; Tan, L.; Yang, Y.; Zhang, Y.; Ma, M.; Xu, Y.; Liao, L.; Sun, G.; Liang, D.; et al. Effects of interstocks on growth and photosynthetic characteristics in ‘Yuanxiaochun’ citrus seedlings. Funct. Plant Biol. 2020, 47, 977–987. [Google Scholar]
- Gil-Izquierdo, A.; Riquelme, M.T.; Porras, I.; Ferreres, F. Effect of the rootstock and interstock grafted in lemon tree (Citrus limon (L.) Burm.) on the flavonoid content of lemon juice. J. Agric. Food Chem. 2004, 52, 324–331. [Google Scholar] [PubMed]
- Gaona-Ponce, M.; Almaguer-Vargas, G.; Barrientos-Priego, A.F.; Borja-De la Rosa, A.M. The interstock effect on the initial growth of the ‘Tahiti’ Persian lime (Citrus x latifolia Tanaka ex Q. Jiménez). Rev. Chapingo Ser. Agric. Trop. 2021, 1, 37–49. [Google Scholar]
- Caristi, C.; Bellocco, E.; Panzera, V.; Toscano, G.; Vadalà, R.; Leuzzi, U. Flavonoids detection by HPLC-DAD-MS-MS in lemon juices from Sicilian cultivars. J. Agric. Food Chem. 2003, 51, 3528–3534. [Google Scholar]
- El-Sayed, S. Effect of rootstock and interstock on growth, yield and fruit quality of some orange varieties. Menoufia J. Plant Product. 2017, 2, 235–248. [Google Scholar]
- Chiaiese, P.; Corrado, G.; Minutolo, M.; Barone, A.; Errico, A. Transcriptional regulation of ascorbic acid during fruit ripening in pepper (Capsicum annuum) varieties with low and high antioxidants content. Plants 2019, 8, 206. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. Postharvest Biol. Technol. 2015, 99, 99–104. [Google Scholar]
- Chen, J.; Guang-Yan, Z.; Shi-Ping, Z.; Yan-Yan, M.; Shu-Tang, Y. Advances in the studies on citrus rootstock evaluation and application. Acta Hortic. Sin. 2013, 40, 1669–1678. [Google Scholar]
- Cantuarias-Avilés, T.; Mourão Filho, F.d.A.A.; Stuchi, E.S.; Silva, S.R.d.; Espinoza-Núñez, E. Tree performance and fruit yield and quality of ‘Okitsu’ Satsuma mandarin grafted on 12 rootstocks. Sci. Hort. 2010, 123, 318–322. [Google Scholar] [CrossRef]
