Response of Tomato Quality Parameters to Water Deficit Under Soil Salinity and Simulation Based on Stem Water Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Treatments
2.3. Measurements
2.4. Modeling of Fruit Quality Function Under Water–Salt Stress
2.5. Statistical Analyses
2.6. Evaluation of Model Performance
3. Results
3.1. Response of Fruit Quality, Fruit Fresh Weight, and Fruit Water Content to Different Stages of Water Deficit Under Soil Salinity
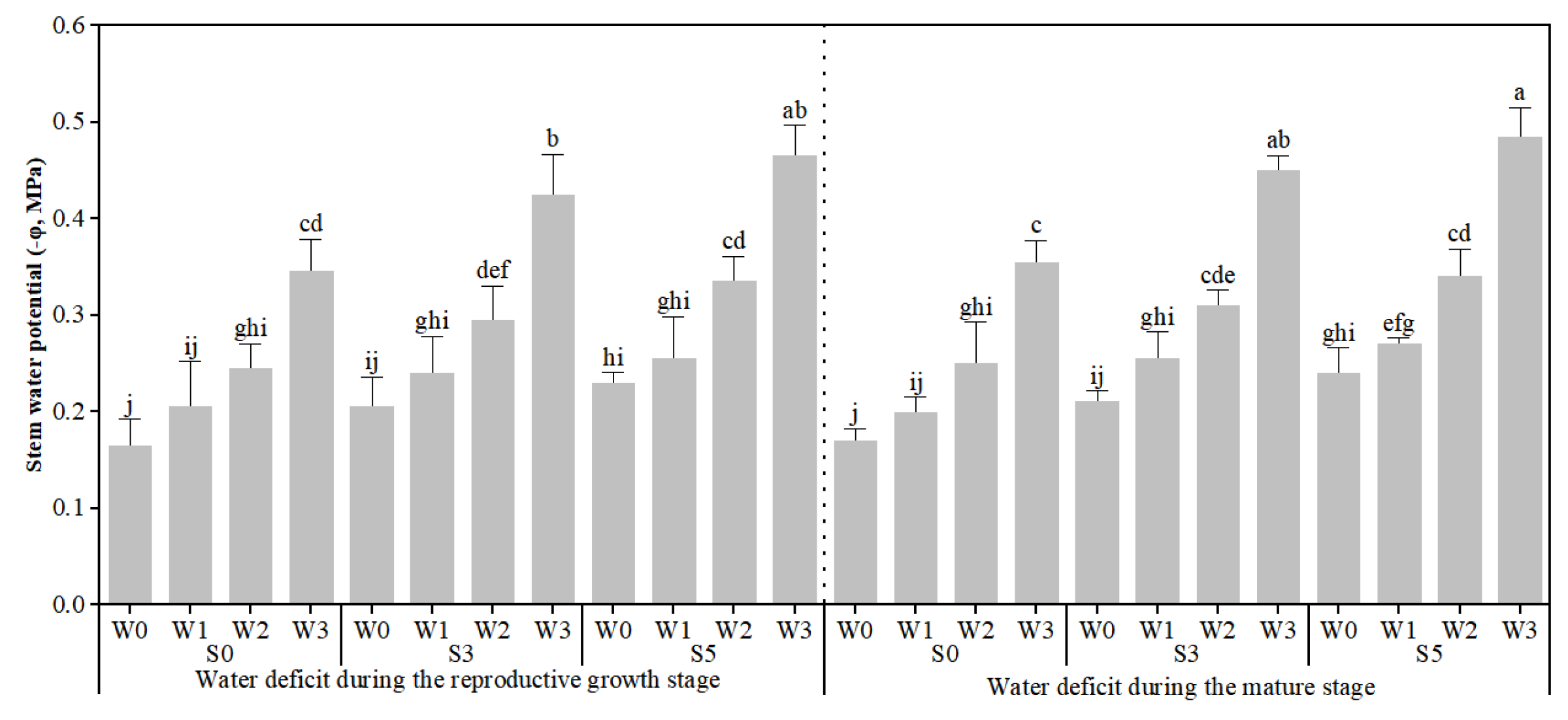
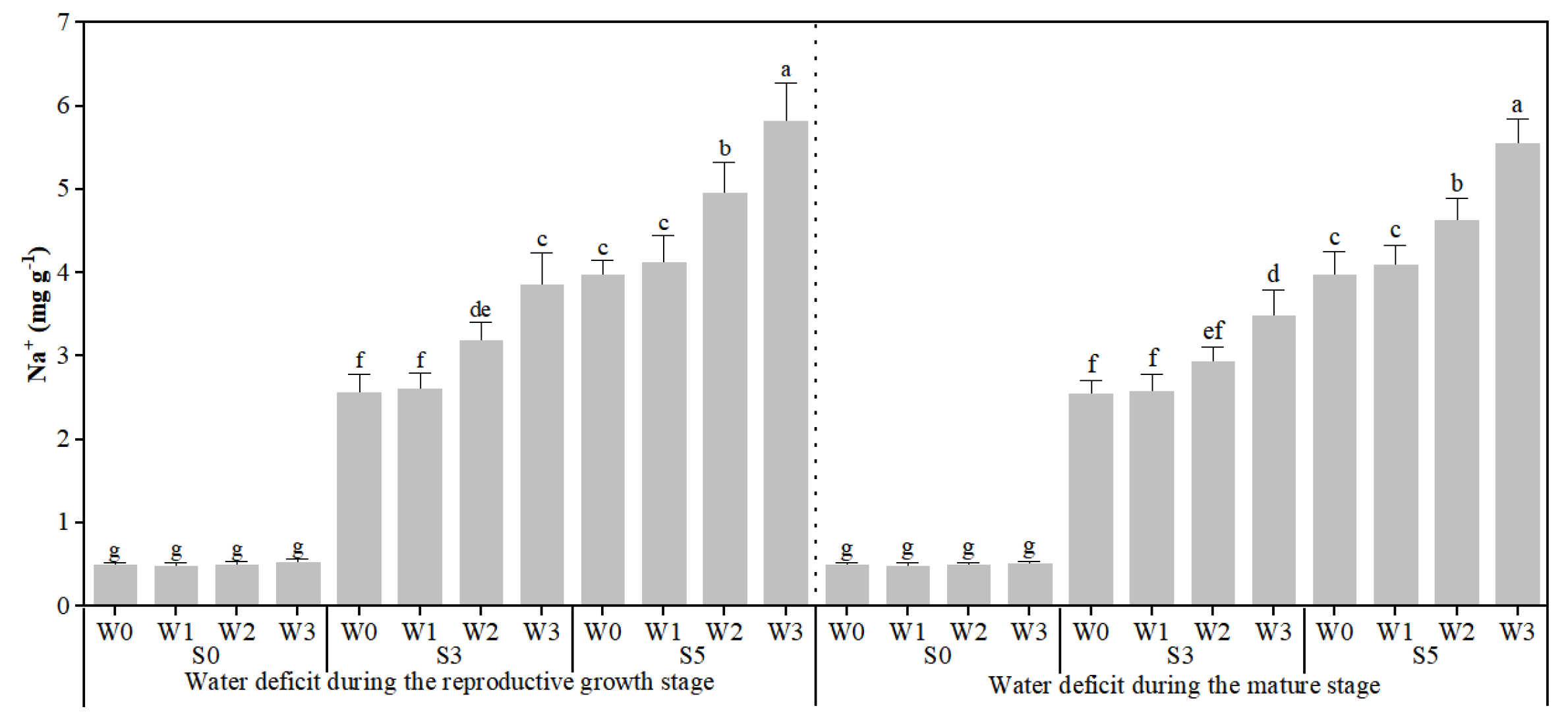
3.2. Response of Plant Stem Water Potential, Fruit Osmotic Potential, and Fruit Sodium Content to Different Stages of Water Deficit Under Soil Salinity
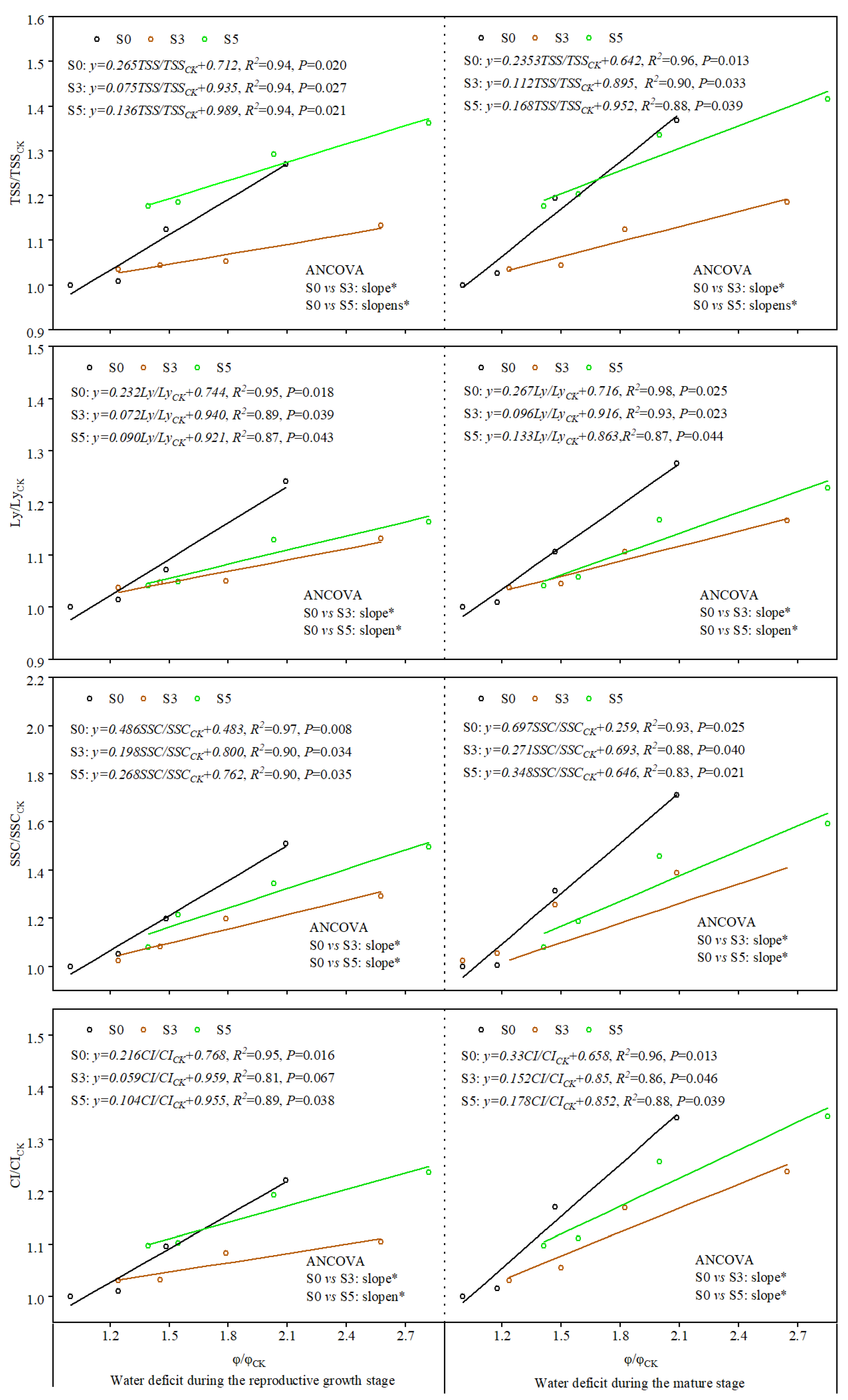
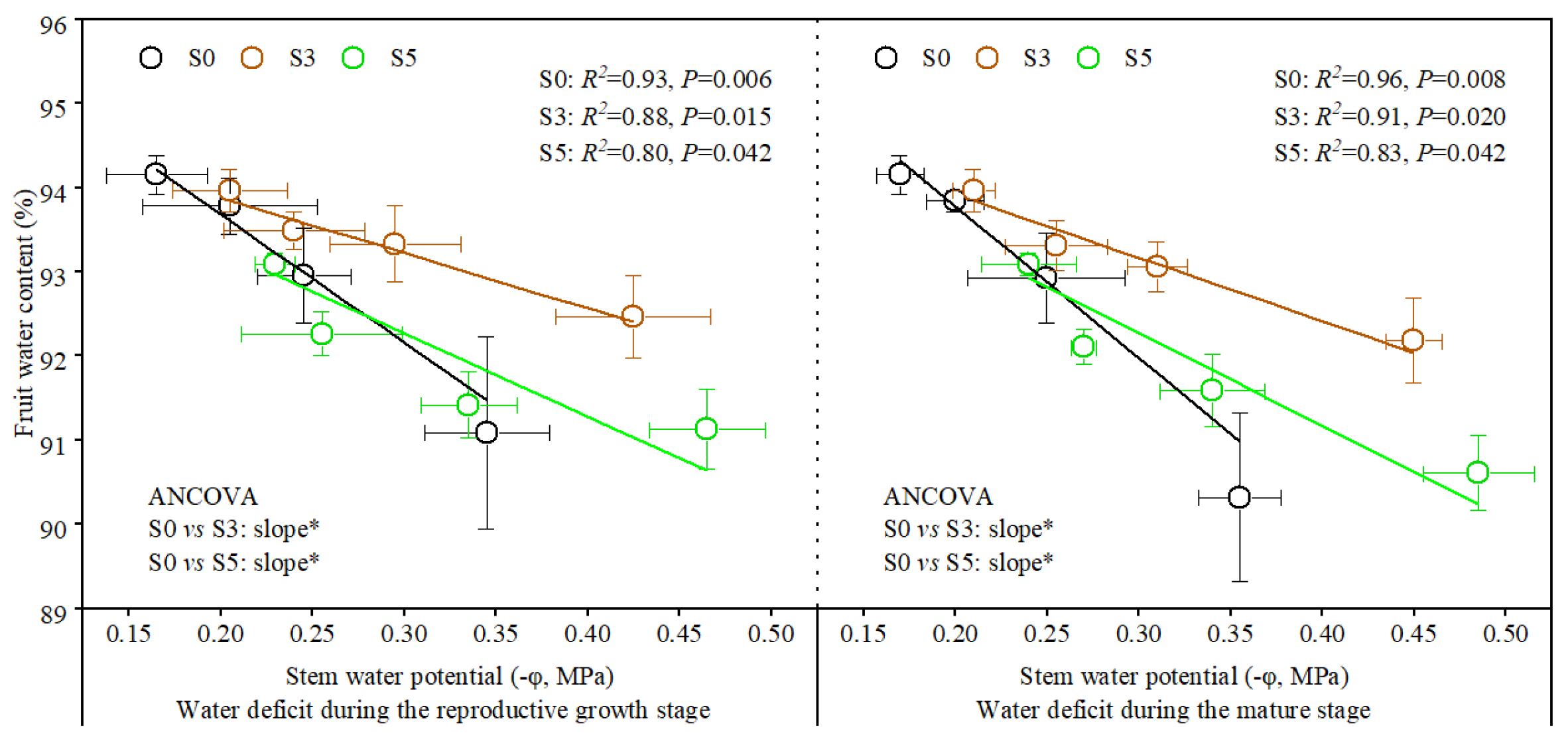
3.3. Relationship of Plant Stem Water Potential with Fruit Quality Parameters and Water Content
3.4. Calibration and Validation of the Tomato Fruit Quality Function Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| θf | field capacity |
| φ | plant stem water potential |
| φπ | fruit osmotic potential |
| TSS | total soluble solids |
| Ly | lycopene |
| SSC | soluble sugars content |
| CI | color index |
| EC | soil electrical conductivity of 1:5 soil-water extract |
| S0 | soil salinity treatment of no salt added |
| S3 | soil salinity treatment of 3 g mixed salt added to 1000 g air-dried soil |
| S5 | soil salinity treatment of 5 g mixed salt added to 1000 g air-dried soil |
| W0 | irrigation treatment of 95% of field capacity as the upper limit |
| W1 | irrigation treatment of 80% of field capacity as the upper limit |
| W2 | irrigation treatment of 70% of field capacity as the upper limit |
| W3 | irrigation treatment of 60% of field capacity as the upper limit |
| RW | water deficit was imposed at reproductive growth stage |
| MW | water deficit was imposed at maturity growth stage |
References
- Yong, K.T.; Yong, P.H.; Ng, Z.X. Tomato and human health: A perspective from post-harvest processing, nutrient bio-accessibility, and pharmacological interaction. Food Front. 2023, 4, 1702–1719. [Google Scholar] [CrossRef]
