Optical and Chemical Profiling of Japanese Strawberries: Fluorescence Fingerprints, Imaging Features, and Quality Attributes Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Source
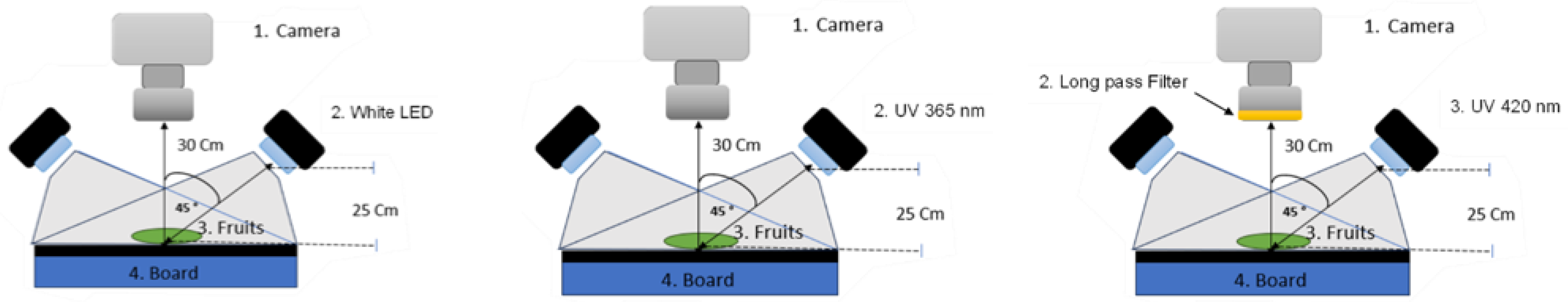
2.2. RGB Image Acquisition
2.3. Image Processing
2.4. Fluorescence Excitation-Emission (EEM) Measurement
2.5. Physicochemical Properties Assessment
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Parameters
3.2. Colorimetric Attributes and Physical Properties of GLCM Based on Fluorescence Image
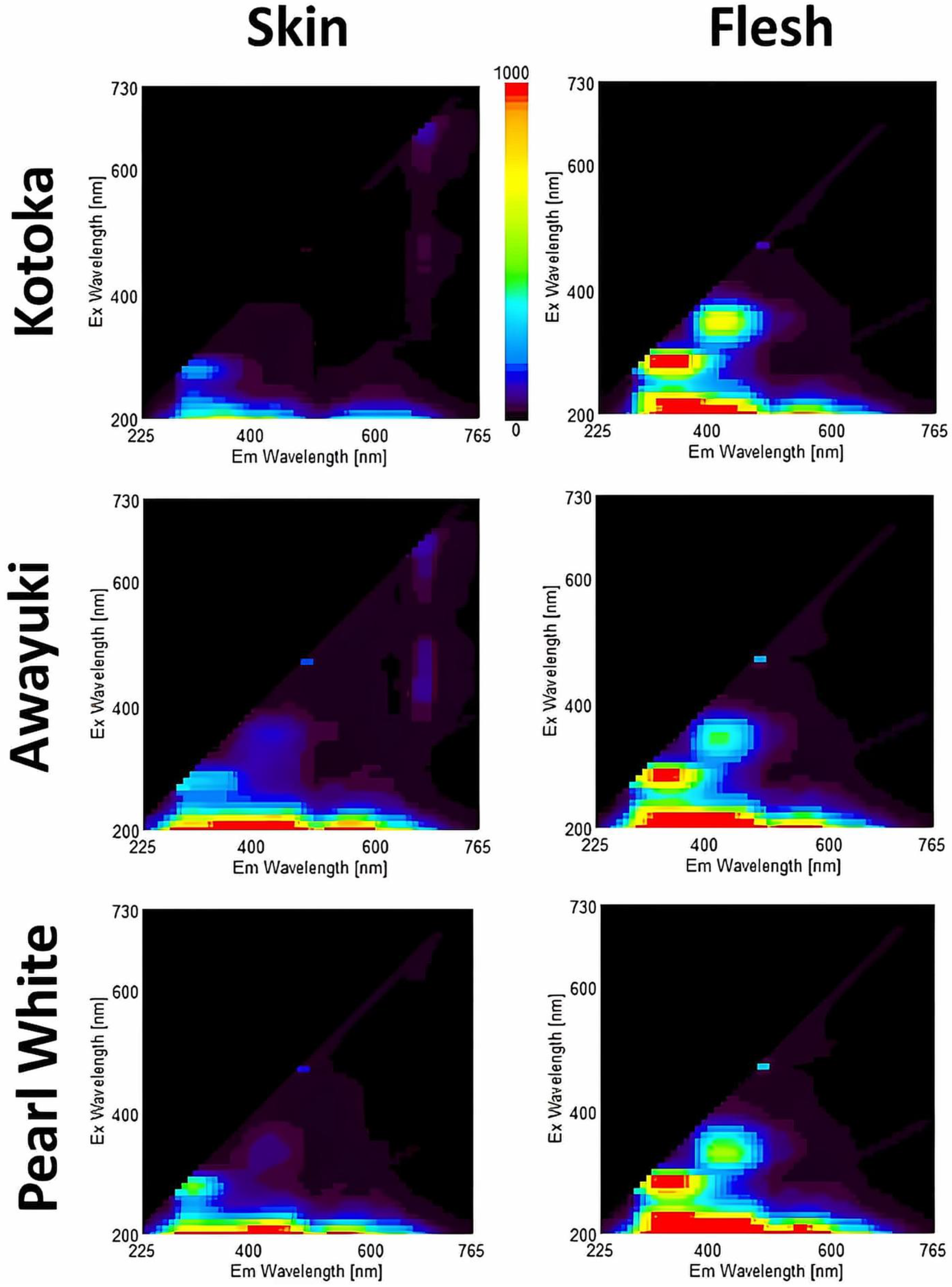
3.3. EEM of the Three Studied Varieties of Strawberry
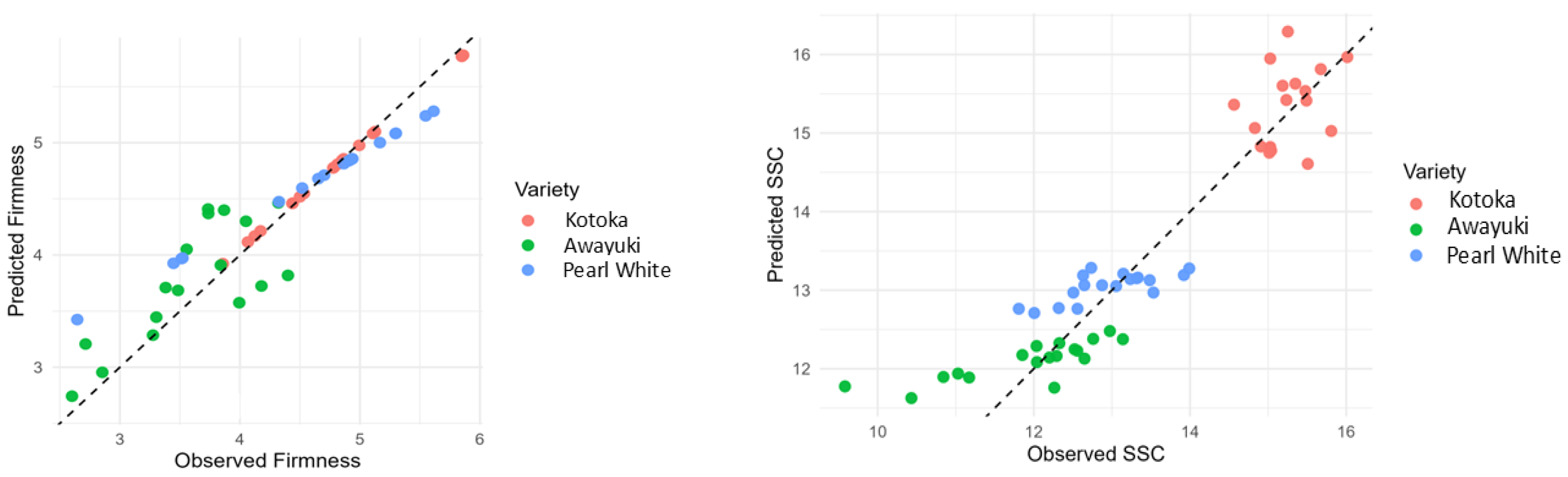
3.4. Prediction of Firmness, SSC, and TA Using EEM Fluorescence
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parvez, S.; Wani, I.A. Postharvest Biology and Technology of Strawberry. In Postharvest Biology and Technology of Temperate Fruits; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 331–348. [Google Scholar] [CrossRef]
- De Simone, N.; Scauro, A.; Fatchurrahman, D.; Russo, P.; Capozzi, V.; Spano, G.; Fragasso, M. Inclusion of Antifungal and Probiotic Lactiplantibacillus plantarum Strains in Edible Alginate Coating as a Promising Strategy to Produce Probiotic Table Grapes and Exploit Biocontrol Activity. Horticulturae 2024, 10, 4. [Google Scholar] [CrossRef]
- De Simone, N.; Scauro, A.; Fatchurrahman, D.; Amodio, M.L.; Capozzi, V.; Colelli, G.; Spano, G.; Fragasso, M.; Russo, P. Probiotic Lactiplantibacillus plantarum strains showing anti-Botrytis activity: A food-grade approach to improve the overall quality of strawberry in post-harvest. Postharvest Biol. Technol. 2024, 218, 113125. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 5 September 2025).