- NMC Stenzel, C.N. Rootstocks for ‘Tahiti’ lime. Sci. Agric. 2004, 61, 151–155. [Google Scholar] [CrossRef]
- Fallahi, E.; Rodney, D.R. Tree size, yield, fruit quality, and leaf mineral nutrient concentration of ‘Fairchild’ mandarin on six rootstock. J. Am. Soc. Hortic. Sci. 1992, 117, 28–31. [Google Scholar] [CrossRef]
- Ahmed, W.; Nawaz, M.A.; Iqbal, M.A.; Khan, M.M. Effect of different rootstocks on plant nutrient status and yield in Kinnow mandarin (Citrus reticulata blanco). Pak. J. Bot. 2007, 39, 1779–1786. [Google Scholar]
- Forner-Giner, M.A.; Alcaide, A.; Primo-Millo, E.; Forner, J.B. Performance of ‘Navelina’ orange on 14 rootstocks in Northern Valencia (Spain). Sci. Hort. 2003, 98, 223–232. [Google Scholar]
- Al-Jaleel, A.; Zekri, M.; Hammam, Y. Yield, fruit quality, and tree health of ‘Allen Eureka’ lemon on seven rootstocks in Saudi Arabia. Sci. Hort. 2005, 105, 457–465. [Google Scholar] [CrossRef]
- Tazima, Z.; Neves, C.S.; Yada, I.; Leite, R. Performance of ‘Okitsu’ satsuma mandarin on nine rootstocks. Sci. Agric. 2013, 70, 422–427. [Google Scholar] [CrossRef]
- Bassal, M.A. Growth, yield and fruit quality of ‘Marisol’ clementine grown on four rootstocks in Egypt. Sci. Hort. 2009, 119, 132–137. [Google Scholar]
- Sharma, J.N.; Josan, J.S.; Thatai, S.K. Effect of rootstocks on tree health, yield and quality of Kinnow mandarin under arid-irrigated region of Punjab. Indian J. Hortic. 2002, 59, 373–377. [Google Scholar]
- Smith, M.W.; Shaw, R.G.; Chapman, J.C.; Owen-Turner, J.; Slade Lee, L.; Bruce McRae, K.; Jorgensen, K.R.; Mungomery, W.V. Long-term performance of ‘Ellendale’ mandarin on seven commercial rootstocks in sub-tropical Australia. Sci. Hort. 2004, 102, 75–89. [Google Scholar] [CrossRef]
- Mourão Filho, F.d.A.A.; Espinoza-Núñez, E.; Stuchi, E.S.; Ortega, E.M.M. Plant growth, yield, and fruit quality of ‘Fallglo’ and ‘Sunburst’ mandarins on four rootstocks. Sci. Hort. 2007, 114, 45–49. [Google Scholar] [CrossRef]
