1. Introduction
As a well-known tropical and subtropical fruit, the mango fruit (
Mangifera indica L., Anacardiaceae) is known as the “king of tropical fruits” and is one of the top five tropical fruits in the world (bananas, pineapples, litchi fruits, mangoes, and longans). Its high sugar, acid, vitamin, and dietary fiber contents, coupled with low amounts of protein, fat, and minerals, make it the most preferred fruit among people. The cultivated area of mangoes in 2022 was nearly 381,000 ha, and the production was about 3.8 million tons of the world [
1]. China is the second largest country in mango production and is rich in mango germplasm (about 900 accessions) [
2], but only a few varieties with a strong, sweet texture are popularized. Most varieties with appealing appearances exhibit limited sweetness, thereby reducing their values to be used as the primary commercial varieties. To a certain extent, the selection and breeding of new mango varieties with the excellent germplasm resources has also been restricted.
Sugar, the most significant source of chemical energy as well as the end product of photosynthesis, is a key indicator of fruit quality, and the perceived sweetness largely depends on the type and proportion of sugars in a fruit [
3]. Much research has given attention to sugar metabolism in fruits, such as peaches [
4], strawberries [
5], apples [
6], tomatoes [
7], and other horticultural crops [
8]. In the case of mango, the content and proportion of soluble sugars—such as sucrose (S), fructose (F), and glucose (G)—in mango fruits considerably contribute to sweetness, flavor, quality, and commercial value [
9,
10].
Among the methods for improving sugar accumulation, using various nontoxic plant growth regulators to explore potential technologies to improve mango sugar biosynthesis and thus increase the sweetness value is an effective and green strategy [
11]. The common fruit sweeteners available in markets include citrus sweeteners, sweetening liquids, and phytoalexins among others [
12,
13,
14,
15], which contain phosphorus, calcium, and various trace elements. However, such sweeteners function by nourishing the tree or as plant stimulants, and their safety in fruits and the ecological environment is difficult to investigate. Furthermore, the effect of the sweeteners on different fruits varies, and no study has identified effective sweeteners for mangoes.
The nontoxic plant growth regulators used in this study as sweeteners, including sucrose-based polymers (SBP), potassium fulvic acid, taurine, and seaweed oligosaccharide peptide [
16], are well-known green and nontoxic compounds. Taurine is a green biogenic compound that has been patented for its sweetening effect on fruits [
17]. Researchers have conducted several studies to increase the sweetening effect of SBP on fruits. For instance, aqueous foliar sprays of SBP during the fruit-bearing stage can effectively increase sucrose synthase (SS) and sucrose phosphate synthase (SPS) activities in longan (
Dimocarpus longan Lour.) leaves, in turn promoting sugar accumulation during the fruit-ripening stage [
18,
19,
20,
21]. In addition, the sweetening effect of SBP has been observed in Sanyuehong litchi (
Litchi chinensis Sonn.), netted melon (
Cucumis melo L.), sweet orange (
Citrus sinensis L. Osbeck), pawpaw (
Carica papaya L.), and cherry tomato (
Solanum lycopersicum (L.) var.
cerasiforme Mill.) [
22,
23]. Potassium fulvic acid is a highly effective macromolecular organic compound characterized by its low molecular weight and easy absorption and utilization by crops to promote crop growth and to increase chlorophyll, vitamin C, and sugar contents. Moreover, the acid enhances resistance to cold, drought, and diseases in plants [
24,
25], making it the most active organic compound among soil humic acids [
26,
27,
28].
The mango cultivar “Renong-1” has high quality and yield; however, it has a low sugar content and low sweetness. Thus, this study aimed to screen a highly efficient and nontoxic plant growth regulator formulation and establish an effective supporting technology system for improving sugar biosynthesis and sweetness values of mango fruits. The results of this study could provide crucial insights for improving the quality of mangoes through the application of eco-friendly plant growth regulators and technological support systems. Furthermore, it could lay the groundwork for developing superior mango varieties that satisfy consumer preferences, utilizing the rich mango germplasm resources in China.
2. Materials and Methods
2.1. Chemicals and Reagents
All chemicals and reagents for soluble sugar extraction and high-performance liquid chromatography (HPLC) analysis were of the highest available purity. Chromatographically pure acetonitrile and ethyl alcohol were respectively from Thermo Fisher Scientific Company (Waltham, MA, USA) and Shanghai Anpel Experimental Technology Co., Ltd., Shanghai, China. For reference standards, sucrose (57-50-1), glucose (50-99-7), and fructose (57-48-7) were purchased from Shanghai Anpel Experimental Technology Co., Ltd., Shanghai, China). Also used were potassium dihydrogen phosphate (7778-77-0, Shanghai Anpel Experimental Technology Co., Ltd., Shanghai, China), microelement fertilizer (Anyang City Xi Mandi fertilizer Co., Ltd., Anyang, China), Taurine (107-35-7, Shanghai Anpel Experimental Technology Co., Ltd., Shanghai, China), and potassium fulvic acid (Anyang City Xi Mandi fertilizer Co., Ltd., Anyang, China). Water was purified using a Milli-Q deionization unit (Millipore, Bedford, MA, USA).
