Gray Mold in Blueberry: Current Research on Pathogenesis, Host Resistance, and Control Strategies
Abstract
1. Introduction
2. Pathological Mechanism of Gray Mold in Blueberry
3. Resistance Mechanisms of Blueberries to Gray Mold
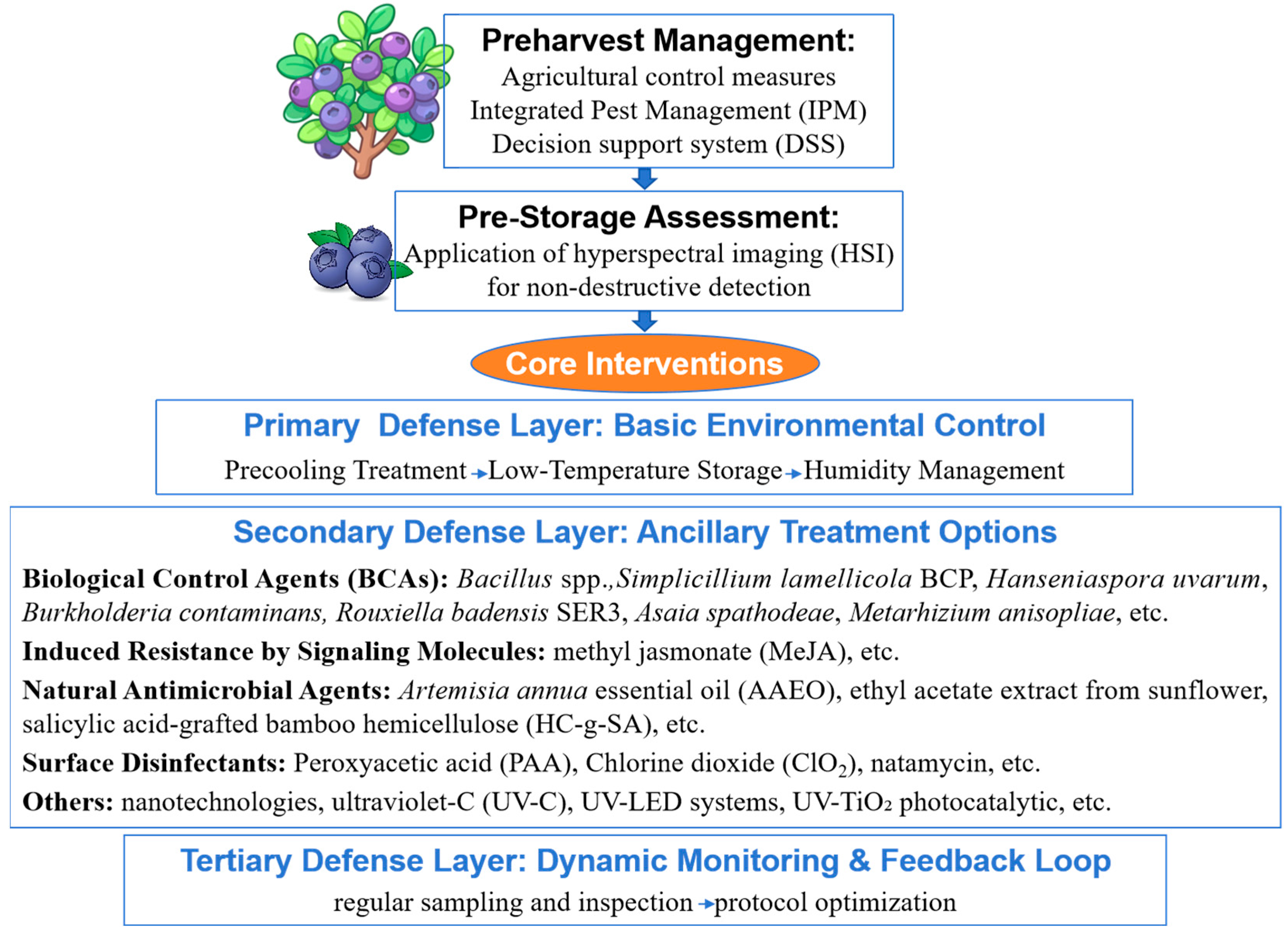
4. Research on the Control of Gray Mold in Blueberry
4.1. Chemical Control
4.2. Physical Control Measures
4.3. Biological Control Strategies
4.3.1. Antagonistic Microorganisms
4.3.2. Plant-Derived Antimicrobial Substances
5. Integrated Control and Sustainable Management
6. Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDA | potato dextrose agar |
| PAL | phenylalanine ammonia-lyase |
| C4H | cinnamic acid 4-hydroxylase |
| MeJA | methyl jasmonate |
| HC-g-SA | salicylic acid-grafted bamboo hemicellulose |
| NO | nitric oxide |
| H2O2 | hydrogen peroxide |
| PGIP | polygalacturonase-inhibiting protein |
| SDH | succinate dehydrogenase |
| SDHI | succinate dehydrogenase inhibitors |
| PAA | Peroxyacetic acid |
| UV | ultraviolet |
| VOCs | volatile organic compounds |
| dsRNA | double-stranded RNA |
| SIGS | spray-induced gene silencing |
| AAEO | Artemisia annua essential oil |
| SOD | superoxide dismutase |
| CAT | catalase |
| MDA | malondialdehyde |
| NOS | nitric oxide synthase |
References
- Wang, C.; Duan, T.; Shi, L.; Zhang, X.; Fan, W.; Wang, M.; Wang, J.; Ren, L.; Zhao, X.; Wang, Y. Characterization of volatile organic compounds produced by Bacillus siamensis YJ15 and their antifungal activity against Botrytis cinerea. Plant Dis. 2022, 106, 2321–2329. [Google Scholar] [CrossRef]
- Neugebauer, K.A.; Mattupalli, C.; Hu, M.; Oliver, J.E.; VanderWeide, J.; Lu, Y.; Sullivan, K.; Stockwell, V.O.; Oudemans, P.; Miles, T.D. Managing fruit rot diseases of Vaccinium corymbosum. Front. Plant Sci. 2024, 15, 1428769. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Wu, L.; Chen, P.; Li, X.; Wen, G.; Tangtrakulwanich, K.; Chethana, K.W.T.; Al-Otibi, F.; Hyde, K.D.; et al. Characterization of Fungal Pathogens Causing Blueberry Fruit Rot Disease in China. Pathogens 2025, 14, 201. [Google Scholar] [CrossRef]
- Stamelou, M.-L.; Sperdouli, I.; Pyrri, I.; Adamakis, I.-D.S.; Moustakas, M. Hormetic Responses of Photosystem II in Tomato to Botrytis cinerea. Plants 2021, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.; Hills, P.; Moore, J.P. Strigolactones GR-24 and Nijmegen Applications Result in Reduced Susceptibility of Tobacco and Grapevine Plantlets to Botrytis cinerea Infection. Plants 2023, 12, 3202. [Google Scholar] [CrossRef]
- Chang, N.; Liu, R.; Lu, C.; Lai, Y.; Xu, Q.; Yang, Y.; Li, Y.; Ling, J.; Xie, B.; Zhao, W.; et al. Role of Methyl thiobutyrate to Botrytis cinerea on cucumber. Front. Plant Sci. 2025, 16, 1551274. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, L.; Zhu, J.; Ren, J.; Liu, R.; Li, X.; Chen, Y.; Hou, H.; Tu, H. Inhibitory effect of 2-methylvaleraldehyde on Botrytis cinerea and its application in postharvest strawberry. Postharvest Biol. Technol. 2025, 230, 113775. [Google Scholar] [CrossRef]