- Georgiou, A. Performance of ‘Nova’ mandarin on eleven rootstocks in Cyprus. Sci. Hort. 2000, 84, 115–126. [Google Scholar] [CrossRef]
- Rong, Y.; Luo, J.; Liao, L.; Wang, Z. Effects of endogenous hormone metabolism on pericarp thickness after grafting on different rootstocks of citrus cv. ‘Kiyomi tangor’. AIP Conf. Proc. 2019, 2110, 020037. [Google Scholar]
- Tracy, L.; Kahn, O.J.B.a.R.J.B. New late-season navel orange varieties evaluated for quality characteristics. Calif. Agric. 2007, 61, 138–143. [Google Scholar]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Haider, M.S.; Shafqat, W.; Asif, M.; Mehmood, A. Influence of different rootstocks on physico-chemical quality attributes of Kinnow mandarin. Pak. J. Agric. Sci. 2021, 58, 929–935. [Google Scholar]
- Mccollum, T.G.; Bowman, K.D.; Castle, W.S. Effects of rootstock on fruit quality and postharvest behavior of ‘Marsh’ grapefruit. Proc. Fla. State Hort. Soc. 2003, 115, 10–13. [Google Scholar]
- Sultan, M. Effect of some citrus rootstocks on fruit quality and storability of Washington navel orange under cold storage conditions. Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 1266–1273. [Google Scholar]
- Emmanouilidou, M.G.; Kyriacou, M.C. Rootstock-modulated yield performance, fruit maturation and phytochemical quality of ‘Lane Late’ and ‘Delta’ sweet orange. Sci. Hort. 2017, 225, 112–121. [Google Scholar]
- Hussain, S.; Curk, F.; Anjum, M.A.; Pailly, O.; Tison, G. Performance evaluation of common clementine on various citrus rootstocks. Sci. Hort. 2013, 150, 278–282. [Google Scholar] [CrossRef]
- Chaparro-Zambrano, H.N.; Velásquez-Ramírez, H.A.; Ordúz-Rodríguez, J.O. Evaluation of ‘Arrayana’ tangerine (Citrus reticulata Blanco) grafted onto different rootstocks in tropical lowlands of Colombian Orinoquia, 2005–2011 (second cycle). Agron. Colomb. 2017, 35, 29–34. [Google Scholar] [CrossRef]
