Diversity, Utilization, and Conservation Status of Araceae in Kalasin Province, Northeastern Thailand
Abstract
1. Introduction
2. Materials and Methods
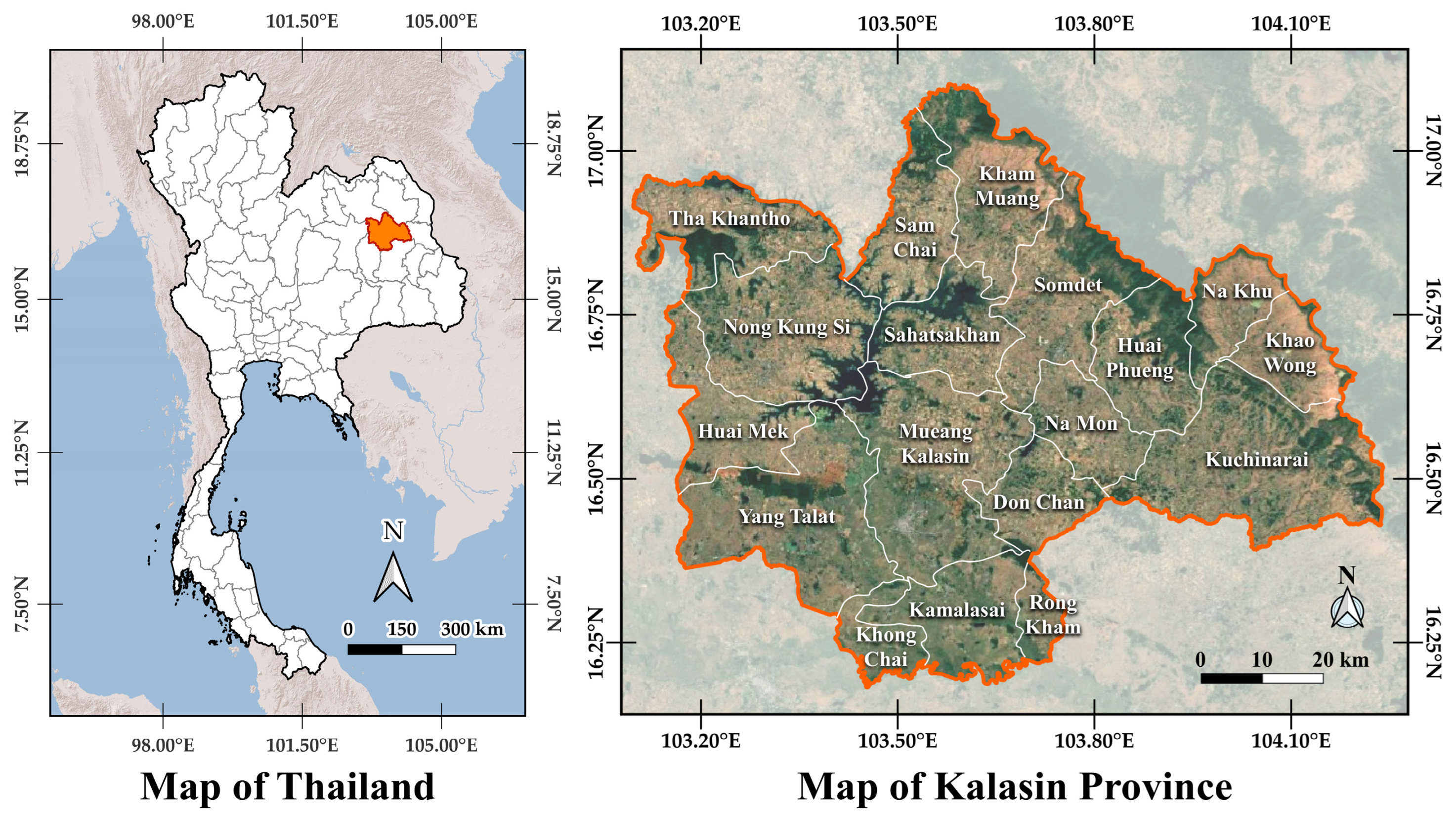
2.1. Study Area
2.2. Plant Material Collection
2.3. Species Identification
2.4. Distribution and Ecological Study
2.5. Phenological Observations
2.6. Study on Utilization
2.7. Conservation Status and Threats
2.8. Statistical Analysis
Jaccard’s Similarity Index
3. Results
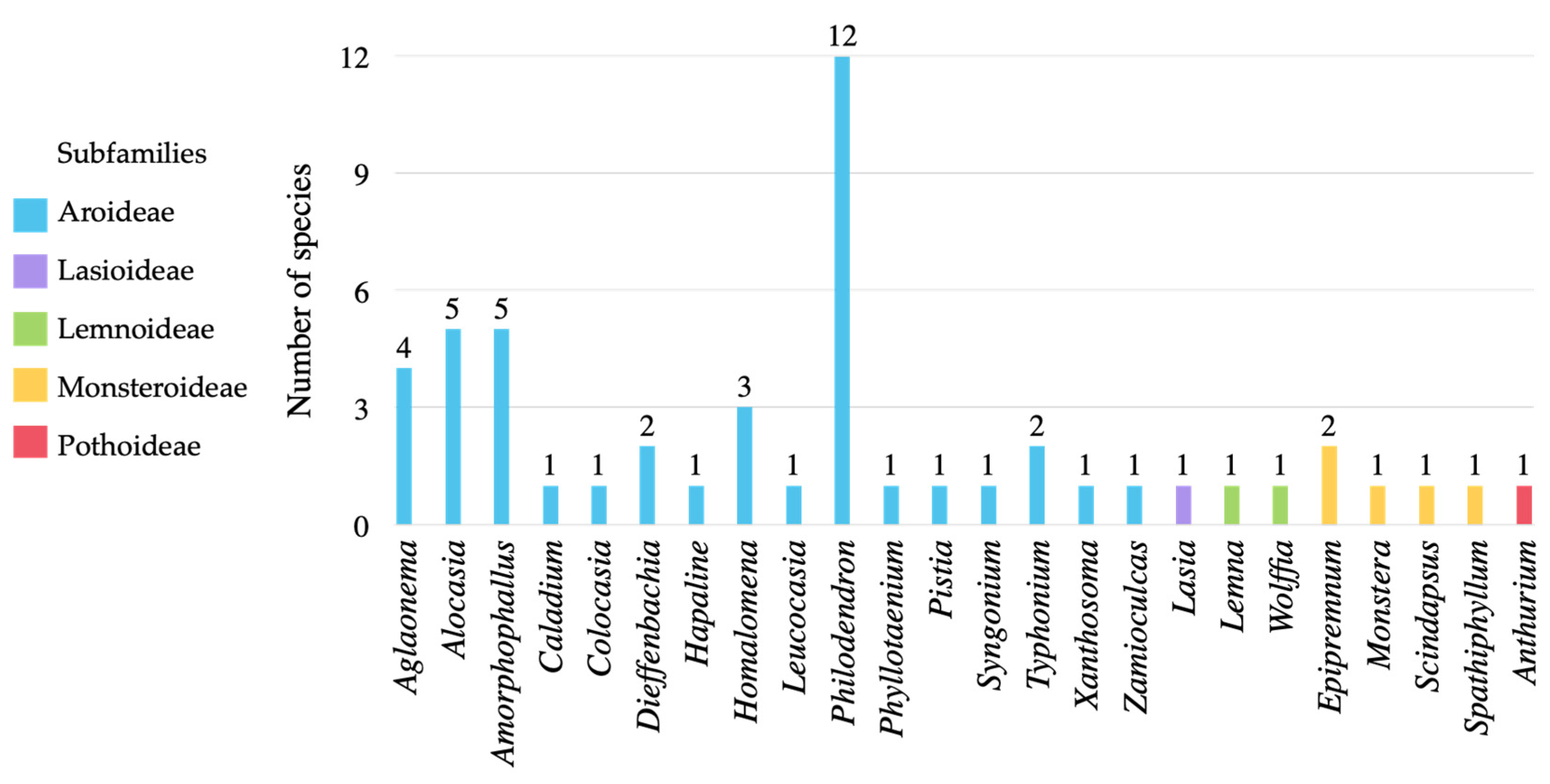
3.1. Diversity of Araceae in Kalasin Province
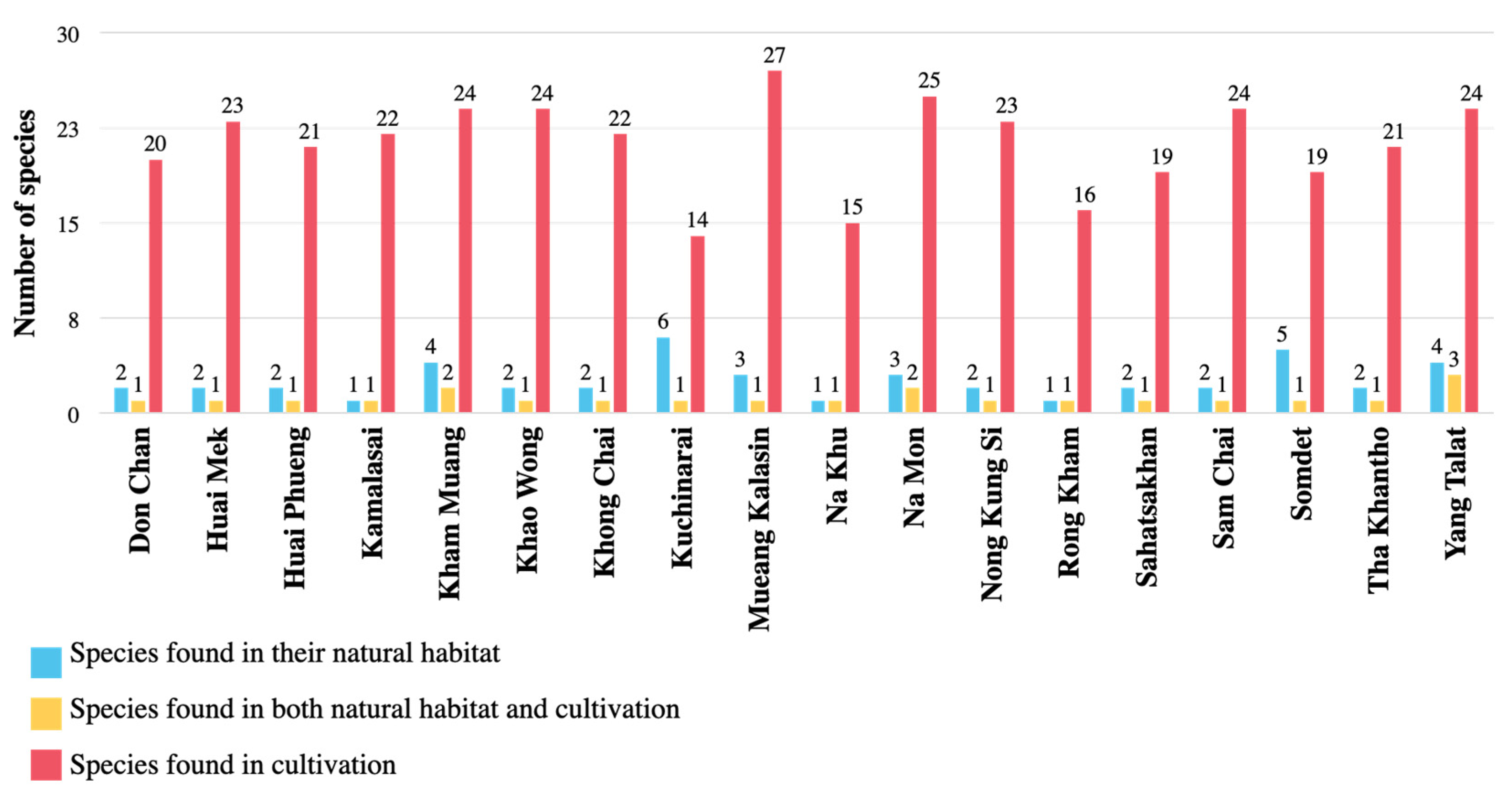
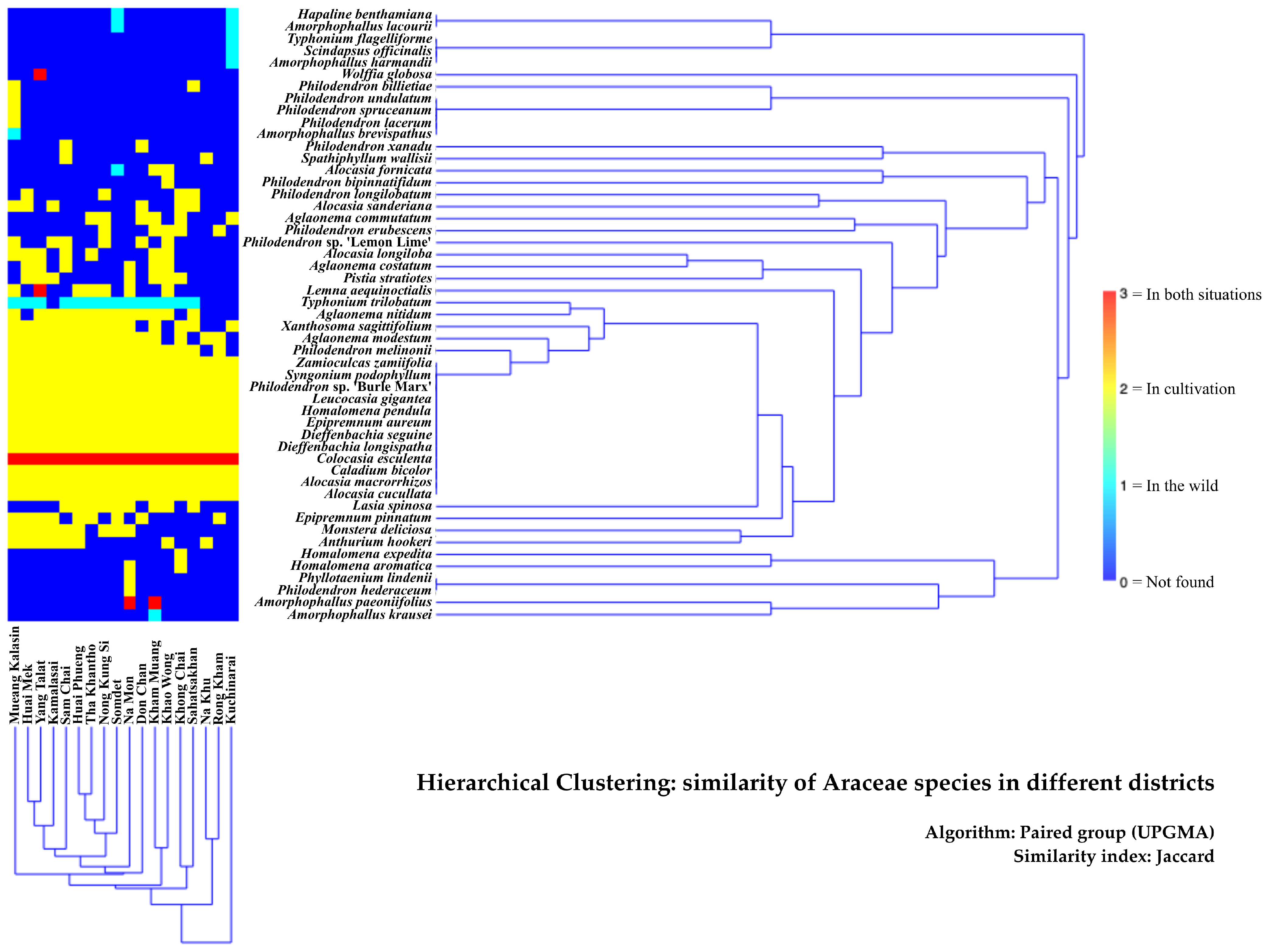
3.2. Distribution of Araceae in Kalasin Province
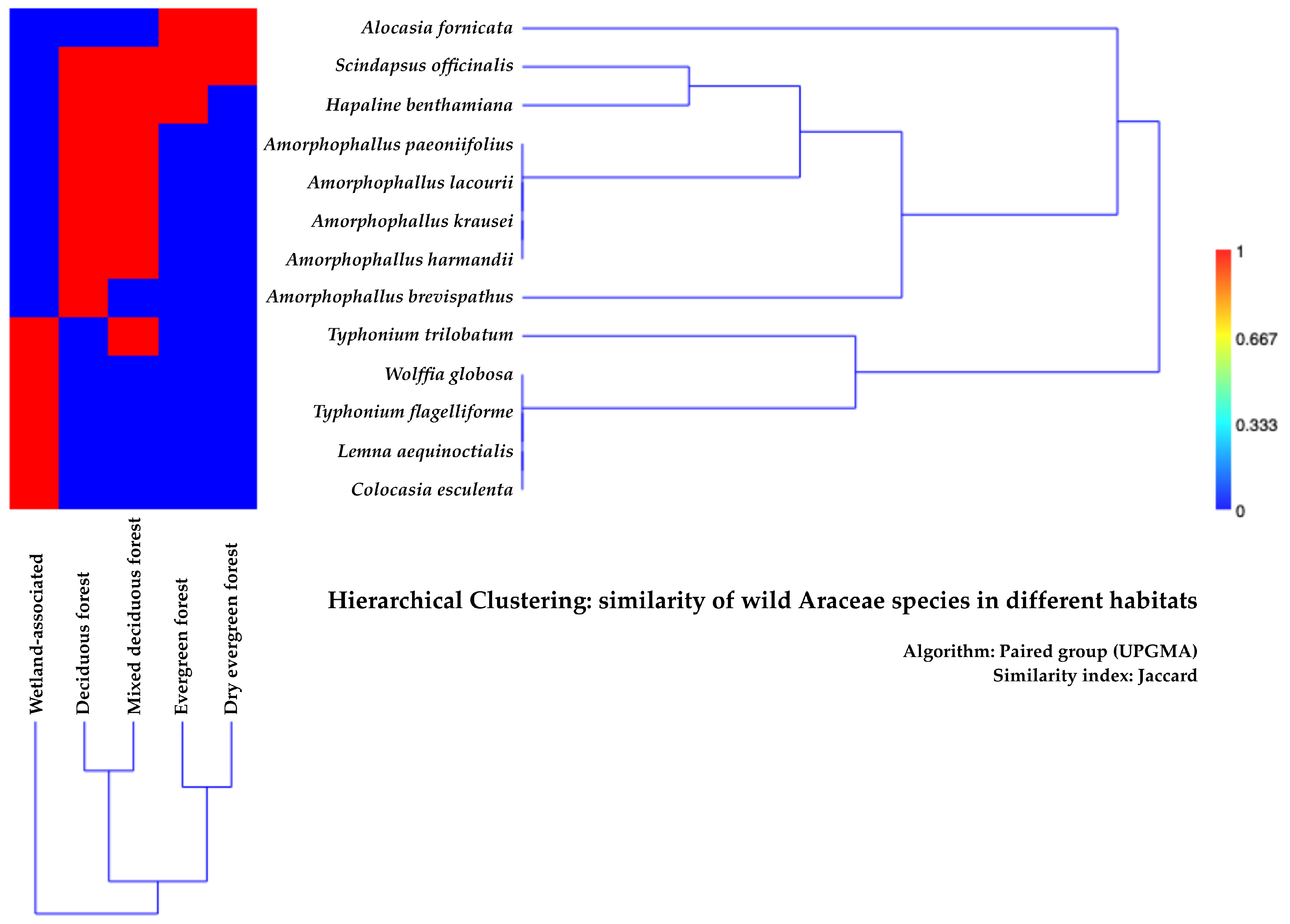
3.3. Ecology and Habitat Preferences of Wild Araceae Species in Kalasin Province
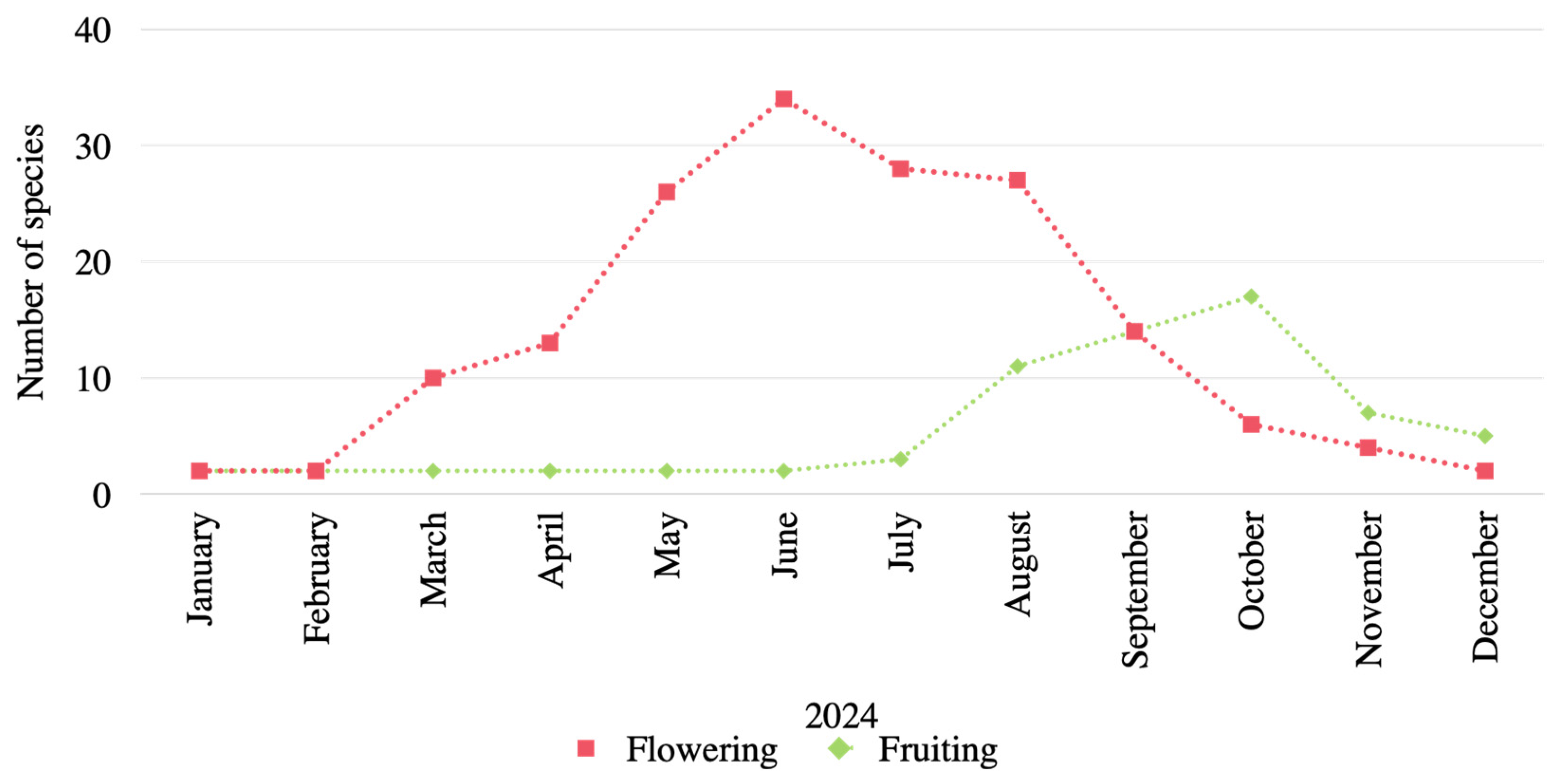
3.4. Phenology of Araceae in Kalasin Province Under Monsoonal Influence
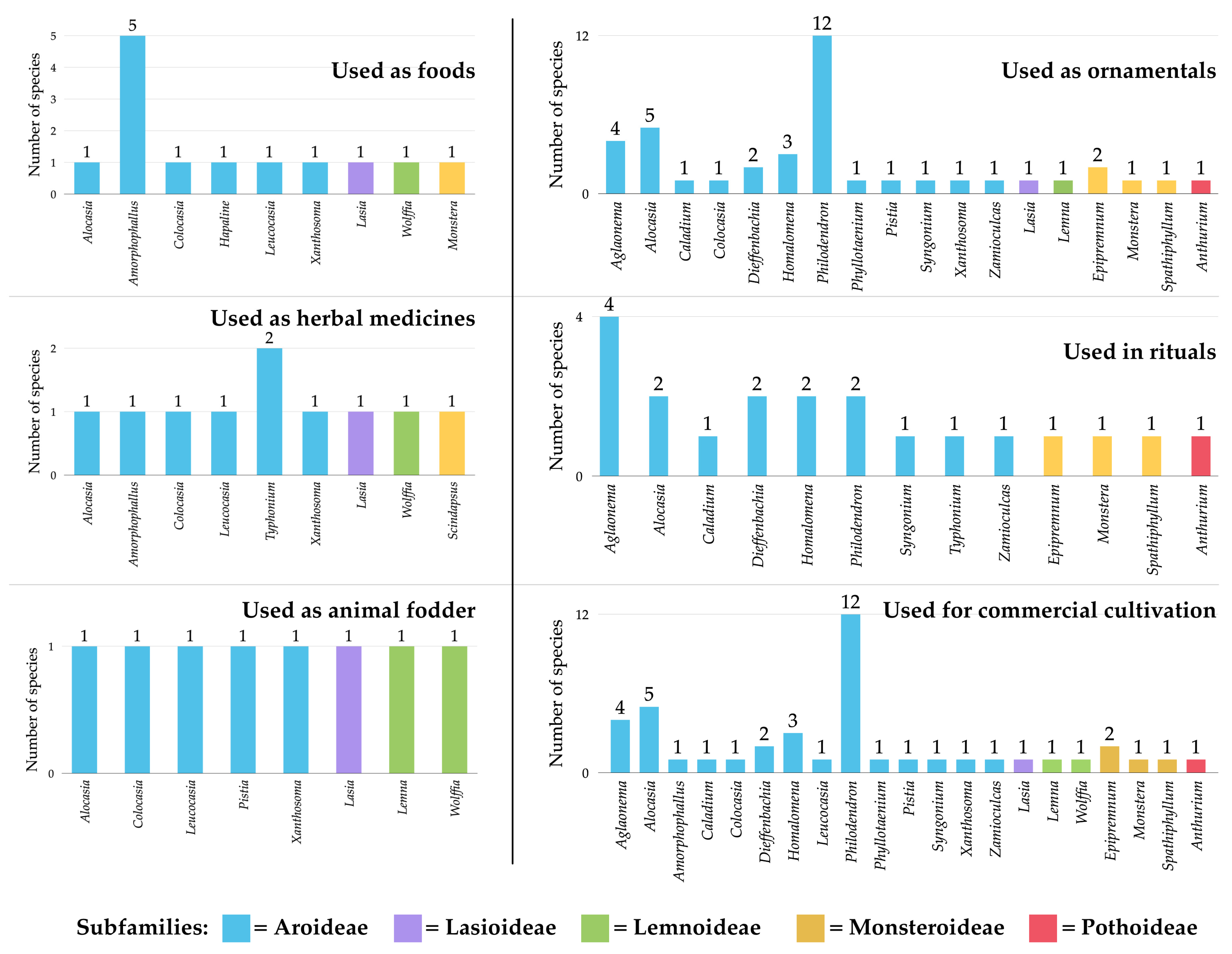
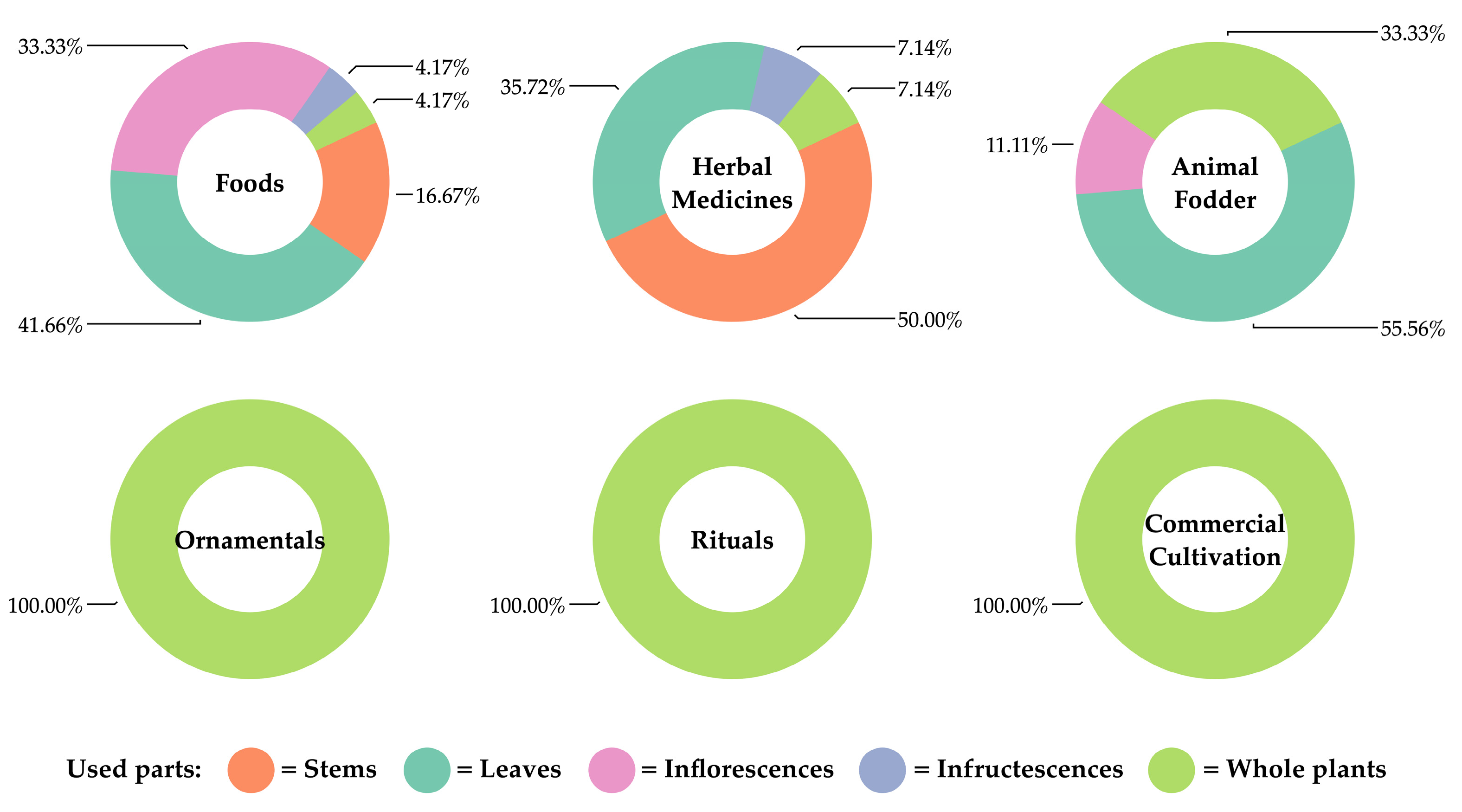
3.5. Utilization of Araceae Species in Kalasin Province
