1. Introduction
Toona sinensis (Juss.) M. Roem. [synonym:
Cedrela sinensis Juss.; Xiangchun in Chinese], a deciduous woody plant of the family Meliaceae, commonly known as Chinese toon or Chinese mahogany, is native to eastern and southeastern Asia and has been cultivated for over 2000 years for use as a vegetable in China and Malaysia and as animal fodder in India [
1,
2]. Young leaves and buds of plants are rich in flavonoids, terpenoids, and phenylpropanoids, which offer numerous health benefits, including antiviral, antioxidant, anticancer, and anti-inflammatory properties [
2]. In recent years,
T. sinensis has attracted increasing attention because of its value in nutritional composition, medicinal applications, and advances in storage, preservation, gene- and omics-based studies [
3,
4]. Fresh buds of
T. sinensis are highly perishable because of their high water content (up to 90%), active metabolism, and susceptibility to postharvest deterioration, leading to a short shelf life. Therefore, effective preservation strategies are essential to extend shelf life and maintain quality.
Various preservation approaches have been explored, including physical, chemical, and biological technologies [
1,
5,
6,
7]. Temperature-controlled preservation is the cornerstone of
T. sinensis storage, with low-temperature storage at approximately 0 °C significantly slowing metabolic activity and allowing buds to be preserved for nearly three days [
1]. Low-temperature storage, when combined with high humidity reduces nutrient loss, enzymatic browning and maintains sensory and nutritional quality [
8,
9]. Quick freezing provides an even longer preservation period of up to one year by minimizing tissue damage while retaining the natural color, aroma, and flavor of the buds [
10]. Despite the various preservation methods that have been developed, most studies have reported limited storage durations for fresh leaves and buds, with specialized techniques such as modified atmosphere packaging achieving a shelf life of up to 25 days [
7,
11]. Furthermore, although low-temperature preservation can prolong shelf life and maintain the commercial and nutritional integrity of
T. sinensis, it can also cause chilling injuries in some cases [
7,
11].
Chemical preservation methods can also extend the storage life of food. Ethylene adsorbents delay post-ripening processes, helping to maintain freshness [
5,
12]. In addition, senescence-inhibiting regulators, such as 6-benzylaminopurine, are highly effective at maintaining bud color, fragrance, and market value for over 45 days [
1,
2]. Although these approaches are effective, concerns regarding food safety and environmental impact necessitate careful regulation and optimization for consumer health. Other methods, including dehydration (e.g., vacuum freeze-drying, which can extend shelf life to two years) [
13] and modified atmosphere packaging (which reduces respiration and defoliation) [
11,
14], have also been effective. Recently, biological preservation has been achieved using natural compounds, such as exogenous betaine [
2,
5]. However, compared to low-temperature and chemical methods, biological preservation is less standardized for large-scale application and cannot easily deal with postharvest challenges, including rapid respiration, water loss, and decay [
5]. Various preservation methods have been developed to overcome these issues. Chlorine dioxide treatment at moderate concentrations (0.4–0.8 mg/L) has shown promise in maintaining sensory quality, inhibiting weight loss, and slowing nutrient depletion [
15].
Salicylic acid (SA), a well-characterized plant hormone, also functions as a preservative and has been widely reported to delay postharvest senescence in horticultural products, such as fruits, leaves, and cut flowers [
16,
17]. When combined with calcium chloride, SA outperformed peppermint oil in preserving fresh mint by preventing chlorophyll degradation, water loss, and membrane damage, thus extending the shelf life from 3 days up to 6 days [
18]. SA concentrations ranging from 100 μM to 1500 μM have been effective in leaf preservation, with 100 μM maintaining photosynthetic function in strawberry plants under stress and 1500 μM delaying senescence in Chinese flowering cabbage [
19,
20]. Even though highly effective, cold-chain systems require high energy consumption, limiting their large-scale application [
6,
7]. Although a previous study reported the effects of low-temperature storage with SA, the possibility of preserving
T. sinensis at room temperature has not been explored [
6]. Therefore, in this study, we investigated the effects of 250 μM exogenous SA on the postharvest quality and physiological characteristics of
T. sinensis buds during room-temperature storage (20 °C) over 7 d, compared to low-temperature storage (4 °C) and untreated controls, by characterizing key parameters: visual appearance, water loss, chlorophyll and carotenoid content, total soluble solids, soluble sugars, reactive oxygen species (ROS) production, antioxidant enzyme activities, non-enzymatic antioxidants such as vitamin C (VC), polyphenols, and flavonoids, oxidative damage indicators, amino acid content, and nitrogen metabolism indicators. The findings provide a comprehensive assessment of the potential of SA as an energy-efficient alternative to low-temperature storage for maintaining the commercial value and extending the shelf life of
T. sinensis.
2. Materials and Methods
2.1. Plant Materials and Treatments
Fresh shoots of
T. sinensis cv. ‘Deinosuchus’ were harvested on 5 April 2024, from a commercial plantation in Taihe County, Anhui Province, China (33°12′17″ N, 115°33′55″ E). Shoots exhibiting uniform color and size and free from pests, diseases, or mechanical damage were selected as experimental materials. The harvested shoots were divided into three treatment groups: (1) low-temperature storage (4 °C); shoots were placed in food preservation bags and stored at 4 °C. (2) Room temperature storage (20 °C); shoots were placed in food preservation bags and stored at 20 °C. (3) Room temperature storage with SA treatment (20 °C + SA): shoots were immersed in a 250 μmol/L SA solution (Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China) for 30 min, drained, and placed in food preservation bags for storage at 20 °C. The 250 μM dose was selected as a mid-range concentration to balance efficacy and potential phytotoxicity for
T. sinensis buds based on previous studies [
19,
21]. All treatments were maintained at a relative humidity of 85 ± 5%. Each treatment included three biological replicates consisting of five shoots. Phenotypic characteristics were observed on days 1, 3, 5, and 7 of the storage periods. Weight loss and total soluble solid (TSS) content were measured at each time point. Following these tests, samples were rapidly frozen in liquid nitrogen and stored at −80 °C for subsequent physiological and biochemical analyses.
