Foliar Selenium Application During Flowering and Fruiting Alleviates Drought-Induced Oxidative Damage and Promotes Tomato Growth
Abstract
1. Introduction
2. Materials and Methods
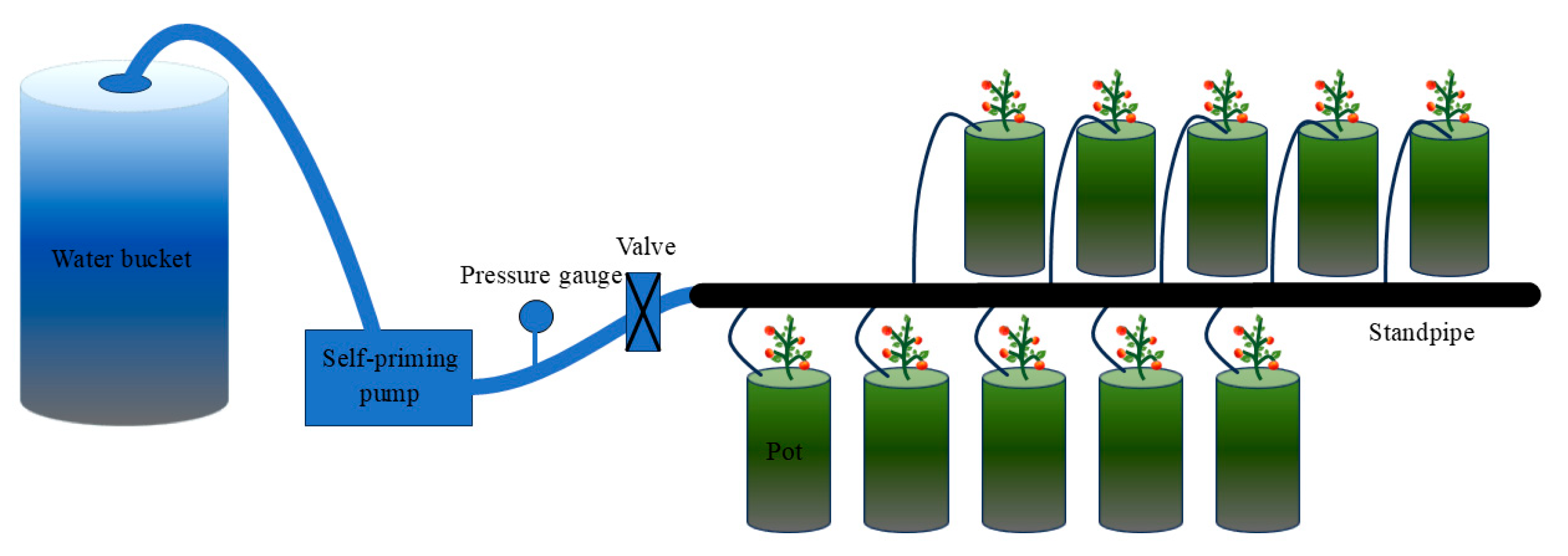
2.1. Overview of the Experimental Site
2.2. Agronomic Management
2.3. Experimental Design
2.4. Main Observations and Methods
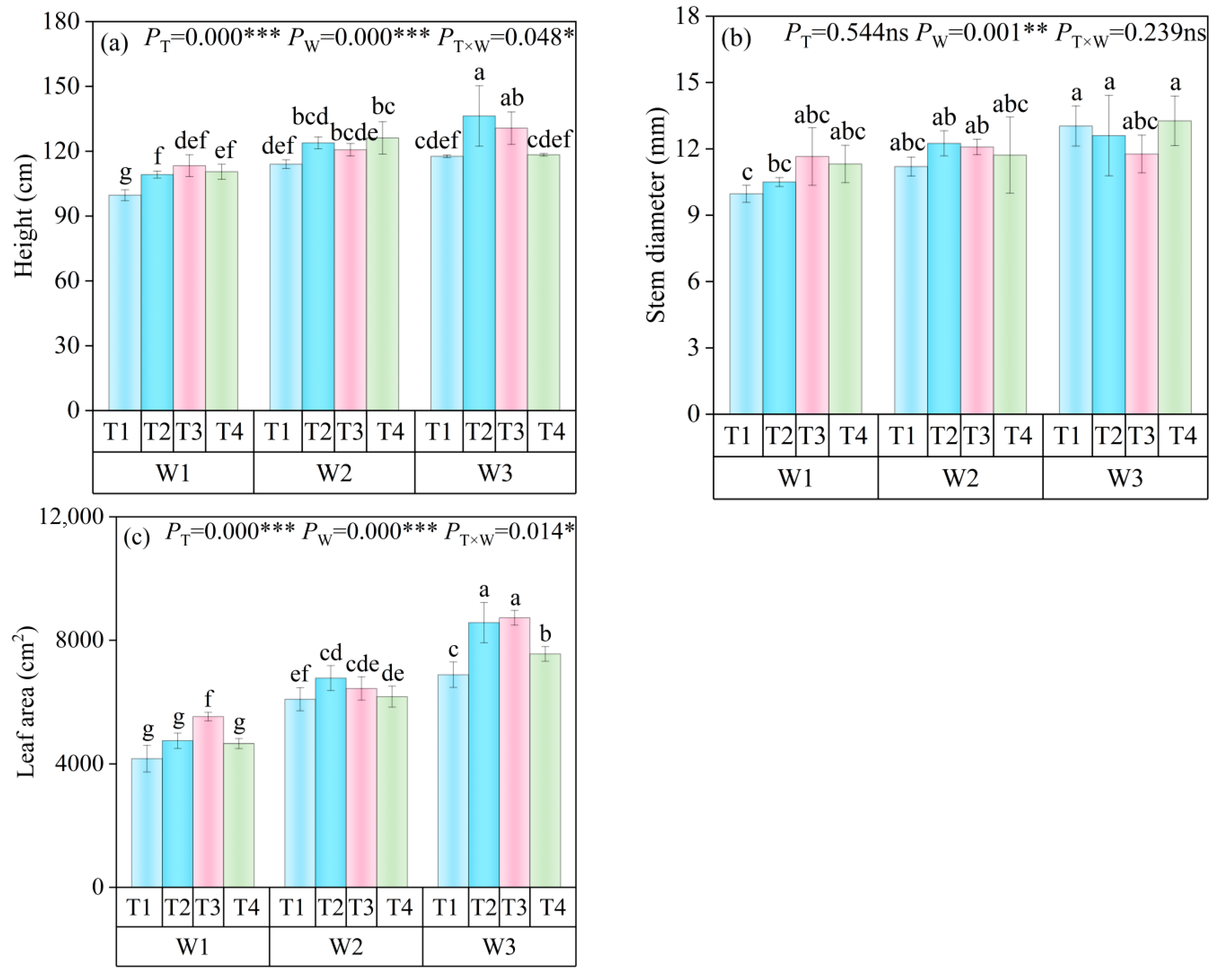
2.4.1. Plant Height, Stem Diameter, and Leaf Area
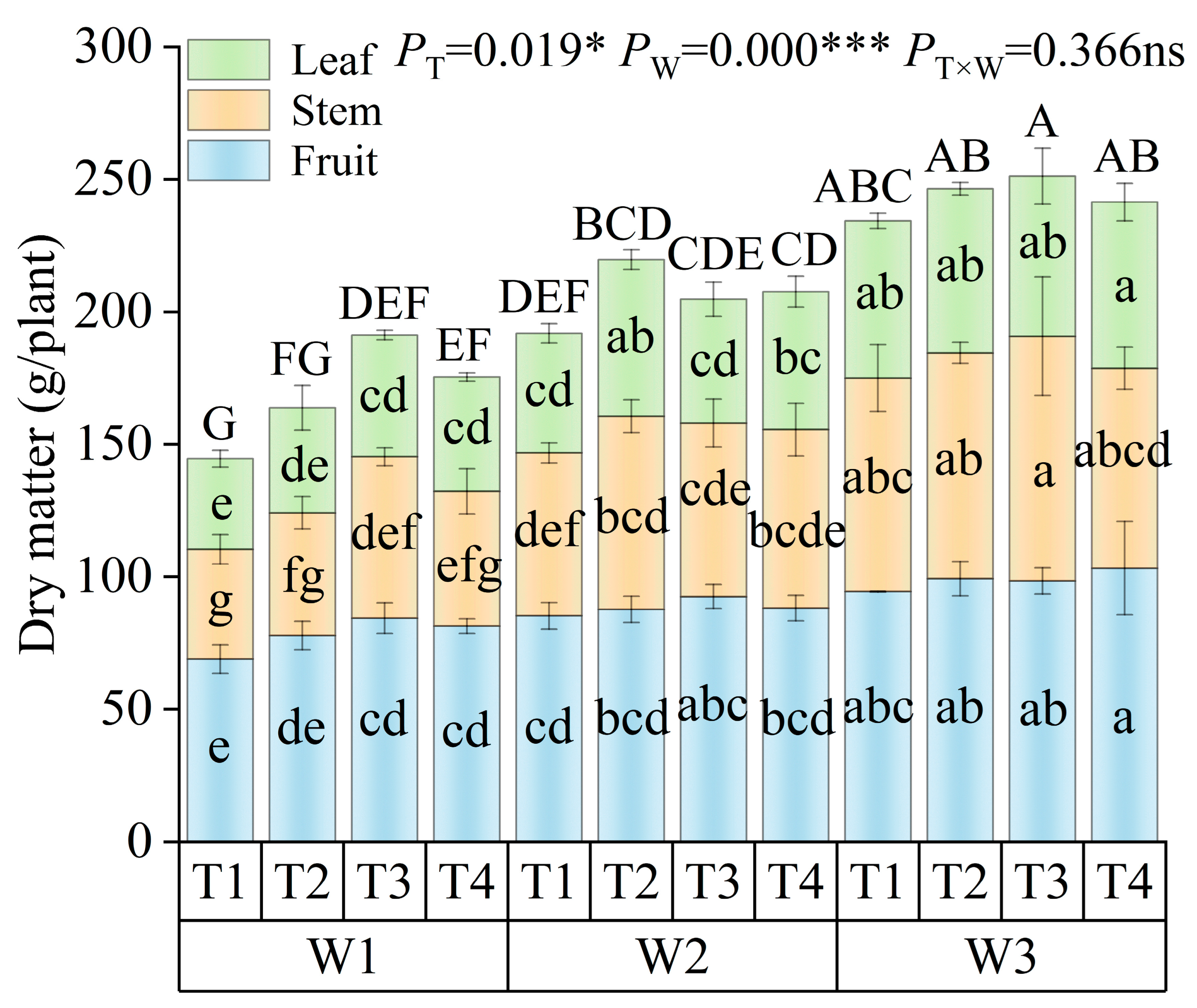
2.4.2. Aboveground Dry Matter
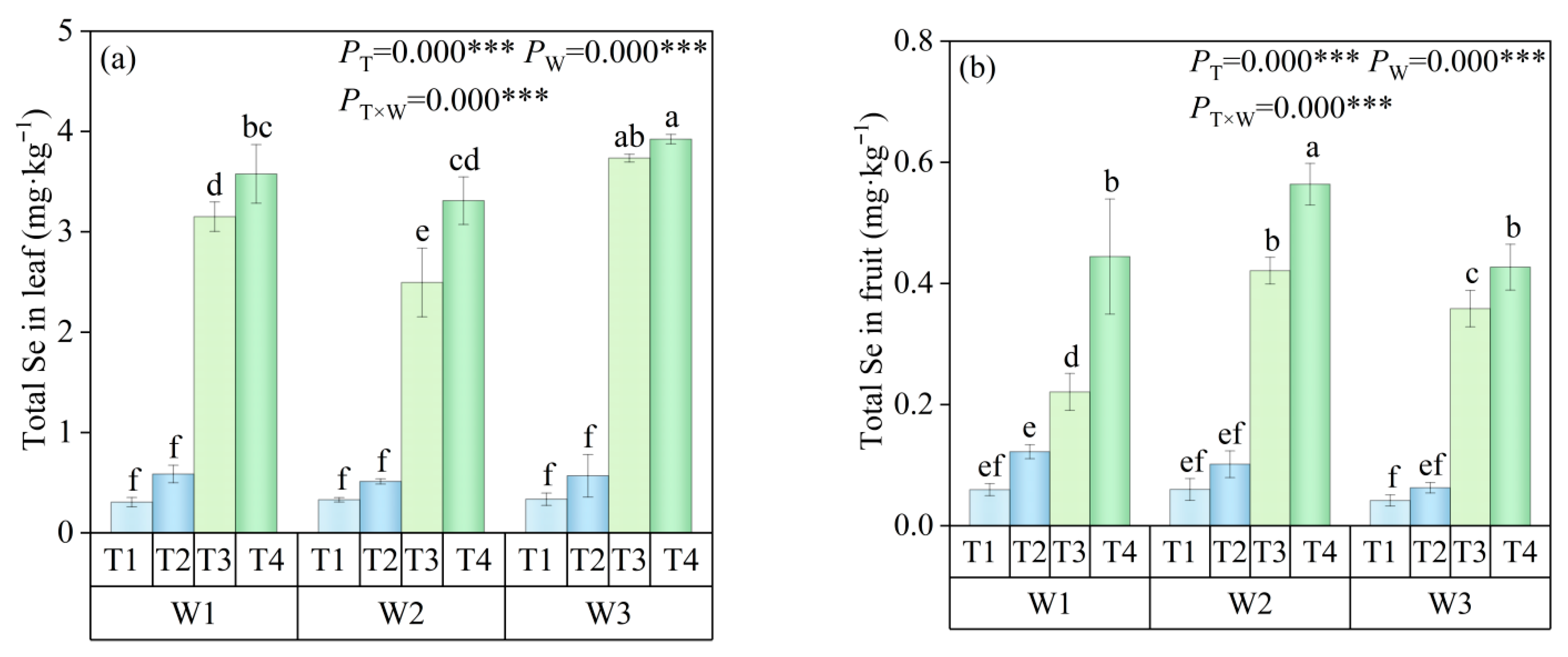
2.4.3. Se Content
2.4.4. Photosynthesis and Related Physiological Parameters
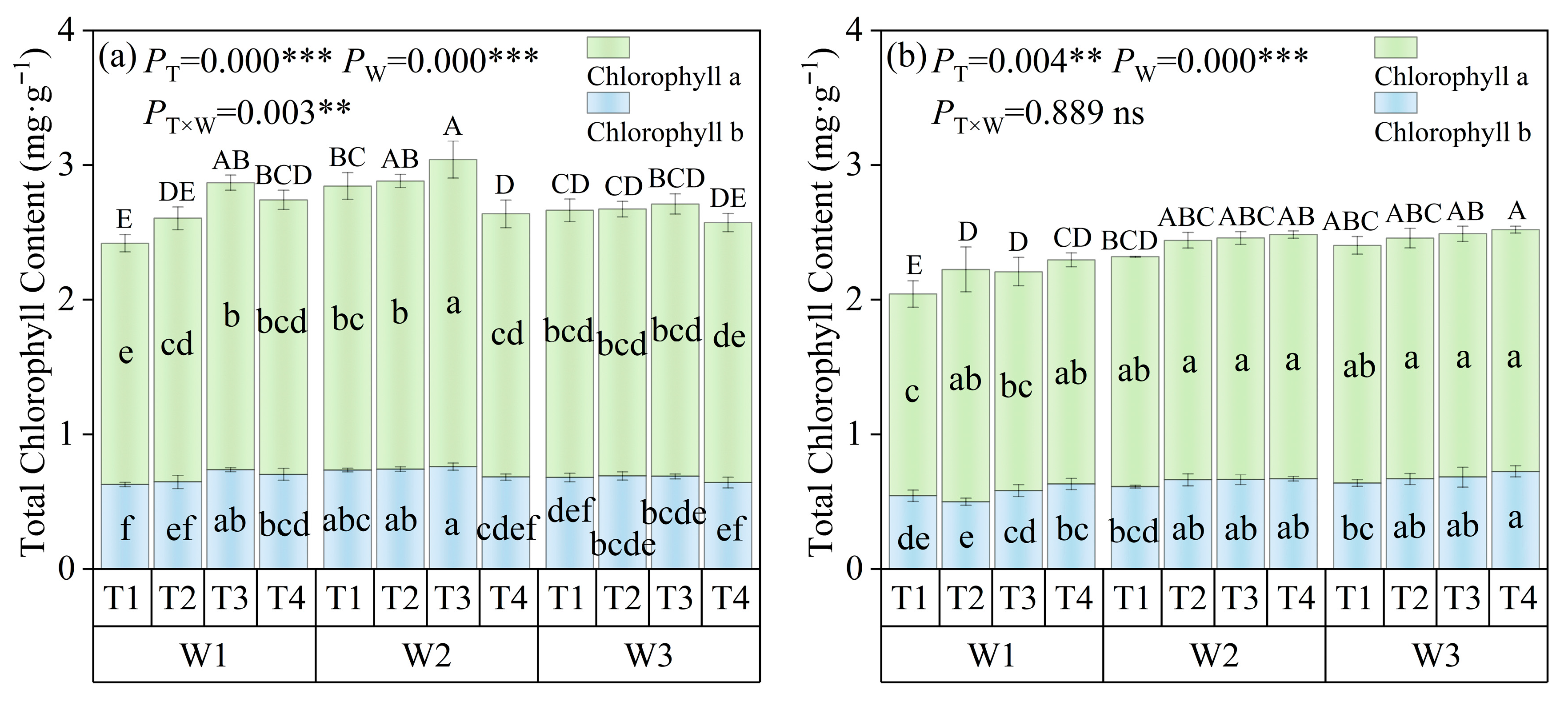
2.4.5. Photosynthetic Pigment Content
2.4.6. Peroxidation Products and Osmoregulatory Substances
2.5. Data Analysis
3. Results
3.1. Growth Status of Tomato
3.1.1. Shoot Dry Biomass in Tomato
3.1.2. Se Content in Different Tomato Organs
3.2. Effects of Se on Tomato Photosynthetic Characteristics
3.2.1. Leaf Photosynthesis
3.2.2. Chlorophyll Changes
3.3. Antioxidant Capacity and Osmoregulatory Substances in Tomato Leaves
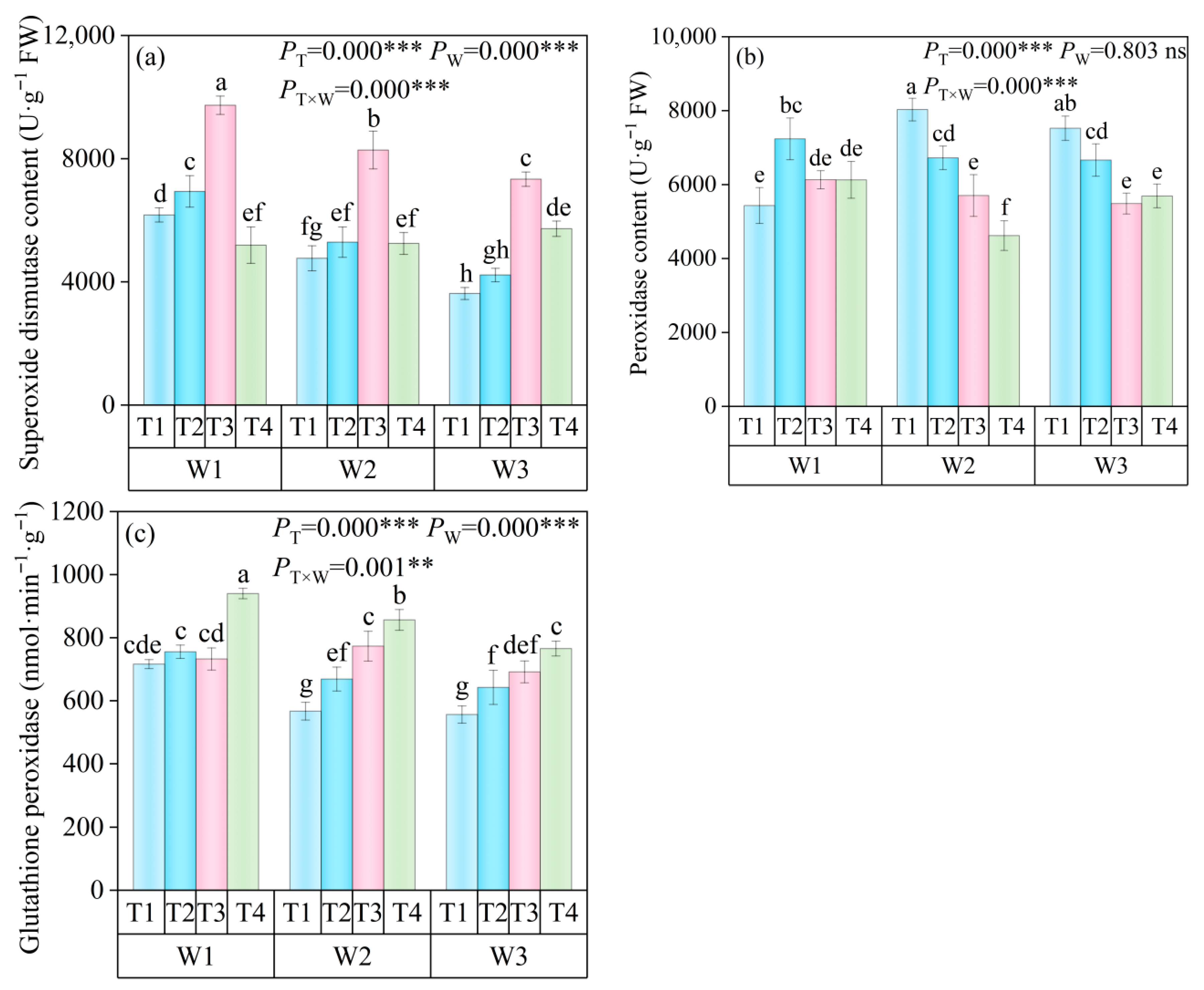
3.3.1. Activities of Superoxide Dismutase (SOD), Peroxidase (POD), and Glutathione Peroxidase (GSH-PX) in Leaves
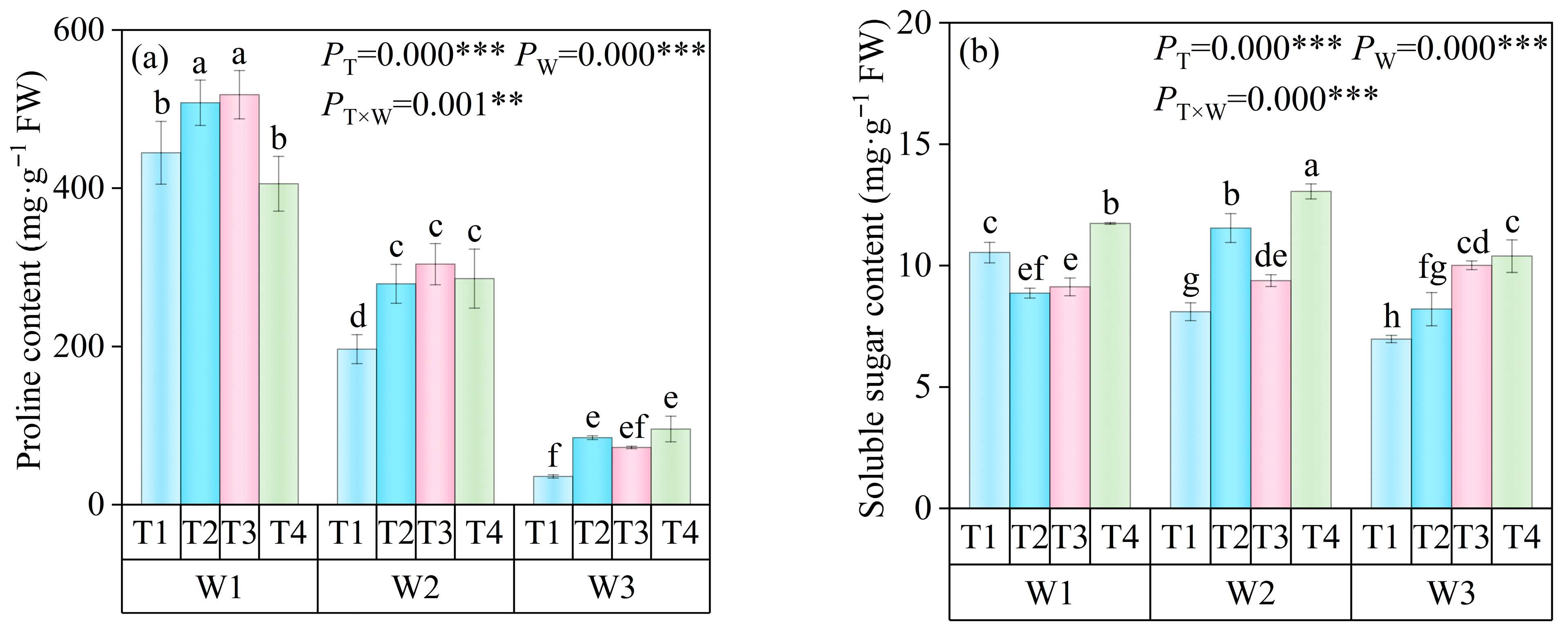