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodríguez, M.A.; Vázquez-Odériz, M.L. Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria × ananassa Duch, cv Selva). J. Food Compos. Anal. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Palma, C.; Morales-Quintana, L.; Ramos, P. Phenolic Content, Color Development, and Pigment−Related Gene Expression: A Comparative Analysis in Different Cultivars of Strawberry During the Ripening Process. Agronomy 2020, 10, 588. [Google Scholar] [CrossRef]
- Chen, Y.; Belwal, T.; Xu, Y.; Ma, Q.; Li, D.; Li, L.; Xiao, H.; Luo, Z. Updated insights into anthocyanin stability behavior from bases to cases: Why and why not anthocyanins lose during food processing. Crit. Rev. Food Sci. Nutr. 2023, 63, 8639–8671. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.; Lyons, T. Strawberry As a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Schwieterman, M.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J.; et al. Strawberry Flavor: Diverse Chemical Compositions, a Seasonal Influence, and Effects on Sensory Perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef]
- Šamec, D.; Maretić, M.; Lugarić, I.; Mešić, A.; Salopek-Sondi, B.; Duralija, B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2016, 194, 828–834. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Mazzoni, L.; Forbes, T.; Gasparrini, M.; Sabbadini, S.; Giampieri, F. The effects of pre-harvest and post-harvest factors on the nutritional quality of strawberry fruits: A review. J. Berry Res. 2014, 4, 10. [Google Scholar] [CrossRef]
- Błaszczyk, J.; Bieniasz, M.; Nawrocki, J.; Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Zaleski, T.; Knaga, J.; Bogdał, S. The Effect of Harvest Date and Storage Conditions on the Quality of Remontant Strawberry Cultivars Grown in a Gutter System under Covers. Agriculture 2022, 12, 1193. [Google Scholar] [CrossRef]
- Mancini, M.; Mazzoni, L.; Gagliardi, F.; Balducci, F.; Duca, D.; Toscano, G.; Mezzetti, B.; Capocasa, F. Application of the Non-Destructive NIR Technique for the Evaluation of Strawberry Fruits Quality Parameters. Foods 2020, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Yu, S.; Guo, B.; Tang, P.; Liang, D. Non-Destructive Detection of Strawberry Quality Using Multi-Features of Hyperspectral Imaging and Multivariate Methods. Sensors 2020, 20, 3074. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar] [CrossRef]
- Fathi-Najafabadi, A.; Besada, C.; Gil, R.; Calatayud, M.A.; Salvador, A. Chlorophyll fluorescence imaging as a tool to evaluate calyx senescence during the ripening of persimmon fruit treated with gibberellic acid. Postharvest Biol. Technol. 2021, 179, 111582. [Google Scholar] [CrossRef]
- Momin, A.; Kondo, N.; Al Riza, D.; Ogawa, Y.; Obenland, D. A Methodological Review of Fluorescence Imaging for Quality Assessment of Agricultural Products. Agriculture 2023, 13, 1433. [Google Scholar] [CrossRef]
- Sikorska, E.; Nowak, P.; Pawlak-Lemanska, K.; Sikorski, M. Characterization and Classification of Direct and Commercial Strawberry Beverages Using Absorbance–Transmission and Fluorescence Excitation–Emission Matrix Technique. Foods 2022, 11, 2143. [Google Scholar] [CrossRef]
- Aparicio, R.; Sayago, A.; Morales, M. Detection of hazelnut oil in virgin olive oil by a spectrofluorimetric method. Eur. Food Res. Technol. 2004, 218, 480–483. [Google Scholar] [CrossRef]
- Lötze, E.; Huybrechts, C.; Sadie, A.; Theron, K.; Valcke, R. Fluorescence imaging as a non-destructive method for pre-harvest detection of bitter pit in apple fruit (Malus domestica Borkh.). Postharvest Biol. Technol. 2006, 40, 287–294. [Google Scholar] [CrossRef]
- Fatchurrahman, D. Identification of UV-Fluorescence Components Associated with and Detection of Surface Damage in Green Pepper (Capsicum annum L). 2015. Available online: https://www.researchgate.net/publication/301285323 (accessed on 19 May 2025).