2.2. Determination of Endogenous SA Content
Endogenous SA content was quantified using high-performance liquid chromatography (HPLC) according to a previously described method [
22], with minor modifications. Briefly, 0.2 g of frozen
T. sinensis sample was homogenized in 90% (
v/
v) methanol (1.5 mL) on ice. The homogenate was then extracted in the dark at 4 °C for 12 h, followed by centrifugation at 8000 rpm for 20 min at 4 °C. The supernatant was collected in a clean 10 mL centrifuge tube and stored at 4 °C for 2 h. The resulting pellet was re-suspended in 1.0 mL of 90% methanol and subjected to a second extraction in the dark at 4 °C for 2 h. After centrifugation at 8000 rpm for 20 min at 4 °C, the supernatants were combined and evaporated to approximately 0.3 mL of aqueous solution. Subsequently, 10 mL of 1 mg/mL trichloroacetic acid was added to each concentrate. The mixture was vortexed vigorously and extracted twice with 1.0 mL of a cyclohexane-ethyl acetate mixture (1:1,
v/
v). The upper organic phases from both extractions were combined in a clean tube and evaporated to dryness. The residue was re-dissolved in methanol (0.5 mL) and filtered to obtain the free SA fraction. To the remaining aqueous phase, 0.5 mL of 2 M hydrochloric acid was added. After vigorous shaking, the mixture was hydrolyzed in a water bath at 80 °C for 1 h. After cooling to room temperature, the hydrolyzed solution was extracted twice using a cyclohexane-ethyl acetate mixture (1:1,
v/
v). The combined organic phases were dried and re-dissolved in methanol (0.5 mL), followed by filtration to yield the conjugated SA fraction. HPLC (1200 series HPLC system; Agilent Technologies Inc., Palo Alto, CA, USA) analysis was performed using a Sepax HP-C18 column (250 mm × 4.6 mm, 5 μm) (Sepax Technologies, Inc., Newark, DE, USA). The column temperature was maintained at 25 °C. A mobile phase consisting of a 65:35 (
v/
v) mixture of acetonitrile and methanol was used for isocratic elution at a flow rate of 1.0 mL/min. The injection volume was 20 μL, and SA was detected at a wavelength of 280 nm. HPLC reagents and standards were purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.3. Determination of Water Loss
The water loss rate of
T. sinensis shoots was determined using the gravimetric method, as described previously [
23]. Fresh shoots were harvested, and their initial fresh weight (W
i) was recorded. The shoots were then placed under controlled conditions, and their weight was measured at regular intervals to determine the final weight (W
f) after a specified period. The water loss rate was calculated as the percentage of weight loss relative to the initial weight using the formula:
where W
i is the initial fresh weight (g) and W
f is the final weight (g) of the shoots.
2.4. Determination of Chlorophyll and Carotenoid Content
Chlorophyll and carotenoid were quantified according to previously described methods [
24,
25,
26], with minor modifications. Briefly, a mixed extraction solution was prepared by blending acetone, absolute ethanol, and distilled water at a volume ratio of 4.5:4.5:1. Approximately 0.5 g of
T. sinensis tissue was weighed, finely chopped, and transferred to a volumetric flask. Next, 10 mL of the extraction solution was added. The samples were extracted in the dark for 3–12 h until the tissues turned completely white. The mixture was subsequently filtered, and the absorbance of the filtrate was measured at 663, 645, and 470 nm using a spectrophotometer (UV-1800; Shimadzu Corporation, Tokyo, Japan). All reagents were purchased from Shanghai China National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China).
2.5. Determination of TSS Content
The TSS content was determined using a portable refractometer (model: DR-10; Ebro Electronic GmbH, Ingolsadt, Germany). Briefly, three T. sinensis leaves were randomly selected from each treatment group and homogenized using a mortar and pestle. The homogenate was then filtered through a cheesecloth to obtain a clear extract. The TSS content of the filtrate was immediately measured using a refractometer and expressed as a percentage (%).
2.6. Determination of Soluble Sugar and Malondialdehyde Content
Soluble sugar and malondialdehyde (MDA) were quantified using the thiobarbituric acid reaction method. Briefly, 0.2 g of T. sinensis sample was homogenized in 1.0 mL of 10% (w/v) trichloroacetic acid. An additional 4.0 mL of trichloroacetic acid was added, and homogenization continued. The homogenate was transferred to a centrifuge tube and centrifuged at 4000 rpm for 10 min. Then, 2.0 mL of the supernatant was collected. A blank control was prepared by adding 2.0 mL of distilled water instead of the supernatant. To each tube, 2.0 mL of 0.6% (w/v) thiobarbituric acid solution was added. After thorough mixing, the mixture was boiled in a water bath for 15 min. After cooling to room temperature, the tubes were centrifuged again to remove any precipitates. The absorbance of the resulting supernatant was measured at 450 nm to determine the soluble sugar content. For MDA quantification, absorbance was measured at 450, 532, and 600 nm using a spectrophotometer (UV-1800, Shimadzu Corporation). All reagents were purchased from Shanghai China National Pharmaceutical Group Chemical Reagent Co., Ltd. The soluble sugar content is expressed as milligram sucrose equivalents per gram fresh weight (mg g−1 FW). MDA content is expressed as micromoles per gram of fresh weight (μmol g−1 FW).
2.7. Determination of Superoxide Anion (O2•−) Content and Superoxide Dismutase and Peroxidase Activities
Approximately 0.2 g of T. sinensis leaf tissue was homogenized in 2.0 mL of ice-cold extraction buffer (0.05 mol/L phosphate buffer, pH 7.8) using a pre-chilled mortar and pestle. The homogenate was transferred to a 5 mL centrifuge tube, and the mortar was rinsed with an additional 3.0 mL of phosphate buffer, which was combined with the homogenate. The mixture was centrifuged at 12,000 rpm for 10 min at 4 °C. The resulting supernatant was collected and used for assays of O2•− content, superoxide dismutase (SOD) activity, and peroxidase (POD) activity.
2.7.1. O2•− Content
The O
2•
− content was determined using the hydroxylamine oxidation method [
25], with minor modifications. Briefly, four test tubes were prepared: one tube contained 1.0 mL of distilled water (blank control), and the other three tubes each contained 1.0 mL of the extracted supernatant. To each tube, 0.5 mL of phosphate buffer (pH 7.8) and 0.5 mL of 10 mmol/L hydroxylamine hydrochloride were added. The mixtures were incubated at 37 °C for 15 min. Subsequently, 1.0 mL of 17 mmol/L sulfanilic acid and 1.0 mL of 7 mmol/L α-naphthylamine were added to each tube and mixed thoroughly. The absorbance was measured at 530 nm using a spectrophotometer (UV-1800, Shimadzu Corporation) against a blank control. All reagents were purchased from Shanghai China National Pharmaceutical Group Chemical Reagent Co., Ltd.
2.7.2. SOD Activity
SOD activity was assayed by measuring its ability to inhibit the photochemical reduction of nitrotetrazolium blue chloride at 560 nm in the presence of riboflavin and l-methionine, using a spectrophotometer (UV-1800, Shimadzu Corporation). One unit of SOD activity (U) was defined as the amount of enzyme required to cause 50% inhibition of nitrotetrazolium blue chloride reduction per gram of fresh weight.
2.7.3. POD Activity
The POD activity was determined using the guaiacol method [
26]. The reaction mixture consisted of 0.05 M phosphate buffer (pH 6.4), guaiacol, and the enzyme extract. The reaction was initiated by adding the enzyme extract, and the increase in absorbance at 470 nm was immediately recorded at 20 s intervals for 5 min using a spectrophotometer (UV-1800; Shimadzu Corporation). One unit of POD activity (U) was defined as the amount of enzyme that caused a change in absorbance of 0.01 per minute under the assay conditions.