3.3.2. Effects of Exogenous Se on Osmoregulatory Substances in Tomato Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kizito, J. Evaluating the Influence of Two Organic Manures on the Productivity of Five Tomato Varieties. OALib 2024, 11, e12632. [Google Scholar] [CrossRef]
- Dawid, J. The Role of Tomato Products for Human Health (Solanum lycopersicum)—A Review. J. Health Med. Nurs. 2016, 33, 66–74. [Google Scholar]
- An, C.-H.; Ri, H.-J.; Han, T.-U.; Kim, S.-I.; Ju, U.-S. Feasibility of Winter Cultivation of Fruit Vegetables in a Solar Greenhouse in Temperate Zone; Experimental and Numerical Study. Sol. Energy 2022, 233, 18–30. [Google Scholar] [CrossRef]
- Li, H.; Song, J.; Sun, J.; Wang, J.; Qiang, X.; Liu, H.; Zheng, M.; Lou, Y. Effects of Different Water and Nitrogen Supply on Fruit Nutrients and Yield Components of Greenhouse Tomato at Different Truss Levels. J. Irrig. Drain. 2023, 42, 1–9. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Xiao, M.; Chao, J.; Dai, Y.; Liu, G.; Senge, M. Effects of Organic Mulching on Soil Moisture and Temperature in Greenhouse Tomato Production under Unheated Greenhouse Cultivation in the Cold Zone of China. Food Sci. Nutr. 2023, 11, 4829–4842. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production Under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Song, J.; Li, H.; Xin, L.; Shen, J.; Liu, H.; Wang, X.; Zhong, Y. Effects of Foliar Selenium Application and Soil Moisture Interaction on Growth and Water Use Efficiency of Greenhouse Tomato. J. Irrig. Drain. 2023, 42, 52–59. (In Chinese) [Google Scholar] [CrossRef]