- Fatchurrahman, D.; Kuramoto, M.; Al Riza, D.F.; Ogawa, Y.; Suzuki, T.; Kondo, N. Fluorescence time series monitoring of different parts of green pepper (Capsicum annuum L.) under different storage temperatures. Comput. Electron. Agric. 2020, 179, 105850. [Google Scholar] [CrossRef]
- Fatchurrahman, D.; Castillejo, N.; Hilaili, M.; Russo, L.; Fathi-Najafabadi, A.; Rahman, A. A Novel Damage Inspection Method Using Fluorescence Imaging Combined with Machine Learning Algorithms Applied to Green Bell Pepper. Horticulturae 2024, 10, 1336. [Google Scholar] [CrossRef]
- Fatchurrahman, D.; Hilaili, M.; Nurwahyuningsih; Russo, L.; Jahari, M.B.; Fathi-Najafabadi, A. Utilizing RGB imaging and machine learning for freshness level determination of green bell pepper (Capsicum annuum L.) throughout its shelf-life. Postharvest Biol. Technol. 2025, 222, 113359. [Google Scholar] [CrossRef]
- Nurwahyuningsih; Fatchurrahman, D.; Hilaili, M.; Chosa, T. Assessment of fluorescence fingerprint in Hayward and Ruby-Red kiwi fruit as a potential approach to monitor quality changes during storage. Food Control 2025, 178, 111528. [Google Scholar] [CrossRef]
- Lawaetz, A.; Stedmon, C. Fluorescence Intensity Calibration Using the Raman Scatter Peak of Water. Appl. Spectrosc. 2009, 63, 936–940. [Google Scholar] [CrossRef]
- Fathi-Najafabadi, A.; Besada, C.; Gil, R.; Salvador, A. Harvest time and postharvest behavior of six japanese nonastringent persimmon cultivars grown under mediterranean conditions. HortScience 2020, 55, 1766–1771. [Google Scholar] [CrossRef]
- Fatchurrahman, D.; Amodio, M.L.; de Chiara, M.L.V.; Chaudhry, M.M.A.; Colelli, G. Early discrimination of mature-and immature-green tomatoes (Solanum lycopersicum L.) using fluorescence imaging method. Postharvest Biol. Technol. 2020, 169, 111287. [Google Scholar] [CrossRef]
- Corvino, A.; Romaniello, R.; Palumbo, M.; Ricci, I.; Cefola, M.; Pelosi, S.; Pace, B. Image analysis to predict the maturity index of strawberries. Adv. Hortic. Sci. 2023, 37, 83–87. [Google Scholar] [CrossRef]
- Patel, H.; Taghavi, T.; Samtani, J.B. Fruit Quality of Several Strawberry Cultivars during the Harvest Season under High Tunnel and Open Field Environments. Horticulturae 2023, 9, 1084. [Google Scholar] [CrossRef]
- Wozniak, W.; Radajewka, B.; Reszelska-Sieciechowicz, A.; Dejwor, I. Sugar and acid content influence organoleptic evaluation of fruits of six strawberry cultivars from controlled cultivation. Acta Hortic. 1997, 439, 333–336. [Google Scholar] [CrossRef]
- Ikegaya, A. Composition of free sugars and organic acids in Japanese strawberry cultivars and their influence on the perception of sweetness and sourness. J. Food Sci. 2024, 89, 614–624. [Google Scholar] [CrossRef]
- Zubair, A.R.; Alo, O.A. Grey Level Co-occurrence Matrix (GLCM) Based Second Order Statistics for Image Texture Analysis. Int. J. Sci. Eng. Investig. 2019, 8, 93. [Google Scholar]