- Rui, L.; Kong, W.-L.; Sun, X.-R.; Wu, X.-Q. First report of Botrytis leaf blight caused by Botrytis cinerea on Aucuba japonica in China. For. Pathol. 2023, 53, e12787. [Google Scholar] [CrossRef]
- Roca-Couso, R.; Flores-Félix, J.D.; Rivas, R. Mechanisms of Action of Microbial Biocontrol Agents against Botrytis cinerea. J. Fungi 2021, 7, 1045. [Google Scholar] [CrossRef]
- Li, Q.; Hou, Z.; Yu, J. First Report of Botrytis californica Causing Gray Mold on Blueberry in China. Plant Dis. 2023, 107, 3318. [Google Scholar] [CrossRef]
- Mamode, A.N.; Neetoo, H.; Ranghoo-Sanmukhiya, V.M.; Hardowar, S.; Vally, V.; Gungoosingh-Bunwaree, A.; Maudarbaccus, F.; Coutinho, T.A.; Vojvodić, M.; Bulajić, A. First Report of Botrytis cinerea Causing Gray Mold on Greenhouse-Grown Tomato Plants in Mauritius. Plant Dis. 2021, 105, 2725. [Google Scholar] [CrossRef]
- Kwon, J.H.; Cheon, M.G.; Choi, O.; Kim, J. First Report of Botrytis cinerea as a Postharvest Pathogen of Blueberry in Korea. Mycobiology 2011, 39, 52–53. [Google Scholar] [CrossRef]
- Cheon, W.; Jeon, Y.H. First Report of Gray Mold Caused by Botrytis cinerea on Greenhouse-Grown Zucchini in Korea. Plant Dis. 2013, 97, 1116. [Google Scholar] [CrossRef]
- Li, X.; Schnabel, G. First Report of Gray Mold of Blackberry Caused by Botrytis cinerea in South Carolina. Plant Dis. 2011, 95, 1592. [Google Scholar] [CrossRef]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef]
- Wang, H.C.; Li, L.C.; Cai, B.; Cai, L.T.; Chen, X.J.; Yu, Z.H.; Zhang, C.Q. Metabolic Phenotype Characterization of Botrytis cinerea, the Causal Agent of Gray Mold. Front. Microbiol. 2018, 9, 470. [Google Scholar] [CrossRef]
- Rivera, S.A.; Zoffoli, J.P.; Latorre, B.A. Infection Risk and Critical Period for the Postharvest Control of Gray Mold (Botrytis cinerea) on Blueberry in Chile. Plant Dis. 2013, 97, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Zuniga, A.I.; Peres, N.A. Potential Impact of Populations Drift on Botrytis Occurrence and Resistance to Multi- and Single-Site Fungicides in Florida Southern Highbush Blueberry Fields. Plant Dis. 2018, 102, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, L.; Xiao, S.; Chen, H.; Han, Y.; Niu, B.; Wu, W.; Gao, H. Ursolic acid, the main component of blueberry cuticular wax, inhibits Botrytis cinerea growth by damaging cell membrane integrity. Food Chem. 2023, 415, 135753. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kou, X.; Wu, C.; Fan, G.; Li, T. Methyl jasmonate induces the resistance of postharvest blueberry to gray mold caused by Botrytis cinerea. J. Sci. Food Agric. 2020, 100, 4272–4281. [Google Scholar] [CrossRef]
- Du, R.; Deng, J.; Huang, E.; Chen, L.; Tang, J.; Liu, Y.; Shi, Z.; Wang, F. Effects of salicylic acid-grafted bamboo hemicellulose on gray mold control in blueberry fruit: The phenylpropanoid pathway and peel microbial community composition. Int. J. Biol. Macromol. 2023, 251, 126303. [Google Scholar] [CrossRef]