- Chołuj, D.; Karwowska, R.; Ciszewska, A.; Jasińska, M. Influence of Long-Term Drought Stress on Osmolyte Accumulation in Sugar Beet (Beta vulgaris L.) Plants. Acta Physiol. Plant 2008, 30, 679–687. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and Silica Nanostructure-Based Recovery of Strawberry Plants Subjected to Drought Stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in Cancer Prevention: A Review of the Evidence and Mechanism of Action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Zhang, X.; Li, M. Effect of Foliar Treatment of Sodium Selenate on Postharvest Decay and Quality of Tomato Fruits. Sci. Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Saldaña-Sánchez, W.D.; León-Morales, J.M.; López-Bibiano, Y.; Hernández-Hernández, M.; Langarica-Velázquez, E.C.; García-Morales, S. Effect of V, Se, and Ce on Growth, Photosynthetic Pigments, and Total Phenol Content of Tomato and Pepper Seedlings. J. Soil Sci. Plant Nutr. 2019, 19, 678–688. [Google Scholar] [CrossRef]
- Ramasamy, S.; Nandagopal, J.G.T.; Balasubramanian, M.; Girija, S. Effect of Abscisic Acid and Selenium Foliar Sprays on Drought Mitigation in Tomato (Solanum lycopersicum L.). In Materials Today-Proceedings; Elsevier: Amsterdam, The Netherlands, 2022; Volume 48, pp. 191–195. [Google Scholar]