- Lu, J.; Shao, G.; Wang, W.; Gao, Y.; Wang, Z.; Zhang, Y.; Wang, J.; Song, E. The role of hydraulic lift in tomato yield and fruit quality under different water and salt stresses. Agric. Water Manag. 2024, 299, 108899. [Google Scholar] [CrossRef]
- Wang, X.; Tian, W.; Zheng, W.; Shah, S.; Li, J.; Wang, X.; Zhang, X. Quantitative relationships between salty water irrigation and tomato yield, quality, and irrigation water use efficiency: A meta-analysis. Agric. Water Manag. 2023, 280, 108213. [Google Scholar] [CrossRef]
- Kuscu, H.; Turhan, A.; Ozmen, N.; Aydinol, P.; Demir, A.O. Optimizing levels of water and nitrogen applied through drip irrigation for yield, quality, and water productivity of processing tomato (Lycopersicon esculentum Mill.). Hortic. Environ. Biotechnol. 2014, 55, 103–114. [Google Scholar] [CrossRef]
- Nangare, D.D.; Singh, Y.; Kumar, P.S.; Minhas, P.S. Growth, fruit yield and quality of tomato (Lycopersicon esculentum Mill.) as affected by deficit irrigation regulated on phenological basis. Agric. Water Manag. 2016, 171, 73–79. [Google Scholar] [CrossRef]
- Chen, J.; Kang, S.; Du, T.; Guo, P.; Qiu, R.; Chen, R.; Gu, F. Modeling relations of tomato yield and fruit quality with water deficit at different growth stages under greenhouse condition. Agric. Water Manag. 2014, 146, 131–148. [Google Scholar] [CrossRef]
- Kuscu, H.; Turhan, A.; Demir, A.O. The response of processing tomato to deficit irrigation at various phenological stages in a sub-humid environment. Agric. Water Manag. 2014, 133, 92–103. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, W.; Du, T.; Kang, S.; Davies, W.J. Responses of water accumulation and solute metabolism in tomato fruit to water scarcity and implications for main fruit quality variables. J. Exp. Bot. 2020, 71, 1249–1264. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Brunel, B.; Bertin, N. Water deficit effects on tomato quality depend on fruit developmental stage and genotype. J. Plant Physiol. 2016, 190, 26–35. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits-making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef]