- Ladika, G.; Strati, I.; Tsiaka, T.; Cavouras, D.; Sinanoglou, V. On the Assessment of Strawberries’ Shelf-Life and Quality, Based on Image Analysis, Physicochemical Methods, and Chemometrics. Foods 2024, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, R.; Watanabe, T.; Nakamura, N.; Koseki, S.; Koyama, K. Investigating the relationship between strawberry maturity and glossiness on the surface during storage through digital image analysis. J. Food Meas. Charact. 2023, 18, 1776–1785. [Google Scholar] [CrossRef]
- Voća, S.; Žlabur, J.Š.; Dobričević, N.; Jakobek, L.; Šeruga, M.; Galić, A.; Pliestić, S. Variation in the Bioactive Compound Content at Three Ripening Stages of Strawberry Fruit. Molecules 2014, 19, 10370–10385. [Google Scholar] [CrossRef]
- Razgonova, M.; Zinchenko, Y.; Pikula, K.; Tekutyeva, L.; Son, O.; Zakharenko, A.; Kalenik, T.; Golokhvast, K. Spatial Distribution of Polyphenolic Compounds in Corn Grains (Zea mays L. var. Pioneer) Studied by Laser Confocal Microscopy and High-Resolution Mass Spectrometry. Plants 2022, 11, 630. [Google Scholar] [CrossRef]
- Kapoor, L.; Simkin, A.J.; Doss, C.G.P.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Melø, T.B.; Naqvi, K.R. Effect of anthocyanins, carotenoids, and flavonols on chlorophyll fluorescence excitation spectra in apple fruit: Signature analysis, assessment, modelling, and relevance to photoprotection. J. Exp. Bot. 2008, 59, 349–359. [Google Scholar] [CrossRef]
- Collings, D.A. Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules. Plants 2019, 8, 596. [Google Scholar] [CrossRef]
- Morales, F.; Cartelat, A.; Álvarez-Fernández, A.; Moya, I.; Cerovic, Z.G. Time-Resolved Spectral Studies of Blue−Green Fluorescence of Artichoke (Cynara cardunculus L. Var. Scolymus) Leaves: Identification of Chlorogenic Acid as One of the Major Fluorophores and Age-Mediated Changes. J. Agric. Food Chem. 2005, 53, 9668–9678. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Nakayama, M.; Noguchi, Y.; Horie, H. Use of image analysis to estimate anthocyanin and UV-excited fluorescent phenolic compound levels in strawberry fruit. Breed. Sci. 2013, 63, 211–217. [Google Scholar] [CrossRef]
- Ma, T.; Inagaki, T.; Tsuchikawa, S. Development of a sensitivity-enhanced chlorophyll fluorescence lifetime spectroscopic method for nondestructive monitoring of fruit ripening and postharvest decay. Postharvest Biol. Technol. 2023, 198, 112231. [Google Scholar] [CrossRef]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and Identification of Strawberry Phenolics with Antioxidant and Human Cancer Cell Antiproliferative Properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef]