- McCollum, G.; Bowman, K.D. Rootstock effects on fruit quality among ‘Ray Ruby’ grapefruit trees grown in the indian river district of florida. HortScience 2017, 52, 541–546. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from citrus fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Shafqat, W.; Ashraf, E.; Albaayit, S.F.A. Rootstock influence on performance of different citrus scion cultivars: A review. J. Glob. Innov. Agric. Sci. 2023, 11, 273–283. [Google Scholar] [CrossRef]
- Machado, F.L.D.C.; Costa, J.D.P.D.; Teixeira, A.D.S.; Costa, J.M.C.D. The influence of rootstock and time of harvest on the fruit quality during storage of in two grapefruit cultivars. Acta Sci. Agron. 2015, 37, 339–346. [Google Scholar] [CrossRef]
- Md Othman, S.N.A.; Hassan, M.A.; Nahar, L.; Basar, N.; Jamil, S.; Sarker, S.D. Essential oils from the Malaysian citrus (Rutaceae) medicinal plants. Medicines 2016, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mas, M.C.; Rambla, J.L.; Lopez-Gresa, M.P.; Blazquez, M.A.; Granell, A. Volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Dugo, G.; Bonaccorsi, I.; Sciarrone, D.; Costa, R.; Dugo, P.; Mondello, L.; Santi, L.; Fakhry, H.A. Characterization of oils from the fruits, leaves and flowers of the bitter orange tree. J. Essent. Oil Res. 2011, 23, 45–59. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Bonaccorsi, I.; Dugo, P.; Mondello, L.; Dugo, G. Analysis of citrus essential oils: State of the art and future perspectives. A review. Flavour Fragr. J. 2012, 27, 98–123. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef]
- Zhang, H.; Wen, H.; Chen, J.; Peng, Z.; Shi, M.; Chen, M.; Yuan, Z.; Liu, Y.; Zhang, H.; Xu, J. Volatile compounds in fruit peels as novel biomarkers for the identification of four citrus species. Molecules 2019, 24, 4550. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.E.; Cruz, P.S.; Cruz, D.T.C.; Bugayong, A.M.S.; Castillo, A.L. Chemical composition and cytotoxicity of Philippine calamansi essential oil. Ind. Crops Prod. 2019, 128, 108–114. [Google Scholar]