- Qi, H.; Li, L.; Zhang, J.; Wang, L.; Chen, Y. Effects of irrigation on sucrose metabolism, dry matter distribution and fruit quality of tomato under water deficit. Sci. Agric. Sin. 2004, 37, 1045–1049. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Du, T. Coupled mechanisms of water deficit and soil salinity affecting tomato fruit growth. Agric. Water Manag. 2024, 295, 108747. [Google Scholar] [CrossRef]
- Du, Y.; Niu, W.; Gu, X.; Zhang, Q.; Cui, B. Water- and nitrogen-saving potentials in tomato production: A meta-analysis. Agric. Water Manag. 2018, 210, 296–303. [Google Scholar] [CrossRef]
- Blanco, V.; Kalcsits, L. Long-term validation of continuous measurements of trunk water potential and trunk diameter indicate different diurnal patterns for pear under water limitations. Agric. Water Manag. 2023, 281, 108257. [Google Scholar] [CrossRef]
- Christenson, C.G.; Gohardoust, M.R.; Calleja, S.; Thorp, K.R.; Tuller, M.; Pauli, D. Monitoring cotton water status with microtensiometers. Irrig. Sci. 2024, 42, 995–1011. [Google Scholar] [CrossRef]
- Katerji, N.; Tardieu, F.; Bethenod, O.; Quetin, P. Behavior of maize stem diameter during drying cycles: Comparison of two methods for detecting water stress. Crop Sci. 1994, 34, 165–169. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; He, P.; Chen, D.; Dai, X.; Jin, Q.; Su, X. The optimal irrigation water salinity and salt component for high-yield and good-quality of tomato in Ningxia. Agric. Water Manag. 2022, 274, 107940. [Google Scholar] [CrossRef]
- Wang, W.; Shi, F.; Gong, Y.; Ding, D.; Li, K.; Xiong, G. Effect of water and salt synergistic regulation at the different growth stages on quality and sucrose-metabolizing enzyme activities of tomato. J. Plant Nutr. 2023, 46, 1730–1744. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Alhagdow, M.; Mounet, F.; Gilbert, L.; Nunes-Nesi, A.; Garcia, V.; Just, D.; Petit, J.; Beauvoit, B.; Fernie, A.R.; Rothan, C.; et al. Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007, 145, 1408–1422. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Cruz, A.M.S.; Bolarin, M.C.; PerezAlfocea, F. Sucrolytic activities in relation to sink strength and carbohydrate composition in tomato fruit growing under salinity. Plant Sci. 1996, 118, 47–55. [Google Scholar] [CrossRef]