- Qu, G.; Wu, W.; Ba, L.; Ma, C.; Ji, N.; Cao, S. Melatonin Enhances the Postharvest Disease Resistance of Blueberries Fruit by Modulating the Jasmonic Acid Signaling Pathway and Phenylpropanoid Metabolites. Front. Chem. 2022, 10, 957581. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, H.; Kou, X.; Wu, C.; Fan, G.; Li, T.; Zhou, D. Metabolomics analysis reveals that MeJA treatment induces postharvest blueberry resistance to Botrytis cinerea. Postharvest Biol. Technol. 2022, 194, 112075. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Wu, C.; Fan, G.; Li, T. Nitric Oxide and Hydrogen Peroxide Are Involved in Methyl Jasmonate-Regulated Response against Botrytis cinerea in Postharvest Blueberries. J. Agric. Food Chem. 2020, 68, 13632–13640. [Google Scholar] [CrossRef]
- Tao, N.; Liu, Y.; Zhang, B.; Guo, Y.; Wang, Q.; Li, Q. SlABCG9 Functioning as a Jasmonic Acid Transporter Influences Tomato Resistance to Botrytis cinerea. J. Agric. Food Chem. 2025, 73, 3897–3907. [Google Scholar] [CrossRef]
- Su, D.; Wu, M.; Wang, H.; Shu, P.; Song, H.; Deng, H.; Yu, S.; Garcia-Caparros, P.; Bouzayen, M.; Zhang, Y.; et al. Bi-functional transcription factor SlbHLH95 regulates fruits flavonoid metabolism and grey mould resistance in tomato. Plant Biotechnol. J. 2025, 23, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Qu, J.; Niu, H.; Lai, L.; Yuan, P.; Wang, Y.; Yang, N.; Wang, X.; Xi, Z.; Wang, X. VvATG18a participates in grape resistance to gray mold induced by BR signaling pathway. Int. J. Biol. Macromol. 2025, 297, 139877. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Y.; Lin, L.; Ying, S.; Yu, C. Cucumber PGIP2 is involved in resistance to gray mold disease. Gene 2024, 923, 148588. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhao, Y.; Ma, Z. Advances in Understanding Fungicide Resistance in Botrytis cinerea in China. Phytopathology 2021, 111, 455–463. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Amiri, A.; Zuniga, A.I.; Peres, N.A. Sources of Primary Inoculum of Botrytis cinerea and Their Impact on Fungicide Resistance Development in Commercial Strawberry Fields. Plant Dis. 2017, 101, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Cui, K.; Song, Y.; Mu, W.; Liu, F. High-Efficiency Control of Gray Mold by the Novel SDHI Fungicide Benzovindiflupyr Combined with a Reasonable Application Approach of Dipping Flower. J. Agric. Food Chem. 2018, 66, 6692–6698. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.; Roberto, S.R.; de Oliveira, A.G. Ultra-Structural Alterations in Botrytis cinerea—The Causal Agent of Gray Mold—Treated with Salt Solutions. Biomolecules 2019, 9, 582. [Google Scholar] [CrossRef]
- An, J.; Lan, W.; Fei, Q.; Li, P.; Wu, W. Synthesis, Antifungal, and Antibacterial Activities of Novel Benzoylurea Derivatives Containing a Pyrimidine Moiety. Molecules 2023, 28, 6498. [Google Scholar] [CrossRef]