3.5.1. Utilization of Araceae as Food
3.5.2. Utilization of Araceae as Animal Fodder
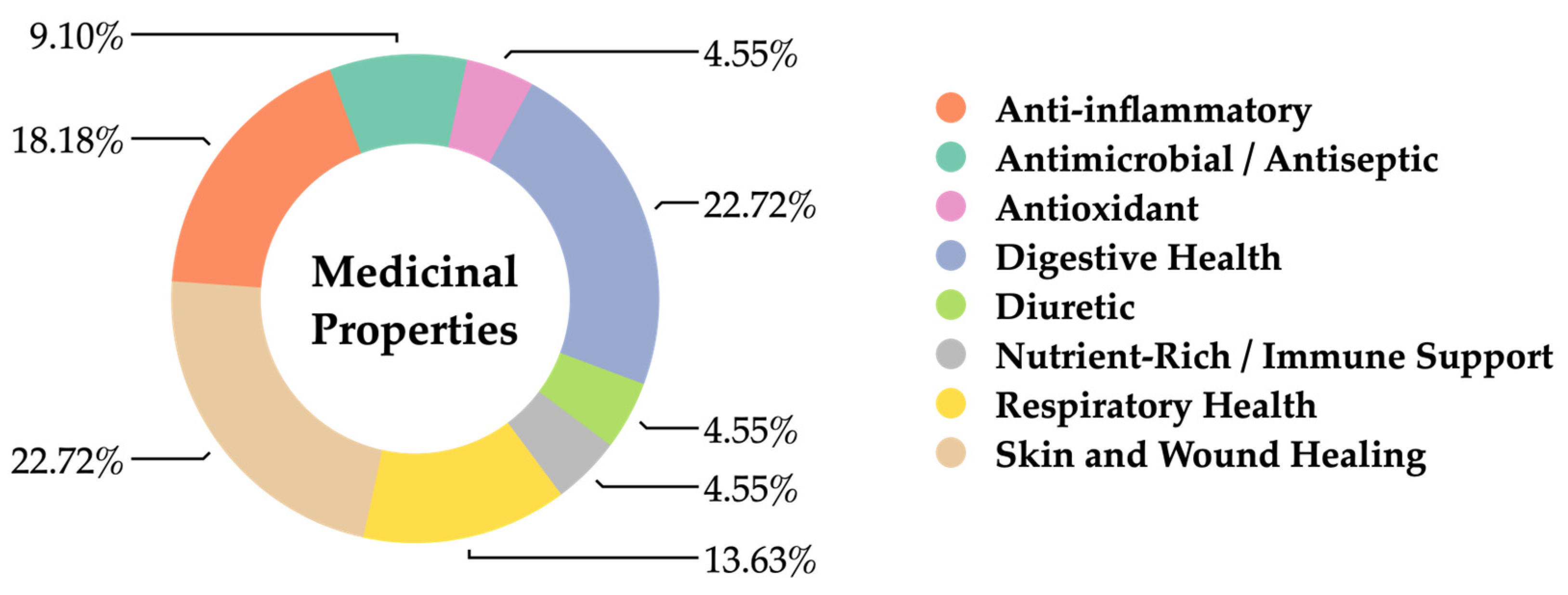
3.5.3. Utilization of Araceae as Herbal Medicine
3.5.4. Utilization of Araceae as Ornamental Foliage
3.5.5. Utilization of Araceae in Rituals
3.5.6. Utilization of Araceae in Commercial Cultivation
3.6. Conservation Status and Threats to Wild Araceae in Kalasin Province
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyce, P.C.; Croat, T.B. The Überlist of Araceae, Totals for Published and Estimated Number of Species in Aroid Genera. Available online: https://www.researchgate.net/publication/369741356_Uberlist_-_April_2023 (accessed on 3 January 2025).
- Wong, S.Y.; Boyce, P.C. Schismatoglottideae (Araceae) of Borneo LXXVII-Circumscribing Schismatoglottis sensu stricto, and Seven New Genera. Webbia J. Plant Taxon. Geogr. 2024, 79, 255–289. [Google Scholar]
- Hein, K.Z.; Prehsler, D.; Saensouk, S.; Low, S.L. Hayarum mirispathum (Araceae—Aroideae): A New Genus and Species from Thailand. Taiwania 2025, 70, 65–74. [Google Scholar]