2.8. Determination of Hydrogen Peroxide (H2O2) Content
The H
2O
2 content was determined according to the method described in [
27] with minor modifications. Briefly, 0.2 g of
T. sinensis shoots was homogenized in 5.0 mL of ice-cold acetone using a pre-chilled mortar and pestle. The homogenate was centrifuged at 10,000 rpm for 15 min at 4 °C. Then, 0.5 mL of supernatant was mixed with 0.5 mL distilled water, 0.2 mL of 5% (
w/
v) titanium sulfate, and 0.2 mL concentrated ammonia solution. The mixture was incubated for 10 min to form a precipitate, which was dissolved by adding 3.0 mL of 2 mmol·L
−1 sulfuric acid. The absorbance was measured at 415 nm using a spectrophotometer (UV-1800, Shimadzu Corporation,). H
2O
2 content was calculated from a standard curve and expressed as μmol·g
−1 FW. Reagents (H
2O
2, sulfuric acid, titanium sulfate) were purchased from Shanghai CNPC Reagent Co., Ltd. (Shanghai, China).
2.9. Determination of Hydroxyl Radical (•OH) and DPPH Radical Scavenging Rates
Approximately 0.2 g of T. sinensis sample was homogenized in 6 mL of 70% (v/v) ethanol. The homogenate was extracted at 4 °C for 2 h, followed by centrifugation at 10,000 rpm for 10 min at 4 °C. The supernatant was used for hydroxyl radical (•OH) and DPPH radical scavenging rate assays.
2.9.1. Hydroxyl Radical (•OH) Scavenging Rate
The •OH scavenging rate was measured according to [
28] with minor modifications. Briefly, 0.02 mL of sample supernatant was mixed with 0.98 mL distilled water, 1.0 mL of 9 mmol·L
−1 FeSO
4, 1.0 mL of 9 mmol·L
−1 salicylic acid, and 1.0 mL of 8.8 mmol·L
−1 H
2O
2. The mixture was incubated at 37 °C for 30 min, and absorbance was measured at 510 nm (UV-1800, Shimadzu Corporation). The •OH scavenging rate was calculated using the formula:
where
2.9.2. DPPH Radical Scavenging Rate
The DPPH scavenging rate was measured according to [
29].
With minor modifications. Briefly, 0.2 mL supernatant was mixed with 0.9 mL 70% (
v/
v) ethanol, and 3.0 mL of 0.1 mmol·L
−1 DPPH in ethanol (total 4.0 mL). A control was prepared by replacing the supernatant with 70% ethanol. The mixture was incubated in the dark at 25 °C for 30 min. Absorbance was measured at 517 nm (UV-1800, Shimadzu Corporation). The scavenging rate was calculated as:
2.10. Determination of Total Soluble Protein Content
The total soluble protein content was quantified using the Bradford method [
30]. For each biological replicate, 0.2 g of
T. sinensis tissue was homogenized with 10 mL of ice-cold 0.1 M sodium phosphate buffer (pH 7.0) containing 2% (
w/
v) Polyvinylpolypyrrolidone (PVPP). The homogenate was centrifuged at 12,000 rpm for 30 min at 4 °C. The supernatant was used for protein quantification. Protein concentration was determined by mixing 0.1 mL supernatant with 5.0 mL Bradford reagent and measuring absorbance at 595 nm (UV-1800, Shimadzu Corporation, Tokyo, Japan), with bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA) as standard. Results were expressed as mg·g
−1 FW.
2.11. Determination of Ascorbate Peroxidase (APX) Activity
APX activity was determined according to [
31] with minor modifications. Briefly, 0.1 g of
T. sinensis tissue was homogenized in 3.0 mL of ice-cold 50 mM phosphate buffer (pH 7.5) containing 0.1 mM EDTA, 1 mM ascorbic acid, and 2% (
w/
v) PVPP. The homogenate was centrifuged at 12,000 rpm for 20 min at 4 °C. Then, 0.01 mL supernatant was added to 2.69 mL of 50 mM phosphate buffer (pH 7.5) containing 0.1 mM EDTA and 0.5 mM ascorbic acid, plus 0.30 mL of 10 mM H
2O
2 (total 3.0 mL). The decrease in absorbance at 290 nm was recorded at 30 s intervals for 5 min (UV-1800). One unit (U) was defined as the amount causing ΔA = 0.01/min. Specific activity was expressed as U·mg
−1 protein. Protein determined by Bradford method [
32] with BSA (Sigma) as standard.
2.12. Determination of Vitamin C Content
Vitamin C content was determined using the molybdenum blue colorimetric method. Briefly, 0.2 g of T. sinensis sample was homogenized in 10 mL oxalic acid-EDTA solution (2% oxalic acid, 0.1% EDTA). The homogenate was filtered, and 6.0 mL filtrate was collected. Subsequently, 2.0 mL metaphosphoric acid-acetic acid solution, 2.0 mL 5% (v/v) sulfuric acid, and 4.0 mL ammonium molybdate solution were added and vortexed. Volume adjusted to 15 mL with distilled water. Absorbance measured at 723 nm (UV-1800, Shimadzu Corporation). Reagents from Aladdin. Standard curve prepared using L-ascorbic acid; content expressed as μg·g−1 FW.
2.13. Determination of Phenylalanine Ammonia-Lyase (PAL) Activity
PAL activity was determined according to a method described in [
32] with minor modifications. Briefly, 0.1 g of
T. sinensis tissue was homogenized in 3.0 mL of ice-cold 50 mM borate buffer (pH 8.5) containing 5 mM β-mercaptoethanol, 2 mM EDTA, and 4% (
w/
v) PVPP. The homogenate was centrifuged at 12,000 rpm for 20 min at 4 °C. Then, 0.05 mL supernatant was mixed with 0.55 mL of 20 mM L-phenylalanine (total 0.6 mL). The mixture was incubated at 37 °C for 1 h, and the reaction was terminated by adding 0.1 mL of 6 mol·L
−1 HCl (final 0.7 mL). Absorbance was measured at 290 nm (UV-1800, Shimadzu Corporation). A control used boiled supernatant. One unit (U) was defined as the amount causing ΔA = 0.01/min. Reagents from Aladdin.
2.14. Determination of Polyphenol Content
Total polyphenol content was determined using the Folin–Ciocalteu method. Briefly, 0.2 g of T. sinensis tissue was extracted with 10 mL 60% (v/v) ethanol at 40 °C for 25 min in water bath. 4.0 mL filtrate transferred to flask. Then, 1.0 mL Folin–Ciocalteu reagent (Aladdin) added, mixed. After 5 min, 4.0 mL 7.5% (w/v) sodium carbonate added; volume to 25 mL with water. Incubated dark, room temp 60 min. Absorbance measured at 765 nm (UV-1800, Shimadzu Corporation). Content calculated from gallic acid standard curve, expressed as μg·g−1 FW.
2.15. Determination of Polyphenol Oxidase (PPO) Activity
PPO activity was determined according to the method in [
31] with minor modifications. Briefly, 0.1 g of
T. sinensis leaf tissue was homogenized in 5 mL of ice-cold 0.05 mol/L phosphate buffer (pH 6.8) using a pre-chilled mortar and pestle. The homogenate was centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant served as the crude enzyme extract. The reaction was initiated by adding 0.1 mL supernatant to 4 mL 0.02 mol/L catechol solution in the same buffer (pH 6.8). The mixture was vortexed and transferred to a cuvette. The increase in absorbance at 420 nm was recorded at 20 s intervals for 3 min using a spectrophotometer (UV-1800). One unit of PPO activity (U) was defined as the amount of enzyme causing a change in absorbance of 0.001 per minute under the assay conditions. PPO specific activity was expressed as U mg
−1 protein.
