Non-Destructive Monitoring of Postharvest Hydration in Cucumber Fruit Using Visible-Light Color Analysis and Machine-Learning Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Relative Water Content
2.3. Colorimetric Analysis
2.4. Imaging Deployment Standards: Lighting and Defect Pre-Screening
2.5. Statistical Analysis and Model Development
3. Results
3.1. Performance of Linear Regression Models
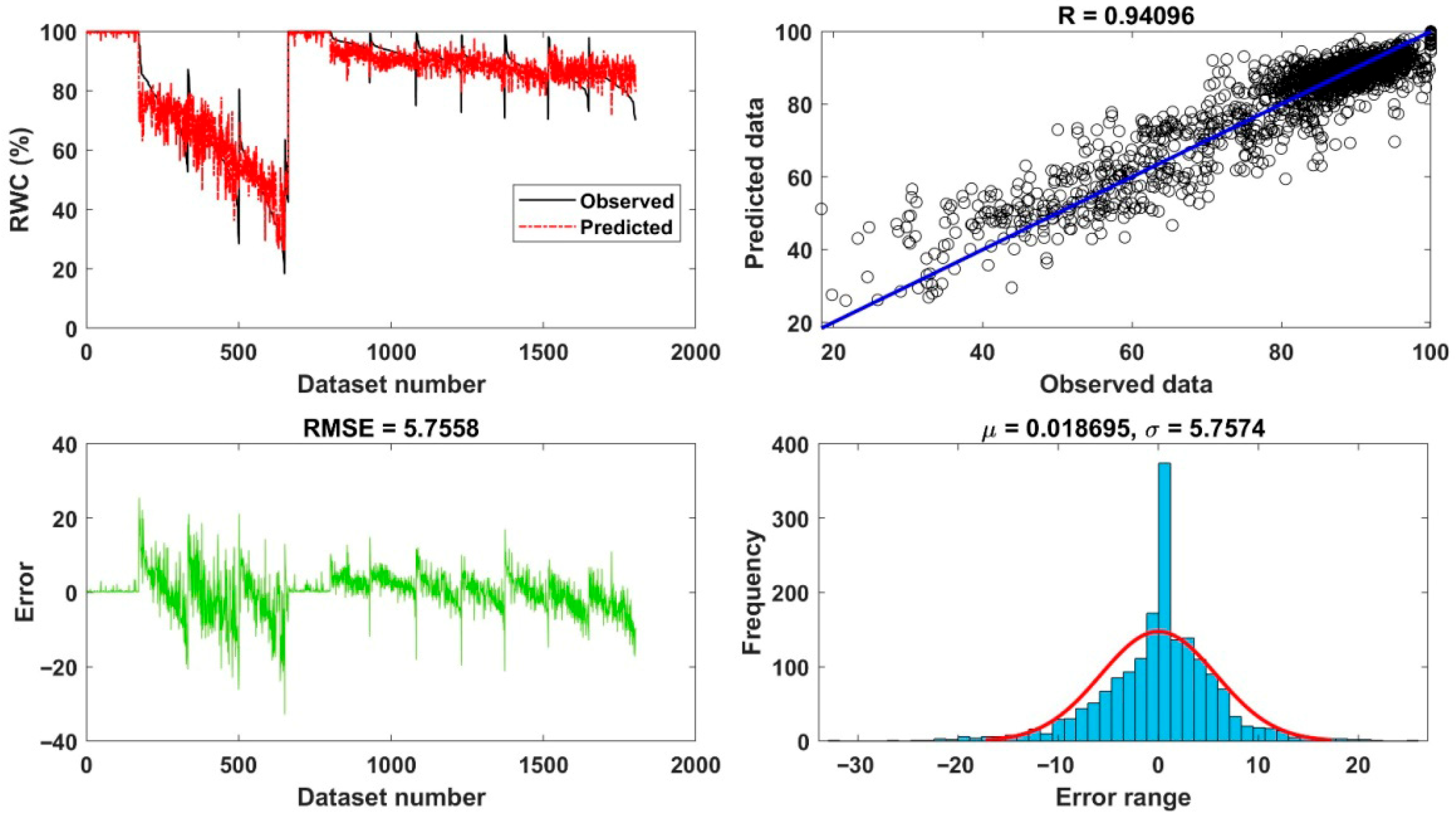
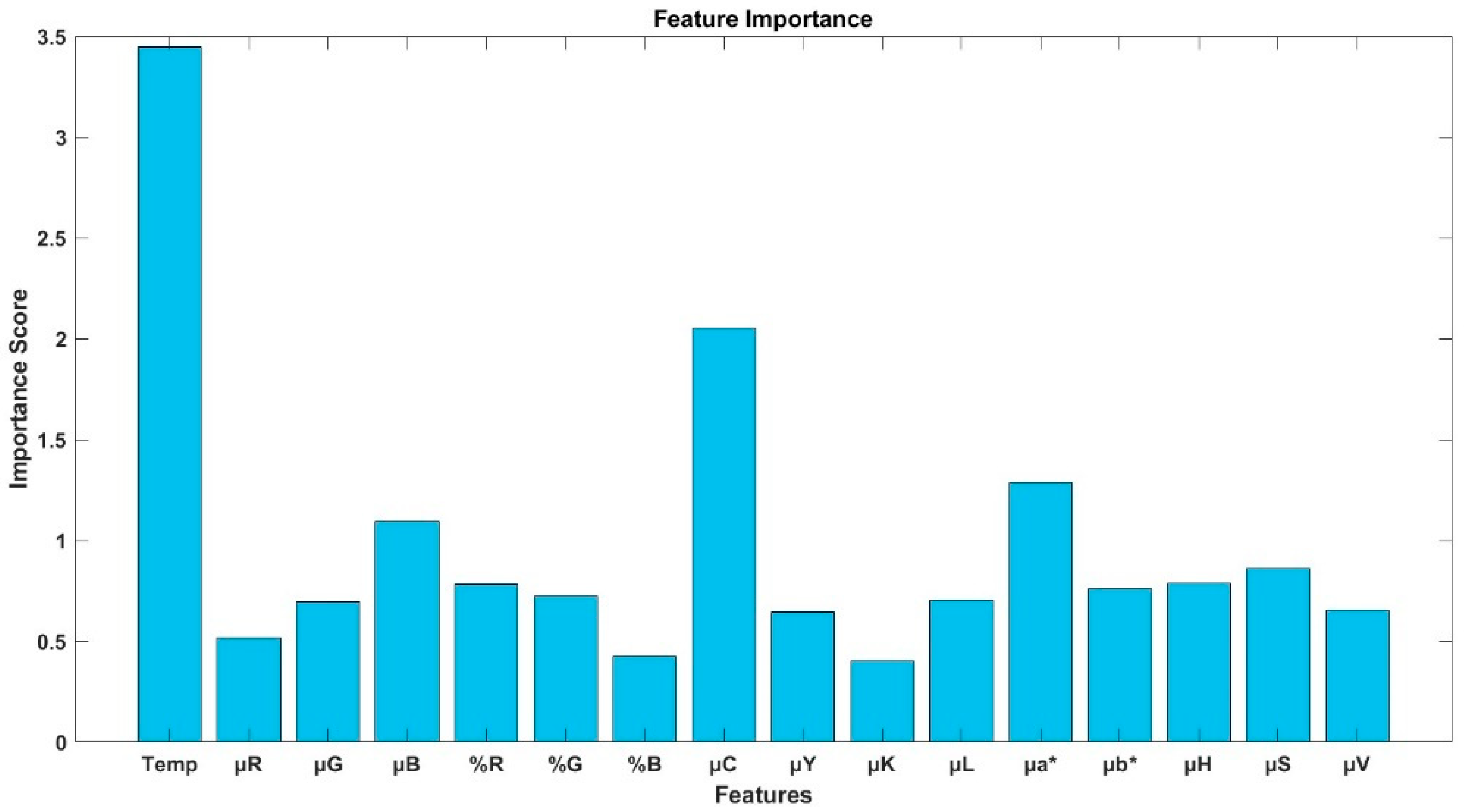
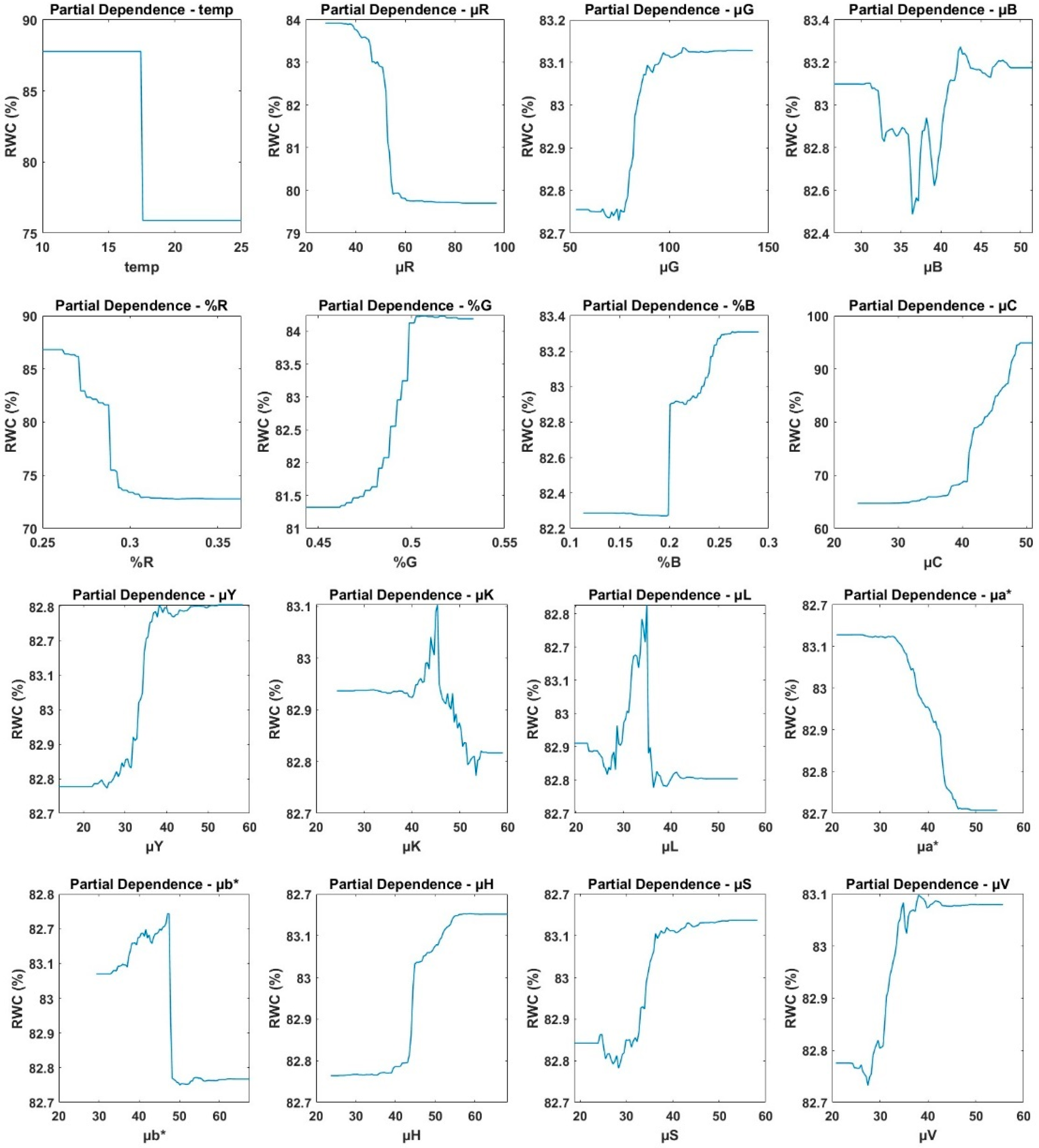
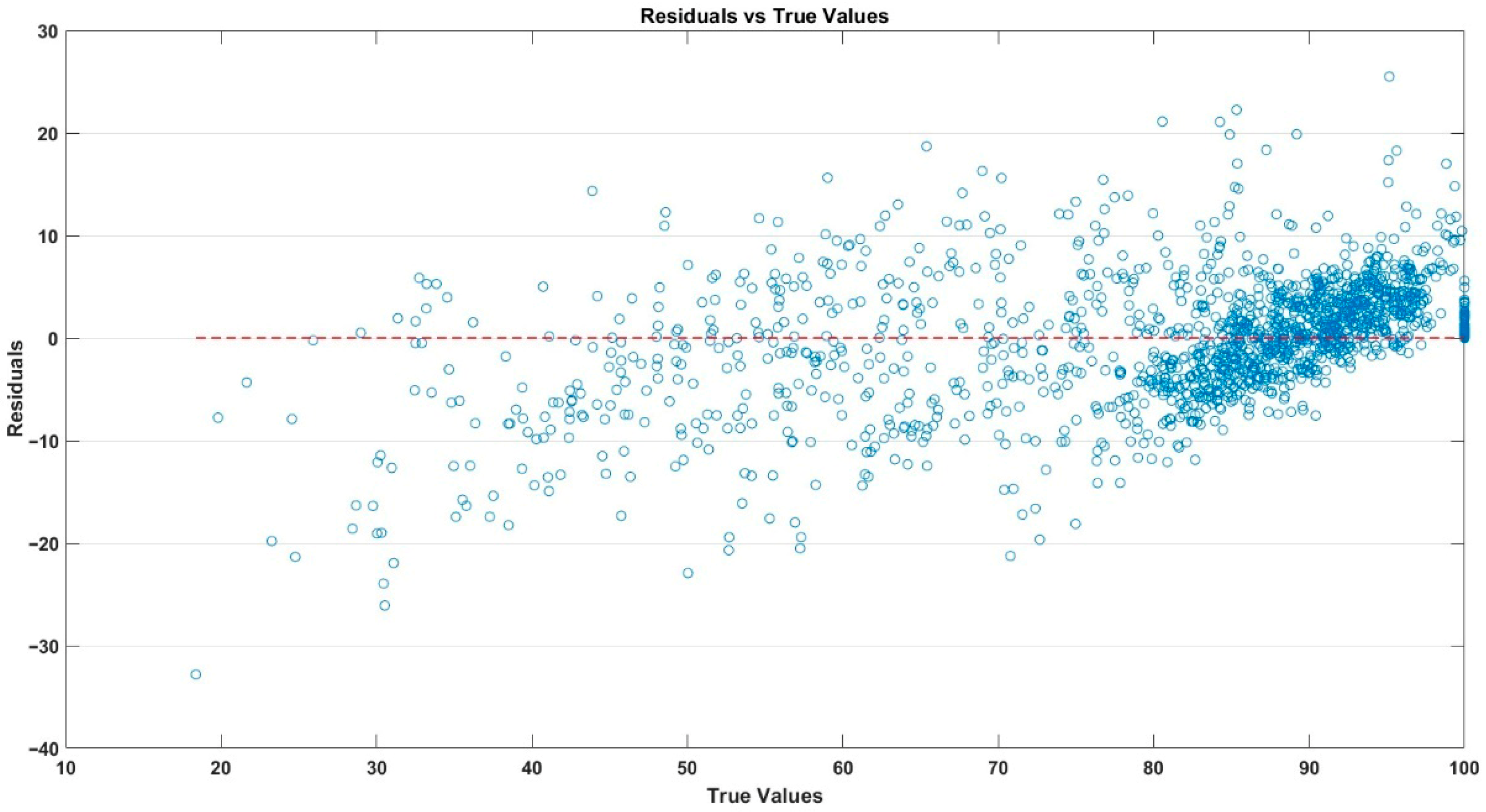
3.2. Performance of the Random Forest Model
4. Discussion
Limitations and Future Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of Postharvest Water Loss in Fruits: Mechanisms, Influencing Factors, and Effective Control Strategies—A Comprehensive Review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Galindo, F.G.; Herppich, W.; Gekas, V.; Sjöholm, I. Factors Affecting Quality and Postharvest Properties of Vegetables: Integration of Water Relations and Metabolism. Crit. Rev. Food Sci. Nutr. 2004, 44, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water Loss of Fresh Fruit: Influencing Pre-Harvest, Harvest and Postharvest Factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Li, L.; Jia, X.; Fan, K. Recent Advance in Nondestructive Imaging Technology for Detecting Quality of Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2025, 65, 5181–5199. [Google Scholar] [CrossRef]