- Pan, N.; Wang, H.; An, J.; Liu, C.; Chen, H.; Fei, Q.; Li, P.; Wu, W. Discovery of Novel Compounds for Combating Rising Severity of Plant Diseases Caused by Fungi and Viruses. ACS Omega 2024, 9, 1424–1435. [Google Scholar] [CrossRef]
- Meng, K.; Deng, T.; Liu, M.; Pu, H.; Zhang, Y.; Zou, H.; Xing, Y.; Xue, W. Novel flavonoid derivatives containing 1,2,4-triazole Schiff bases as potential antifungal agents: Design, synthesis, and biological evaluation. Bioorg. Chem. 2024, 153, 107965. [Google Scholar] [CrossRef]
- Liu, C.; Fei, Q.; Pan, N.; Wu, W. Design, Synthesis, and Antifungal Activity of Novel 1,2,4-Triazolo [4,3-c] trifluoromethylpyrimidine Derivatives Bearing the Thioether Moiety. Front. Chem. 2022, 10, 939644. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Li, G.T.; Chen, Y. Synthesis and Antifungal Activity of Aldehydes-Thiourea Derivatives as Promising Antifungal Agents Against Postharvest Gray Mold Disease. Chem. Biodivers. 2025, 22, e202403131. [Google Scholar] [CrossRef]
- Abbey, J.A.; Alzohairy, S.A.; Neugebauer, K.A.; Hatlen, R.J.; Miles, T.D. Fungicide resistance in Botrytis cinerea and identification of Botrytis species associated with blueberry in Michigan. Front. Microbiol. 2024, 15, 1425392. [Google Scholar] [CrossRef]
- Baral, R.; DeLong, J.A.; McGhee, G.C.; Stockwell, V.; Mattupalli, C. Molecular Characterization of Botrytis Isolates from Blueberry and Red Raspberry in the Pacific Northwest with Resistance to SDHI Fungicides. Plant Dis. 2025. [Google Scholar] [CrossRef]
- Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide Resistance Profiling in Botrytis cinerea Populations from Blueberry in California and Washington and Their Impact on Control of Gray Mold. Plant Dis. 2016, 100, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Wang, F.; Obenland, D.; Xiao, C.L. Effects of Peroxyacetic Acid on Postharvest Diseases and Quality of Blueberries. Plant Dis. 2021, 105, 3231–3237. [Google Scholar] [CrossRef]
- Hatamzadeh, S.; Akbari Oghaz, N.; Rahnama, K.; Noori, F. Comparison of the Antifungal Activity of Chlorine Dioxide, Peracetic Acid and Some Chemical Fungicides in Post-harvest Management of Penicillium digitatum and Botrytis cinerea Infecting Sweet Orange and Strawberry Fruits. Agric. Res. 2024, 13, 72–84. [Google Scholar] [CrossRef]
- Saito, S.; Wang, F.; Xiao, C.-L. Natamycin as a postharvest treatment to control gray mold on stored blueberry fruit caused by multi-fungicide resistant Botrytis cinerea. Postharvest Biol. Technol. 2022, 187, 111862. [Google Scholar] [CrossRef]
- Li, T.; Zhou, J.; Yuan, Z.; Liu, R.Y.; Li, J. Intermittent changes in temperature and humidity repress gray mold in tomato. Plant Dis. 2023, 107, 306–314. [Google Scholar] [CrossRef]
- Falagán, N.; Miclo, T.; Terry, L.A. Graduated Controlled Atmosphere: A Novel Approach to Increase ‘Duke’ Blueberry Storage Life. Front. Plant Sci. 2020, 11, 00221. [Google Scholar] [CrossRef]
