Hydrogen Sulfide Mitigates Manganese-Induced Toxicity in Malus hupehensis Plants by Regulating Osmoregulation, Antioxidant Defense, Mineral Homeostasis, and Glutathione Ascorbate Cycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatments
2.2. Estimation of Growth and Photosynthetic Pigment Parameters
2.3. Estimation of Leaf RWC
2.4. Estimation of Soluble Sugar and Proline Contents
2.5. Estimation of Metal Element Content
2.6. Evaluation of MDA and H2O2 Contents
2.7. Determination of Electrolyte Leakage
2.8. RNA Extraction and RT-qPCR Analysis
2.9. Estimation of Enzymatic and Non-Enzymatic Antioxidant Activity
2.10. Statistical Analysis
3. Results
3.1. Effect of H2S on Plant Biomass and Leaf RWC Under Manganese Stress
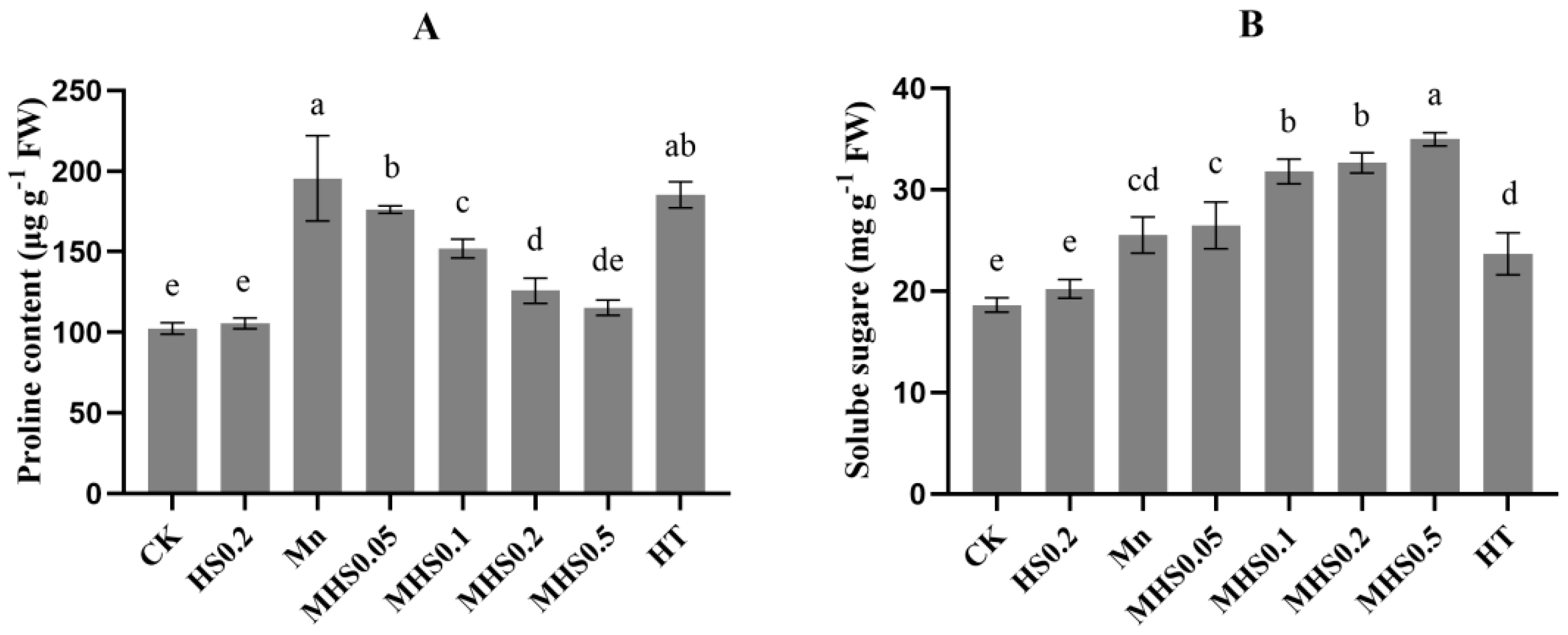
3.2. Effect of H2S on Proline and Soluble Sugar Contents Under Manganese Stress
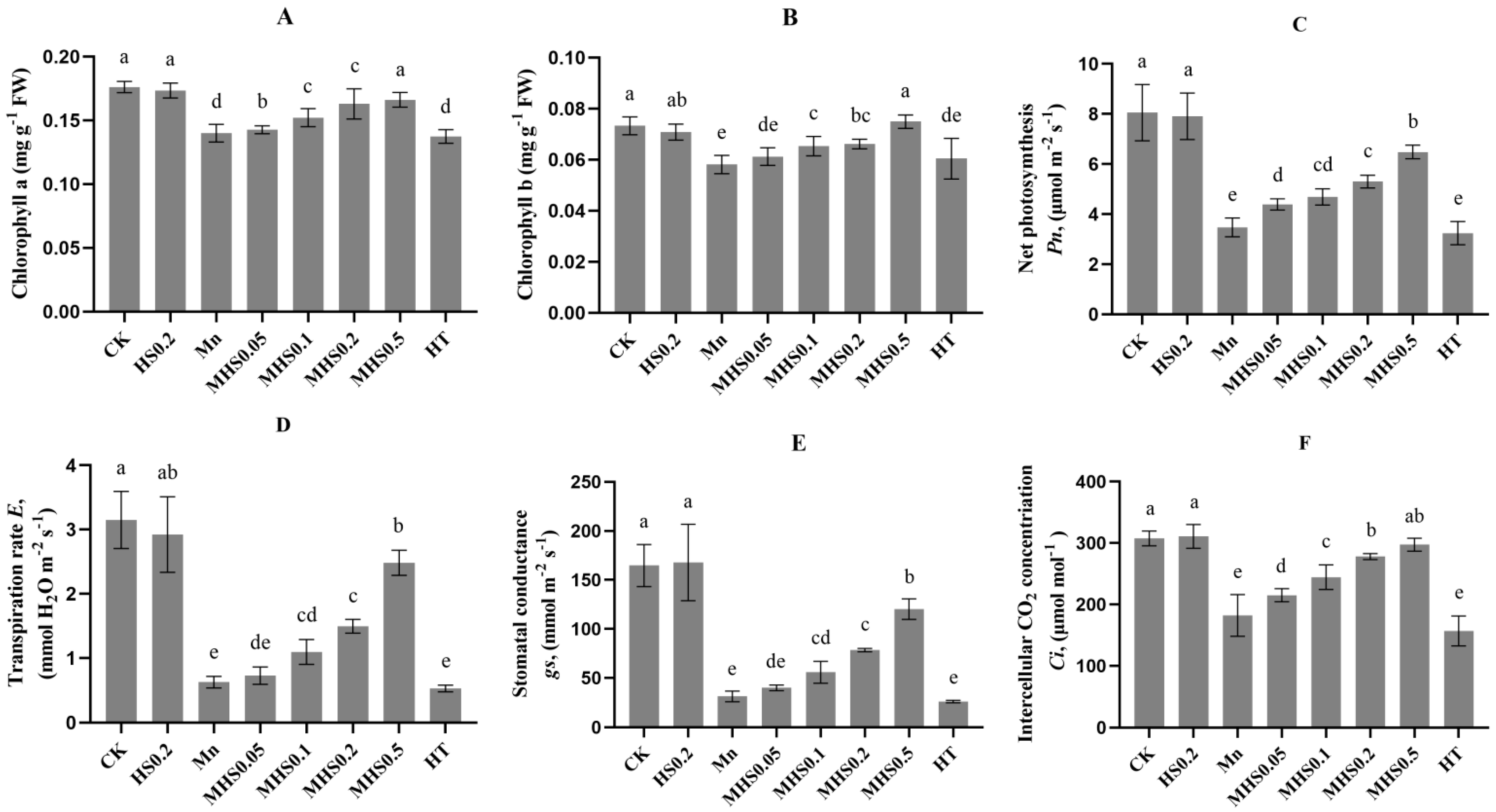
3.3. Effect of H2S on Chlorophyll Content and Gas Exchange Parameters Under Manganese Stress
3.4. Effect of H2S on Mineral Homeostasis and Manganese Accumulation Under Mn Toxicity
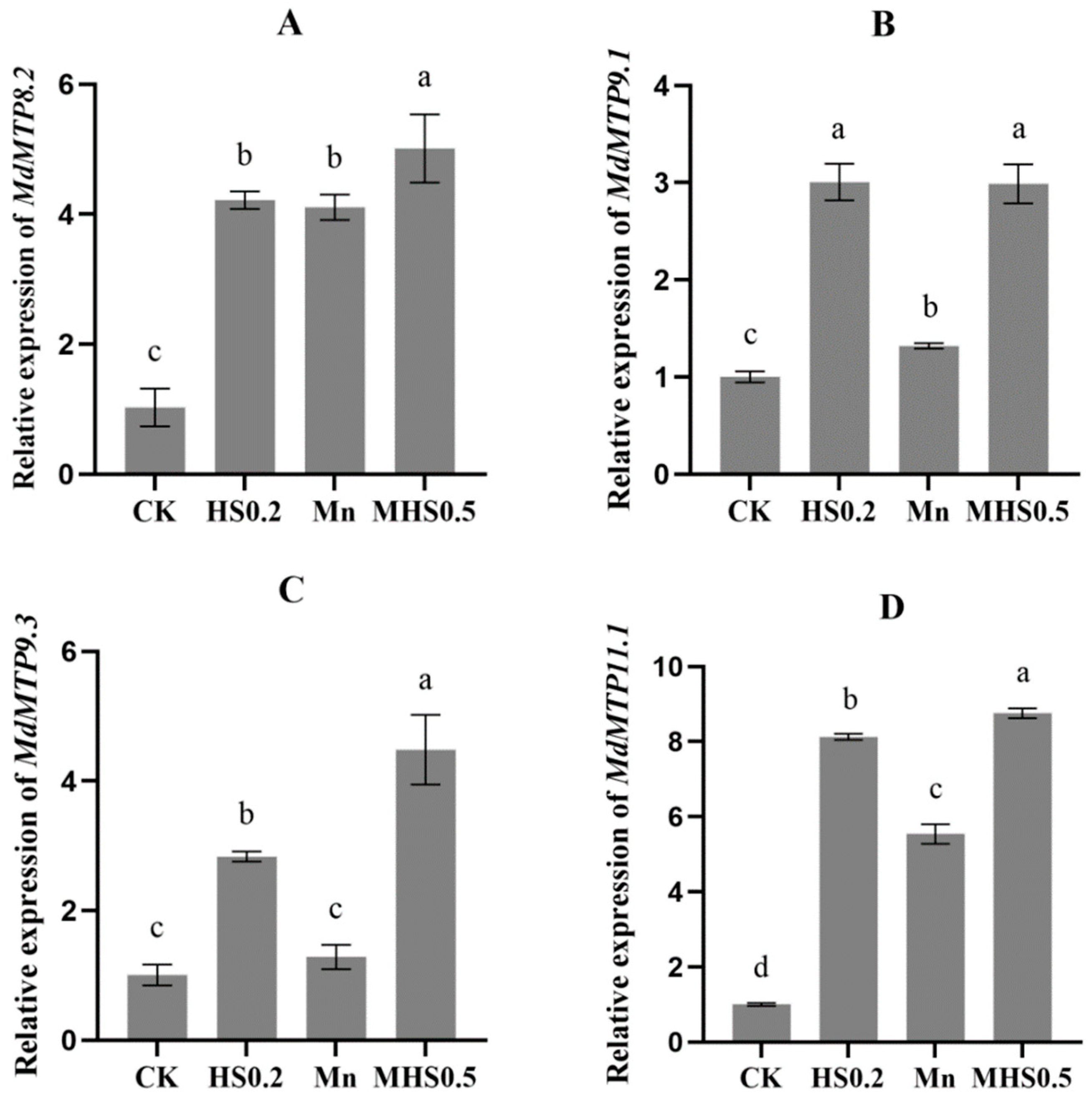
3.5. Effect of H2S on the Expression of Manganese Tolerance Protein Genes Under Mn Stress
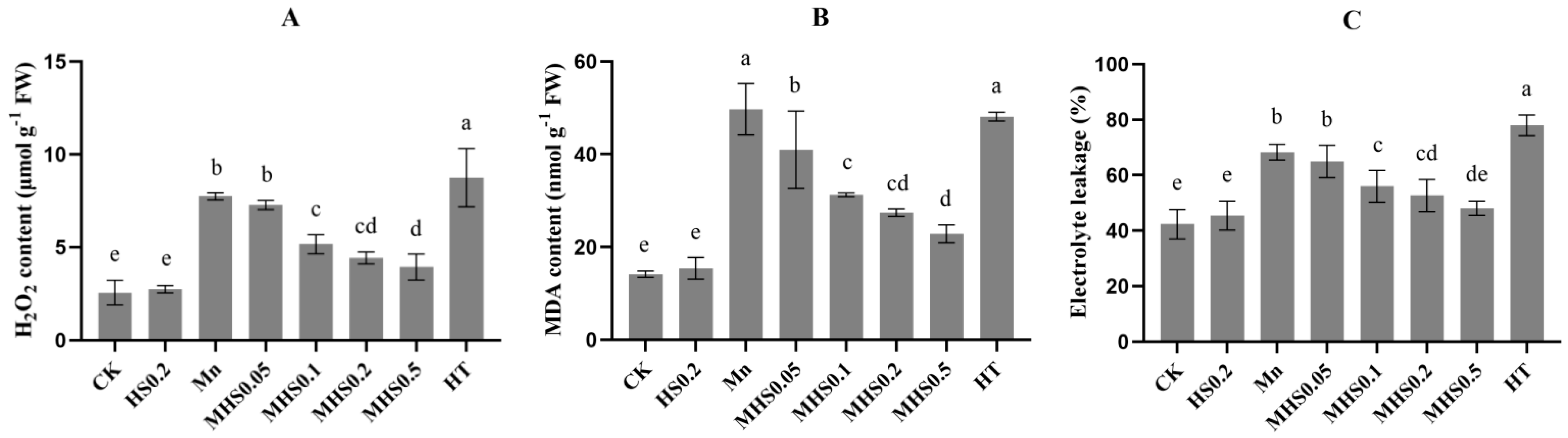
3.6. Effect of H2S on ROS Accumulation and Electrolyte Leakage Under Mn Stress
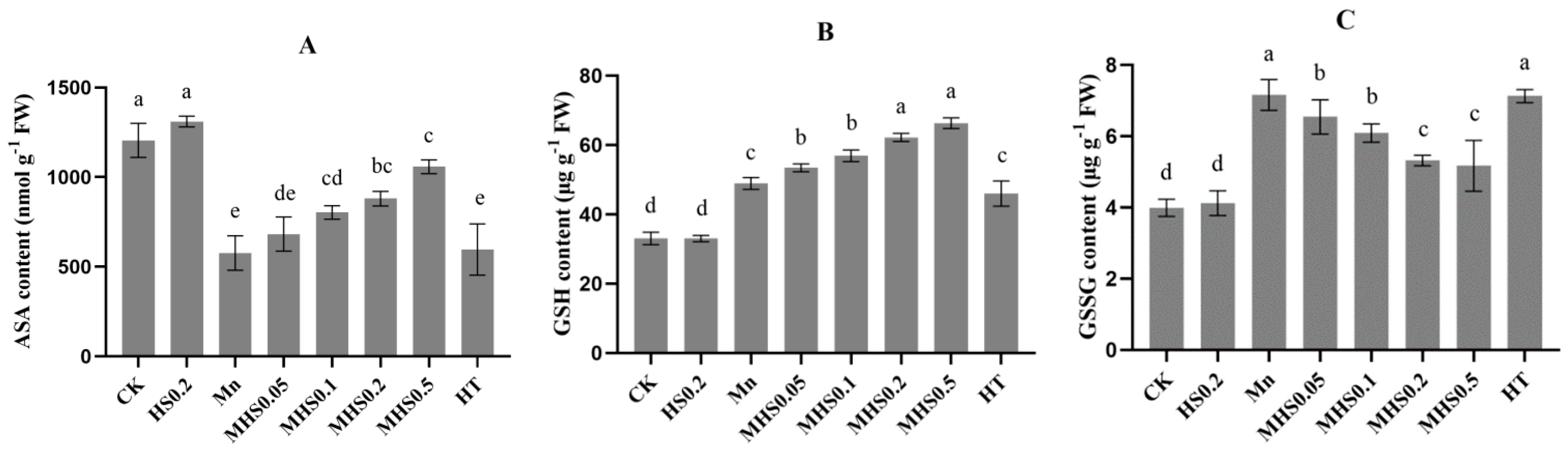
3.7. Effect of H2S on Antioxidant Enzyme Activities and Antioxidant Content Under Mn Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the Mechanisms of Plant Tolerance to Manganese Toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Yang, L.-T.; Lu, Y.-B.; Li, H.; Zhang, S.-Q.; Chen, L.-S. Proteomic changes of Citrus roots in response to long-term manganese toxicity. Trees 2014, 28, 1383–1399. [Google Scholar] [CrossRef]
- Millaleo, R.; Alvear, M.; Aguilera, P.; González-Villagra, J.; de la Luz Mora, M.; Alberdi, M.; Reyes-Díaz, M. Mn Toxicity Differentially Affects Physiological and Biochemical Features in Highbush Blueberry (Vaccinium corymbosum L.) Cultivars. J. Soil. Sci. Plant Nutr. 2019, 20, 795–805. [Google Scholar] [CrossRef]