2.16. Determination of Total Flavonoid Content
The total flavonoid content was quantified according to the method of our previous work [
33]. Briefly, 0.1 g of
T. sinensis tissue was homogenized in 10 mL water, filtered. Then, 4.0 mL filtrate + 1.0 mL water. Next, 0.2 mL 5% (
w/
v) sodium nitrite added, vortexed, 6 min. Then, 0.2 mL 10% (
w/
v) aluminum nitrate added, vortexed, 6 min. Then, 1.0 mL 4% (
w/
v) sodium hydroxide added; volume adjusted to 10 mL with 60% (
v/
v) ethanol. Mixture vortexed, incubated at room temperature 15 min. Absorbance measured at 510 nm (UV-1800, Shimadzu Corporation). Reagents were sourced from Shanghai CNPC Reagent Co., Ltd. (Shanghai). Protein content was calculated from a glutamic acid standard curve and expressed as mg·g
−1 fresh weight (FW).
2.17. Total Free Amino Acid Content
Total free amino acid content was determined using the ninhydrin chromogenic method [
34]. Briefly, 0.2 g of
T. sinensis tissue was ground in 25 mL flask. 15 mL 70% ethanol added, ultrasonicated 60 min. Filtered, residue washed with 70% ethanol, combined, diluted to 25 mL. Then, 1.0 mL sample + 0.5 mL 2% ninhydrin + 1.0 mL 0.2 M phosphate buffer (pH 8.0). Heated boiling 15 min, cooled in ice to room temp. Volume adjusted to 10 mL with water. Absorbance measured at 401 nm (UV-1800, Shimadzu Corporation). Reagents were sourced from Shanghai CNPC Reagent Co., Ltd. (Shanghai). Protein content was calculated from a glutamic acid standard curve and expressed as mg·g
−1 fresh weight (FW).
2.18. Amino Acid Composition
Nine amino acids quantified by pre-column derivatization [
35]. Separated on Seepax HP-C18 column (250 × 4.6 mm, 5 μm, Sepax) at 40 °C. Mobile phases: A: 0.1 mol·L
−1 sodium acetate-acetonitrile; B: acetonitrile-water (80:20,
v/
v). Gradient: (0–4 min, 95% A; 4.01–31 min, 95 → 67% A; 31.01–40 min, 67 → 65% A; 40.01–45 min, 65 → 10% A; 45.01–53 min, 10% A; 53.01–55 min, 10 → 95% A; 55.01–67 min, 95% A). Flow: 1.0 mL/min; injection: 20 μL. Detection: 254 nm. Quantified vs. standards. Reagents/standards from Sigma.
2.19. Determination of Nitrate Reductase (NR) Activity
Nitrate reductase (NR) activity was determined using commercial assay kit (E-BC-K035-S, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Briefly, 0.2 g T. sinensis tissue homogenized in kit buffer. Centrifuged 12,000 rpm, 10 min, 4 °C. Supernatant used per kit instructions. NR activity measured at 540 nm (UV-1800, Shimadzu Corporation), expressed as U·g−1 FW. 1 U = 1 μmol nitrite/min.
2.20. Determination of Glutamate Dehydrogenase (GDH) Activity
Glutamate dehydrogenase (GDH) activity was determined according to the method in a previous study [
36] with minor modifications. Briefly, 0.1 g
T. sinensis tissue homogenized in 2.0 mL ice-cold buffer: 165 mM Tris-HCl (pH 7.5), 0.4 M sucrose, 10 mM KCl, 10 mM MgCl
2, 10 mM EDTA, 10 mM β-mercaptoethanol. Centrifuged 10,000 rpm, 30 min, 4 °C. Supernatant + pellet resuspended in 1 mL 50 mM potassium phosphate (pH 7.5), 0.25 M sucrose. Then, 0.01 mL sample + 0.80 mL 25 mM phosphate (pH 7.5) + 9.9 mM α-ketoglutarate + 0.80 mL + 300 mM NH
4Cl + 0.80 mL + 0.9 mM NADH. ΔA 340 nm recorded at 20 s intervals for 5 min, 25 °C (UV-1800, Shimadzu Corporation). Reagents were sourced from Shanghai CNPC Reagent Co., Ltd. (Shanghai). Enzyme activity was defined as one unit (U) equal to 1 nmol of NADH oxidized per minute. Specific activity was calculated and expressed as U·mg
−1 protein.
2.21. Determination of Nitrite Content
The nitrite content was determined using the ultraviolet spectrophotometric method, with reagents and standard solutions prepared according to the Chinese National Food Safety Standard [
37]. Sample processing and quantification steps were slightly modified. Briefly, 0.2 g of
T. sinensis tissue was homogenized and transferred to a 25 mL beaker. Then, 15 mL of distilled water was added, followed by 0.5 mL of potassium ferrocyanide solution. After thorough mixing, 0.5 mL of zinc acetate solution was added to precipitate proteins. The mixture was allowed to stand for 30 min and then filtered to remove precipitated proteins. The filtrate was collected for subsequent analysis.
A volume of 2.0 mL of the filtrate was precisely transferred to a 5 mL test tube. Then, 0.2 mL of sulfanilic acid solution was added, mixed well, and allowed to stand for 3–5 min. Subsequently, 0.1 mL of N-(1-naphthyl) ethylenediaminedihydrochloride solution was added, and the volume was adjusted to 10 mL with distilled water. After thorough mixing, the solution was incubated for 15 min at room temperature. The absorbance was measured at 538 nm using a spectrophotometer (UV-1800, Shimadzu Corporation). The potassium ferrocyanide, zinc acetate, and N-(1-naphthyl) ethylenediaminedihydrochloride were purchased from Shanghai China National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China). The nitrite content was quantified based on a standard curve prepared with sodium nitrite and expressed as μg·g−1 FW.
2.22. Statistical Analysis
Data were analyzed using SPSS v26.0 software and visualized using OriginPro 2021. Differences between parameters were tested using a one-way analysis of variance (ANOVA) to compare between treatments within the given sampling day. All experiments were conducted in triplicate, and the data were presented as mean ± standard deviation. p < 0.05 were considered statistically significant.
4. Discussion
SA has emerged as an effective natural compound for the postharvest preservation of various fruits and vegetables. SA treatments effectively delay senescence by maintaining higher chlorophyll levels and reducing chlorophyll-degrading enzyme activity in green onions and Chinese flowering cabbage [
40,
41]. This mechanism involves decreasing ROS levels and enhancing antioxidant enzyme activity [
40]. Weight loss is a major indicator of storage quality of
T. sinensis bud storage quality. Transpiration-driven water loss is a key factor [
42,
43]. In our study, even though the SA levels under 20 °C + SA were reduced to similar levels as observed in other treatments, water loss was significantly reduced in 20 °C + SA. Similarly, SA treatments reduced fruit weight loss in peaches during short storage, with higher SA concentrations (1.5 mM) being more effective than lower SA concentrations [
44].