- Cubero, S.; Aleixos, N.; Moltó, E.; Gómez-Sanchis, J.; Blasco, J. Advances in Machine Vision Applications for Automatic Inspection and Quality Evaluation of Fruits and Vegetables. Food Bioproc. Technol. 2011, 4, 487–504. [Google Scholar] [CrossRef]
- Quemada, C.; Pérez-Escudero, J.M.; Gonzalo, R.; Ederra, I.; Santesteban, L.G.; Torres, N.; Iriarte, J.C. Remote Sensing for Plant Water Content Monitoring: A Review. Remote Sens. 2021, 13, 2088. [Google Scholar] [CrossRef]
- Van As, H.; Scheenen, T.; Vergeldt, F.J. MRI of Intact Plants. Photosynth. Res. 2009, 102, 213–222. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Defraeye, T.; De Ketelaere, B.; Herremans, E.; Hertog, M.L.A.T.M.; Saeys, W.; Torricelli, A.; Vandendriessche, T.; Verboven, P. Nondestructive Measurement of Fruit and Vegetable Quality. Annu. Rev. Food Sci. Technol. 2014, 5, 285–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, J.; Wang, Y.; Liu, X.; Zhang, Y.; Fu, H. Non-Destructive Detection of Fruit Quality: Technologies, Applications and Prospects. Foods 2025, 14, 2137. [Google Scholar] [CrossRef] [PubMed]
- Aline, U.; Bhattacharya, T.; Faqeerzada, M.A.; Kim, M.S.; Baek, I.; Cho, B.-K. Advancement of Non-Destructive Spectral Measurements for the Quality of Major Tropical Fruits and Vegetables: A Review. Front. Plant Sci. 2023, 14, 1240361. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Rezaei Nejad, A.; Fanourakis, D.; Fatahi, S.; Ahmadi Majd, M. Employment of Artificial Neural Networks for Non-Invasive Estimation of Leaf Water Status Using Color Features: A Case Study in Spathiphyllum wallisii. Acta Physiol. Plant 2021, 43, 78. [Google Scholar] [CrossRef]
- Camarillo-Castillo, F.; Huggins, T.D.; Mondal, S.; Reynolds, M.P.; Tilley, M.; Hays, D.B. High-Resolution Spectral Information Enables Phenotyping of Leaf Epicuticular Wax in Wheat. Plant Methods 2021, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf Life Potential and the Fruit Cuticle: The Unexpected Player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Muñoz, R.; Heredia, A.; Domínguez, E. The Role of Cuticle in Fruit Shelf-Life. Curr. Opin. Biotechnol. 2022, 78, 102802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Perkins-Veazie, P.; Ma, G.; Gunter, C. Muskmelon Fruit Quality in Response to Postharvest Essential Oil and Whey Protein Sprays. HortScience 2017, 52, 887–891. [Google Scholar] [CrossRef]
- Reitz, N.F.; Mitcham, E.J. Validation and Demonstration of a Pericarp Disc System for Studying Blossom-End Rot of Tomatoes. Plant Methods 2021, 17, 28. [Google Scholar] [CrossRef]
- Woltering, E.; Mensink, M.; Nijenhuis-De Vries, M.; Harchioui, N.E.; Hogeveen-Van Echtelt, E. Determining Water Loss Characteristics in Cucumber Cultivars Breeding for Post-Harvest Quality Work Package 1, Year 1; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2021. [Google Scholar]