- APG IV [Angiosperm Phylogeny Group IV]; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Givnish, T.J.; Zuluaga, A.; Spalink, D.; Soto Gomez, M.; Lam, V.K.Y.; Saarela, J.M.; Sass, C.; Iles, W.J.D.; de Sousa, D.J.L.; Leebens-Mack, J.; et al. Monocot Plastid Phylogenomics, Timeline, Net Rates of Species Diversification, the Power of Multi-Gene Analyses, and a Functional Model for the Origin of Monocots. Am. J. Bot 2018, 105, 1888–1910. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, P.R.; Wafula, E.K.; Barrett, C.F.; Ayyampalayam, S.; McNeal, J.R.; Rentsch, J.D.; McKain, M.R.; Heyduk, K.; Harkess, A.; Villegente, M.; et al. Phylogenomic Resolution of Order- and Family-Level Monocot Relationships Using 602 Single-Copy Nuclear Genes and 1375 BUSCO Genes. Front. Plant Sci. 2022, 13, 876779. [Google Scholar] [CrossRef]
- Haigh, A.L.; Gibernau, M.; Maurin, O.; Bailey, P.; Carlsen, M.M.; Hay, A.; Leempoel, K.; McGinnie, C.; Mayo, S.; Morris, S.; et al. Target Sequence Data Shed New Light on the Infrafamilial Classification of Araceae. Am. J. Bot. 2023, 110, e16117. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Henny, R.J.; Liao, F. Aroids Are Important Medicinal Plants. Acta Hortic. 2007, 756, 346–354. [Google Scholar] [CrossRef]
- Nauheimer, L.; Metzler, D.; Renner, S.S. Global History of the Ancient Monocot Family Araceae Inferred with Models Accounting for Past Continental Positions and Previous Ranges Based on Fossils. New Phytol. 2012, 195, 938–950. [Google Scholar] [CrossRef]
- Asharo, R.K.; Novitasari, A.; Azizah, S.D.N.; Saraswati, R.A.; Setyaningsih, F.; Apriliani, P.; Priambodo, R.; Pasaribu, P.O.; Rizkawati, V.; Usman, U.; et al. Araceae Floristic and Potential Study in Bogor Botanical Gardens, West Java, Indonesia. J. Ris. Biol. Apl. 2022, 4, 9–18. [Google Scholar] [CrossRef]
- Croat, T.B. The Ecology and Life Forms of Araceae. Aroideana 1988, 11, 4–56. [Google Scholar]
- Mayo, S.J.; Bogner, J.; Boyce, P.C. The Genera of Araceae; Royal Botanic Gardens: Kew, UK, 1997; 370p. [Google Scholar]