- Chu, H.H.; Car, S.; Socha, A.L.; Hindt, M.N.; Punshon, T.; Guerinot, M.L. The Arabidopsis MTP8 transporter determines the localization of manganese and iron in seeds. Sci. Rep. 2017, 7, 11024. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, L.A.; Uren, N.C. Manganese oxidation and reduction in soils: Effects of temperature, water potential, pH and their interactions. Soil. Res. 2014, 52, 483–494. [Google Scholar] [CrossRef]
- Wang, F.; Ge, S.; Lyu, M.; Liu, J.; Li, M.; Jiang, Y.; Xu, X.; Xing, Y.; Cao, H.; Zhu, Z.; et al. DMPP reduces nitrogen fertilizer application rate, improves fruit quality, and reduces environmental cost of intensive apple production in China. Sci. Total Environ. 2022, 802, 149813. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, R.; Xie, Y.; Jiang, C.; Yu, Y. Recent Advances in Understanding Mechanisms of Plant Tolerance and Response to Aluminum Toxicity. Sustainability 2021, 13, 1782. [Google Scholar] [CrossRef]
- Wan, H.; Yang, F.; Zhuang, X.; Cao, Y.; He, J.; Li, H.; Qin, S.; Lyu, D. Malus rootstocks affect copper accumulation and tolerance in trees by regulating copper mobility, physiological responses, and gene expression patterns. Environ. Pollut. 2021, 287, 117610. [Google Scholar] [CrossRef]
- Min, S.F.; Chang, Z.X.; Chen, L.; Feng, L.Z.; Tang, B.; Ping, Z.Z. Effects of manganese pollution on soil mincrobial community fuxtion and plant growth. J. Environ. Prot. Ecol. 2019, 20, 63–74. [Google Scholar]
- Noor, I.; Sohail, H.; Zhang, D.; Zhu, K.; Shen, W.; Pan, J.; Hasanuzzaman, M.; Li, G.; Liu, J. Silencing of PpNRAMP5 improves manganese toxicity tolerance in peach (Prunus persica) seedlings. J. Hazard. Mater. 2023, 454, 131442. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Fahad, S.; Haider, I.; Ahmed, N.; Ahmad, S.; Hussain, S.; Arshad, M. Oxidative Stress and Antioxidant Defense in Plants Exposed to Metal/Metalloid Toxicity. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 353–370. [Google Scholar]
- Liu, Y.; Chen, J.; Li, X.; Yang, S.; Wu, Z.; Xue, Y.; Chen, J. Physiological Mechanisms in Which Manganese Toxicity Inhibits Root Growth in Soybean. J. Soil Sci. Plant Nutr. 2023, 23, 4141–4156. [Google Scholar] [CrossRef]