- Yang, C.; Guo, Z.; Fernandes Barbin, D.; Dai, Z.; Watson, N.; Povey, M.; Zou, X. Hyperspectral Imaging and Deep Learning for Quality and Safety Inspection of Fruits and Vegetables: A Review. J. Agric. Food Chem. 2025, 73, 10019–10035. [Google Scholar] [CrossRef]
- Rahman, A.; Kandpal, L.; Lohumi, S.; Kim, M.; Lee, H.; Mo, C.; Cho, B.-K. Nondestructive Estimation of Moisture Content, PH and Soluble Solid Contents in Intact Tomatoes Using Hyperspectral Imaging. Appl. Sci. 2017, 7, 109. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests for Classification in Ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Abdelkader, M.F.M.; Mahmoud, M.H.; Lo’ay, A.A.; Abdein, M.A.; Metwally, K.; Ikeno, S.; Doklega, S.M.A. The Effect of Combining Post-Harvest Calcium Nanoparticles with a Salicylic Acid Treatment on Cucumber Tissue Breakdown via Enzyme Activity during Shelf Life. Molecules 2022, 27, 3687. [Google Scholar] [CrossRef]
- Hussain, N.; Azizan, A.H.; Ghazali, A.M. Impact of Minimal Processing on Quality and Shelf Life of Cucumbers (Cucumis Sativus). J. Biochem. Microbiol. Biotechnol. 2025, 13, 42–48. [Google Scholar] [CrossRef]
- Kitinoja, L. Innovative Approaches to Food Loss and Waste Issues. 2016. Available online: https://www.researchgate.net/profile/Lisa-Kitinoja/publication/303185499_Innovative_Approaches_to_Food_Loss_and_Waste_Issues/links/573881f308ae9f741b2bcbec/Innovative-Approaches-to-Food-Loss-and-Waste-Issues.pdf (accessed on 1 September 2025).