- Boyce, P.C.; Wong, S.Y. The Araceae of Malesia I: Introduction. Malay. Nat. J. 2012, 64, 33–67. [Google Scholar]
- Boyce, P.C.; Wong, S.Y. Borneo and Its Disproportionately Large Rheophytic Aroid Flora. Gard. Bull. Singap. 2019, 71 (Suppl. 2), 497–524. [Google Scholar]
- Zotz, G. Life Forms in Aroids—Natural Variability vs. Terminological Confusion. Aroideana 2020, 43, 315–333. [Google Scholar]
- Croat, T.B.; Ortiz, O.O. Distribution of Araceae and the Diversity of Life Forms. Acta Soc. Bot. Pol. 2020, 89, 1–23. [Google Scholar]
- Gandawijaja, D.; Idris, S.; Nasution, R.; Nyman, L.P.; Arditti, J. Amorphophallus titanum Becc.: A Historical Review and Some Recent Observations. Ann. Bot. 1983, 51, 269–278. [Google Scholar]
- Boyce, P.C.; Sookchaloem, D.; Hetterscheid, W.L.A.; Gusman, G.; Jacobsen, N.; Idei, T.; Du, N.V. Araceae. Flora Thail. 2012, 11, 101–321. [Google Scholar]
- POWO. Plants of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 3 January 2025).