- Zhong, Y.; Cui, H.; Li, H.; Qiang, X.; Han, Q.; Liu, H. Foliar Application of Selenium Enhances Drought Tolerance in Tomatoes by Modulating the Antioxidative System and Restoring Photosynthesis. Agronomy 2024, 14, 1184. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium Application in Two Methods Promotes Drought Tolerance in Solanum lycopersicum Plant by Inducing the Antioxidant Defense System. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Ragályi, P.; Takács, T.; Füzy, A.; Uzinger, N.; Dobosy, P.; Záray, G.; Szűcs-Vásárhelyi, N.; Rékási, M. Effect of Se-Enriched Irrigation Water on the Biomass Production and Elemental Composition of Green Bean, Cabbage, Potato and Tomato. Plants 2021, 10, 2086. [Google Scholar] [CrossRef] [PubMed]
- Dima, S.-O.; Neamțu, C.; Desliu-Avram, M.; Ghiurea, M.; Capra, L.; Radu, E.; Stoica, R.; Faraon, V.-A.; Zamfiropol-Cristea, V.; Constantinescu-Aruxandei, D.; et al. Plant Biostimulant Effects of Baker’s Yeast Vinasse and Selenium on Tomatoes Through Foliar Fertilization. Agronomy 2020, 10, 133. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Stibilj, V.; Urbanc-Berčič, O. Combined Effects of Selenium and Drought on Photosynthesis and Mitochondrial Respiration in Potato. Plant Physiol. Biochem. 2007, 45, 162–167. [Google Scholar] [CrossRef]
- Kostopoulou, P.; Barbayiannis, N.; Noitsakis, B. Water Relations of Yellow Sweetclover Under the Synergy of Drought and Selenium Addition. Plant Soil. 2010, 330, 65–71. [Google Scholar] [CrossRef]