- Onwude, D.I.; Chen, G.; Eke-emezie, N.; Kabutey, A.; Khaled, A.Y.; Sturm, B. Recent Advances in Reducing Food Losses in the Supply Chain of Fresh Agricultural Produce. Processes 2020, 8, 1431. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, Pestered Outside? Differences and Similarities between Plants Growing in Controlled Conditions and in the Field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Tsaniklidis, G.; Makraki, T.; Papadimitriou, D.; Nikoloudakis, N.; Taheri-Garavand, A.; Fanourakis, D. Non-Destructive Estimation of Area and Greenness in Leaf and Seedling Scales: A Case Study in Cucumber. Agronomy 2025, 15, 2294. [Google Scholar] [CrossRef]
- Kirchgessner, N.; Hodel, M.; Studer, B.; Patocchi, A.; Broggini, G.A.L. FruitPhenoBox—A Device for Rapid and Automated Fruit Phenotyping of Small Sample Sizes. Plant Methods 2024, 20, 74. [Google Scholar] [CrossRef]
- Tjandra, A.D.; Heywood, T.; Chandrawati, R. Trigit: A Free Web Application for Rapid Colorimetric Analysis of Images. Biosens. Bioelectron. X 2023, 14, 100361. [Google Scholar] [CrossRef]
- Dai, W.; Hamasaki, T. Statistics in Medicine, 4th ed.; Riffenburgh, R.H., Gillen, D.L., Eds.; Academic Press: Oxford, UK, 2020; Volume 76. [Google Scholar]
- Keller, C.A.; Evans, M.J. Application of Random Forest Regression to the Calculation of Gas-Phase Chemistry within the GEOS-Chem Chemistry Model V10. Geosci. Model. Dev. 2019, 12, 1209–1225. [Google Scholar] [CrossRef]
- Cheng, L.; De Vos, J.; Zhao, P.; Yang, M.; Witlox, F. Examining Non-Linear Built Environment Effects on Elderly’s Walking: A Random Forest Approach. Transp. Res. D Transp. Environ. 2020, 88, 102552. [Google Scholar] [CrossRef]
- Moalemiyan, M.; Ramaswamy, H.S. Quality Retention and Shelf-Life Extension in Mediterranean Cucumbers Coated with a Pectin-Based Film. J. Food Res. 2012, 1, 159. [Google Scholar] [CrossRef]
- Manjunatha, M.; Anurag, R.K. Effect of Modified Atmosphere Packaging and Storage Conditions on Quality Characteristics of Cucumber. J. Food Sci. Technol. 2014, 51, 3470–3475. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-Z.; Sun, D.-W. Application of Hyperspectral Imaging in Food Safety Inspection and Control: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 1039–1058. [Google Scholar] [CrossRef]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and Future Perspectives of Multi-/Hyperspectral Thermal Infrared Remote Sensing for Crop Water-Stress Detection: A Review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef]
- Mertens, S.; Verbraeken, L.; Sprenger, H.; De Meyer, S.; Demuynck, K.; Cannoot, B.; Merchie, J.; De Block, J.; Vogel, J.T.; Bruce, W.; et al. Monitoring of Drought Stress and Transpiration Rate Using Proximal Thermal and Hyperspectral Imaging in an Indoor Automated Plant Phenotyping Platform. Plant Methods 2023, 19, 132. [Google Scholar] [CrossRef]
- Vásquez, R.A.R.; Heenkenda, M.K.; Nelson, R.; Segura Serrano, L. Developing a New Vegetation Index Using Cyan, Orange, and Near Infrared Bands to Analyze Soybean Growth Dynamics. Remote Sens. 2023, 15, 2888. [Google Scholar] [CrossRef]
- Suárez, L.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Pérez-Priego, O.; Miller, J.R.; Jiménez-Muñoz, J.C.; Sobrino, J. Assessing Canopy PRI for Water Stress Detection with Diurnal Airborne Imagery. Remote Sens. Environ. 2008, 112, 560–575. [Google Scholar] [CrossRef]