- Mashhor, M.; Boyce, P.C.; Othman, A.; Suleiman, B. The Araceae of Peninsular Malaysia; Penerbitan Univ. Sains Malaysia: Pulau Pinang, Malaysia, 2012; 146p. [Google Scholar]
- Low, S.L.; Yu, C.C.; Ooi, I.H.; Eiadthong, W.; Galloway, A.; Zhou, Z.K.; Xing, Y.W. Extensive Miocene Speciation in and out of Indochina: The Biogeographic History of Typhonium sensu stricto (Araceae) and Its Implication for the Assembly of Indochina Flora. J. Syst. Evol. 2020, 59, 419–428. [Google Scholar]
- Choenkwan, S.; Fox, J.M.; Rambo, A.T. Agriculture in the Mountains of Northeastern Thailand: Current Situation and Prospects for Development. Mt. Res. Dev. 2014, 34, 95–106. [Google Scholar]
- Phatlamphu, N.; Saensouk, S.; Saensouk, P.; Jungsongduang, A. Ethnobotany of Edible Plants in Muang District, Kalasin Province, Thailand. Biodiversitas 2021, 22, 5432–5444. [Google Scholar]
- Phatlamphu, N.; Saensouk, S.; Saensouk, P.; Junsongduang, A.; Setyawan, A.D. Economic Value Assessment of Edible Plants in Muang District, Kalasin Province, Thailand. Biodiversitas 2023, 24, 3960–3967. [Google Scholar]
- Niamngon, P.; Saensouk, S.; Saensouk, P.; Junsongduang, A. Ethnobotanical Knowledge of Isaan Laos Tribe in Khong Chai District, Kalasin Province, Thailand with Particular Focus on Medicinal Uses. Biodiversitas 2023, 24, 6793–6824. [Google Scholar] [CrossRef]
- Topographic Map. Kalasin Province. Available online: https://en-nz.topographic-map.com/map-cqrxcz/Kalasin-Province/?center=53.11051%2C139.04297&zoom=2 (accessed on 3 January 2025).
- Jitpromma, T.; Saensouk, S.; Saensouk, P.; Boonma, T. Diversity, Traditional Uses, Economic Values, and Conservation Status of Zingiberaceae in Kalasin Province, Northeastern Thailand. Horticulturae 2025, 11, 247. [Google Scholar] [CrossRef]
- van Welzen, P.C.; Madern, A.; Raes, N.; Parnell, J.A.N.; Simpson, D.A.; Byrne, C.; Curtis, T.; Macklin, J.; Trias-Blasi, A.; Prajaksood, A.; et al. The Current and Future Status of Floristic Provinces in Thailand. In Land Use, Climate Change and Biodiversity Modeling: Perspectives and Applications; IGI Global: Hershey, PA, USA, 2011; pp. 219–247. [Google Scholar]
- Suwanarat, S.; Chiangga, S.; Yupapin, P. Rainfall Variability Study in Bangkok and Songkhla Province, Thailand Using Cross Wavelet Coherence Stationary Oscillation. Ramkhamhaeng Int. J. Sci. Technol. 2021, 4, 1–10. [Google Scholar]
- Weather-and-Climate.com. Average Monthly Rainfall, Temperature, Sunshine, Kalasin, Kalasin Province, Thailand. Available online: https://weather-and-climate.com/average-monthly-Rainfall-Temperature-Sunshine,kalasin-kalasin-province-th,Thailand#:~:text=Kalasin%20experiences%20significant%20rainfall%20throughout,recorded%20across%2018%20rainy%20days (accessed on 3 January 2025).
- Wongko, K.; Buffetaut, E.; Khamha, S.; Lauprasert, K. Spinosaurid Theropod Teeth from the Redbeds of the Khok Kruat Formation (Early Cretaceous) in Northeastern Thailand. Trop. Nat. Hist. 2019, 19, 8–20. [Google Scholar]
- JSTOR Global Plants. Available online: https://plants.jstor.org/ (accessed on 3 January 2025).
- Global Biodiversity Information Facility (GBIF). Available online: https://www.gbif.org/ (accessed on 3 January 2025).
- IUCN. The IUCN Red List of Threatened Species, Version 2024-2. 2024. Available online: https://www.iucnredlist.org (accessed on 3 January 2025).
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria Version 16. 2024. Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf (accessed on 3 January 2025).
- Bachman, S.; Moat, J.; Hill, A.; de la Torre, J.; Scott, B. Supporting Red List Threat Assessments with GeoCAT: Geospatial Conservation Assessment Tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 3 January 2025).
- Ito, Y.; Barfod, A.S. An Updated Checklist of Aquatic Plants of Myanmar and Thailand. Biodivers. Data J. 2014, 2, e1019. [Google Scholar]
- Lacoul, P.; Freedman, B. Environmental Influences on Aquatic Plants in Freshwater Ecosystems. Environ. Rev. 2006, 14, 89–136. [Google Scholar] [CrossRef]
- Boonma, T.; Saensouk, S.; Saensouk, P. Biogeography, Conservation Status, and Traditional Uses of Zingiberaceae in Saraburi Province, Thailand, with Kaempferia chaveerachiae sp. nov. Horticulturae 2024, 10, 934. [Google Scholar] [CrossRef]
- Kaplan, A.; Zelicha, H.; Tsaban, G.; Meir, A.Y.; Rinott, E.; Kovsan, J.; Novack, L.; Thiery, J.; Ceglarek, U.; Burkhardt, R.; et al. Protein Bioavailability of Wolffia globosa Duckweed, a Novel Aquatic Plant—A Randomized Controlled Trial. Clin. Nutr. 2019, 38, 2576–2582. [Google Scholar] [CrossRef]
- Zubair, M.W.; Imran, A.; Islam, F.; Afzaal, M.; Saeed, F.; Zahra, S.M.; Akhtar, M.N.; Noman, M.; Ateeq, H.; Aslamet, M.A.; et al. Functional Profile and Encapsulating Properties of Colocasia esculenta (Taro). Food Sci. Nutr. 2023, 11, 2440–2449. [Google Scholar] [PubMed]
- Ali, H.; Yaqoob, U. Traditional Uses, Phytochemistry, Pharmacology and Toxicity of Arisaema (Areaceae): A Review. Bull. Natl. Res. Cent. 2021, 45, 47. [Google Scholar]
- Setyawan, H.B.; Yulianto, R.; Zelin, O.; Purnamasari, L. Potential of Three Taro (Colocasia esculenta L.) Cultivars as Animal Feed. ASEAN J. Sci. Technol. Dev. 2021, 38, 97–102. [Google Scholar]
- Saswati, R.; Choudhury, M.D.; Paul, S.B. Antibacterial Activity of Araceae: An Overview. Int. J. Res. Ayurveda Pharm. 2013, 4, 15–17. [Google Scholar]
- Tyagi, R.S.; Pachute, A.P.; Singh, A.; Baghel, A.; Patel, B.D. Antibacterial Activity of Aqueous and Ethanolic Extracts of Scindapsus officinales (Roxb.) Schott. Adv. Biol. Res. 2011, 5, 77–80. [Google Scholar]