- Lu, S.; Li, T.; Jiang, J. Tomato Key Sucrose Metabolizing Enzyme Activities and Gene Expression Under NaCl and PEG Iso-Osmotic Stresses. Agric. Sci. China 2009, 8, 1046–1052. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N. Metabolic profile of organoleptic and health-promoting qualities in two tomato cultivars subjected to salt stress and their interactions using correlation network analysis. Sci. Hortic. 2015, 184, 8–17. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Du, T. Osmotic adjustment of tomato under mild soil salinity can enhance drought resistance. Environ. Exp. Bot. 2022, 202, 105004. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Shukla, M.K.; Du, T. Proposing a crop-water-salt production function based on plant response to stem water potential. Agric. Water Manag. 2023, 278, 108162. [Google Scholar] [CrossRef]
- Thomas, T.R.; Matthews, M.A.; Shackel, K.A. Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant Cell Environ. 2006, 29, 993–1001. [Google Scholar] [CrossRef]
- Colle, I.J.P.; Lemmens, L.; Van Buggenhout, S.; Met, K.; Van Loey, A.M.; Hendrickx, M.E. Processing tomato pulp in the presence of lipids: The impact on lycopene bioaccessibility. Food Res. Int. 2013, 51, 32–38. [Google Scholar] [CrossRef]
- Moriasi, D.N.; Arnold, J.G.; Van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Trans. Asabe 2007, 50, 885–900. [Google Scholar] [CrossRef]
- De Pascale, S.; Martino, A.; Raimondi, G.; Maggio, A. Comparative analysis of water and salt stress-induced modifications of quality parameters in cherry tomatoes. J. Hortic. Sci. Biotechnol. 2007, 82, 283–289. [Google Scholar] [CrossRef]
- Guichard, S.; Bertin, N.; Leonardi, C.; Gary, C. Tomato fruit quality in relation to water and carbon fluxes. Agronomie 2001, 21, 385–392. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Shennan, C.; Grattan, S.R. Developmental-changes in tomato fruit composition in response to water deficit and salinity. Physiol. Plant. 1991, 83, 177–185. [Google Scholar] [CrossRef]