- Rosas, A.; Rengel, Z.; de la Luz Mora, M. Manganese supply and pH influence growth, carboxylate exudation and peroxidase activity of ryegrass and white clover. J. Plant Nutr. 2007, 30, 253–270. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate Synthesis and Secretion Mediated by a Manganese-Enhanced Malate Dehydrogenase Confers Superior Manganese Tolerance in Stylosanthes guianensis. Plant Physiol. 2015, 167, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Li, Z.; Su, X.; Liang, M.; Li, W.; Tang, X.; Li, J.; Qiao, X. The Malus domestica metal tolerance protein MdMTP11.1 was involved in the detoxification of excess manganese in Arabidopsis thaliana. J. Plant Physiol. 2023, 288, 154056. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Fan, Y.; Wang, Y.; Ma, Y.; Gu, D.; Lu, Y.; Zhang, S.; Chen, X.; Zhang, W. Pear metal transport protein PbMTP8.1 confers manganese tolerance when expressed in yeast and Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2021, 208, 111687. [Google Scholar] [CrossRef]
- Ueno, D.; Sasaki, A.; Yamaji, N.; Miyaji, T.; Fujii, Y.; Takemoto, Y.; Moriyama, S.; Che, J.; Moriyama, Y.; Iwasaki, K.; et al. A polarly localized transporter for efficient manganese uptake in rice. Nat. Plants 2015, 1, 15170. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, M.; Madawala, H.M.S.P.; Ok, Y.S.; Vithanage, M. Correction to: Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health 2019, 41, 1813–1831. [Google Scholar] [CrossRef]
- Arif, Y.; Hayat, S.; Yusuf, M.; Bajguz, A. Hydrogen sulfide: A versatile gaseous molecule in plants. Plant Physiol. Biochem. 2021, 158, 372–384. [Google Scholar] [CrossRef] [PubMed]
- da-Silva, C.J.; Modolo, L.V. Hydrogen sulfide: A new endogenous player in an old mechanism of plant tolerance to high salinity. Acta Bot. Bras. 2017, 32, 150–160. [Google Scholar] [CrossRef]
- Li, Z.G.; Fang, J.R.; Bai, S.J. Hydrogen sulfide signaling in plant response to temperature stress. Front. Plant Sci. 2024, 15, 1337250. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Wang, Z.; Zhang, W.; Yang, H. H2S pretreatment mitigates the alkaline salt stress on Malus hupehensis roots by regulating Na+/K+ homeostasis and oxidative stress. Plant Physiol. Biochem. 2020, 156, 233–241. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; He, L.F. The central role of hydrogen sulfide in plant responses to toxic metal stress. Ecotoxicol. Environ. Saf. 2018, 157, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Dong, J.; Li, R.; Zhao, X.; Zhu, Z.; Zhang, F.; Zhou, K.; Lin, X. Sodium hydrosulfide alleviates aluminum toxicity in Brassica napus through maintaining H2S, ROS homeostasis and enhancing aluminum exclusion. Sci. Total Environ. 