SA-treated
T. sinensis leaves had lower chlorophyll content on a fresh weight basis. However, considering the greater water loss in other treatments, chlorophyll was evidently better preserved under the 20 °C + SA treatment (
Figure 1d). Carotenoids exhibited a declining trend under the 20 °C + SA treatment, suggesting their accelerated depletion and greater utilization in mitigating postharvest stress (
Figure 1e).
TSS reduction was greater in the 20 °C + SA treatment than in other treatments. However, considering the lower water loss under 20 °C + SA, it can be inferred that TSS was actually better preserved in this treatment, despite the higher apparent TSS levels observed under 20 °C alone, where greater water loss likely concentrated the soluble solids (
Figure 1f). Soluble sugar content was initially higher at 4 °C, but by day 7, all treatments exhibited similar levels. Considering the lower water loss, the 20 °C + SA treatment likely retained more soluble sugars, followed by the 4 °C treatment (
Figure 1g). SA reduces sugar accumulation and TSS content in fruit, thereby delaying ripening during storage. These effects are associated with the modulation of sugar metabolism and enhanced antioxidant defenses, which help maintain fruit quality and extend shelf life [
45,
46]. Our results are consistent with those of previous reports.
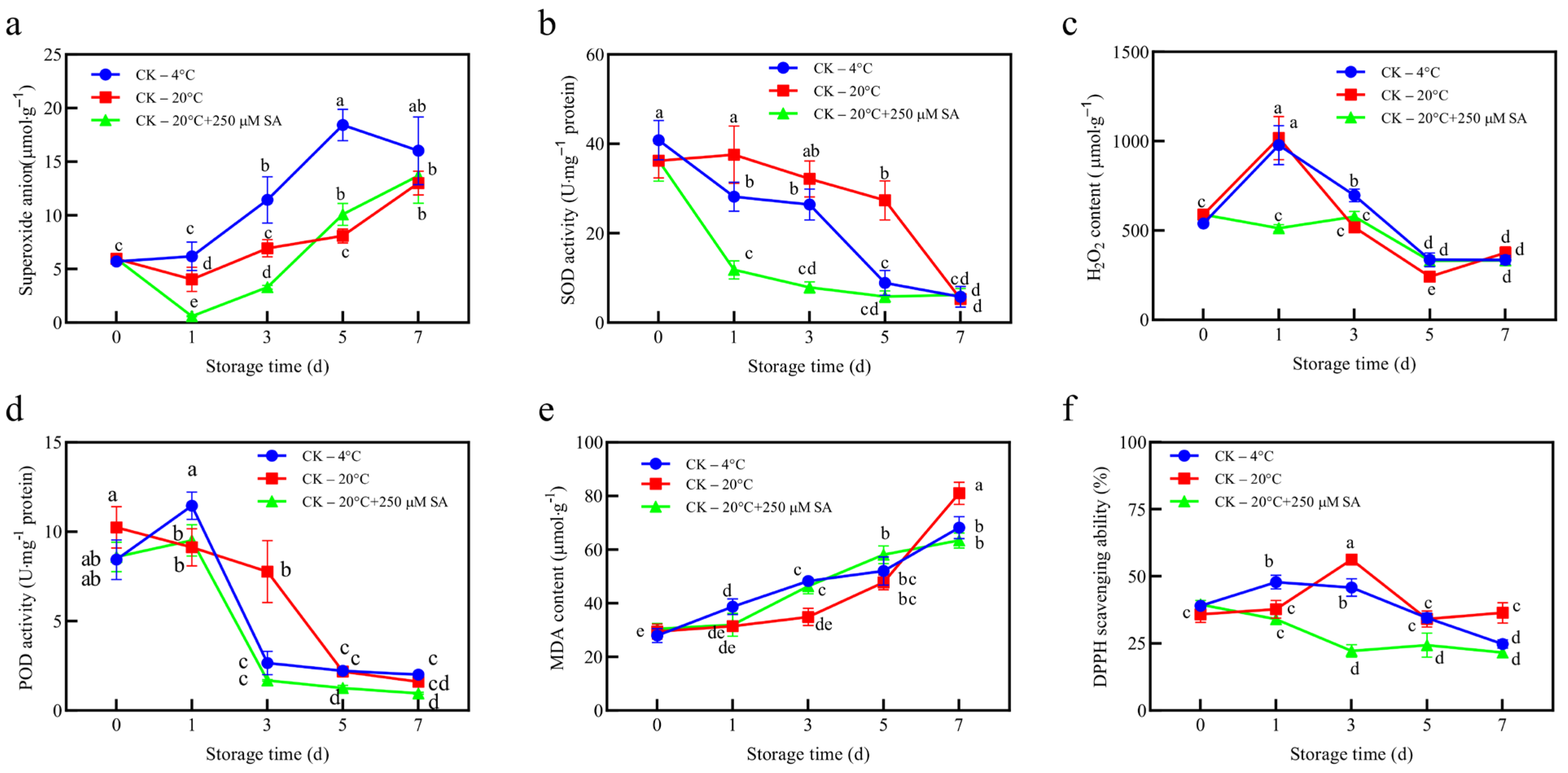
A marked reduction in superoxide anions and SOD activity was observed in the SA-treated group, suggesting that SA attenuated the early stages of storage-associated oxidative processes (
Figure 2a,b). In contrast, Zhang et al. reported that SA application enhanced both SOD and POD activities while reducing ROS accumulation, thereby delaying leaf senescence [
19]. In the present study, the reduction in ROS levels was only evident during the initial stages of treatment; by day 7, ROS levels and antioxidant enzyme activities did not differ significantly among treatments. However, a limitation of this study is that ROS production was not measured directly. The observed reduction in superoxide levels (
Figure 2a) and lower scavenging activities for DPPH and •OH (
Figure 2f and
Figure 3h) provide indirect evidence of attenuated oxidative stress in the SA-treated group. Future studies should aim to directly measure ROS to confirm whether SA reduces ROS production.
Assessment of MDA levels revealed that lipid peroxidation in the 20 °C + SA treatment was comparable to that under 4 °C storage, while the 20 °C control exhibited the lowest MDA content, indicating minimal lipid peroxidation (
Figure 2e). Interestingly, DPPH radical scavenging activity was significantly lower in the 20 °C + SA treatment compared with that in both the 20 °C control and 4 °C treatment, which showed comparable levels (
Figure 2f). This suggests that exogenous SA may have reduced the reliance on non-enzymatic antioxidants by enhancing other antioxidant pathways, thereby lowering the overall demand for radical scavenging metabolites. Alternatively, SA treatment may have influenced the metabolic allocation of phenolic compounds, leading to a reduced contribution of non-enzymatic antioxidants to the total antioxidant capacity. In contrast, the 20 °C control and 4 °C treatment maintained higher DPPH activity, reflecting a greater dependence on non-enzymatic radical scavenging mechanisms under these conditions.