- Meucci, A.; Shiriaev, A.; Rosellini, I.; Malorgio, F.; Pezzarossa, B. Se-Enrichment Pattern, Composition, and Aroma Profile of Ripe Tomatoes After Sodium Selenate Foliar Spraying Performed at Different Plant Developmental Stages. Plants 2021, 10, 1050. [Google Scholar] [CrossRef]
- Villacampa, Y.; Navarro-Gonzalez, J.F.; Reyes, J.A.; Sastre-Vazquez, P. Numerical and Regression Models for the Leaf Area of Tomato Seedlings. In Ecosystems and Sustainable Development Ix; Marinov, A.M., Brebbia, C.A., Eds.; Wit Press: Southampton, UK, 2013; Volume 175, pp. 3–10. [Google Scholar]
- Vacchina, V.; Dumont, J. Total Selenium Quantification in Biological Samples by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). In Selenoproteins: Methods and Protocols; Chavatte, L., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1661, pp. 145–152. ISBN 978-1-4939-7258-6. [Google Scholar]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise Estimation of Chlorophyll a, b and Carotenoid Content by Deconvolution of the Absorption Spectrum and New Simultaneous Equations for Chlorophyll Determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Sun, X.; Xing, S.; Cong, W.; Liu, X. Study on Dormancy Mechanism and Breaking Dormancy Method of Viburnum Sargentii Seeds. Am. J. Plant Sci. 2019, 10, 65–78. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Wu, S.; Rao, S.; Li, L.; Cheng, S.; Cheng, H. Morphological and Physiological Indicators and Transcriptome Analyses Reveal the Mechanism of Selenium Multilevel Mitigation of Cadmium Damage in Brassica Juncea. Plants 2023, 12, 1583. [Google Scholar] [CrossRef]
- Kar, R.K. Plant Responses to Water Stress Role of Reactive Oxygen Species. Plant Signal. Behav. 2011, 6, 1741–1745. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants Under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive Effects of Drought and Heat Stresses on Morpho-Physiological Attributes, Yield, Nutrient Uptake and Oxidative Status in Maize Hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in Leaf Morphology, Antioxidant Activity and Photosynthesis Capacity in Two Different Drought-Tolerant Cultivars of Chrysanthemum During and After Water Stress. Sci. Hortic. 2013, 161, 249–258. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The Roles of Selenium in Protecting Plants Against Abiotic Stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Ghorbanli, M.; Gafarabad, M.; Amirkian, T.; Allahverdi, B. Investigation of Proline, Total Protein, Chlorophyll, Ascorbate and Dehydroascorbate Changes Under Drought Stress in Akria and Mobil Tomato Cultivars. Available online: https://journals.iau.ir/article_540675_e99838d78036ac679c11f1e0382758d0.pdf (accessed on 3 September 2025).