- Renquist, A.R.; Reid, J.B. Processing tomato fruit quality: Influence of soil water deficits at flowering and ripening. Aust. J. Agric. Res. 2001, 52, 793–799. [Google Scholar] [CrossRef]
- Shameer, S.; Vallarino, J.G.; Fernie, A.R.; Ratcliffe, R.G.; Sweetlove, L.J. Flux balance analysis of metabolism during growth by osmotic cell expansion and its application to tomato fruits. Plant J. 2020, 103, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Balibrea, M.E.; Parra, M.; Bolarin, M.C.; Perez-Alfocea, F. Cytoplasmic sucrolytic activity controls tomato fruit growth under salinity. Aust. J. Plant Physiol. 1999, 26, 561–568. [Google Scholar] [CrossRef]
- Yin, Y.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef]
- Yang, X.; Borno, M.L.; Wei, Z.; Liu, F. Combined effect of partial root drying and elevated atmospheric CO2 on the physiology and fruit quality of two genotypes of tomato plants with contrasting endogenous ABA levels. Agric. Water Manag. 2021, 254, 106987. [Google Scholar] [CrossRef]
- Borawska-Jarmulowicz, B.; Mastalerczuk, G.; Gozdowski, D.; Maluszynska, E.; Szydlowska, A. The sensitivity of Lolium perenne and Poa pratensis to salinity and drought during the seed germination and under different photoperiod conditions. Zemdirbyste 2017, 104, 71–78. [Google Scholar] [CrossRef]
- Martinez, J.P.; Kinet, J.M.; Bajji, M.; Lutts, S. NaCl alleviates polyethylene glycol-induced water stress in the halophyte species Atriplex halimus L. J. Exp. Bot. 2005, 56, 2421–2431. [Google Scholar] [CrossRef]
- Albacete, A.A.; Martinez-Andujar, C.; Perez-Alfocea, F. Hormonal and metabolic regulation of source-sink relations under salinity and drought: From plant survival to crop yield stability. Biotechnol. Adv. 2014, 32, 12–30. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Hessini, K.; Martinez, J.P.; Gandour, M.; Albouchi, A.; Soltani, A.; Abdelly, C. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ. Exp. Bot. 2009, 67, 312–319. [Google Scholar] [CrossRef]