- Zhang, C.; Filella, I.; Liu, D.; Ogaya, R.; Llusià, J.; Asensio, D.; Peñuelas, J. Photochemical Reflectance Index (PRI) for Detecting Responses of Diurnal and Seasonal Photosynthetic Activity to Experimental Drought and Warming in a Mediterranean Shrubland. Remote Sens. 2017, 9, 1189. [Google Scholar] [CrossRef]
- Sui, X.; Shan, N.; Hu, L.; Zhang, C.; Yu, C.; Ren, H.; Turgeon, R.; Zhang, Z. The Complex Character of Photosynthesis in Cucumber Fruit. J. Exp. Bot. 2017, 68, 1625–1637. [Google Scholar] [CrossRef]
- Thompson, R.L.; Fleming, H.P.; Hamann, D.D.; Monroe, R.J. Method for Determination of Firmness in Cucumber Slices 1. J. Texture Stud. 1982, 13, 311–324. [Google Scholar] [CrossRef]
- Fanourakis, D.; Papadakis, V.M.; Machado, M.; Psyllakis, E.; Nektarios, P.A. Non-invasive Leaf Hydration Status Determination through Convolutional Neural Networks Based on Multispectral Images in Chrysanthemum. Plant Growth Regul. 2024, 102, 485–496. [Google Scholar] [CrossRef]
- Falcioni, R.; Gonçalves, J.V.F.; de Oliveira, K.M.; de Oliveira, C.A.; Reis, A.S.; Crusiol, L.G.T.; Furlanetto, R.H.; Antunes, W.C.; Cezar, E.; de Oliveira, R.B.; et al. Chemometric Analysis for the Prediction of Biochemical Compounds in Leaves Using UV-VIS-NIR-SWIR Hyperspectroscopy. Plants 2023, 12, 3424. [Google Scholar] [CrossRef]
- Amoriello, T.; Ciorba, R.; Ruggiero, G.; Masciola, F.; Scutaru, D.; Ciccoritti, R. Vis/NIR Spectroscopy and Vis/NIR Hyperspectral Imaging for Non-Destructive Monitoring of Apricot Fruit Internal Quality with Machine Learning. Foods 2025, 14, 196. [Google Scholar] [CrossRef]
- Rett-Cadman, S.; Colle, M.; Mansfeld, B.; Barry, C.S.; Wang, Y.; Weng, Y.; Gao, L.; Fei, Z.; Grumet, R. QTL and Transcriptomic Analyses Implicate Cuticle Transcription Factor SHINE as a Source of Natural Variation for Epidermal Traits in Cucumber Fruit. Front. Plant Sci. 2019, 10, 1536. [Google Scholar] [CrossRef]
- Liu, X.; Ge, X.; An, J.; Liu, X.; Ren, H. CsCER6 and CsCER7 Influence Fruit Glossiness by Regulating Fruit Cuticular Wax Accumulation in Cucumber. Int. J. Mol. Sci. 2023, 24, 1135. [Google Scholar] [CrossRef]
- Tovar, J.C.; Hoyer, J.S.; Lin, A.; Tielking, A.; Callen, S.T.; Elizabeth Castillo, S.; Miller, M.; Tessman, M.; Fahlgren, N.; Carrington, J.C.; et al. Raspberry Pi–Powered Imaging for Plant Phenotyping. Appl. Plant Sci. 2018, 6, e1031. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.B.; Davies, M.; Hobbs, M.J.; Pering, T.D.; McGonigle, A.J.S.; Willmott, J.R. High-Resolution Hyperspectral Imaging Using Low-Cost Components: Application within Environmental Monitoring Scenarios. Sensors 2022, 22, 4652. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An Artificial Neural Network Approach for Non-Invasive Estimation of Essential Oil Content and Composition through Considering Drying Processing Factors: A Case Study in Mentha Aquatica. Ind. Crops Prod. 2021, 171, 113985. [Google Scholar] [CrossRef]
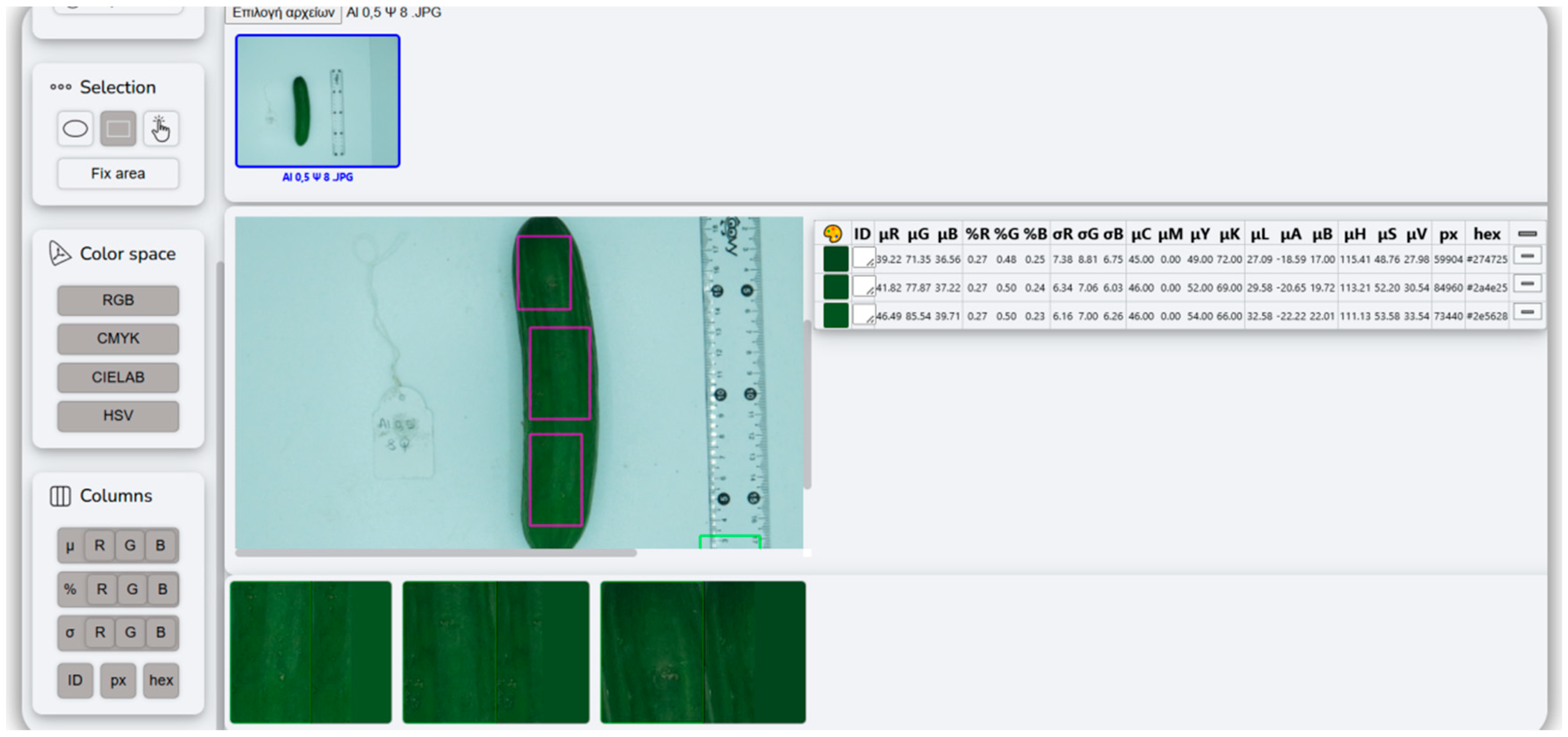

| Color Parameter | Formula | Range (Unit) |
|---|---|---|
| red (R) 1 | amount of red | 0–255 (−) |
| green (G) 1 | amount of green | |
| blue (B) 1 | amount of blue | |
| %R | 0≤ ≤100 (%) | |
| %G | ||
| %B | ||
| cyan (C) 2 | 0–100 (%) | |
| magenta (M) 2 | ||
| yellow (Y) 2 | ||
| black (K) 2 | ||
| lightness (L*) 3 | 0 specifies black, 100 specifies white | |
| red/green (a*) 3 | amount of red or green tones | −110–110 (−) |