- Chen, W.; Deng, J.; Wang, D.; Yang, H.; Yang, J.; Puangsin, B.; He, X.; Shi, Z. Slow-release antimicrobial preservation composite coating based on bamboo-derived xylan—A new way to preserve blueberry freshness. Food Chem. 2025, 463, 141291. [Google Scholar] [CrossRef]
- Haley, O.C.; Pliakoni, E.D.; Rivard, C.; Nwadike, L.; Bhullar, M. The Attenuation of Microbial Reduction in Blueberry Fruit Following UV-LED Treatment. J. Food Prot. 2023, 86, 100056. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Shahbaz, H.M.; Kim, J.U.; Lee, H.; Lee, D.-U.; Park, J. Efficacy of UV-TiO2 photocatalysis technology for inactivation of Escherichia coli K12 on the surface of blueberries and a model agar matrix and the influence of surface characteristics. Food Microbiol. 2018, 76, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Cai, J.; Tang, S.; Pang, C.; Luo, J.; Wang, X. Carboxylated cellulose nanocrystals mediated flower-like zinc oxide for antimicrobial without activation of light. J. Colloid Interface Sci. 2025, 683, 906–917. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Lv, J.; Zhang, X.; Li, Y.; Han, X.; Zhang, W. Development of starch-based films reinforced with curcumin-loaded nanocomplexes: Characterization and application in the preservation of blueberries. Int. J. Biol. Macromol. 2024, 264, 130464. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, H.; Zhang, L.; Xu, Y. Clove essential oil loaded chitosan nanocapsules on quality and shelf-life of blueberries. Int. J. Biol. Macromol. 2023, 249, 126091. [Google Scholar] [CrossRef]
- Bouhadi, M.; Javed, Q.; Jakubus, M.; Elkouali, M.; Fougrach, H.; Ansar, A.; Ban, S.G.; Ban, D.; Heath, D.; Černe, M. Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks. Agronomy 2025, 15, 1131. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Valencia, A.L.; Olivares, D.; Poblete-Morales, M.; Silva-Moreno, E.; Defilippi, B.G. Antifungal effect of volatile organic compounds (VOCs) release from Antarctic bacteria under postharvest conditions. Food Packag. Shelf Life 2023, 39, 101160. [Google Scholar] [CrossRef]
- Chacón, F.I.; Sineli, P.E.; Mansilla, F.I.; Pereyra, M.M.; Diaz, M.A.; Volentini, S.I.; Poehlein, A.; Meinhardt, F.; Daniel, R.; Dib, J.R. Native Cultivable Bacteria from the Blueberry Microbiome as Novel Potential Biocontrol Agents. Microorganisms 2022, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cedeño, L.R.; de los Santos-Villalobos, S.; Santoyo, G. Functional and Genomic Analysis of Rouxiella badensis SER3 as a Novel Biocontrol Agent of Fungal Pathogens. Front. Microbiol. 2021, 12, 709855. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Rivadeneira, M.F.; Bello, F.; Giudici, V.N.; Musumeci, M.A. Burkholderia contaminans isolated from root of blueberry shrub produces occidiofungins and pyrrolnitrin useful to prevent postharvest decays of blueberry fruits caused by Botrytis cinerea. Pest Manag Sci. 2025, 81, 6265–6279. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.; Aminian, H.; Remize, F.; Sheikh, M.; Ebrahimi, L. Integrated control of blue and gray molds of apples with antagonistic yeasts combined with carbon dioxide or ozone. J. Plant Pathol. 2021, 103, 943–953. [Google Scholar] [CrossRef]