2023, 858, 160073. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant 2020, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Kharbech, O.; Sakouhi, L.; Mahjoubi, Y.; Ben Massoud, M.; Debez, A.; Zribi, O.T.; Djebali, W.; Chaoui, A.; Mur, L.A.J. Nitric oxide donor, sodium nitroprusside modulates hydrogen sulfide metabolism and cysteine homeostasis to aid the alleviation of chromium toxicity in maize seedlings (Zea mays L.). J. Hazard. Mater. 2022, 424, 127302. [Google Scholar] [CrossRef]
- Hongqiang, Y.; Kaixuan, D.; Wei, Z. Biology and Physiology of Malus Hupehensis for the Apogamic Plant Resource. Acta Hortic. 2008, 769, 441–447. [Google Scholar] [CrossRef]
- DI, A. Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar]
- Lazcano-Ferrat, I.; Lovatt, C.J. Relationship between relative water content, nitrogen pools, and growth of Phaseolus vulgaris L. and P. acutifolius A. Gray during water deficit. Crop Sci. 1999, 39, 467–475. [Google Scholar] [CrossRef]
- Maribel, L.; Dionisio-Sese, S.T. Antioxidant responses of rice seedlings to salinity stress. Antioxid. Responses Rice Seedl. Salin. Stress 1998, 135, 1–9. [Google Scholar]
- Fang, T.; Cao, Z.; Li, J.; Shen, W.; Huang, L. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol. Biochem. 2014, 76, 44–51. [Google Scholar] [CrossRef]
- Sheng, H.; Zeng, J.; Yan, F.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Effect of exogenous salicylic acid on manganese toxicity, mineral nutrients translocation and antioxidative system in polish wheat (Triticum polonicum L.). Acta Physiol. Plant. 2015, 37, 32. [Google Scholar] [CrossRef]
- Rajpoot, R.; Srivastava, R.K.; Rani, A.; Pandey, P.; Dubey, R.S. Manganese-induced oxidative stress, ultrastructural changes, and proteomics studies in rice plants. Protoplasma 2021, 258, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, S.; Poschenrieder, C.; Stoyanova, Z.; Georgieva, K.; Velichkova, M.; Barceló, J. Silicon amelioration of manganese toxicity in Mn-sensitive and Mn-tolerant maize varieties. Environ. Exp. Bot. 2009, 65, 189–197. [Google Scholar] [CrossRef]