- Khan, R.; Ma, X.; Zhang, J.; Wu, X.; Iqbal, A.; Wu, Y.; Zhou, L.; Wang, S. Circular Drought-Hardening Confers Drought Tolerance via Modulation of the Antioxidant Defense System, Osmoregulation, and Gene Expression in Tobacco. Physiol. Plant. 2021, 172, 1073–1088. [Google Scholar] [CrossRef]
- Neysanian, M.; Iranbakhsh, A.; Ahmadvand, R.; Ardebili, Z.O.; Ebadi, M. Selenium Nanoparticles Conferred Drought Tolerance in Tomato Plants by Altering the Transcription Pattern of microRNA-172 (miR-172), bZIP, and CRTISO Genes, Upregulating the Antioxidant System, and Stimulating Secondary Metabolism. Protoplasma 2024, 261, 735–747. [Google Scholar] [CrossRef]
- Hussain, S.; Ahmed, S.; Akram, W.; Li, G.; Yasin, N.A. Selenium Seed Priming Enhanced the Growth of Salt-Stressed Brassica rapa L. Through Improving Plant Nutrition and the Antioxidant System. Front. Plant Sci. 2023, 13, 1050359. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Sánchez-Aspeytia, D.; González-Morales, S. Chitosan-PVA and Copper Nanoparticles Improve Growth and Overexpress the SOD and JA Genes in Tomato Plants Under Salt Stress. Agronomy 2018, 8, 175. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C. Water Stress Tolerance of Wheat (Triticum aestivum L.): Variations in Hydrogen Peroxide Accumulation and Antioxidant Activity in Tolerant and Susceptible Genotypes. J. Agron. Crop Sci. 2001, 186, 63–70. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed Under NaCl Stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Kumar, S.; Thakur, P.; Sharma, S.; Kaur, N.; Kaur, R.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Singh, K.; et al. Promotion of Growth in Mungbean (Phaseolus aureus Roxb.) by Selenium Is Associated with Stimulation of Carbohydrate Metabolism. Biol. Trace Elem. Res. 2011, 143, 530–539. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; dos Reis, A.R. Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses Against Abiotic Stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W. Proline Accumulation Is a General Response to Abiotic Stress in the Date Palm Tree (Phoenix dactylifera L.). Genet. Mol. Res. 2015, 14, 9943–9950. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of Proline Biosynthesis, Degradation, Uptake and Transport in Higher Plants: Its Implications in Plant Growth and Abiotic Stress Tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Verbruggen, N.; Hermans, C. Proline Accumulation in Plants: A Review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Villamor, J.G.; Verslues, P.E. Essential Role of Tissue-Specific Proline Synthesis and Catabolism in Growth and Redox Balance at Low Water Potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef]
- Khan, T.A.; Ahmad, A.; Saeed, T.; Yusuf, M.; Faisal, M.; Alatar, A.A. Investigating the Influence of Selenium and Epibrassinolide on Antioxidant Activity, Proline Accumulation, and Protein Expression Profiles in Wheat Plants Experiencing Heat and Drought Stress. Front. Plant Sci. 2024, 15, 1441483. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; Silva, V.M.; Montanha, G.S.; Lavres, J.; de Carvalho, H.W.P.; dos Reis, A.R. Assessment of Selenium Spatial Distribution Using μ-XFR in Cowpea (Vigna unguiculata (L.) Walp.) Plants: Integration of Physiological and Biochemical Responses. Ecotox. Environ. Safe. 2021, 207, 111216. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and Sulfur Influence Ethylene Formation and Alleviate Cadmium-Induced Oxidative Stress by Improving Proline and Glutathione Production in Wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Cheng, H.; Kong, W.; Tang, T.; Ren, K.; Zhang, K.; Wei, H.; Lin, T. Identification of Key Gene Networks Controlling Soluble Sugar and Organic Acid Metabolism During Oriental Melon Fruit Development by Integrated Analysis of Metabolic and Transcriptomic Analyses. Front. Plant Sci. 2022, 13, 830517. [Google Scholar] [CrossRef]
- Nezamivand Chegini, S.; Jafarinia, M.; Ghotbi-Ravandi, A.A. Unraveling the Impacts of Progressive Drought Stress on the Photosynthetic Light Reaction of Tomato: Assessed by Chlorophyll-a Fluorescence and Gene Expression Analysis. Cell. Mol. Biol. 2024, 70, 176–184. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chlorophyll Metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline Under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, M. Chlorophyll Modifications and Their Spectral Extension in Oxygenic Photosynthesis. In Annual Review of Biochemistry; Kornberg, R.D., Ed.; Annual Reviews: Palo Alto, CA, USA, 2014; Volume 83, pp. 317–340. ISBN 978-0-8243-0883-4. [Google Scholar]