- Rodrigues, A.C.A.; Andrade, I.M.; Costa, C.C.; Santos, S.S.O.; Souza, R.T.B. Araceae Juss. Used as an Ornamental in Northeastern Brazil. Comun. Sci. 2024, 16, e4220. [Google Scholar]
| No. | Scientific Name | Vernacular Name | Native Status in Thailand | Distribution Of Species Across the Districts of Kalasin Province | Conservation Status | Proposed by Authors | Ecology of Wild Species | Phenology | Utilization | Voucher | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Don Chan | Huai Mek | Huai Phueng | Kamalasai | Kham Muang | Khao Wong | Khong Chai | Kuchinarai | Mueang Kalasin | Na Khu | Na Mon | Nong Kung Si | Rong Kham | Sahatsakhan | Sam Chai | Somdet | Tha Khantho | Yang Talat | Flowering | Fruiting | Purposes | Used Parts | ||||||||
| 1 | Aglaonema commutatum Schott | Kaew Kanjana | ITD | CT | - | - | - | CT | - | CT | CT | - | - | - | CT | - | - | - | - | CT | - | 6–9 | 10–12 | Or, Ri, Co | P | KLS001 | |||
| 2 | Aglaonema costatum N.E.Br. | Po Num Ngern | NTV | - | CT | - | - | CT | CT | - | - | - | - | CT | - | - | - | CT | - | - | CT | 3–6 | 8–10 | Or, Ri, Co | P | KLS002 | |||
| 3 | Aglaonema modestum Schott ex Engl. | Keaw Muen Pee | NTV | CT | CT | CT | CT | CT | - | CT | - | CT | CT | CT | CT | CT | - | CT | CT | CT | CT | 3–5 | 7–9 | Or, Ri, Co | P | KLS003 | |||
| 4 | Aglaonema nitidum (Jack) Kunth | Rio Ngern | NTV | CT | - | CT | CT | CT | CT | - | - | CT | - | CT | CT | - | CT | CT | CT | CT | CT | 1–12 | 1–12 | Or, Ri, Co | P | KLS004 | |||
| 5 | Alocasia cucullata (Lour.) G.Don | Nangkwak | NTV | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 5 | Not seen | Or, Ri, Co | P | KLS005 | |||
| 6 | Alocasia fornicata (Kunth) Schott | Kradat Dong | NTV | - | - | - | - | CT | CT | - | - | - | - | - | - | - | - | - | WD | - | - | LC | VU | DEF, EF | 6–8 | 10–12 | Or, Co | P | KLS006 |
| 7 | Alocasia longiloba Miq. | Wan Phaya Chong Ang | NTV | - | CT | - | - | CT | CT | - | - | CT | - | - | - | - | - | CT | - | CT | CT | 8–10 | Not seen | Or, Co | P | KLS007 | |||
| 8 | Alocasia macrorrhizos (L.) G.Don | Kra Dat Dam | NTV | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 6–8 | 10–12 | Fo, Me, Or, An, Co | S, P | KLS008 | |||
| 9 | Alocasia sanderiana W.Bull | Kaew Sarapat Nuek | ITD | CT | CT | - | CT | - | - | CT | - | CT | - | - | - | - | CT | - | - | - | - | 6–8 | Not seen | Or, Ri, Co | P | KLS009 | |||
| 10 | Amorphophallus brevispathus Gagnep. | Buk | NTV | - | - | - | - | - | - | - | - | WD | - | - | - | - | - | - | - | - | - | NE | EN | DF | 5–6 | 8–10 | Fo | L, Fl | KLS010 |
| 11 | Amorphophallus harmandii Engl. & Gehrm. | Buk | NTV | - | - | - | - | - | - | - | WD | - | - | - | - | - | - | - | - | - | - | NE | VU | DF, MDF | 5–7 | 8–10 | Fo | L, Fl | KLS011 |
| 12 | Amorphophallus krausei Engl. | Buk | NTV | - | - | - | - | WD | - | - | - | - | - | - | - | - | - | - | - | - | - | NE | DD | DF, MDF | 5–6 | 8–10 | Fo | L, Fl | KLS012 |
| 13 | Amorphophallus lacourii Linden & André | Buk | NTV | - | - | - | - | - | - | - | WD | - | - | - | - | - | - | - | WD | - | - | NE | VU | DF, MDF | 5–8 | 8–10 | Fo | L, Fl | KLS013 |
| 14 | Amorphophallus paeoniifolius (Dennst.) Nicolson | Buk Khangkhok | NTV | - | - | - | - | BT | - | - | - | - | - | BT | - | - | - | - | - | - | - | LC | LC | DF, MDF | 5–6 | 8–10 | Fo, Me, Co | S, L, Fl, P | KLS014 |
| 15 | Anthurium hookeri Kunth | Anthurium Rang Nok | ITD | - | CT | CT | CT | - | CT | - | - | CT | CT | - | - | - | - | CT | - | - | CT | 5–6 | 7–9 | Or, Ri, Co | P | KLS015 | |||
| 16 | Caladium bicolor (Aiton) Vent. | Bon Si | ITD | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 8–9 | 10–11 | Or, Ri, Co | P | KLS016 | |||
| 17 | Colocasia esculenta (L.) Schott | Phueak | NTV | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | BT | LC | LC | WAH | 8–9 | 10–11 | Fo, Me, Or, An, Co | S, L, Fl, P | KLS017 |
| 18 | Dieffenbachia longispatha Engl. & K.Krause | Sait Tee Wilson | ITD | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 6–8 | Not seen | Or, Ri, Co | P | KLS018 | |||
| 19 | Dieffenbachia seguine (Jacq.) Schott | Saow Noi Pra Paeng | ITD | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 3–9 | Not seen | Or, Ri, Co | P | KLS019 | |||
| 20 | Epipremnum aureum (Linden & André) G.S.Bunting | Phlu Daang | ITD | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | Not seen | Not seen | Or, Ri, Co | P | KLS020 | |||
| 21 | Epipremnum pinnatum (L.) Engl. | Phlu Chang | NTV | CT | CT | CT | CT | - | - | - | - | CT | - | - | - | CT | - | - | CT | CT | CT | 4–7 | 9–11 | Or, Co | P | KLS021 | |||
| 22 | Hapaline benthamiana Schott | Bon Tao | NTV | - | - | - | - | - | - | - | WD | - | - | - | - | - | - | - | WD | - | - | NE | VU | EF, DF, MDF | 5–8 | 9–10 | Fo | L, Fl | KLS022 |
| 23 | Homalomena aromatica (Spreng.) Schott | Tao Kiat | NTV | - | - | - | - | - | - | CT | - | - | - | CT | - | - | - | - | - | - | - | 6–9 | Not seen | Or, Ri, Co | P | KLS023 | |||
| 24 | Homalomena expedita A.Hay & Hersc. | Phat Bok Riow | ITD | - | - | - | - | - | - | CT | - | - | - | - | - | - | - | - | - | - | - | 5–7 | Not seen | Or, Co | P | KLS024 | |||
| 25 | Homalomena pendula (Blume) Bakh.f. | Sa Neh Jan Daeng | ITD | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 6–9 | Not seen | Or, Ri, Co | P | KLS025 | |||
| 26 | Lasia spinosa (L.) Thwaites | Phak Naam | NTV | - | - | CT | - | CT | CT | - | - | - | - | CT | CT | - | CT | CT | CT | CT | - | 7–9 | 10–12 | Fo, Me, Or, An, Co | L, Fl, P | KLS026 | |||
| 27 | Lemna aequinoctialis Welw. | Nhae | NTV | - | - | CT | - | - | CT | - | - | CT | - | CT | CT | - | - | - | - | CT | BT | LC | LC | WAH | 1–12 | 1–12 | Or, An, Co | P | KLS027 |