- Alamri, S.; Ali, H.M.; Khan, M.I.R.; Singh, V.P.; Siddiqui, M.H. Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol. Biochem. 2020, 155, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Rahman, A.; Ansary, M.M.; Watanabe, A.; Fujita, M.; Tran, L.S. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 2015, 5, 14078. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shah, A.A.; Khan, W.U.; Yasin, N.A.; Ahmad, A.; Abbas, M.; Ali, A.; Safdar, N. Hydrogen sulfide mitigates cadmium induced toxicity in Brassica rapa by modulating physiochemical attributes, osmolyte metabolism and antioxidative machinery. Chemosphere 2021, 263, 127999. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Ma, Y.; Jiang, M.; Yang, F.; Ni, L.; Lu, W. Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 2014, 75, 33–44. [Google Scholar] [CrossRef]
- Luo, S.; Liu, Z.; Wan, Z.; He, X.; Lv, J.; Yu, J.; Zhang, G. Foliar Spraying of NaHS Alleviates Cucumber Salt Stress by Maintaining N+/K+ Balance and Activating Salt Tolerance Signaling Pathways. Plants 2023, 12, 2450. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Turan, M.; Ors, S.; Dursun, A.; Yildirim, E. Improving salt tolerance of bean (Phaseolus vulgaris L.) with hydrogen sulfide. Photosynthetica 2023, 61, 25–36. [Google Scholar] [CrossRef]
- Alejandro, S.; Holler, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Hou, L.; Wang, Z.; Gong, G.; Zhu, Y.; Ye, Q.; Lu, S.; Liu, X. Hydrogen Sulfide Alleviates Manganese Stress in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 5046. [Google Scholar] [CrossRef]
- Messant, M.; Hani, U.; Hennebelle, T.; Guerard, F.; Gakiere, B.; Gall, A.; Thomine, S.; Krieger-Liszkay, A. Manganese concentration affects chloroplast structure and the photosynthetic apparatus in Marchantia polymorpha. Plant Physiol. 2023, 192, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Zambrosi, F.C.B.; Mesquita, G.L.; Marchiori, P.E.R.; Tanaka, F.A.O.; Machado, E.C.; Ribeiro, R.V. Anatomical and physiological bases of sugarcane tolerance to manganese toxicity. Environ. Exp. Bot. 2016, 132, 100–112. [Google Scholar] [CrossRef]
- Costa, G.B.; Simioni, C.; Ramlov, F.; Maraschin, M.; Chow, F.; Bouzon, Z.L.; Schmidt, É.C. Effects of manganese on the physiology and ultrastructure of Sargassum cymosum. Environ. Exp. Bot. 2017, 133, 24–34. [Google Scholar] [CrossRef]