- Babazadeh Darjazi, B.; Rustaiyan, A.; Talaei, A.; Khalighi, A.; Larijani, K.; Golein, B.; Hayatbakhsh, E.; Taghizad, R. The Effects of rootstock on the volatile flavour components of Page mandarin [(C. reticulata var dancy C. Paradisi var dancan) C. clemantina] juice and peel. Iran. J. Chem. Chem. Eng. 2009, 28, 99–111. [Google Scholar]
- Saini, M.K.; Capalash, N.; Kaur, C.; Singh, S.P. Comprehensive metabolic profiling to decipher the influence of rootstocks on fruit juice metabolome of Kinnow (C. nobilis × C. deliciosa). Sci. Hort. 2019, 257, 108673. [Google Scholar]
- Forner-Giner, M.A.; Ballesta-de Los Santos, M.; Melgarejo, P.; Martinez-Nicolas, J.J.; Nunez-Gomez, D.; Continella, A.; Legua, P. Influence of different rootstocks on fruit quality and primary and secondary metabolites content of blood oranges cultivars. Molecules 2023, 28, 4176. [Google Scholar] [CrossRef]

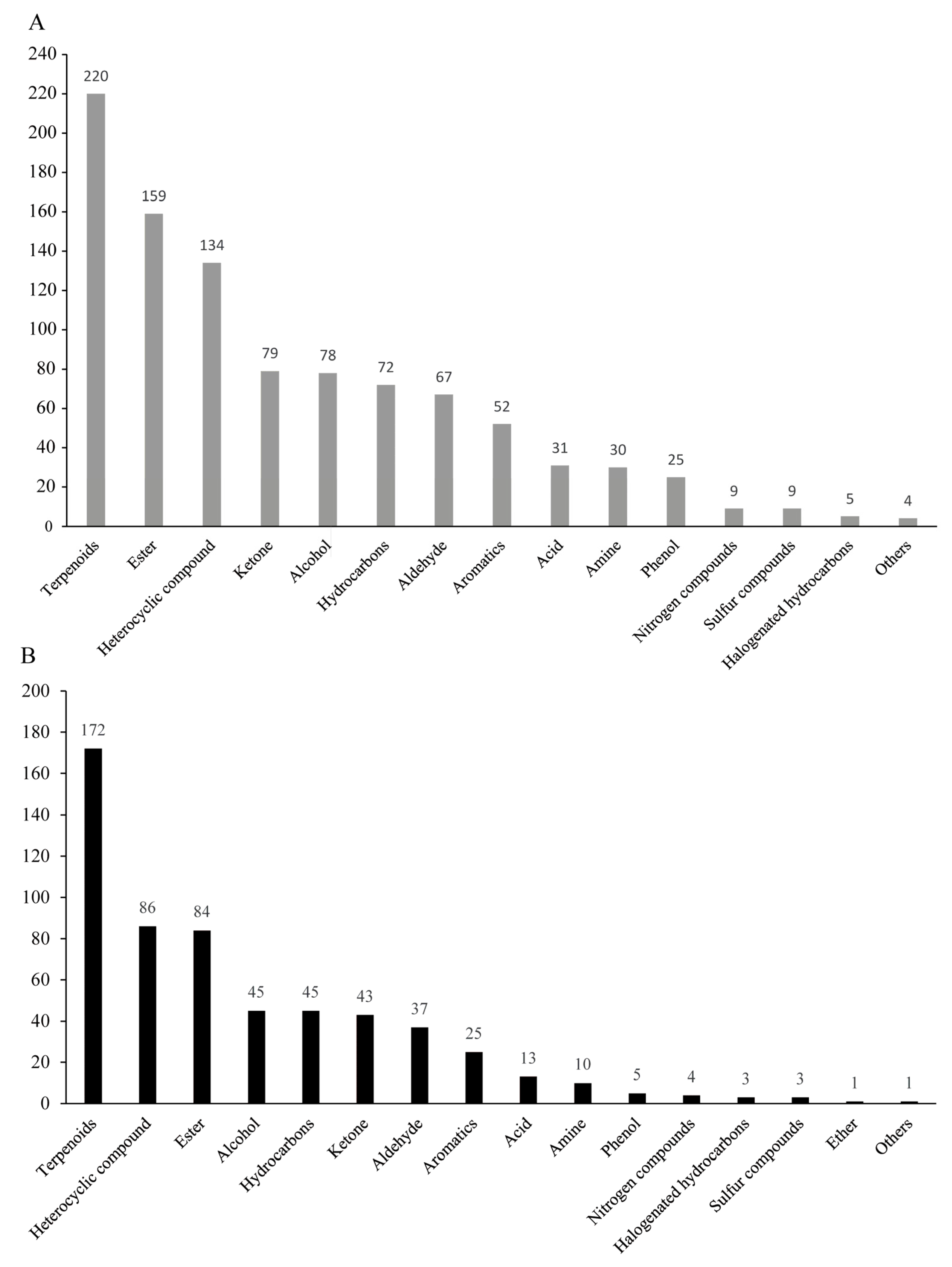
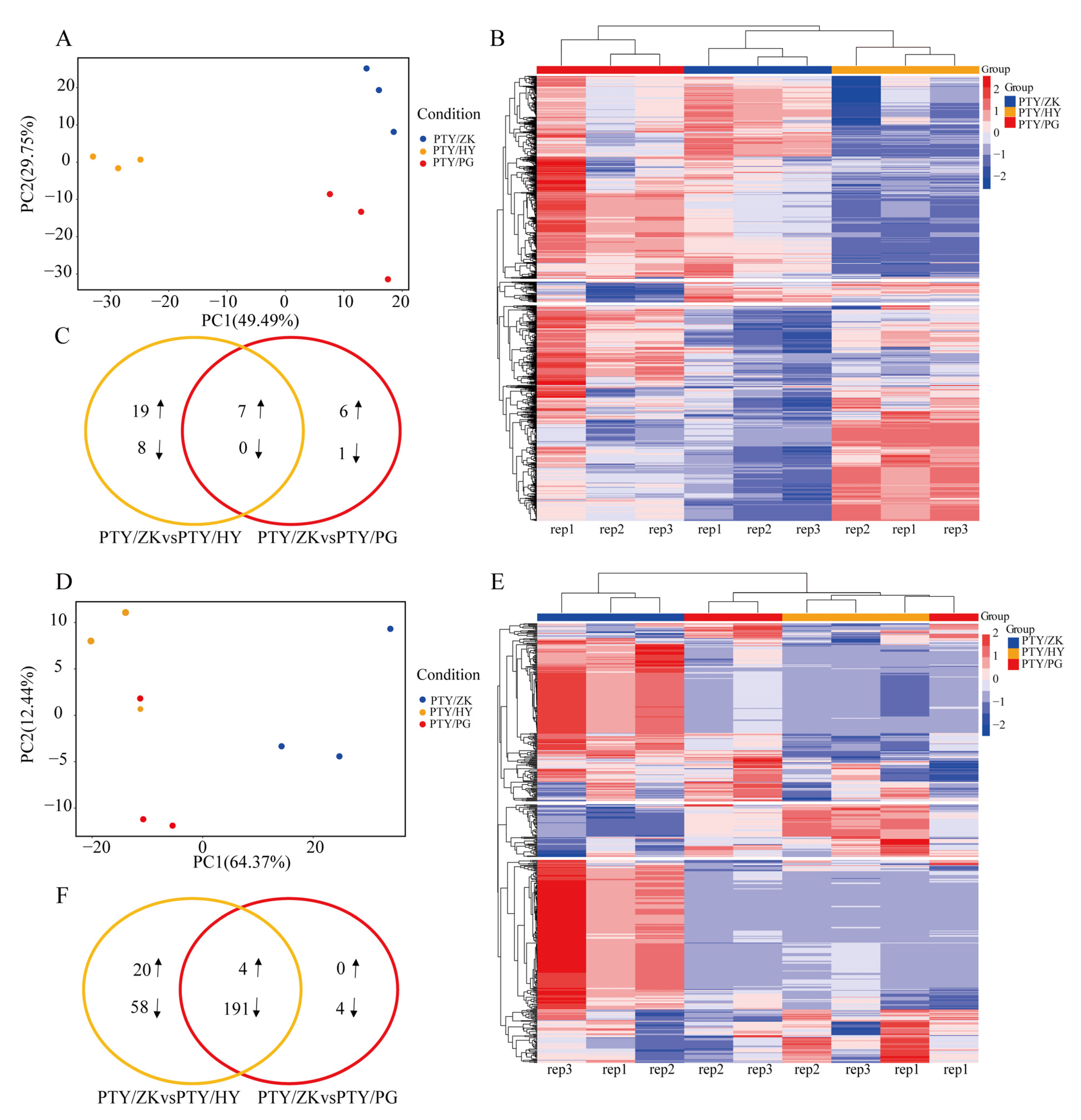
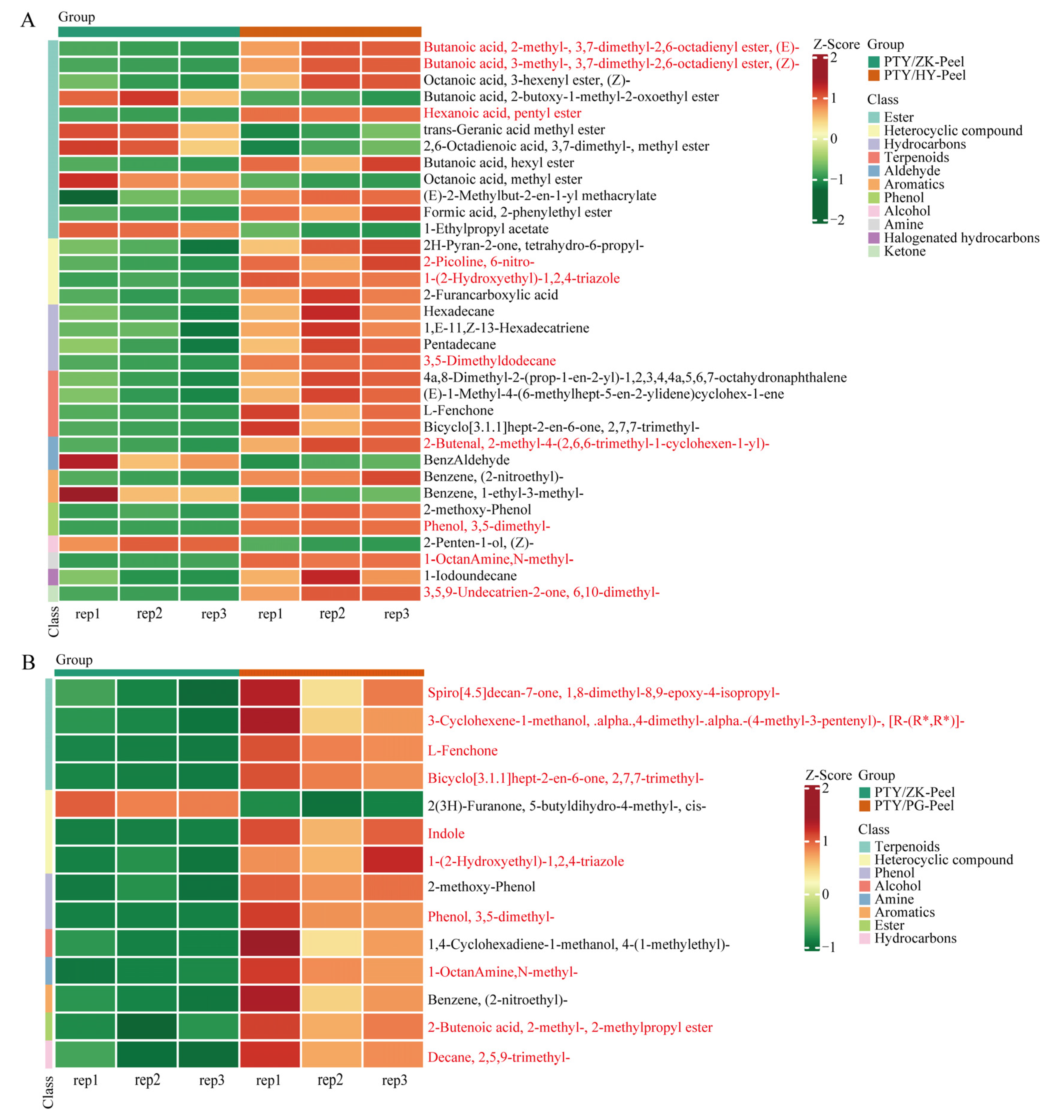
| Index | Single Fruit Weight (g) | Equatorial Diameter (cm) | Fruit Height (cm) | Fruit Shape Index | Peel Thickness (mm) | TSS (Brix) | Acidity (%) |
|---|---|---|---|---|---|---|---|
| PTY/ZK | 484.11 ± 12.91 a | 10.39 ± 0.19 a | 8.98 ± 0.15 a | 0.87 ± 0.015 a | 4.25 ± 0.97 a | 11.7 ± 0.14 b | 0.53 ± 0.001 c |
| PTY/HY | 474.12 ± 21.10 a | 10.39 ± 0.17 a | 8.61 ± 0.13 b | 0.83 ± 0.017 b | 3.27 ± 0.53 b | 12.3 ± 0.08 a | 0.57 ± 0.001 b |
| PTY/PK | 488.91 ± 16.79 a | 10.57 ± 0.17 a | 8.81 ± 0.15 a | 0.83 ± 0.006 b | 3.75 ± 0.43 a | 11.6 ± 0.12 b | 0.64 ± 0.002 a |
| Index | L* | a* | b* | C* | H° | CCI |
|---|---|---|---|---|---|---|
| PTY/ZK | 69.05 ± 1.97 b | 1.25 ± 3.40 b | 67.02 ± 4.171 b | 67.12 ± 4.30 b | 87.36 ± 1.67 a | 0.22 ± 0.80 b |
| PTY/HY | 76.15 ± 0.97 a | 6.89 ± 2.20 a | 72.01 ± 1.01 a | 72.36 ± 1.16 a | 84.55 ± 1.67 b | 1.26 ± 0.40 a |