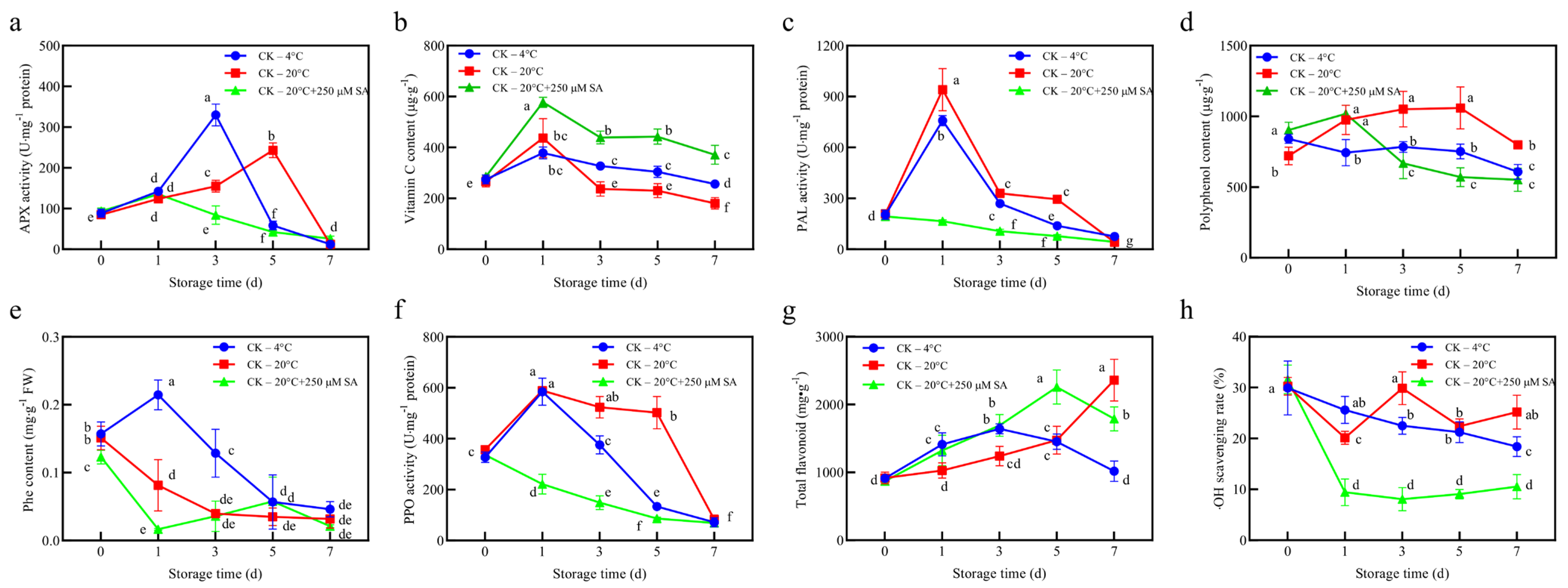
To further clarify the reason for these differences, we examined the activities of enzymatic and non-enzymatic antioxidant components (
Figure 3). The 20 °C + SA treatment reduced APX activity while maintaining higher VC levels in
T. sinensis leaves, whereas other treatments showed higher APX activity accompanied by lower VC content, indicating that the APX–VC system was underutilized under SA treatment (
Figure 3a,b). PAL activity, phenolic content, and total flavonoids were measured to evaluate the phenylpropanoid pathway because PAL catalyzes the entry step into this pathway, and phenolics and flavonoids serve as major non-enzymatic antioxidants [
47,
48]. Surprisingly, PAL activity was also low in SA-treated leaves; by day 7, it was comparable to that under 4 °C storage, whereas 20 °C storage led to the highest PAL activity and polyphenol content (
Figure 3c,d). Phenolic content was significantly lower in SA-treated leaves during the initial stages but became comparable to other treatments from day 5 onward (
Figure 3e). PPO activity was also monitored because it influences the effective pool of antioxidant phenolics by catalyzing oxidative turnover. PPO activity was lower in SA-treated leaves, whereas 20 °C storage led to the highest activity (
Figure 3f). Conversely, the rapid increase in total flavonoid content in SA-treated leaves indicated that flavonoid biosynthesis was active (
Figure 3g).
Finally, the •OH scavenging capacity was determined because hydroxyl radicals are highly reactive ROS, and their neutralization provides a direct measure of radical scavenging efficiency beyond DPPH. Surprisingly, the SA treatment maintained a low •OH scavenging capacity (
Figure 3h). The reduced •OH scavenging capacity in the 20 °C + SA treatment, compared to the higher scavenging rates observed in the 20 °C control and 4 °C treatment (
Figure 3h) aligns with the lower DPPH scavenging activity in SA-treated leaves (
Figure 2f). This suggests that SA may suppress non-enzymatic antioxidant mechanisms, particularly those involving phenolic compounds and flavonoids, which are key contributors to •OH and DPPH scavenging. The lower PAL and PPO activities in the 20 °C + SA treatment, coupled with reduced phenolic content during the initial storage stages (
Figure 3c,d,f), likely contributed to the diminished •OH scavenging capacity. In contrast, the 20 °C control and 4 °C treatment exhibited higher PAL and PPO activities and elevated polyphenol and flavonoid content, supporting their enhanced non-enzymatic scavenging capabilities (
Figure 3c,d,g). Notably, the higher ascorbate (VC) content in the SA-treated leaves (
Figure 3b) suggests that SA may prioritize ascorbate accumulation or recycling over phenolic-based scavenging, potentially through enhanced enzymatic pathways that do not directly contribute to •OH neutralization. This shift in antioxidant strategy could explain the reduced •OH scavenging capacity in the 20 °C + SA treatment, reflecting the broader modulation of the antioxidant system by SA.
Taken together, these results indicated that SA treatment reprogrammed antioxidant metabolism in T. sinensis leaves. The reduction in APX activity, despite the higher VC content, suggests that the ascorbate glutathione cycle was underutilized, limiting enzymatic detoxification of H2O2. At the same time, SA treatment suppressed PAL activity and initially inhibited phenolic accumulation, indicating a weaker contribution of the phenylpropanoid pathway, whereas the reduced PPO activity suggested that phenolic depletion through oxidative turnover was not the main factor. Interestingly, flavonoid levels increased rapidly under SA treatment, yet this did not translate into a stronger •OH scavenging capacity. This suggests either that the qualitative profile of flavonoids favored compounds that were less reactive toward •OH, or that other radical-quenching pools were insufficient. An alternative explanation is that ROS production is lower under SA treatment, reducing the need for strong enzymatic or non-enzymatic defenses. Overall, the consistently low •OH scavenging under SA treatment indicates that despite shifts in phenolic and flavonoid metabolism, SA treatment may have limited both the demand for and the effectiveness of non-enzymatic antioxidants capable of neutralizing highly reactive ROS.