- Li, J.; Yang, T.; Yuan, F.; Lv, X.; Zhou, Y. Inhibitory Effect and Potential Antagonistic Mechanism of Isolated Epiphytic Yeasts against Botrytis cinerea and Alternaria alternata in Postharvest Blueberry Fruits. Foods 2024, 13, 1334. [Google Scholar] [CrossRef]
- Du, Q.; Li, R.; Liu, L.; Chen, L.; Tang, J.; Deng, J.; Wang, F. Application of Bacillus tequilensis for the control of gray mold caused by Botrytis cinerea in blueberry and mechanisms of action: Inducing phenylpropanoid pathway metabolism. Front. Microbiol. 2024, 15, 1455008. [Google Scholar] [CrossRef]
- Romero-Contreras, Y.J.; Gonzalez-Serrano, F.; Formey, D.; Aragón, W.; Chacón Florencia, I.; Torres, M.; Cevallos, M.Á.; Dib, J.R.; Rebollar, E.A.; Serrano, M. Amphibian skin bacteria display antifungal activity and induce plant defense mechanisms against Botrytis cinerea. Front. Plant Sci. 2024, 15, 1392637. [Google Scholar] [CrossRef]
- Sarven, M.S.; Hao, Q.; Deng, J.; Yang, F.; Wang, G.; Xiao, Y.; Xiao, X. Biological Control of Tomato Gray Mold Caused by Botrytis Cinerea with the Entomopathogenic Fungus Metarhizium anisopliae. Pathogens 2020, 9, 213. [Google Scholar] [CrossRef]
- Islam, M.T.; Sherif, S.M. RNAi-Based Biofungicides as a Promising Next-Generation Strategy for Controlling Devastating Gray Mold Diseases. Int. J. Mol. Sci. 2020, 21, 2072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Z.; Wang, C.; Tan, B.; Luo, X. New and Antifungal Diterpenoids of Sunflower against Gray Mold. J. Agric. Food Chem. 2023, 71, 16647–16656. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Wang, L.; Li, M.; Wang, Y.; Liang, J.; Wang, D.; Zhang, H. Investigating the antifungal mechanism of Artemisia argyi essential oil against Botrytis cinerea, Alternaria alternata, and Penicillium digitatum and its application in extending blueberry shelf life. Int. J. Food Microbiol. 2025, 439, 111262. [Google Scholar] [CrossRef]
- Santoro, K.; Maghenzani, M.; Chiabrando, V.; Bosio, P.; Gullino, M.L.; Spadaro, D.; Giacalone, G. Thyme and Savory Essential Oil Vapor Treatments Control Brown Rot and Improve the Storage Quality of Peaches and Nectarines, but Could Favor Gray Mold. Foods 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Valeria, A.; Vittorio, R.; Giorgia, F. Efficacy of preharvest application of biocontrol agents against gray mold in grapevine. Front. Plant Sci. 2023, 14, 1154370. [Google Scholar] [CrossRef]
- Amiri, A.; Zuniga, A.I.; Peres, N.A. Prevalence of Botrytis Cryptic Species in Strawberry Nursery Transplants and Strawberry and Blueberry Commercial Fields in the Eastern United States. Plant Dis. 2018, 102, 398–404. [Google Scholar] [CrossRef]
- Romanazzi, G.; Gabler, F.M.; Margosan, D.; Mackey, B.E.; Smilanick, J.L. Effect of chitosan dissolved in different acids on its ability to control postharvest gray mold of table grape. Phytopathology 2009, 99, 1028–1036. [Google Scholar] [CrossRef]