- Alsahli, A.A.; Bhat, J.A.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Hydrogen Sulfide (H2S) Mitigates Arsenic (As)-Induced Toxicity in Pea (Pisum sativum L.) Plants by Regulating Osmoregulation, Antioxidant Defense System, Ascorbate Glutathione Cycle and Glyoxalase System. J. Plant Growth Regul. 2020, 40, 2515–2531. [Google Scholar] [CrossRef]
- González, A.; Lynch, J.P. Effects of manganese toxicity on leaf CO2 assimilation of contrasting common bean genotypes. Physiol. Plant. 2006, 101, 872–880. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Zhou, Y.; Adeel, M.; Mehmood, S.; Ahmad, M.A.; Javed, R.; Imtiaz, M.; Aziz, O.; et al. Hydrogen sulfide enhances rice tolerance to nickel through the prevention of chloroplast damage and the improvement of nitrogen metabolism under excessive nickel. Plant Physiol. Biochem. 2019, 138, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, F.H.; Wang, W.H.; Zheng, C.J.; Lin, G.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cui, H.; Lv, X.; Yang, Y.; Wang, Y.; Lu, X. Exogenous CaCl2 reduces salt stress in sour jujube by reducing Na+ and increasing K+, Ca2+, and Mg2+ in different plant organs. J. Hortic. Sci. Biotechnol. 2016, 92, 98–106. [Google Scholar] [CrossRef]
- Haider, M.U.; Hussain, M.; Farooq, M.; Nawaz, A. Zinc Nutrition for Improving the Productivity and Grain Biofortification of Mungbean. J. Soil Sci. Plant Nutr. 2020, 20, 1321–1335. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, D.; Sun, Z.; Ju, C.; Miao, C.; Wang, Z.; Xie, D.; Ma, L.; Gong, Z.; Wang, C. Tonoplast-associated calcium signaling regulates manganese homeostasis in Arabidopsis. Mol. Plant 2021, 14, 805–819. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Natasha; Khalid, S.; Abbas, G.; Niazi, N.K.; Murtaza, B.; Rashid, M.I.; Bibi, I. Redox Mechanisms and Plant Tolerance Under Heavy Metal Stress: Genes and Regulatory Networks. In Plant Metallomics and Functional Omics: A System-Wide Perspective; Sablok, G., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 71–105. [Google Scholar]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated Actions of Glyoxalase and Antioxidant Defense Systems in Conferring Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2017, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: Possible involvement of nitric oxide. J. Plant Physiol. 2015, 181, 20–29. [Google Scholar] [CrossRef] [PubMed]