2.2. Mango Variety and Treatment
“Renong-1” mango fruits were collected from a mango orchard at the South Subtropical Crop Research Institute, Chinese Academy of Tropical Agricultural Sciences, Zhanjiang city, Guangdong province, China (20°18′ N, 101°18′ E). “Renong-1” is a conventional excellent high-yielding variety independently cultivated by the mango research group at the institute, although it has the disadvantages of low sugar content and low sweet taste.
Field trials were conducted from April to July 2022, where the natural conditions were generally 20–35 °C and 75–100% relativity humidity. Five solutions—SBP, sucrose-potassium dihydrogen phosphate-microelement fertilizer mixture solution, taurine-potassium dihydrogen phosphate-microelement fertilizer mixture solution, potassium fulvic acid solution, and a seaweed oligosaccharide peptide solution—were applied at different fruit growth stages. A total of 63 well-grown “Renong-1” mango trees with consistent growth at the blooming stage were selected and tagged. The trees were divided into 21 groups of 3; 20 groups were sprayed by the 5 solutions at different fruit development stages using a spray gun, and 1 group was blank processed as a control (CK) (
Table 1).
SBP was supplied by the School of Environmental and Life Sciences, Nanning Normal of University, Nanning, China and is a new environmentally friendly efficient plant growth regulator. Sucrose crystals were pre-irradiated by γ-rays of 60 Co and turned into an activated form. Then, the activated sucrose was polymerized directly with another monomer (e.g., acrylic acid) to yield SBP.
Based on our prior experiments, the SBP solution (A1) was prepared by diluting the original SBP liquid 100-fold with water. SPM solution (A2) was prepared by mixing 5000 ppm S, 0.4% potassium dihydrogen phosphate, and 500 ppm microelement fertilizer (i.e., 50 g S, 40 g potassium dihydrogen phosphate, 5 g microelement fertilizer, and 10 L water). TPM solution (A3) was prepared by mixing 500 ppm taurine, 0.4% potassium dihydrogen phosphate, and 500 ppm microelement fertilizer (i.e., 5 g taurine, 40 g potassium dihydrogen phosphate, 5 g microelement fertilizer, and 10 L water). PFA solution (A4) was prepared by mixing 20 g potassium fulvic acid and 10 L water. SOP solution (A5) was prepared by mixing 8.3 mL seaweed oligosaccharide peptide solution and 10 L water.
Sampling method: At the physiological maturity stage (about 125 days after flowering), five fruits which were well-shaped, disease-free, and of uniform size and maturity were picked from Renong-1 mango trees from each of five directions (east, south, west, north, and middle). More than half of the harvested fruits were subjected to post-ripening treatment at room temperature (25 °C) until they became edible (i.e., reached the full ripening stage). For both stages, 10 fruits were ground, weighed, extracted, and used to quantify their contents of S, G, and F as well as enzyme activities. The tests were repeated three times, and the average value was calculated.
2.3. Determination of Total Soluble Solid Content
The total soluble solid content of fruit pulps was determined using a digital portable refractometer (PAL-1; Atago Co., Ltd., Tokyo, Japan).
2.4. Determination of Soluble Sugar Contents
The contents of S, F, and G were determined using HPLC coupled with mass spectrometry (HPLC-MS) equipped with an auto sampler, an ultraviolet detector (LC-20A, Shimadzu Inc., Kyoto, Japan), and integration software (v1.5.2). The column was 250 mm × 4.6 mm, i.d., 5 μm ZORBAX SB-C18 (PerkinElmer Inc., Waltham, MA USA, the same below). Briefly, 1 g of fruit pulp was extracted with 85% alcohol and centrifuged, and the supernatant was evaporated in a water bath at 85 °C and then dissolved in 4 mL of water. Afterward, 1 mL of the resulting solution was filtered through a 0.4 μm membrane filter for subsequent liquid-phase analysis under the following chromatographic conditions: mobile phase consisted of acetonitrile and water (72:28, v/v), chromatographic column was an amino column, flow rate was 1.0 mL/min, column temperature was set to 27 °C, injection volume was 10 μL, and the run time was 15 min. The soluble sugar content was calculated with reference to the peak areas of the samples and the corresponding standard curves of the sugars.