| PTY/PK | 69.84 ± 2.27 b | 1.02 ± 3.57 b | 64.99 ± 4.00 b | 65.07 ± 4.04 b | 87.36 ± 1.29 a | 0.19 ± 0.77 b |
| Group | Compounds Name | Classification | Log2FC | Type |
|---|---|---|---|---|
| PTY/HY vs. PTY/ZK_peel | 2-Butenal, 2-methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | Aldehyde | 1.62 | up |
| 1-Octanamine,N-methyl- | Amine | 2.00 | up | |
| Butanoic acid, 2-methyl-, 3,7-dimethyl-2,6-octadienyl ester, (E)- | Ester | 2.51 | up | |
| Butanoic acid, 3-methyl-, 3,7-dimethyl-2,6-octadienyl ester, (Z)- | Ester | 2.48 | up | |
| Hexanoic acid, pentyl ester | Ester | 1.47 | up | |
| 1-(2-Hydroxyethyl)-1,2,4-triazole | Heterocyclic compound | 1.55 | up | |
| 2-Picoline, 6-nitro- | Heterocyclic compound | 1.49 | up | |
| 3,5-Dimethyldodecane | Hydrocarbons | 1.63 | up | |
| 3,5,9-Undecatrien-2-one, 6,10-dimethyl- | Ketone | 2.49 | up | |
| Phenol, 3,5-dimethyl- | Phenol | 10.36 | up | |
| 2-Penten-1-ol, (Z)- | Alcohol | −1.17 | down | |
| Benzaldehyde | Aldehyde | −1.42 | down | |
| Benzene, 1-ethyl-3-methyl- | Aromatics | −1.29 | down | |
| trans-Geranic acid methyl ester | Ester | −1.26 | down | |
| 2,6-Octadienoic acid, 3,7-dimethyl-, methyl ester | Ester | −1.19 | down | |
| Butanoic acid, 2-butoxy-1-methyl-2-oxoethyl ester | Ester | −1.18 | down | |
| 1-Ethylpropyl acetate | Ester | −1.16 | down | |
| Octanoic acid, methyl ester | Ester | −1.03 | down | |
| PTY/PG vs. PTY/ZK_peel | Phenol, 3,5-dimethyl- | Phenol | 9.93 | up |
| Indole | Heterocyclic compound | 4.88 | up | |
| Bicyclo[3.1.1]hept-2-en-6-one, 2,7,7-trimethyl- | Terpenoids | 2.08 | up | |
| L-Fenchone | Terpenoids | 2.05 | up | |
| 3-Cyclohexene-1-methanol, .alpha.,4-dimethyl-.alpha.-(4-methyl-3-pentenyl)-, [R-(R*,R*)]- | Terpenoids | 1.62 | up | |
| 1-Octanamine,N-methyl- | Amine | 1.56 | up | |
| Spiro[4.5]decan-7-one, 1,8-dimethyl-8,9-epoxy-4-isopropyl- | Terpenoids | 1.25 | up | |
| Decane, 2,5,9-trimethyl- | Hydrocarbons | 1.24 | up | |