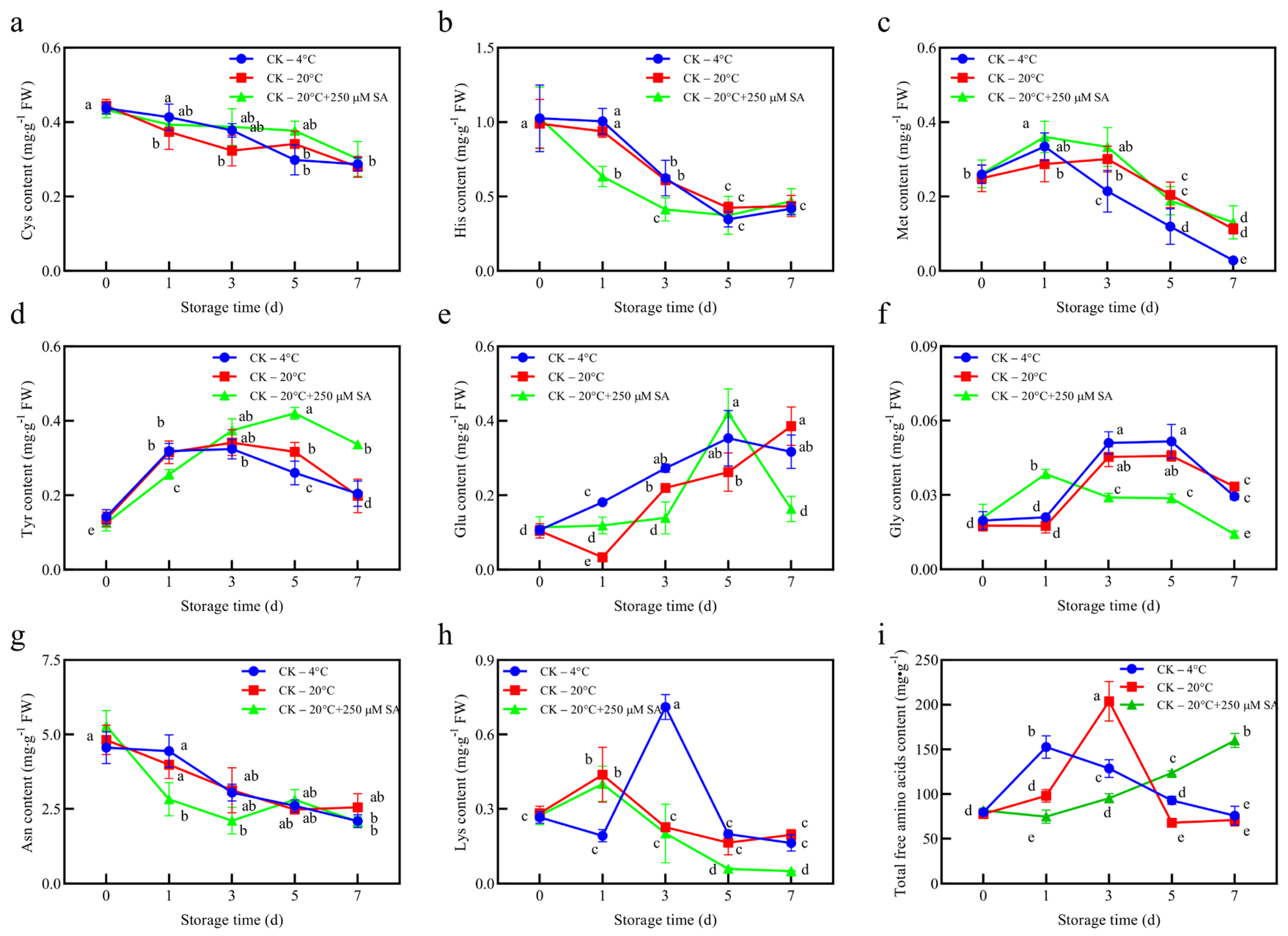
The amino acid profiles provided additional insights into how SA treatment modulates stress responses and metabolic allocation in
T. sinensis leaves. cys and his progressively declined in all treatments, but the faster reduction of His under 20 °C + SA suggests that SA may accelerate its utilization in redox regulation [
49]. Met showed an early increase under 4 °C and 20 °C + SA conditions, likely reflecting an initial protective response, but its later decline—particularly under 4 °C—indicates reduced synthesis or increased incorporation into proteins or secondary metabolites as part of stress adaptation.
Tyr accumulation under SA treatment throughout the storage period contrasted with the transient peaks in the other treatments, implying that SA may redirect Tyr toward the biosynthesis of specific secondary metabolites or flavonoids, which is consistent with the observed rapid flavonoid increase (
Figure 3) [
50]. Fluctuations in Glu and Gly suggest the dynamic regulation of nitrogen metabolism, which could influence glutathione synthesis and indirectly affect ROS detoxification. The subsequent decline in Glu and Gly may reflect their utilization in maintaining basal antioxidant capacity rather than large-scale ROS scavenging [
50]. The Asn and Lys patterns indicated differential nitrogen allocation under SA treatment, with reduced early levels possibly supporting osmotic adjustment or secondary metabolism, whereas total free amino acids gradually accumulated, reaching the highest level by day 7. This accumulation may reflect a stress-mitigating strategy, storing nitrogen-rich compounds for osmoprotection, redox buffering, and metabolic flexibility rather than direct ROS detoxification [
51,
52,
53]. Overall, the amino acid results support the interpretation shown in
Figure 3 that SA treatment modulates metabolic allocation rather than strongly inducing classical antioxidant pathways. Reduced enzymatic activity (APX) and phenolic synthesis (PAL) under SA, together with altered amino acid dynamics, suggest that leaves experience lower ROS pressure and maintain a controlled adaptive metabolic state, balancing antioxidant defense, osmoprotection, and nitrogen storage during preservation.