- Sikorska, E.; Włodarska, K.; Khmelinskii, I. Application of multidimensional and conventional fluorescence techniques for classification of beverages originating from various berry fruit. Methods Appl. Fluoresc. 2020, 8, 015006. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Yu, O.; Tang, J.; Gu, X.; Wan, X.; Fang, C. Metabolic profiling of strawberry (Fragaria × ananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011, 62, 1103–1118. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic Acids in Berries, Fruits, and Beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef]
- Sano, H.; Kawaguchi, S.; Iimori, T.; Kuragano, M.; Tokuraku, K.; Uwai, K. Using fluorescence spectra to quantitatively evaluate the constituent content and functionality of hydroponically grown Fragaria × Ananassa. Eur. Food Res. Technol. 2025, 251, 3087–3103. [Google Scholar] [CrossRef]


| Cultivar | TSS (°Brix) | TA (%) | MI | Firmness (N) |
|---|---|---|---|---|
| Kotoka | 15.53 ± 0.23 a | 0.96 ± 0.01 a | 16.37 ± 0.51 c | 4.74 ± 0.4 a |
| Awayuki | 11.66 ± 0.31 c | 0.61 ± 0.01 b | 19.62 ± 1.11 b | 4.01 ± 0.2 b |
| Pearl White | 13.11 ± 0.25 b | 0.61 ± 0.01 b | 22.28 ± 0.96 a | 4.85 ± 0.3 a |
| Cultivar | B Channel | G Channel | R Channel | L | a | b | Contrast | Dissimilarity | Homogeneity | Energy | Correlation | ASM | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | 365 LED | ‘Kotoka’ | 36.87 ± 17.17 c | 18.95 ± 4.72 c | 32.85 ± 5.28 c | 22.32 ± 7.19 c | 138.82 ± 4.14 a | 119.41 ± 7.63 a | 32.01 ± 9.48 c | 3.66 ± 0.45 c | 0.26 ± 0.01 a | 0.05 ± 0.01 a | 0.84 ± 0.09 b | 0.01 ± 0.01 a |
| ‘Awayuki’ | 160.17 ± 21.22 a | 111.28 ± 15.75 b | 64.85 ± 6.83 a | 115.91 ± 15.07 b | 128.09 ± 2.06 b | 97.11 ± 3.78 b | 64.86 ± 23.6 b | 4.42 ± 0.57 b | 0.26 ± 0.01 a | 0.03 ± 0.01 b | 0.98 ± 0.01 a | 0.01 ± 0.01 a | ||
| ‘Pearl White’ | 139.49 ± 16.95 b | 125.67 ± 13.25 a | 48.21 ± 5.01 b | 123.14 ± 12.33 a | 110.12 ± 2.54 c | 113.97 ± 4.21 a | 115.46 ± 28.35 a | 5.41 ± 0.37 a | 0.25 ± 0.01 a | 0.03 ± 0.01 b | 0.97 ± 0.01 a | 0.01 ± 0.01 a | ||
| 420 LED | ‘Kotoka’ | 2.57 ± 0.24 b | 26.19 ± 9.37 c | 15.97 ± 1.83 c | 22.46 ± 8.13 c | 120.27 ± 5.19 a | 138.88 ± 3.77 c | 45.81 ± 14.41 a | 3.57 ± 0.77 a | 0.33 ± 0.04 a | 0.08 ± 0.01 a | 0.82 ± 0.05 b | 0.01 ± 0.01 a | |
| ‘Awayuki’ | 1.43 ± 0.19 b | 60.79 ± 7.12 b | 49.34 ± 2.73 a | 59.87 ± 6.83 b | 115.16 ± 3.19 b | 157.86 ± 2.75 b | 27.55 ± 7.91 a | 3.23 ± 0.21 a | 0.31 ± 0.01 a | 0.04 ± 0.01 b | 0.97 ± 0.01 a | 0.01 ± 0.01 b | ||
| ‘Pearl White’ | 10.85 ± 1.73 a | 105.66 ± 8.75 a | 36.15 ± 3.97 b | 98.26 ± 8.21 a | 90.16 ± 2.28 b | 169.05 ± 2.54 a | 47.96 ± 5.27 a | 4.03 ± 0.16 a | 0.27 ± 0.01 a | 0.04 ± 0.01 b | 0.98 ± 0.01 a | 0.01 ± 0.01 b | ||
| White LED | ‘Kotoka’ | 1.36 ± 0.1 c | 9.25 ± 1.23 c | 116.83 ± 4.21 b | 60.54 ± 2.65 c | 170.23 ± 1.11 c | 162.04 ± 1.28 b | 59.15 ± 5.23 c | 3.13 ± 0.16 c | 0.47 ± 0.02 a | 0.06 ± 0.01 a | 0.91 ± 0.01 b | 0.01 ± 0.01 a | |