| 2-Butenoic acid, 2-methyl-, 2-methylpropyl ester | Ester | 1.24 | up | |
| 1-(2-Hydroxyethyl)-1,2,4-triazole | Heterocyclic compound | 1.19 | up | |
| 2(3H)-Furanone, 5-butyldihydro-4-methyl-, cis- | Heterocyclic compound | −1.97 | down | |
| PTY/HY vs. PTY/ZK_pulp | 3,6-Nonadien-1-ol, (E,Z)- | Alcohol | 1.26 | up |
| 6-Nonen-1-ol, (E)- | Alcohol | 1.16 | up | |
| 2,6-Nonadien-1-ol | Alcohol | 1.16 | up | |
| 1-Cyclohexene-1-carboxaldehyde, 4-(1-methylethenyl)-, (S)- | Aldehyde | 17.53 | up | |
| Lilac Aldehyde C | Aldehyde | 1.28 | up | |
| 2-Butenoic acid, 3-hexenyl ester, (E,Z)- | Ester | 2.82 | up | |
| Butanoic acid, 4-hexenyl ester, (Z)- | Ester | 2.47 | up | |
| 2(5H)-Furanone, 5-ethyl-3-hydroxy-4-methyl- | Heterocyclic compound | 3.58 | up | |
| 1-Dodecene | Hydrocarbons | 3.27 | up | |
| 4-Undecene, 3-methyl-, (Z)- | Hydrocarbons | 1.37 | up | |
| 6-Octenoic acid, 3,7-dimethyl- | Acid | −2.51 | down | |
| Benzaldehyde, 4-ethoxy- | Aldehyde | −2.32 | down | |
| N-Benzylformamide | Amine | −2.34 | down | |
| 8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl-, endo- | Heterocyclic compound | −3.89 | down | |
| Phenol, 4-(1,1-dimethylpropyl)- | Phenol | −2.34 | down | |
| Bicyclo[3.1.0]hexane, 4-methylene-1-(1-methylethyl)- | Terpenoids | −2.73 | down | |
| (E)-1-Methyl-4-(6-methylhept-5-en-2-ylidene)cyclohex-1-ene | Terpenoids | −2.48 | down | |
| Ylangene | Terpenoids | −2.41 | down | |
| (2R,8R,8aS)-8,8a-Dimethyl-2-(prop-1-en-2-yl)-1,2,3,7,8,8a-hexahydronaphthalene | Terpenoids | −2.35 | down | |
| .alpha.-Cubebene | Terpenoids | −2.32 | down | |
| PTY/PG vs. PTY/ZK_pulp | 1-Cyclohexene-1-carboxAldehyde, 4-(1-methylethenyl)-, (S)- | Aldehyde | 16.89 | up |
| Butanoic acid, 4-hexenyl ester, (Z)- | Ester | 1.94 | up | |
| 2(5H)-Furanone, 5-ethyl-3-hydroxy-4-methyl- | Heterocyclic compound | 2.95 | up | |
| 1-Dodecene | Hydrocarbons | 2.60 | up | |
| 6-Octenoic acid, 3,7-dimethyl- | Acid | −3.27 | down | |
| Dodecanal | Aldehyde | −2.54 | down | |