| yellow/blue (b*) 3 | amount of yellow or blue tones | |
| hue (H) 4 | location on the color wheel | 0–360 (°) |
| saturation (S) 4 | vividness or dullness | 0–255 (−) |
| value (V) 4 | amount of white |
| Region | Model | a (Constant) | b (Fitted Coefficient) | R2 | RMSE | BC lower | BC Upper | |
|---|---|---|---|---|---|---|---|---|
| Neck (stalk region)–stem end | 1 | RWC = a + b μR | −1.117 | 129.078 | 0.284 | 14.057 | 0.259 | 0.309 |
| 2 | RWC = a + b μG | −0.498 | 119.912 | 0.099 | 15.764 | 0.081 | 0.123 | |
| 3 | RWC = a + b μB | −0.605 | 104.133 | 0.021 | 16.442 | 0.013 | 0.028 | |
| 4 | RWC = a + b%R | −693.185 | 272.421 | 0.485 | 11.923 | 0.464 | 0.504 | |
| 5 | RWC = a + b%G | 340.053 | −84.599 | 0.104 | 15.721 | 0.087 | 0.128 | |
| 6 | RWC = a + b%B | 219.924 | 31.627 | 0.087 | 15.876 | 0.0725 | 0.105 | |
| 7 | RWC = a + b μC | 3.228 | −61.675 | 0.638 | 9.993 | 0.620 | 0.653 | |
| 8 | RWC = a + b μY | 0.021 | 105.526 | 0.021 | 16.438 | 0.013 | 0.032 | |
| 9 | RWC = a + b μK | 1.267 | −6.879 | 0.099 | 15.766 | 0.081 | 0.120 | |
| 10 | RWC = a + b μL | −1.347 | 120.893 | 0.115 | 15.631 | 0.095 | 0.136 | |
| 11 | RWC = a + b μa* | −0.495 | 73.511 | 0.005 | 16.57 | 0.001 | 0.012 | |
| 12 | RWC = a + b μb* | −1.194 | 105.978 | 0.099 | 15.769 | 0.081 | 0.120 | |
| 13 | RWC = a + b μH | 1.356 | −68.146 | 0.305 | 13.848 | 0.281 | 0.329 | |
| 14 | RWC = a + b μS | −0.467 | 107.569 | 0.023 | 16.420 | 0.014 | 0.034 | |
| 15 | RWC = a + b μV | −1.270 | 119.910 | 0.099 | 15.764 | 0.079 | 0.118 | |
| Mid-region | 1 | RWC = a + b μR | −1.243 | 140.759 | 0.373 | 13.159 | 0.350 | 0.391 |
| 2 | RWC = a + b μG | −0.664 | 137.923 | 0.177 | 15.065 | 0.157 | 0.199 | |
| 3 | RWC = a + b μB | −0.848 | 115.656 | 0.042 | 16.259 | 0.031 | 0.053 | |
| 4 | RWC = a + b%R | −752.269 | 290.521 | 0.503 | 11.709 | 0.481 | 0.522 | |
| 5 | RWC = a + b%G | 314.472 | −72.086 | 0.073 | 15.993 | 0.060 | 0.088 | |
| 6 | RWC = a + b%B | 275.875 | 19.354 | 0.121 | 15.576 | 0.116 | 0.159 | |
| 7 | RWC = a + b μC | 3.438 | −69.830 | 0.649 | 9.846 | 0.633 | 0.664 | |
| 8 | RWC = a + b μY | −0.670 | 118.619 | 0.044 | 16.244 | 0.031 | 0.056 | |
| 9 | RWC = a + b μK | 1.684 | −30.723 | 0.177 | 15.071 | 0.157 | 0.199 | |
| 10 | RWC = a + b μL | −1.784 | 139.169 | 0.196 | 14.891 | 0.176 | 0.219 | |
| 11 | RWC = a + b μa* | 0.259 | 88.488 | 0.001 | 16.603 | 0.001 | 0.004 | |
| 12 | RWC = a + b μb* | −1.517 | 115.466 | 0.159 | 15.231 | 0.141 | 0.181 | |
| 13 | RWC = a + b μH | 1.541 | −86.929 | 0.340 | 13.492 | 0.319 | 0.365 | |
| 14 | RWC = a + b μS | −0.696 | 120.006 | 0.045 | 16.232 | 0.032 | 0.057 | |
| 15 | RWC = a + b μV | −1.692 | 137.921 | 0.178 | 15.065 | 0.155 | 0.197 | |
| Blossom region–Blossom end | 1 | RWC = a + b μR | −1.066 | 137.436 | 0.402 | 12.847 | 0.379 | 0.421 |
| 2 | RWC = a + b μG | −0.659 | 142.138 | 0.235 | 14.534 | 0.216 | 0.258 | |
| 3 | RWC = a + b μB | −0.511 | 103.418 | 0.017 | 16.471 | 0.011 | 0.025 | |
| 4 | RWC = a + b%R | −670.327 | 271.373 | 0.507 | 11.665 | 0.486 | 0.527 | |
| 5 | RWC = a + b%G | 235.327 | −33.799 | 0.037 | 16.301 | 0.028 | 0.048 | |
| 6 | RWC = a + b%B | 319.381 | 11.989 | 0.189 | 14.959 | 0.169 | 0.209 | |
| 7 | RWC = a + b μC | 3.153 | −55.113 | 0.617 | 10.286 | 0.599 | 0.631 | |
| 8 | RWC = a + b μY | −0.988 | 137.515 | 0.102 | 15.743 | 0.085 | 0.119 | |
| 9 | RWC = a + b μK | 1.676 | −25.555 | 0.234 | 14.535 | 0.213 | 0.255 | |