| ‘Awayuki’ | 76.45 ± 3.47 b | 139.27 ± 3.18 b | 208.11 ± 2.01 a | 162.41 ± 2.46 b | 148.49 ± 1.04 b | 171.47 ± 1.08 a | 108.28 ± 6.34 b | 5.04 ± 0.17 b | 0.33 ± 0.01 b | 0.04 ± 0.01 b | 0.98 ± 0.01 a | 0.01 ± 0.01 b | ||
| ‘Pearl White’ | 125.268 ± 4.54 a | 173.57 ± 3.55 a | 204.96 ± 2.52 a | 185.01 ± 3.01 a | 133.41 ± 0.68 c | 157.15 ± 1.46 c | 187.47 ± 11.55 a | 5.79 ± 0.19 a | 0.36 ± 0.01 b | 0.04 ± 0.01 b | 0.98 ± 0.01 a | 0.01 ± 0.01 b | ||
| Flesh | 365 LED | ‘Kotoka’ | 98.04 ± 32.38 c | 87.98 ± 14.63 c | 73.18 ± 7.59 a | 94.12 ± 15.22 c | 130.79 ± 3.29 a | 122.22 ± 10.95 a | 66.09 ± 25.25 a | 3.86 ± 0.34 a | 0.30 ± 0.01 b | 0.04 ± 0.01 a | 0.95 ± 0.01 a | 0.01 ± 0.01 a |
| ‘Awayuki’ | 216.013 ± 6.76 b | 166.30 ± 7.48 b | 94.38 ± 5.45 a | 166.92 ± 6.58 b | 120.55 ± 1.29 b | 96.18 ± 1.20 a | 42.58 ± 3.75 a | 3.16 ± 0.11 a | 0.34 ± 0.01 a | 0.05 ± 0.01 a | 0.99 ± 0.01 a | 0.01 ± 0.01 a | ||
| ‘Pearl White’ | 228.95 ± 5.21 a | 191.22 ± 4.60 a | 87.00 ± 4.44 a | 185.93 ± 4.24 a | 109.31 ± 0.45 c | 100.35 ± 0.54 a | 62.09 ± 2.45 a | 3,12 ± 0,04 a | 0.35 ± 0.01 a | 0.04 ± 0.01 a | 0,99± 0,01 a | 0,01 ± 0.01 a | ||
| 420 LED | ‘Kotoka’ | 2.7 ± 1.51 c | 56.34 ± 1.49 c | 55.93 ± 7.38 | 59.18 ± 7.95 c | 121.56 ± 8.32 a | 157.57 ± 2.75 c | 109.43 ± 12.00 a | 3.95 ± 1.61 a | 0.32 ± 0.03 a | 0.05 ± 0.01 a | 0.92 ± 0.07 a | 0.01 ± 0.01 a | |
| ‘Awayuki’ | 5.01 ± 2.23 b | 79.71 ± 6.69 b | 44.58 ± 9.24 | 77.19 ± 7.05 b | 104.26 ± 3.36 b | 163.58 ± 2.01 b | 66.89 ± 97.05 a | 3.35 ± 1.44 a | 0.34 ± 0.04 a | 0.07 ± 0.02 a | 0.96 ± 0.03 a | 0.01 ± 0.01 a | ||
| ‘Pearl White’ | 14.05 ± 0.91 a | 117.62 ± 2.71 a | 34.17 ± 0.98 | 111.01 ± 2.19 a | 86.81 ± 1.21 c | 172.01 ± 0.56 a | 43.06 ± 3.29 a | 3.56 ± 0.05 a | 0.31 ± 0.03 a | 0.05 ± 0.01 a | 0.98 ± 0.01 a | 0.01 ± 0.01 a | ||
| White LED | ‘Kotoka’ | 79.32 ± 12.97 c | 141.95 ± 11.99 b | 229.60 ± 5.78 a | 173.59 ± 8.54 b | 156.10 ± 2.74 a | 174.51 ± 2.21 a | 83.01 ± 13.25 b | 4.82 ± 0.32 a | 0.33 ± 0.01 b | 0.05 ± 0.01 b | 0.99 ± 0.01 ab | 0.01 ± 0.01 b | |
| ‘Awayuki’ | 139.46 ± 5.34 b | 195.49 ± 4.35 a | 236.41 ± 1.66 a | 207.71 ± 3.28 a | 135.94 ± 1.17 b | 161.77 ± 1.20 b | 89.61 ± 8.13 b | 3.22 ± 0.14 b | 0.47 ± 0.01 a | 0.07 ± 0.01 a | 0.99 ± 0.01 a | 0.01 ± 0.01 a | ||
| ‘Pearl White’ | 173.08 ± 7.91 a | 211.66 ± 5.91 a | 223.32 ± 5.39 a | 216.43 ± 5.34 a | 126.28 ± 0.29 c | 148.39 ± 1.42 c | 159.92 ± 11.54 a | 3.84 ± 0.24 b | 0.47 ± 0.01 a | 0.07 ± 0.01 a | 0.99 ± 0.01 b | 0.01 ± 0.01 ab |
| Skin | Flesh | ||||
|---|---|---|---|---|---|
| Ex/Em | Cultivar | Intensity (R.U.) | Ex/Em | Cultivar | Intensity (R.U.) |
| 290/325 | ‘Kotoka’ | 54.0691 c | 280/235 | ‘Kotoka’ | 274.673 a |
| ‘Awayuki’ | 74.235 b | ‘Awayuki’ | 274.463 a | ||
| ‘Pearl White’ | 112.287 a | ‘Pearl White’ | 274.653 a | ||
| 340/435 | ‘Kotoka’ | 507.675 b | 440/685 | ‘Kotoka’ | 158.718 a |
| ‘Awayuki’ | 1184.78 a | ‘Awayuki’ | 97.2218 c | ||
| ‘Pearl White’ | 73.7794 c | ‘Pearl White’ | 134.736 b | ||