- Verslues, P.E.; Sharma, S. Proline Metabolism and Its Implications for Plant-Environment Interaction. Arab. Book/Am. Soc. Plant Biol. 2010, 8, e0140. [Google Scholar] [CrossRef]
- Bai, B.; Wang, Z.; Gao, L.; Chen, W.; Shen, Y. Effects of Selenite on the Growth of Alfalfa (Medicago sativa L. Cv. Sadie 7) and Related Physiological Mechanisms. Acta Physiol. Plant 2019, 41, 78. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium Uptake, Dynamic Changes in Selenium Content and Its Influence on Photosynthesis and Chlorophyll Fluorescence in Rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Tao, J.; Leng, J.; Lei, X.; Wan, C.; Li, D.; Wu, Y.; Yang, Q.; Wang, P.; Feng, B.; Gao, J. Effects of Selenium (Se) Uptake on Plant Growth and Yield in Common Buckwheat (Fagopyrum esculentum moench). Field Crop. Res. 2023, 302, 109070. [Google Scholar] [CrossRef]
- Mackova, J.; Vaskova, M.; Macek, P.; Hronkova, M.; Schreiber, L.; Santrucek, J. Plant Response to Drought Stress Simulated by ABA Application: Changes in Chemical Composition of Cuticular Waxes. Environ. Exp. Bot. 2013, 86, 70–75. [Google Scholar] [CrossRef]
- Zhu, P.; Zhuang, Q.; Ciais, P.; Welp, L.; Li, W.; Xin, Q. Elevated Atmospheric CO2 Negatively Impacts Photosynthesis Through Radiative Forcing and Physiology-Mediated Climate Feedback. Geophys. Res. Lett. 2017, 44, 1956–1963. [Google Scholar] [CrossRef]
- Cacefo, V.; Ribas, A.F.; Vieira, L.G.E. Proline Metabolism as a Mechanism for the Energy Dissipation in VaP5CSF129A Transgenic Tobacco Plants Under Water Deficit. J. Plant Physiol. 2023, 283, 153964. [Google Scholar] [CrossRef] [PubMed]
- De Vita, P.; Platani, C.; Fragasso, M.; Ficco, D.B.M.; Colecchia, S.A.; Del Nobile, M.A.; Padalino, L.; Di Gennaro, S.; Petrozza, A. Selenium-Enriched Durum Wheat Improves the Nutritional Profile of Pasta Without Altering Its Organoleptic Properties. Food Chem. 2017, 214, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N.; Hussain, R.A. Selenium Supply Methods and Time of Application Influence Spring Wheat (Triticum aestivum L.) Yield Under Water Deficit Conditions. J. Agric. Sci. 2017, 155, 643–656. [Google Scholar] [CrossRef]
| Treatment | Pn (μmolCO2·m−2·s−1) | Gs (molH2O·m−2·s−1) | Tr (molH2O·m−2·s−1) | |
|---|---|---|---|---|
| W1 | T1 | 36.17 ± 0.74 g | 0.56 ± 0.09 cdef | 7.13 ± 0.10 g |
| T2 | 37.20 ± 0.38 f | 0.47 ± 0.04 f | 8.82 ± 0.42 ef | |
| T3 | 38.21 ± 0.86 e | 0.58 ± 0.02 bcde | 9.92 ± 0.60 cd | |
| T4 | 38.47 ± 0.49 e | 0.50 ± 0.07 def | 8.97 ± 0.63 ef | |
| W2 | T1 | 39.81 ± 0.14 d | 0.48 ± 0.07 ef | 8.35 ± 0.38 f |
| T2 | 40.45 ± 0.54 d | 0.63 ± 0.04 bc | 9.58 ± 0.37 de | |
| T3 | 40.72 ± 0.45 d | 0.65 ± 0.09 abc | 10.19 ± 0.62 bcd | |
| T4 | 42.11 ± 0.22 c | 0.60 ± 0.04 bcd | 10.86 ± 0.30 ab | |
| W3 | T1 | 42.49 ± 0.37 bc | 0.69 ± 0.06 ab | 9.38 ± 0.43 de |
| T2 | 42.80 ± 0.89 abc | 0.63 ± 0.05 bc | 10.91 ± 0.34 ab | |
| T3 | 43.37 ± 0.14 ab | 0.75 ± 0.05 a | 11.64 ± 0.52 a | |
| T4 | 43.68 ± 0.66 a | 0.61 ± 0.02 bcd | 10.47 ± 0.49 bc | |
| p | T | 0.000 *** | 0.005 ** | 0.000 *** |
| W | 0.000 *** | 0.000 *** | 0.000 *** | |
| T × W | 0.266 ns | 0.022 * | 0.021 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Zhong, Y.; Li, H.; Qiang, X.; Sun, L.; Gao, F.; Wang, G.; Liu, H. Foliar Selenium Application During Flowering and Fruiting Alleviates Drought-Induced Oxidative Damage and Promotes Tomato Growth. Horticulturae 2025, 11, 1242. https://doi.org/10.3390/horticulturae11101242
Cui H, Zhong Y, Li H, Qiang X, Sun L, Gao F, Wang G, Liu H. Foliar Selenium Application During Flowering and Fruiting Alleviates Drought-Induced Oxidative Damage and Promotes Tomato Growth. Horticulturae. 2025; 11(10):1242. https://doi.org/10.3390/horticulturae11101242
Chicago/Turabian StyleCui, Haixue, Yuan Zhong, Huanhuan Li, Xiaoman Qiang, Lijian Sun, Fukui Gao, Gang Wang, and Hao Liu. 2025. "Foliar Selenium Application During Flowering and Fruiting Alleviates Drought-Induced Oxidative Damage and Promotes Tomato Growth" Horticulturae 11, no. 10: 1242. https://doi.org/10.3390/horticulturae11101242
APA StyleCui, H., Zhong, Y., Li, H., Qiang, X., Sun, L., Gao, F., Wang, G., & Liu, H. (2025). Foliar Selenium Application During Flowering and Fruiting Alleviates Drought-Induced Oxidative Damage and Promotes Tomato Growth. Horticulturae, 11(10), 1242. https://doi.org/10.3390/horticulturae11101242