| 10 | RWC = a + b μL | −1.767 | 143.570 | 0.252 | 14.368 | 0.228 | 0.279 | |
| 11 | RWC = a + b μa* | 0.810 | 101.164 | 0.012 | 16.511 | 0.007 | 0.019 | |
| 12 | RWC = a + b μb* | −1.568 | 120.50 | 0.233 | 14.547 | 0.211 | 0.255 | |
| 13 | RWC = a + b μH | 1.651 | −94.873 | 0.396 | 12.907 | 0.371 | 0.423 | |
| 14 | RWC = a + b μS | −1.004 | 138.380 | 0.104 | 15.731 | 0.086 | 0.122 | |
| 15 | RWC = a + b μV | 142.138 | −1.679 | 0.235 | 14.534 | 0.213 | 0.257 | |
| Whole fruit | 1 | RWC = a + b μR | 141.329 | −1.261 | 0.392 | 12.961 | 0.369 | 0.410 |
| 2 | RWC = a + b μG | 139.624 | −0.689 | 0.189 | 14.959 | 0.171 | 0.211 | |
| 3 | RWC = a + b μB | −0.793 | 112.964 | 0.031 | 16.355 | 0.022 | 0.039 | |
| 4 | RWC = a + b%R | −777.716 | 298.199 | 0.551 | 11.134 | 0.533 | 0.571 | |
| 5 | RWC = a + b%G | 385.268 | −107.382 | 0.090 | 15.851 | 0.076 | 0.106 | |
| 6 | RWC = a + b%B | 315.219 | 10.845 | 0.149 | 15.319 | 0.131 | 0.171 | |
| 7 | RWC = a + b μC | 3.597 | -76.537 | 0.698 | 9.134 | 0.684 | 0.711 | |
| 8 | RWC = a + b μY | −0.813 | 126.561 | 0.059 | 16.116 | 0.046 | 0.074 | |
| 9 | RWC = a + b μK | 1.755 | −35.931 | 0.189 | 14.959 | 0.169 | 0.214 | |
| 10 | RWC = a + b μL | −1.842 | 140.698 | 0.208 | 14.789 | 0.189 | 0.229 | |
| 11 | RWC = a + b μa* | 0.219 | 87.604 | 0.001 | 16.606 | 0.001 | 0.004 | |
| 12 | RWC = a + b μb* | −1.631 | 118.078 | 0.183 | 15.016 | 0.165 | 0.202 | |
| 13 | RWC = a + b μH | 1.648 | −98.003 | 0.377 | 13.114 | 0.354 | 0.401 | |
| 14 | RWC = a + b μS | −0.851 | 128.627 | 0.062 | 16.092 | 0.048 | 0.077 | |
| 15 | RWC = a + b μV | −1.756 | 139.623 | 0.189 | 14.959 | 0.167 | 0.209 | |
| Statistical Index | Neck (Stalk Region)–Stem End | Mid-Region | Blossom Region–Blossom End | Whole Fruit Average |
|---|---|---|---|---|
| R | 0.929 | 0.927 | 0.925 | 0.941 |
| R2 (95% CI) | 0.863 (0.851–0.874) | 0.859 (0.846–0.871) | 0.855 (0.842–0.868) | 0.886 (0.875–0.897) |
| MSE | 39.597 | 39.074 | 40.084 | 33.13 |
| RMSE (95% CI) | 6.293 (6.201–6.385) | 6.251 (6.159–6.343) | 6.331 (6.239–6.423) | 5.756 (5.664–5.848) |
| MAE | 4.385 | 4.321 | 4.212 | 3.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makraki, T.; Tsaniklidis, G.; Papadimitriou, D.M.; Taheri-Garavand, A.; Fanourakis, D. Non-Destructive Monitoring of Postharvest Hydration in Cucumber Fruit Using Visible-Light Color Analysis and Machine-Learning Models. Horticulturae 2025, 11, 1283. https://doi.org/10.3390/horticulturae11111283
Makraki T, Tsaniklidis G, Papadimitriou DM, Taheri-Garavand A, Fanourakis D. Non-Destructive Monitoring of Postharvest Hydration in Cucumber Fruit Using Visible-Light Color Analysis and Machine-Learning Models. Horticulturae. 2025; 11(11):1283. https://doi.org/10.3390/horticulturae11111283
Chicago/Turabian StyleMakraki, Theodora, Georgios Tsaniklidis, Dimitrios M. Papadimitriou, Amin Taheri-Garavand, and Dimitrios Fanourakis. 2025. "Non-Destructive Monitoring of Postharvest Hydration in Cucumber Fruit Using Visible-Light Color Analysis and Machine-Learning Models" Horticulturae 11, no. 11: 1283. https://doi.org/10.3390/horticulturae11111283
APA StyleMakraki, T., Tsaniklidis, G., Papadimitriou, D. M., Taheri-Garavand, A., & Fanourakis, D. (2025). Non-Destructive Monitoring of Postharvest Hydration in Cucumber Fruit Using Visible-Light Color Analysis and Machine-Learning Models. Horticulturae, 11(11), 1283. https://doi.org/10.3390/horticulturae11111283