- Lu, J.; Shao, G.; Gao, Y.; Zhang, K.; Wei, Q.; Cheng, J. Effects of water deficit combined with soil texture, soil bulk density and tomato variety on tomato fruit quality: A meta-analysis. Agric. Water Manag. 2021, 243, 106427. [Google Scholar] [CrossRef]
- Veit-Kohler, U.; Krumbein, A.; Kosegarten, H. Effect of different water supply on plant growth and fruit quality of Lycopersicon esculentum. J. Plant Nutr. Soil Sci. 1999, 162, 583–588. [Google Scholar] [CrossRef]
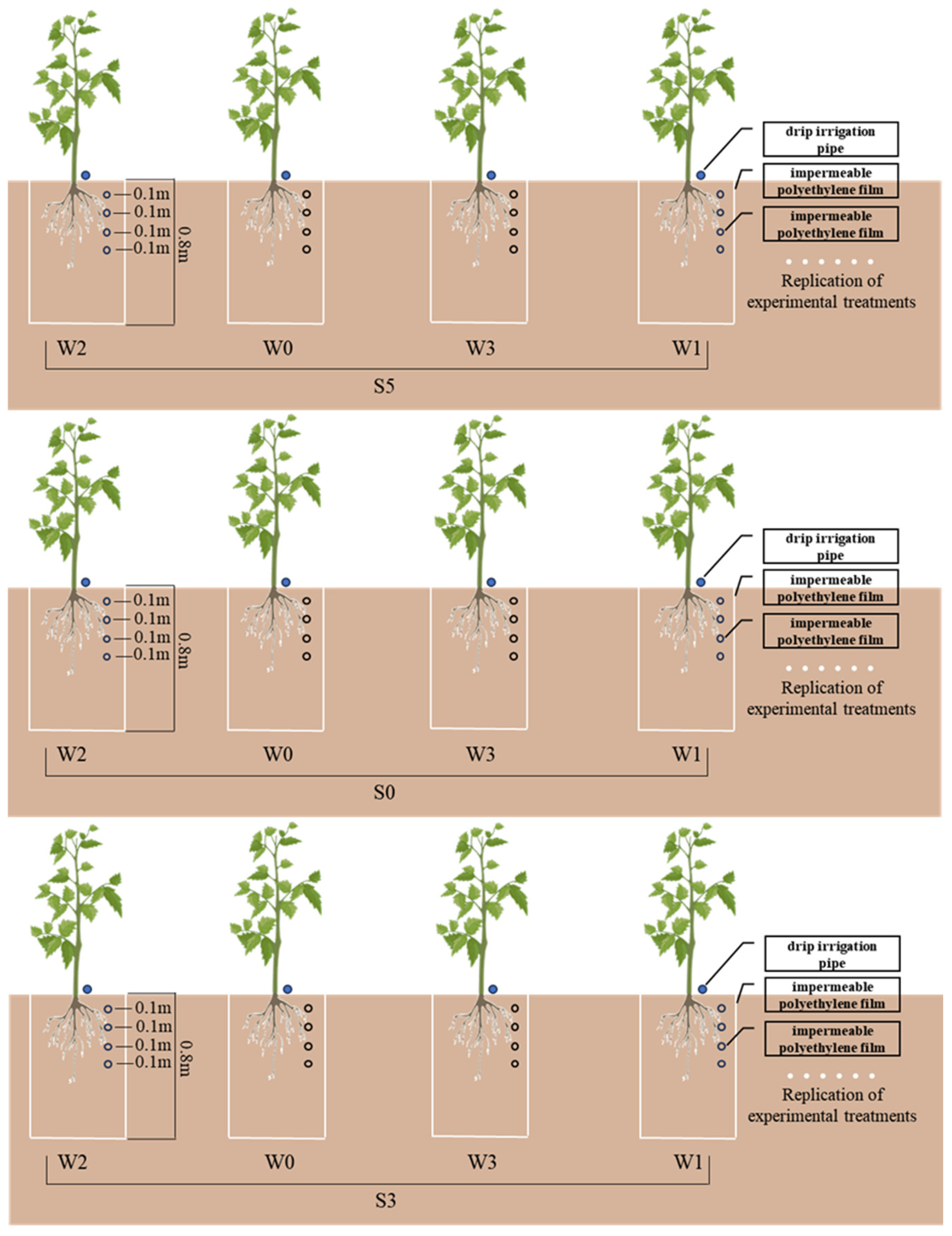
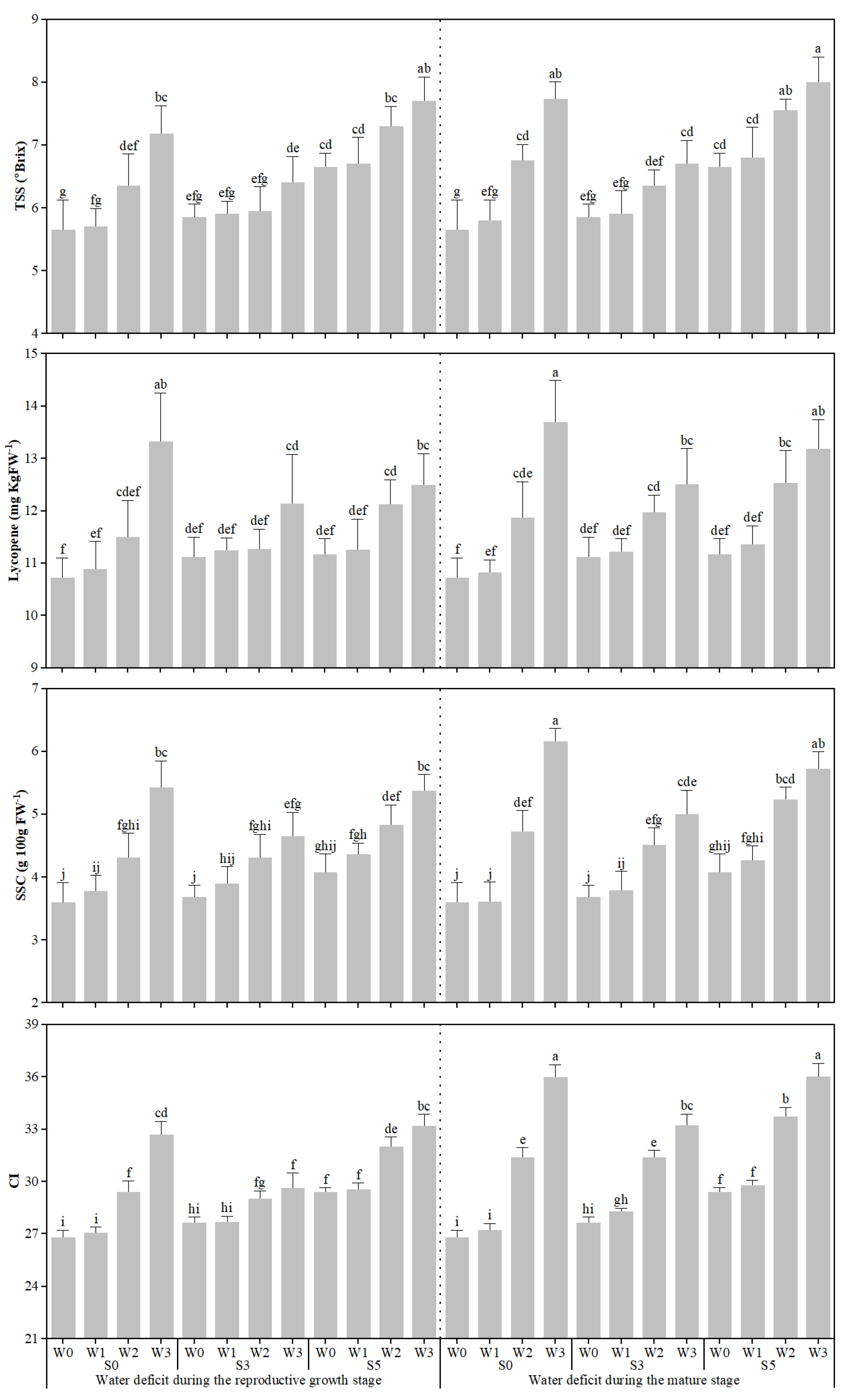
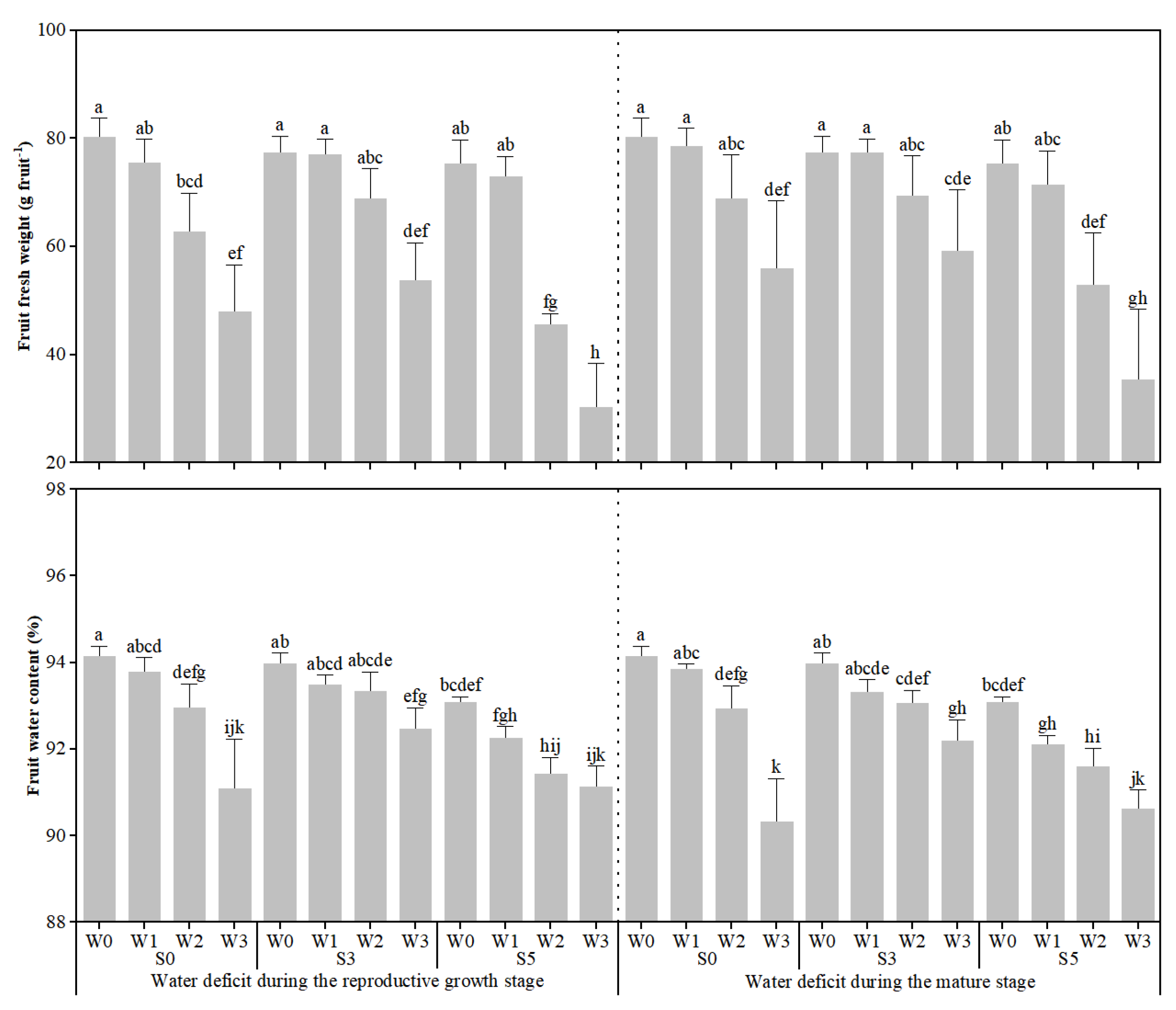
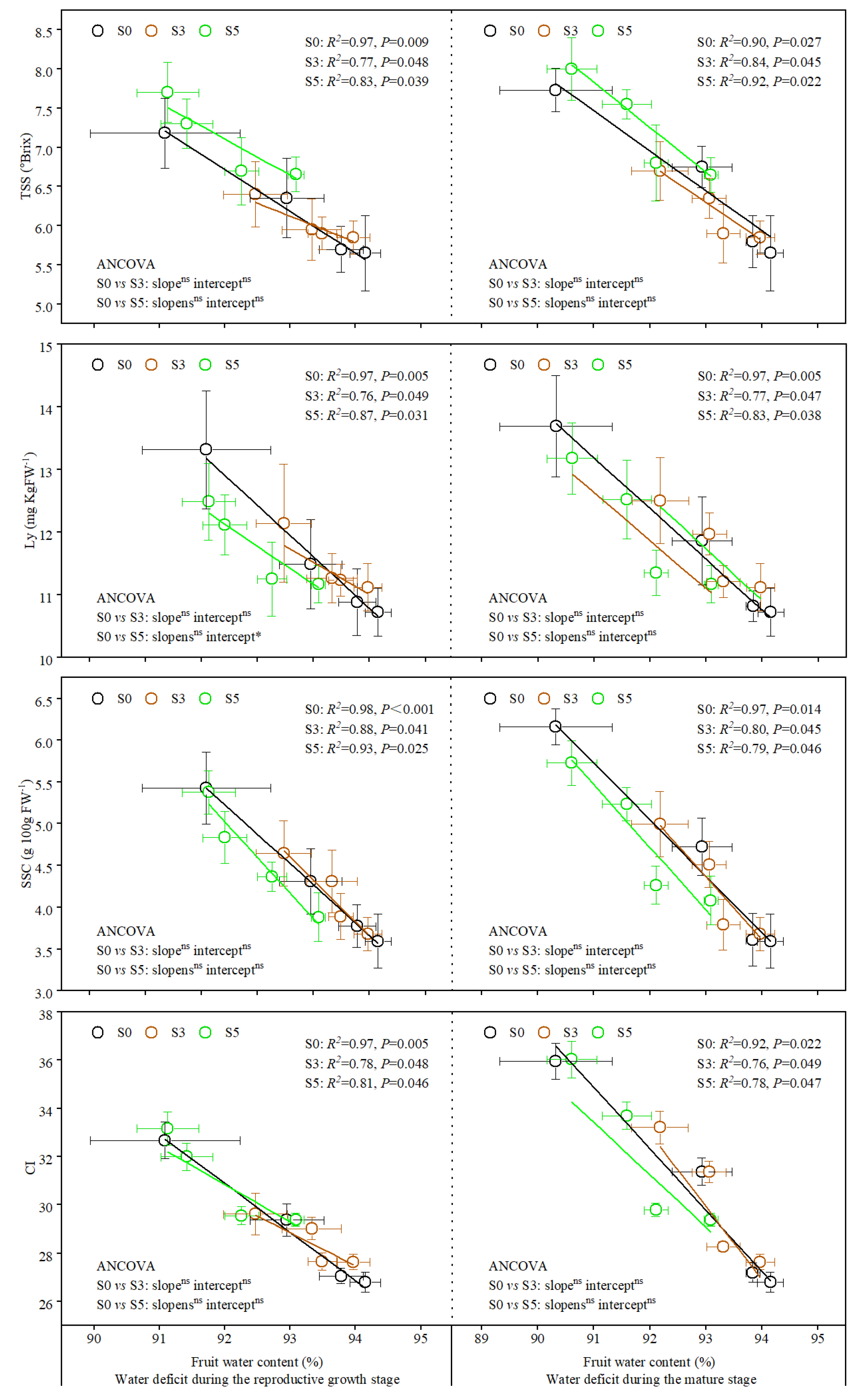


| Year | Growth Stage | Date (MM/DD) | Treatments | Irrigation Amount (mm) and Irrigation Times (No.) | |||
|---|---|---|---|---|---|---|---|
| Seedling Stage | Reproductive Growth | Mature Stage | Whole | ||||
| 2019 | Seedling | 06/01–06/27 | S0W0 | 33.6 (2) | 149.2 (8) | 87.3 (4) | 270.1 (14) |
| Reproductive | 06/28–08/08 | S3W0 | 33.6 (2) | 137.6 (8) | 75.7 (4) | 246.9 (14) | |
| Mature | 08/09–09/08 | S5W0 | 33.6 (2) | 130.4 (8) | 83.2 (4) | 246.8 (14) | |
| Whole | 06/01–09/08 | S0RW1 | 33.6 (2) | 118.5 (8) | 80.2 (4) | 232.3 (14) | |
| S0RW2 | 33.6 (2) | 84.6 (7) | 72.5 (4) | 190.7 (13) | |||
| S0RW3 | 33.6 (2) | 72.8 (6) | 67.3 (4) | 173.6 (12) | |||
| S3RW1 | 33.6 (2) | 109.8 (8) | 79.4 (4) | 222.8 (14) | |||
| S3RW2 | 33.6 (2) | 89.3 (70) | 74.2 (4) | 197.1 (13) | |||
| S3RW3 | 33.6 (2) | 61.5 (6) | 68.3 (4) | 163.4 (12) | |||
| S5RW1 | 33.6 (2) | 93.1 (8) | 79.7 (4) | 206.3 (14) | |||
| S5RW2 | 33.6 (2) | 75.7 (7) | 77.1 (4) | 186.4 (13) | |||
| S5RW3 | 33.6 (2) | 60.8 (6) | 74.3 (4) | 168.8 (12) | |||
| S0MW1 | 33.6 (2) | 149.2 (8) | 83.3 (4) | 266.1 (14) | |||
| S0MW2 | 33.6 (2) | 149.2 (8) | 67.5 (3) | 250.3 (13) | |||
| S0MW3 | 33.6 (2) | 149.2 (8) | 52.5 (2) | 235.3 (12) | |||
| S3MW1 | 33.6 (2) | 137.6 (8) | 78.6 (4) | 249.8 (14) | |||
| S3MW2 | 33.6 (2) | 137.6 (8) | 71.2 (3) | 242.4 (13) | |||
| S3MW3 | 33.6 (2) | 137.6 (8) | 53.4 (2) | 224.6 (12) | |||
| S5MW1 | 33.6 (2) | 130.4 (8) | 64.7 (4) | 228.7 (14) | |||
| S5MW2 | 33.6 (2) | 130.4 (8) | 58.3 (3) | 222.3 (13) | |||