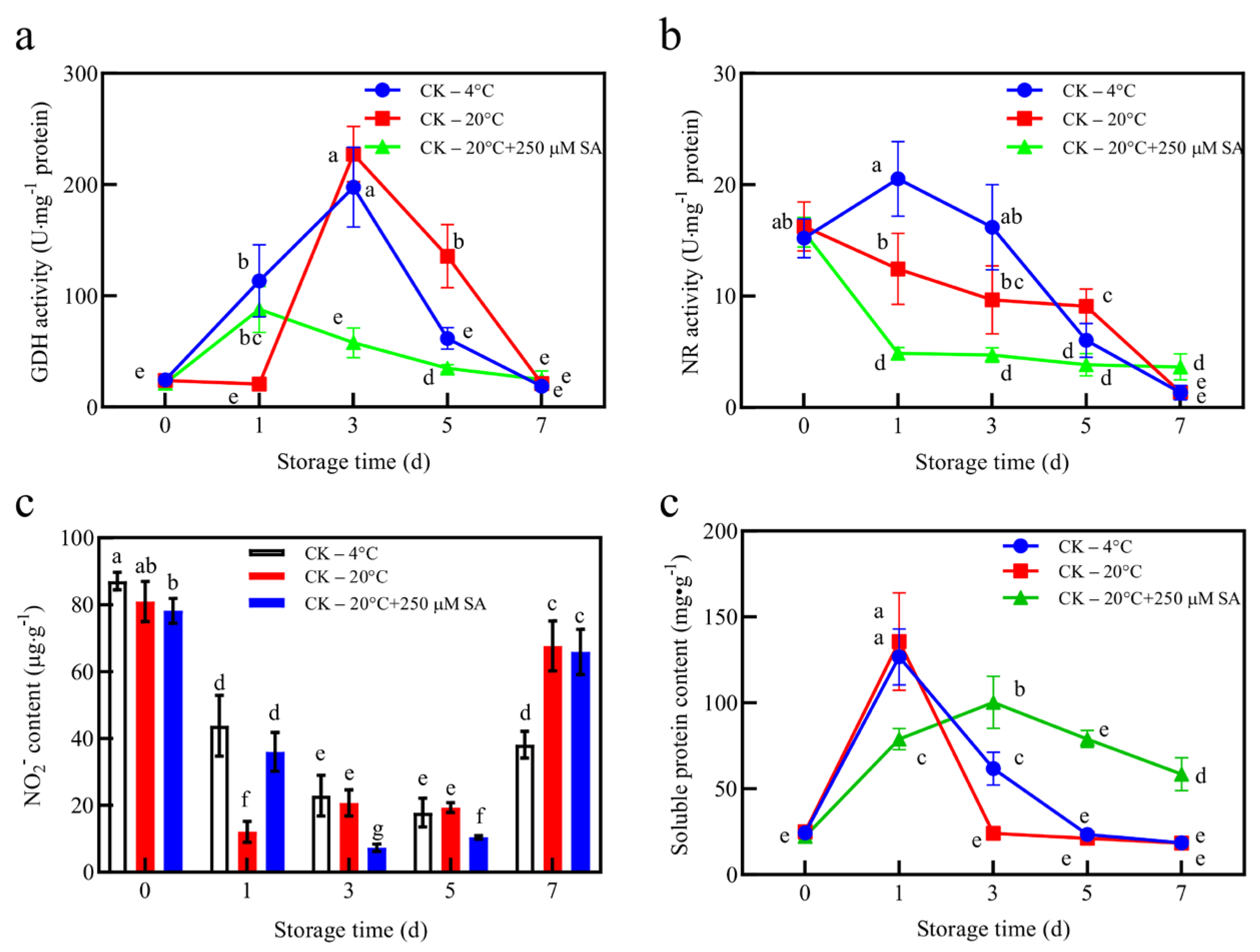
To investigate nitrogen utilization and protein turnover during storage, protein and nitrogen metabolism indicators were analyzed in
T. sinensis leaves (
Figure 5). These metrics provide insights into how storage conditions influence metabolic balance, antioxidant capacity, and overall leaf quality. GDH activity was lower throughout the storage period in 20 °C + SA leaves than it was in the 4 °C treatment and 20 °C control, which exhibited gradual or peak increments at day 3 (
Figure 5a). GDH catalyzes the reversible interconversion of glutamate and α-ketoglutarate, which play key roles in nitrogen assimilation and amino acid homeostasis [
54,
55]. The relatively low GDH activity under the SA treatment suggests reduced nitrogen turnover, consistent with lower ROS stress and a lower need for de novo amino acid synthesis, which may help to conserve metabolic resources and maintain leaf turgor and freshness. NR activity showed a gradual decline in leaves stored at 4 °C and 20 °C, whereas those stored under 20 °C + SA treatment experienced a sudden drop following initial measurements (
Figure 5b). NR catalyzes the nitrate reduction to nitrite, which is a critical step in nitrogen assimilation [
54,
55]. The transient reduction in NR activity in SA-treated leaves may indicate a lower nitrate assimilation demand, possibly because the leaves experienced reduced metabolic stress, which is consistent with higher appearance, quality, and freshness. NO
2•
− content was initially low on treatment day in all treatments and increased on day 1 for all treatments except for 20 °C + SA, which remained comparatively lower in SA-treated leaves on days 3 and 5, suggesting a limited accumulation of reactive nitrogen intermediates (
Figure 5c). This may reflect more efficient nitrogen utilization or a lower rate of oxidative deamination reactions, which would reduce cellular damage and preserve tissue integrity.
Total soluble protein content increased gradually in 20 °C + SA leaves, peaking on day 7 to a higher level than observed in other treatments (
Figure 5d). This contrasts with the early spikes and subsequent declines observed in 4 °C and 20 °C leaves. The sustained protein content in SA-treated leaves could indicate reduced proteolysis and stabilized protein turnover, contributing to the maintenance of the structural and enzymatic proteins necessary for leaf turgor, appearance, and overall freshness. The trends in Lys content mirrored those for total soluble protein, suggesting the coordinated preservation of essential amino acids and proteins under SA treatment (
Figure 4h and
Figure 5d).
Based on the observed changes in nitrogen and protein metabolism, SA treatment appeared to mitigate the metabolic stress in T. sinensis leaves during storage. Lower GDH and NR activities, along with stable nitrite levels, suggested a reduced demand for nitrogen assimilation and amino acid turnover, likely reflecting lower ROS-induced stress. Concurrently, the gradual increase and higher final levels of total soluble protein and Lys under the SA treatment indicated limited proteolysis and a more stable protein turnover, supporting the maintenance of structural and enzymatic proteins essential for leaf turgor, color, and overall quality. This metabolic reallocation, favoring the retention of nitrogen in soluble proteins and amino acids rather than in stress-response pathways, likely contributes to both the nutritional and structural integrity of the leaves. Collectively, these effects help preserve leaf freshness and appearance, explaining the superior visual quality of SA-treated leaves compared with those stored at 20 °C or 4 °C. In contrast, SA treatment at ambient 20 °C eliminates the need for energy-intensive refrigeration infrastructure, relying instead on a simple, low-cost chemical application that can be integrated into existing handling processes. This not only lowers energy consumption and carbon footprint but also makes it more accessible for small-scale farmers or regions with limited access to cold chain facilities, ultimately reducing overall storage costs while maintaining high-quality produce. Future studies should explore optimal SA concentrations and application methods to further refine this approach for commercial viability.
The promising results of the SA treatment for preserving
T. sinensis leaves at room temperature highlight its potential. However, several aspects of the study design and practical implications require further exploration. Future studies should conduct sensory evaluations to assess the flavor, texture, and overall consumer acceptance of SA-treated leaves, as these factors are critical for market success. Additionally, our study was conducted over a 7 d storage period; therefore, future studies should aim to evaluate SA performance over longer periods that are more relevant to commercial storage and distribution. As we tested only one SA concentration, future studies must also explore multiple concentrations and application methods, such as spraying or soaking, to optimize the effectiveness and practicality for farmers. For instance, changes in the phenolic content and higher ascorbate levels in SA-treated leaves (
Figure 3b,d) might alter taste or health benefits, potentially making the leaves less flavorful and reducing consumer appeal. There is also a need to investigate whether SA residues remain on leaves, as this could raise concerns about safety or marketability. Although SA reduces the need for refrigeration, its application may involve high costs or logistical challenges for small-scale farmers, such as purchasing and consistently applying the compound. Uncertainties remain regarding whether SA’s benefits of SA will apply to different
T. sinensis varieties or diverse climatic conditions. Future studies are essential to address these questions through sensory evaluations, extended storage trials, and varied SA concentrations to ensure that SA treatment is safe, effective, and appealing to both farmers and consumers, building on this initial report and enhancing its commercial potential.