| Sample Number | Processing Reagents |
|---|---|
| CK | Mn 0 mmol/L + NaHS 0 mmol/L |
| HS0.2 | Mn 0 mmol/L + NaHS 0.2 mmol/L |
| Mn | Mn 1 mmol/L + NaHS 0 mmol/L |
| MHS0.05 | Mn 1 mmol/L + NaHS 0.05 mmol/L |
| MHS0.1 | Mn 1 mmol/L + NaHS 0.1 mmol/L |
| MHS0.2 | Mn 1 mmol/L + NaHS 0.2 mmol/L |
| MHS0.5 | Mn 1 mmol/L + NaHS 0.5 mmol/L |
| HT | Mn 1 mmol/L + NaHS 0.2 mmol/L + HT 0.2 mmol/L |
| Gene Name | Primers’ Sequences (5′-3′) |
|---|---|
| MdMTP8.2F | TCTTATTTGTTTCGCTGCGTGTAG |
| MdMTP8.2R | GCATTGTGACCGTTCGATTTTC |
| MdMTP9.1F | GACAGTGGACGCCGTCGTTT |
| MdMTP9.1R | ACTGCCATCCGCTCGCTCTT |
| MdMTP9.3F | GGCCTCATGTTGAATTAACCTGTA |
| MdMTP9.3R | GGCCTCATGTTGAATTAACCTGTA |
| MdMTP11.1F | TCCCACACACTCTCTCCTTTTACCT |
| MdMTP11.1R | CGTCGAAGTTCAACCGCCAC |
| Treatments | Aerial Parts | Roots | Leaf RWC (%) | ||
|---|---|---|---|---|---|
| FW (mg) | DW (mg) | FW (mg) | DW (mg) | ||
| control | 1.74 ± 0.08 a | 0.66 ± 0.02 ab | 0.7 ± 0.13 a | 0.18 ± 0.03 a | 91.52 ± 1.88 a |
| HS0.2 | 1.77 ± 0.05 a | 0.69 ± 0.03 a | 0.68 ± 0.08 a | 0.18 ± 0.02 a | 90.4 ± 3.77 a |
| Mn | 0.82 ± 0.07 e | 0.29 ± 0.02 e | 0.33 ± 0.07 e | 0.05 ± 0.01 d | 64.34 ± 5.16 de |
| MHS0.05 | 0.96 ± 0.1 d | 0.32 ± 0.04 e | 0.39 ± 0.04 de | 0.06 ± 0.01 cd | 65.88 ± 4.06 de |
| MHS0.1 | 1.16 ± 0.08 c | 0.47 ± 0.03 d | 0.42 ± 0.1d e | 0.08 ± 0.02 c | 67.66 ± 2.89 d |
| MHS0.2 | 1.35 ± 0.07 b | 0.54 ± 0.04 c | 0.49 ± 0.04 cd | 0.11 ± 0.01 b | 75.01 ± 3 c |
| MHS0.5 | 1.44 ± 0.1 b | 0.63 ± 0.05 b | 0.58 ± 0.07 bc | 0.12 ± 0.02 b | 81.81 ± 2.48 b |
| HT | 0.87 ± 0.04 de | 0.31 ± 0.02 e | 0.35 ± 0.09 e | 0.06 ± 0.02 cd | 60.78 ± 3.93 e |
| Treatments | Fe (mg g−1 DW) | Zn (mg g−1 DW) | Mg (mg g−1 DW) | Ca (mg g−1 DW) |
|---|---|---|---|---|
| CK | 1.82 ± 0.46 a | 0.09 ± 0.01 a | 6.68 ± 0.23 a | 4.89 ± 0.49 ab |
| HS0.2 | 1.71 ± 0.13 ab | 0.09 ± 0.02 a | 6.58 ± 0.5 a | 5.44 ± 1 a |
| Mn | 1.07 ± 0.08 d | 0.05 ± 0 c | 3.16 ± 0.51 c | 3.65 ± 0.5 cd |
| MHS0.05 | 1.36 ± 0.42 bcd | 0.05 ± 0.01 c | 3.4 ± 0.6 bc | 3.89 ± 0.26 cd |
| MHS0.1 | 1.41 ± 0.03 abcd | 0.05 ± 0 c | 3.41 ± 0.61 bc | 4.05 ± 0.35 bcd |
| MHS0.2 | 1.63 ± 0.11 abc | 0.06 ± 0 b | 3.83 ± 0.42 bc | 3.82 ± 0.11 cd |