| S5MW3 | 33.6 (2) | 130.4 (8) | 42.6 (2) | 206.6 (12) | |||
| 2020 | Seedling | 06/01–06/26 | S0W0 | 38.3 (2) | 155.2 (8) | 91.3 (5) | 284.8 (15) |
| Reproductive | 06/27–08/11 | S3W0 | 38.3 (2) | 141.2 (8) | 84.2 (5) | 263.7 (15) | |
| Mature | 08/12–09/15 | S5W0 | 38.3 (8) | 132.2 (8) | 88.3 (5) | 258.8 (15) | |
| Whole | 06/01–09/15 | S0RW1 | 38.3 (8) | 122.4 (8) | 90.2 (5) | 250.9 (15) | |
| S0RW2 | 38.3 (8) | 88.5 (7) | 84.1 (5) | 211.1 (14) | |||
| S0RW3 | 38.3 (8) | 72.8 (6) | 86.3 (5) | 197.4 (13) | |||
| S3RW1 | 38.3 (8) | 110.4 (8) | 89.2 (5) | 237.9 (15) | |||
| S3RW2 | 38.3 (8) | 85.6 (7) | 81.4 (5) | 205.3 (14) | |||
| S3RW3 | 38.3 (8) | 66.2 (6) | 76.3 (5) | 180.8 (13) | |||
| S5RW1 | 38.3 (8) | 112.4 (8) | 90.7 (5) | 241.4 (15) | |||
| S5RW2 | 38.3 (8) | 81.5 (7) | 84.4 (5) | 204.2 (14) | |||
| S5RW3 | 38.3 (8) | 62.8 (6) | 79.3 (5) | 180.4 (13) | |||
| S0MW1 | 38.3 (8) | 155.2 (8) | 85.5 (5) | 240.7 (15) | |||
| S0MW2 | 38.3 (8) | 155.2 (8) | 79.3 (4) | 234.5 (14) | |||
| S0MW3 | 38.3 (8) | 155.2 (8) | 55.3 (3) | 210.5 (13) | |||
| S3MW1 | 38.3 (8) | 141.2 (8) | 80.2 (5) | 221.4 (15) | |||
| S3MW2 | 38.3 (8) | 141.2 (8) | 76.9 (4) | 218.1 (14) | |||
| S3MW3 | 38.3 (8) | 141.2 (8) | 57.5 (3) | 198.7 (13) | |||
| S5MW1 | 38.3 (8) | 132.2 (8) | 70.6 (5) | 202.8 (15) | |||
| S5MW2 | 38.3 (8) | 132.2 (8) | 65.5 (4) | 197.7 (14) | |||
| S5MW3 | 38.3 (8) | 132.2 (8) | 46.2 (3) | 178.4 (13) | |||
| Parameters | θ0 | θr | a | d | C | τ |
|---|---|---|---|---|---|---|
| Value | 0.422 | 0.029 | 0.019 | −2.563 | 0.396 | 1.96 |
| Treatment | φ | FW | WC | TSS | Ly | SSC | CI | φπ | Na+ Content |
|---|---|---|---|---|---|---|---|---|---|
| S (soil salinity) | ** | ** | ** | ** | * | ** | ** | ** | ** |
| WD (water deficit) | ** | ** | ** | ** | ** | ** | ** | ** | ns |
| WDS (water deficit stage) | ns | ns | ns | * | ns | * | ** | ** | ns |
| S × W | * | ** | ** | ** | ** | ** | ** | ** | ** |
| S × WDS | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| W × WDS | ns | ns | ns | ns | ns | * | ** | ** | ns |