| 490/745 | ‘Kotoka’ | 764.048 b | 440/745 | ‘Kotoka’ | 218.083 c |
| ‘Awayuki’ | 1493.76 a | ‘Awayuki’ | 357.413 b | ||
| ‘Pearl White’ | 86.5641 c | ‘Pearl White’ | 442.849 a | ||
| Parameters | Tissue | R2 (%) Cal | Adjusted R2 (%) Cal | RMSE Cal | MAE Cal | R2 (%) Pred | Adjusted R2 (%) Pred | RMSE Pred | MAE Pred |
|---|---|---|---|---|---|---|---|---|---|
| SSC | Skin | 83.48 | 83.07 | 0.61 | 0.49 | 84.84 | 83.93 | 0.59 | 0.45 |
| SSC | Flesh | 81.79 | 81.34 | 0.75 | 0.58 | 81.56 | 80.46 | 0.76 | 0.61 |
| TA | Skin | 90.54 | 90.31 | 0.05 | 0.04 | 88.77 | 88.10 | 0.05 | 0.04 |
| TA | Flesh | 88.84 | 88.57 | 0.06 | 0.04 | 86.99 | 86.21 | 0.07 | 0.05 |
| MI | Skin | 61.25 | 60.30 | 1.91 | 1.37 | 70.73 | 68.59 | 1.85 | 1.42 |
| MI | Flesh | 65.48 | 64.63 | 1.61 | 1.31 | 67.61 | 65.67 | 1.51 | 1.17 |
| Firmness | Skin | 83.51 | 84.85 | 0.41 | 0.28 | 82.23 | 81.16 | 0.33 | 0.21 |
| Firmness | Flesh | 35.03 | 33.43 | 0.56 | 0.42 | 43.77 | 40.39 | 0.62 | 0.51 |
| No | Excitation (nm) | Emission (nm) | Suggested Compound | Part | Variety | References |
|---|---|---|---|---|---|---|
| 1 | 250–300 | 300–400 | Amino acids | Flesh | Troyonoka | [46,47] |
| 2 | 310–395 | 370–565 | Coumaric acid and glycosides (p-coumaric acid, p-hydroxybenzoic, gallic acid) | Flesh | Polka and Jonsok | [43,45,48] |
| 3 | <350 | 260–450 550–650 | polyphenols | Leaves | Yotsuboshi | [49] |
| 4 | >350 | 650–850 | chlorophylls | Leaves | Yotsuboshi | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilaili, M.; Fathi-Najafabadi, A.; Nurwahyuningsih; Castillejo, N.; Russo, L.; Kondo, N.; Fatchurrahman, D. Optical and Chemical Profiling of Japanese Strawberries: Fluorescence Fingerprints, Imaging Features, and Quality Attributes Prediction. Horticulturae 2025, 11, 1291. https://doi.org/10.3390/horticulturae11111291
Hilaili M, Fathi-Najafabadi A, Nurwahyuningsih, Castillejo N, Russo L, Kondo N, Fatchurrahman D. Optical and Chemical Profiling of Japanese Strawberries: Fluorescence Fingerprints, Imaging Features, and Quality Attributes Prediction. Horticulturae. 2025; 11(11):1291. https://doi.org/10.3390/horticulturae11111291
Chicago/Turabian StyleHilaili, Maulidia, Ayoub Fathi-Najafabadi, Nurwahyuningsih, Noelia Castillejo, Lucia Russo, Naoshi Kondo, and Danial Fatchurrahman. 2025. "Optical and Chemical Profiling of Japanese Strawberries: Fluorescence Fingerprints, Imaging Features, and Quality Attributes Prediction" Horticulturae 11, no. 11: 1291. https://doi.org/10.3390/horticulturae11111291
APA StyleHilaili, M., Fathi-Najafabadi, A., Nurwahyuningsih, Castillejo, N., Russo, L., Kondo, N., & Fatchurrahman, D. (2025). Optical and Chemical Profiling of Japanese Strawberries: Fluorescence Fingerprints, Imaging Features, and Quality Attributes Prediction. Horticulturae, 11(11), 1291. https://doi.org/10.3390/horticulturae11111291