2.5. Determination of Total Sugar Content and Calculation of Perceived Sweetness
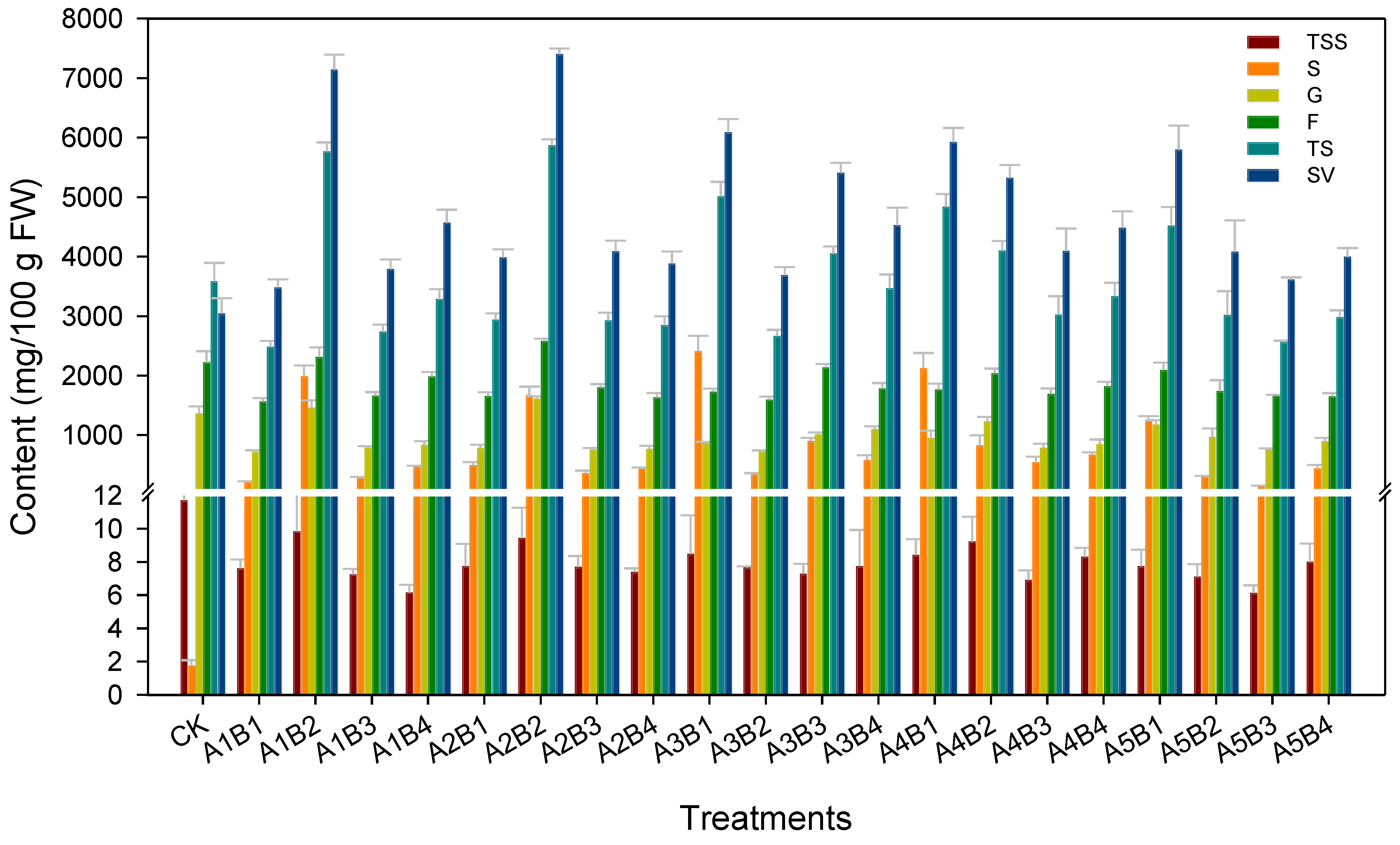
Total sugar (TS) content was calculated by summing the contents of F, G, and S.
Perceived sweetness was calculated according to the method of Yao et al. [
29] as follows: S = 1.00, F = 1.75, and G = 0.75. Therefore, SV = S content × 1.00 + F content × 1.75 + G content × 0.75.
2.6. Determination of Starch Content
Starch content was determined using a micro method test kit (Suzhou Comin Biotechnology Co., Ltd., Suzhou, China), where the experimental procedures were performed according to the manufacturer’s instructions.
2.7. Determination of Activities of Enzymes Associated with Sugar Metabolism
The activities of enzymes associated with sugar metabolism were determined using assay kits. The activities of ADP-glucose pyrophosphorylase (AGP), sucrose synthase (SS), protein kinase (PK), phosphoglucomutase (PGM), α-amylase (α-amy), β-amylase (β-amy), debranching enzyme (DBE), isoamylase (ISA), invertase (INV), acid invertase (AI), and sucrose phosphate synthase (SPS) were measured independently using an AGP assay kit, SS assay kit, PK assay kit, PGM assay kit, α-amylase assay kit, β-amylase test kit, starch DBE test kit, ISA test kit, INV test kit, AI test kit, and SPS assay kit (Enzyme-linked Biotechnology Co., Ltd., Shanghai, China), respectively, according to the manufacturer’s instructions.
2.8. Statistical Analysis
The analysis of soluble sugar contents and processing of data obtained via HPLC-MS were performed using the built-in Analyst 1.5.2 (AB SCIEX, Foster City, CA, USA).
Statistical analysis of the data was performed using MS Excel 2010 (Microsoft Corp., Redmond, WA, USA) and IBM SPSS Statistics 22.0 (IBM Corp., Armonk, NY, USA). Multiple regression analyses were conducted using the LASSO (least absolute shrinkage and selection operator) model, implemented in R (v3.6.1) with the Lars package, and the results were further visually presented with the Igraph package.