| S × W × WDS | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Quality Parameters | Salinity Correction Factor | Sensitivity Index (λqi) | R2 | |
|---|---|---|---|---|
| Reproductive Period | Mature Period | |||
| TSS | −0.115 | −0.267 | −0.334 | 0.91 |
| LY | −0.058 | −0.171 | −0.215 | 0.94 |
| SSC | −0.205 | −0.473 | −0.575 | 0.84 |
| CI | −0.101 | −0.210 | −0.335 | 0.92 |
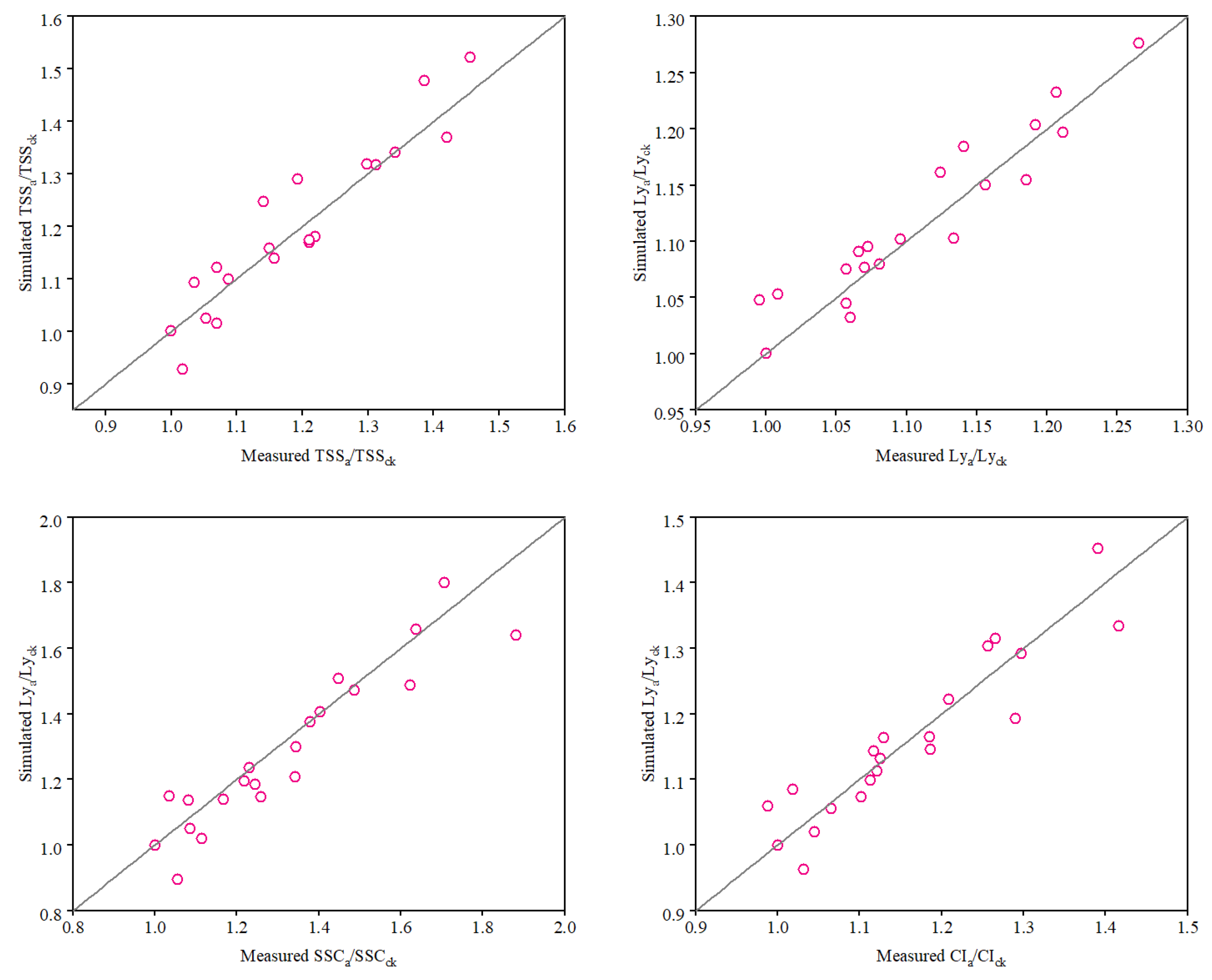
| Quality Parameters | b | R2 | RMSE | AAE | EF | dIA |
|---|---|---|---|---|---|---|
| TSS | 1.013 | 0.862 | 0.056 | 0.045 | 0.957 | 0.959 |
| LY | 1.008 | 0.882 | 0.026 | 0.021 | 0.99 | 0.967 |
| SAR | 0.971 | 0.87 | 0.092 | 0.069 | 0.939 | 0.96 |
| CI | 0.998 | 0.855 | 0.046 | 0.037 | 0.974 | 0.962 |
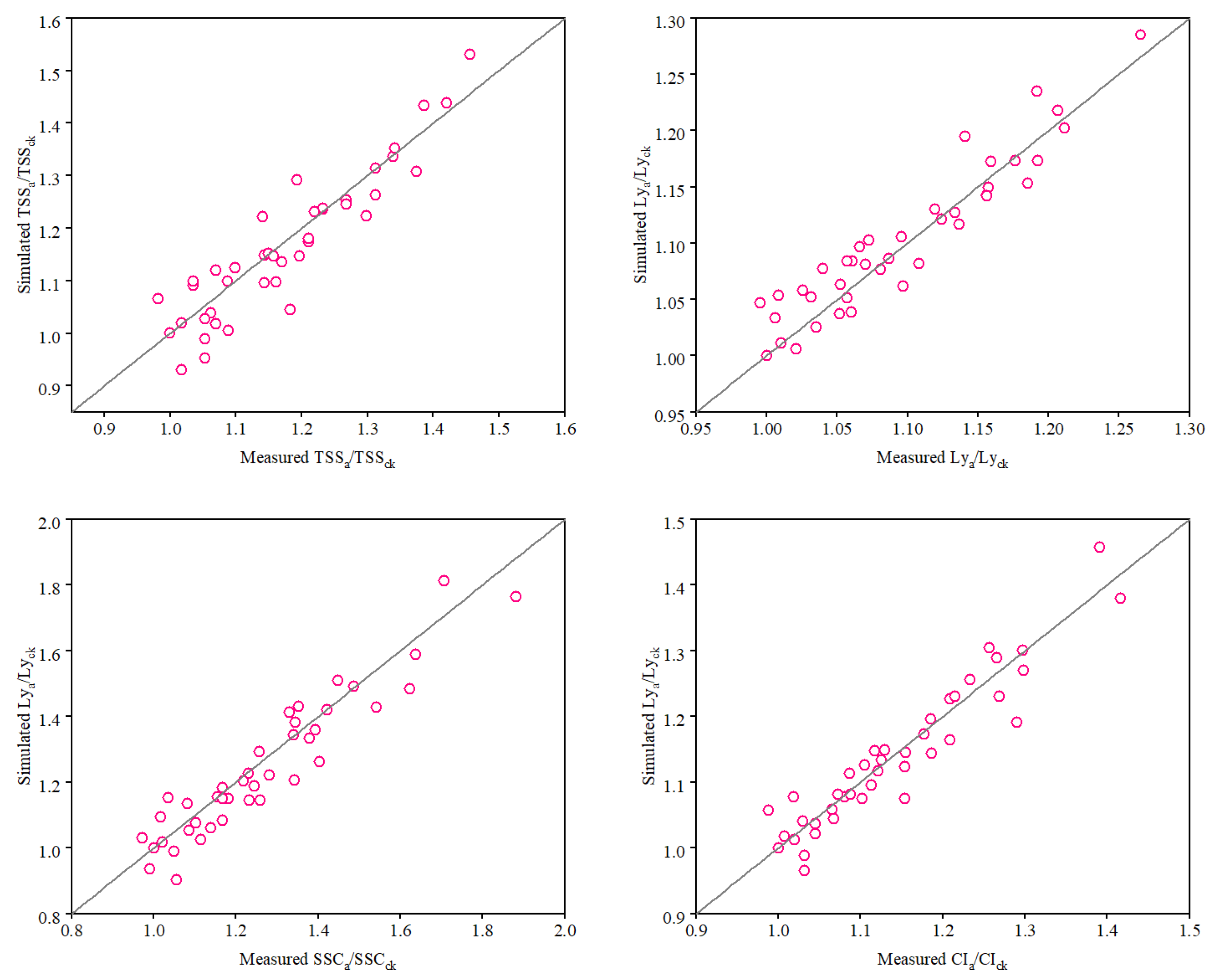
| Quality Parameters | b | R2 | RMSE | AAE | EF | dIA |
|---|---|---|---|---|---|---|
| TSS | 0.991 | 0.832 | 0.053 | 0.041 | 0.933 | 0.957 |
| LY | 1.005 | 0.877 | 0.023 | 0.018 | 0.984 | 0.969 |
| SAR | 0.979 | 0.889 | 0.074 | 0.059 | 0.936 | 0.968 |
| CI | 0.996 | 0.896 | 0.035 | 0.027 | 0.972 | 0.973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, H.; Liu, H.; Wang, J.; Qiang, X. Response of Tomato Quality Parameters to Water Deficit Under Soil Salinity and Simulation Based on Stem Water Potential. Horticulturae 2025, 11, 114. https://doi.org/10.3390/horticulturae11020114
Zhang X, Li H, Liu H, Wang J, Qiang X. Response of Tomato Quality Parameters to Water Deficit Under Soil Salinity and Simulation Based on Stem Water Potential. Horticulturae. 2025; 11(2):114. https://doi.org/10.3390/horticulturae11020114
Chicago/Turabian StyleZhang, Xianbo, Huanhuan Li, Hao Liu, Jinglei Wang, and Xiaoman Qiang. 2025. "Response of Tomato Quality Parameters to Water Deficit Under Soil Salinity and Simulation Based on Stem Water Potential" Horticulturae 11, no. 2: 114. https://doi.org/10.3390/horticulturae11020114
APA StyleZhang, X., Li, H., Liu, H., Wang, J., & Qiang, X. (2025). Response of Tomato Quality Parameters to Water Deficit Under Soil Salinity and Simulation Based on Stem Water Potential. Horticulturae, 11(2), 114. https://doi.org/10.3390/horticulturae11020114