- Qi, X.; Ogden, E.L.; Bostan, H.; Sargent, D.J.; Ward, J.; Gilbert, J.; Iorizzo, M.; Rowland, L.J. High-Density Linkage Map Construction and QTL Identification in a Diploid Blueberry Mapping Population. Front. Plant Sci. 2021, 12, 692628. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Sirohi, R.; Ahluwalia, V.; Zhou, Y.; Sindhu, R.; Binod, P.; Singhnia, R.R.; Patel, A.K.; Juneja, A.; et al. Sustainable blueberry waste recycling towards biorefinery strategy and circular bioeconomy: A review. Bioresour. Technol. 2021, 332, 125181. [Google Scholar] [CrossRef]
- Shin, T.S.; Yu, N.H.; Lee, J.; Choi, G.J.; Kim, J.; Shin, C.S. Development of a Biofungicide Using a Mycoparasitic Fungus Simplicillium lamellicola BCP and Its Control Efficacy against Gray Mold Diseases of Tomato and Ginseng. Plant Pathol. J. 2017, 33, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, M. Improved grey mould management on blueberries using the iLeaf-Botrytis-Blueberry-Model decision support system. Acta Hortic. 2022, 1349, 221–226. [Google Scholar] [CrossRef]
- Haghbin, N.; Bakhshipour, A.; Zareiforoush, H.; Mousanejad, S. Non-destructive pre-symptomatic detection of gray mold infection in kiwifruit using hyperspectral data and chemometrics. Plant Methods 2023, 19, 53. [Google Scholar] [CrossRef]
- Richards, J.K.; Xiao, C.; Jurick, W.M. Botrytis spp.: A Contemporary Perspective and Synthesis of Recent Scientific Developments of a Widespread Genus that Threatens Global Food Security. Phytopathology 2021, 111, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mao, H.; Zhang, X.; Liu, Y.; Du, X. A Rapid Detection Method for Tomato Gray Mold Spores in Greenhouse Based on Microfluidic Chip Enrichment and Lens-Less Diffraction Image Processing. Foods 2021, 10, 3011. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Zhao, Q.; Deng, J.; Cui, L.; Zhang, T.; Yang, Q.; Zhao, S. Gray Mold in Blueberry: Current Research on Pathogenesis, Host Resistance, and Control Strategies. Horticulturae 2025, 11, 1241. https://doi.org/10.3390/horticulturae11101241
Xiao L, Zhao Q, Deng J, Cui L, Zhang T, Yang Q, Zhao S. Gray Mold in Blueberry: Current Research on Pathogenesis, Host Resistance, and Control Strategies. Horticulturae. 2025; 11(10):1241. https://doi.org/10.3390/horticulturae11101241
Chicago/Turabian StyleXiao, Lifeng, Qiuyue Zhao, Jie Deng, Lingyan Cui, Tingting Zhang, Qin Yang, and Sifeng Zhao. 2025. "Gray Mold in Blueberry: Current Research on Pathogenesis, Host Resistance, and Control Strategies" Horticulturae 11, no. 10: 1241. https://doi.org/10.3390/horticulturae11101241
APA StyleXiao, L., Zhao, Q., Deng, J., Cui, L., Zhang, T., Yang, Q., & Zhao, S. (2025). Gray Mold in Blueberry: Current Research on Pathogenesis, Host Resistance, and Control Strategies. Horticulturae, 11(10), 1241. https://doi.org/10.3390/horticulturae11101241