| Butanedioic acid, bis(2-methylpropyl) ester | Ester | −2.54 | down | |
| Acetic acid, decyl ester | Ester | −2.51 | down | |
| Cyclohexanol, 1-methyl-4-(1-methylethylidene)-, acetate | Ester | −2.38 | down | |
| 8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl-, endo- | Heterocyclic compound | −3.62 | down | |
| 2-Tetradecene, (E)- | Hydrocarbons | −2.50 | down | |
| 2-Undecanone, 6,10-dimethyl- | Ketone | −2.45 | down | |
| Phenol, 4-(1,1-dimethylpropyl)- | Phenol | −2.38 | down | |
| .alpha.-Cubebene | Terpenoids | −2.38 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, F.; Nie, Z.; Huang, X.; Cui, C.; Yang, Y.; Xu, J.; Wang, L.; Sun, L. Investigating the Effect of Two Interstocks, Changshanhuyou and Ponkan, on the Fruit Quality and Volatile Flavor of Cocktail Grapefruit (Citrus paradisi Macf. cv. Cocktail). Horticulturae 2025, 11, 403. https://doi.org/10.3390/horticulturae11040403
Ke F, Nie Z, Huang X, Cui C, Yang Y, Xu J, Wang L, Sun L. Investigating the Effect of Two Interstocks, Changshanhuyou and Ponkan, on the Fruit Quality and Volatile Flavor of Cocktail Grapefruit (Citrus paradisi Macf. cv. Cocktail). Horticulturae. 2025; 11(4):403. https://doi.org/10.3390/horticulturae11040403
Chicago/Turabian StyleKe, Fuzhi, Zhenpeng Nie, Xiu Huang, Changjiang Cui, Yi Yang, Jianguo Xu, Luoyun Wang, and Lifang Sun. 2025. "Investigating the Effect of Two Interstocks, Changshanhuyou and Ponkan, on the Fruit Quality and Volatile Flavor of Cocktail Grapefruit (Citrus paradisi Macf. cv. Cocktail)" Horticulturae 11, no. 4: 403. https://doi.org/10.3390/horticulturae11040403
APA StyleKe, F., Nie, Z., Huang, X., Cui, C., Yang, Y., Xu, J., Wang, L., & Sun, L. (2025). Investigating the Effect of Two Interstocks, Changshanhuyou and Ponkan, on the Fruit Quality and Volatile Flavor of Cocktail Grapefruit (Citrus paradisi Macf. cv. Cocktail). Horticulturae, 11(4), 403. https://doi.org/10.3390/horticulturae11040403