| MHS0.5 | 1.71 ± 0.14 ab | 0.08 ± 0.01 a | 4.1 ± 0.52 b | 4.43 ± 0.38 bc |
| HT | 1.15 ± 0.06 cd | 0.05 ± 0 c | 2.27 ± 0.43 d | 3.51 ± 0.22 d |
| Treatments | Mn (mg g−1 DW) | Percentage of Mn in Leaves (%) | |||
|---|---|---|---|---|---|
| Leaf | Root | Stem | Total | Leaf | |
| CK | 0.06 ± 0.01 d | 0.01 ± 0 f | 0.07 ± 0.02 f | 0.14 ± 0.03 e | 0.44 ± 0.07 a |
| HS0.2 | 0.07 ± 0.01 d | 0.01 ± 0.01 f | 0.06 ± 0 f | 0.15 ± 0.02 e | 0.65 ± 0.31 a |
| Mn | 0.79 ± 0.16 ab | 1.7 ± 0.03 a | 0.77 ± 0.03 b | 3.26 ± 0.17 a | 0.24 ± 0.05 c |
| MHS0.05 | 0.74 ± 0.22 ab | 1.2 ± 0.15 c | 0.57 ± 0.02 d | 2.51 ± 0.25 b | 0.3 ± 0.09 bc |
| MHS0.1 | 0.66 ± 0.15 bc | 1.24 ± 0.07 c | 0.69 ± 0.07 c | 2.59 ± 0.29 b | 0.25 ± 0.06 c |
| MHS0.2 | 0.52 ± 0.06 c | 0.59 ± 0.01 d | 0.63 ± 0.02 d | 1.74 ± 0.09 c | 0.3 ± 0.03 bc |
| MHS0.5 | 0.47 ± 0.11 c | 0.42 ± 0.04 e | 0.33 ± 0.01 e | 1.22 ± 0.14 d | 0.39 ± 0.09 ab |
| HT | 0.91 ± 0.05 a | 1.38 ± 0.02 b | 0.9 ± 0 a | 3.18 ± 0.07 a | 0.28 ± 0.02 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Wang, B.; Chen, T.; Zhang, M. Hydrogen Sulfide Mitigates Manganese-Induced Toxicity in Malus hupehensis Plants by Regulating Osmoregulation, Antioxidant Defense, Mineral Homeostasis, and Glutathione Ascorbate Cycle. Horticulturae 2025, 11, 133. https://doi.org/10.3390/horticulturae11020133
Liu B, Wang B, Chen T, Zhang M. Hydrogen Sulfide Mitigates Manganese-Induced Toxicity in Malus hupehensis Plants by Regulating Osmoregulation, Antioxidant Defense, Mineral Homeostasis, and Glutathione Ascorbate Cycle. Horticulturae. 2025; 11(2):133. https://doi.org/10.3390/horticulturae11020133
Chicago/Turabian StyleLiu, Bowen, Baozhu Wang, Tianlnog Chen, and Manrang Zhang. 2025. "Hydrogen Sulfide Mitigates Manganese-Induced Toxicity in Malus hupehensis Plants by Regulating Osmoregulation, Antioxidant Defense, Mineral Homeostasis, and Glutathione Ascorbate Cycle" Horticulturae 11, no. 2: 133. https://doi.org/10.3390/horticulturae11020133
APA StyleLiu, B., Wang, B., Chen, T., & Zhang, M. (2025). Hydrogen Sulfide Mitigates Manganese-Induced Toxicity in Malus hupehensis Plants by Regulating Osmoregulation, Antioxidant Defense, Mineral Homeostasis, and Glutathione Ascorbate Cycle. Horticulturae, 11(2), 133. https://doi.org/10.3390/horticulturae11020133