| 28 | Leucocasia gigantea (Blume) Schott | Bon Yaak | NTV | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 4–6 | 8–10 | Fo, Me, An, Co | S, L, P | KLS028 | |||
| 29 | Monstera deliciosa Liebm. | Phlu Cheek, Phlu Chaek, Monstera | ITD | - | CT | CT | CT | - | - | - | - | CT | - | CT | CT | - | - | CT | CT | - | CT | 3–6 | 8–10 | Fo, Or, Ri, Co | Fr, P | KLS029 | |||
| 30 | Philodendron billietiae Croat | Philo Gaan Som | ITD | - | - | - | - | - | - | - | - | CT | - | - | - | - | CT | - | - | - | - | 7–10 | Not seen | Or, Co | P | KLS030 | |||
| 31 | Philodendron bipinnatifidum Schott ex Endl. | Philo See Lorm | ITD | - | - | - | - | - | CT | - | - | - | - | - | - | - | - | - | - | - | - | 6–8 | Not seen | Or, Co | P | KLS031 | |||
| 32 | Philodendron erubescens K.Koch & Augustin | Morrakot Daeng | ITD | - | - | - | - | CT | CT | CT | - | - | - | - | CT | CT | - | - | - | - | - | 3–6 | Not seen | Or, Co | P | KLS032 | |||
| 33 | Philodendron hederaceum (Jacq.) Schott | Philo Bai Hua Jai | ITD | - | - | - | - | - | - | - | - | - | - | CT | - | - | - | - | - | - | - | 3–8 | Not seen | Or, Co | P | KLS033 | |||
| 34 | Philodendron lacerum (Jacq.) Schott | Philo Bai A-ngoon | ITD | - | - | - | - | - | - | - | - | CT | - | - | - | - | - | - | - | - | - | 5–6 | Not seen | Or, Co | P | KLS034 | |||
| 35 | Philodendron longilobatum Sakur. | Philo Longi Lelano | ITD | - | CT | - | - | - | - | CT | - | - | - | - | CT | - | CT | - | - | - | - | Not seen | Not seen | Or, Co | P | KLS035 | |||
| 36 | Philodendron melinonii Brongn. ex Regel | Philo Sait Tee Ruay Sap, Sait Tee Mee Sap | ITD | CT | CT | CT | CT | CT | CT | CT | - | CT | - | CT | CT | CT | CT | CT | CT | CT | CT | 3–8 | Not seen | Or, Co | P | KLS036 | |||
| 37 | Philodendron sp. ‘Burle Marx’ | Philo Morrakot Yok | ITD | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | Not seen | Not seen | Or, Ri, Co | P | KLS037 | |||
| 38 | Philodendron sp. ‘Lemon Lime’ | Philo Si Thong | ITD | CT | - | - | CT | - | CT | - | - | CT | - | - | CT | - | - | CT | - | - | - | Not seen | Not seen | Or, Ri, Co | P | KLS038 | |||
| 39 | Philodendron spruceanum G.S.Bunting | Philo Nuat Plaa Muek | ITD | - | - | - | - | - | - | - | - | CT | - | - | - | - | - | - | - | - | - | Not seen | Not seen | Or, Co | P | KLS039 | |||
| 40 | Philodendron undulatum Engl. | Philo Bai Kluen | ITD | - | - | - | - | - | - | - | - | CT | - | - | - | - | - | - | - | - | - | Not seen | Not seen | Or, Co | P | KLS040 | |||
| 41 | Philodendron xanadu Croat, Mayo & J.Boos | Xanadu | ITD | CT | - | - | - | - | - | - | - | - | - | - | - | - | - | CT | - | - | - | 5–6 | Not seen | Or, Co | P | KLS041 | |||
| 42 | Phyllotaenium lindenii André | Xanthosoma | ITD | - | - | - | - | - | - | - | - | - | - | CT | - | - | - | - | - | - | - | 6–11 | Not seen | Or, Co | P | KLS042 | |||
| 43 | Pistia stratiotes L. | Jork | NTV | - | CT | - | CT | CT | CT | CT | - | - | - | CT | - | - | - | - | - | - | CT | 5–11 | 6–12 | Or, An, Co | P | KLS043 | |||
| 44 | Scindapsus officinalis (Roxb.) Schott | Phlu Chaang | NTV | - | - | - | - | - | - | - | WD | - | - | - | - | - | - | - | - | - | - | NE | VU | DEF, EF, DF, MDF | 6–8 | 9–10 | Me | Fr | KLS044 |
| 45 | Spathiphyllum wallisii Regel | Nah Wua Thai, Dehlee | ITD | - | - | - | - | - | - | - | - | - | CT | - | - | - | - | CT | - | - | - | 3–8 | Not seen | Or, Ri, Co | P | KLS045 | |||
| 46 | Syngonium podophyllum Schott | Ngern Lai Ma | ITD | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 6–8 | Not seen | Or, Ri, Co | P | KLS046 | |||
| 47 | Typhonium flagelliforme (G.Lodd.) Blume | Wan Phraya Hok Hak | NTV | - | - | - | - | - | - | - | WD | - | - | - | - | - | - | - | - | - | - | LC | LC | WAH | 4–5 | 6–8 | Me | S, L | KLS047 |
| 48 | Typhonium trilobatum (L.) Schott | Utta Phit | NTV | WD | WD | WD | - | WD | WD | WD | - | WD | - | WD | WD | - | WD | WD | WD | WD | WD | NE | LC | MDF, WAH | 5–7 | 8–9 | Me, Ri | S, P | KLS048 |
| 49 | Wolffia globosa (Roxb.) Hartog & Plas | Phaam | NTV | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | BT | LC | LC | WAH | Not seen | Not seen | Fo, Me, An, Co | P | KLS049 |
| 50 | Xanthosoma sagittifolium (L.) Schott | Bon Kradat Dam | ITD | - | CT | CT | CT | CT | - | CT | CT | CT | - | CT | CT | - | CT | CT | CT | CT | CT | 7–8 | Not seen | Fo, Me, Or, An, Co | S, L, P | KLS050 | |||
| 51 | Zamioculcas zamiifolia (G.Lodd.) Engl. | Kwak Morrakot | ITD | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | CT | 7–9 | Not seen | Or, Ri, Co | P | KLS051 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hein, K.Z.; Saensouk, S.; Saensouk, P.; Rakarcha, S.; Chanthavongsa, K.; Boonma, T. Diversity, Utilization, and Conservation Status of Araceae in Kalasin Province, Northeastern Thailand. Horticulturae 2025, 11, 372. https://doi.org/10.3390/horticulturae11040372
Hein KZ, Saensouk S, Saensouk P, Rakarcha S, Chanthavongsa K, Boonma T. Diversity, Utilization, and Conservation Status of Araceae in Kalasin Province, Northeastern Thailand. Horticulturae. 2025; 11(4):372. https://doi.org/10.3390/horticulturae11040372
Chicago/Turabian StyleHein, Khant Zaw, Surapon Saensouk, Piyaporn Saensouk, Sarayut Rakarcha, Khamfa Chanthavongsa, and Thawatphong Boonma. 2025. "Diversity, Utilization, and Conservation Status of Araceae in Kalasin Province, Northeastern Thailand" Horticulturae 11, no. 4: 372. https://doi.org/10.3390/horticulturae11040372
APA StyleHein, K. Z., Saensouk, S., Saensouk, P., Rakarcha, S., Chanthavongsa, K., & Boonma, T. (2025). Diversity, Utilization, and Conservation Status of Araceae in Kalasin Province, Northeastern Thailand. Horticulturae, 11(4), 372. https://doi.org/10.3390/horticulturae11040372








