Integration of Physiological and Transcriptomic Analyses Provides Insights into the Regulatory Mechanisms of Adventitious Root Formation in Phoebe bournei Cuttings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Morphological and Anatomical Observations
2.3. Determination of Physiological Parameters
2.4. Transcriptome Sequencing and Data Analysis
2.5. Weighted Gene Co-Expression Network Analysis (WGCNA) Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Validation
2.7. Statistical Analysis
3. Results
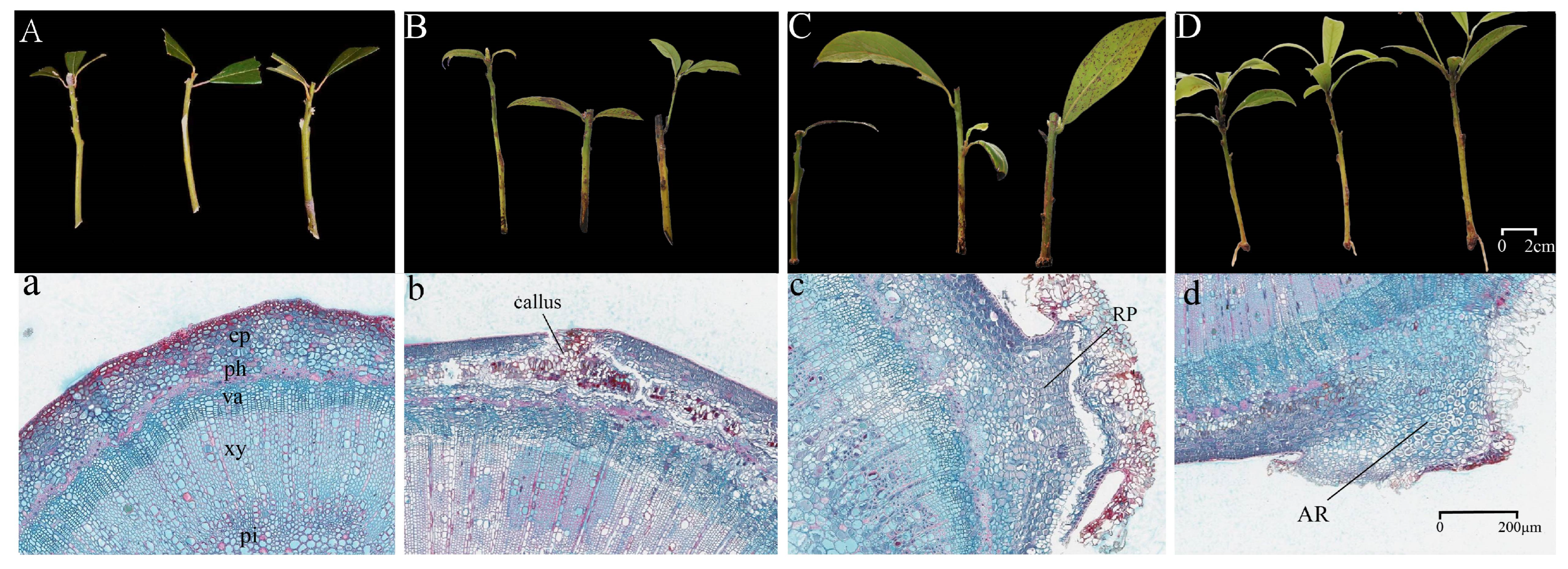
3.1. Morphological and Anatomical Changes During AR Formation
3.2. Changes in Physiological Parameters During AR Formation in P. bournei Cuttings
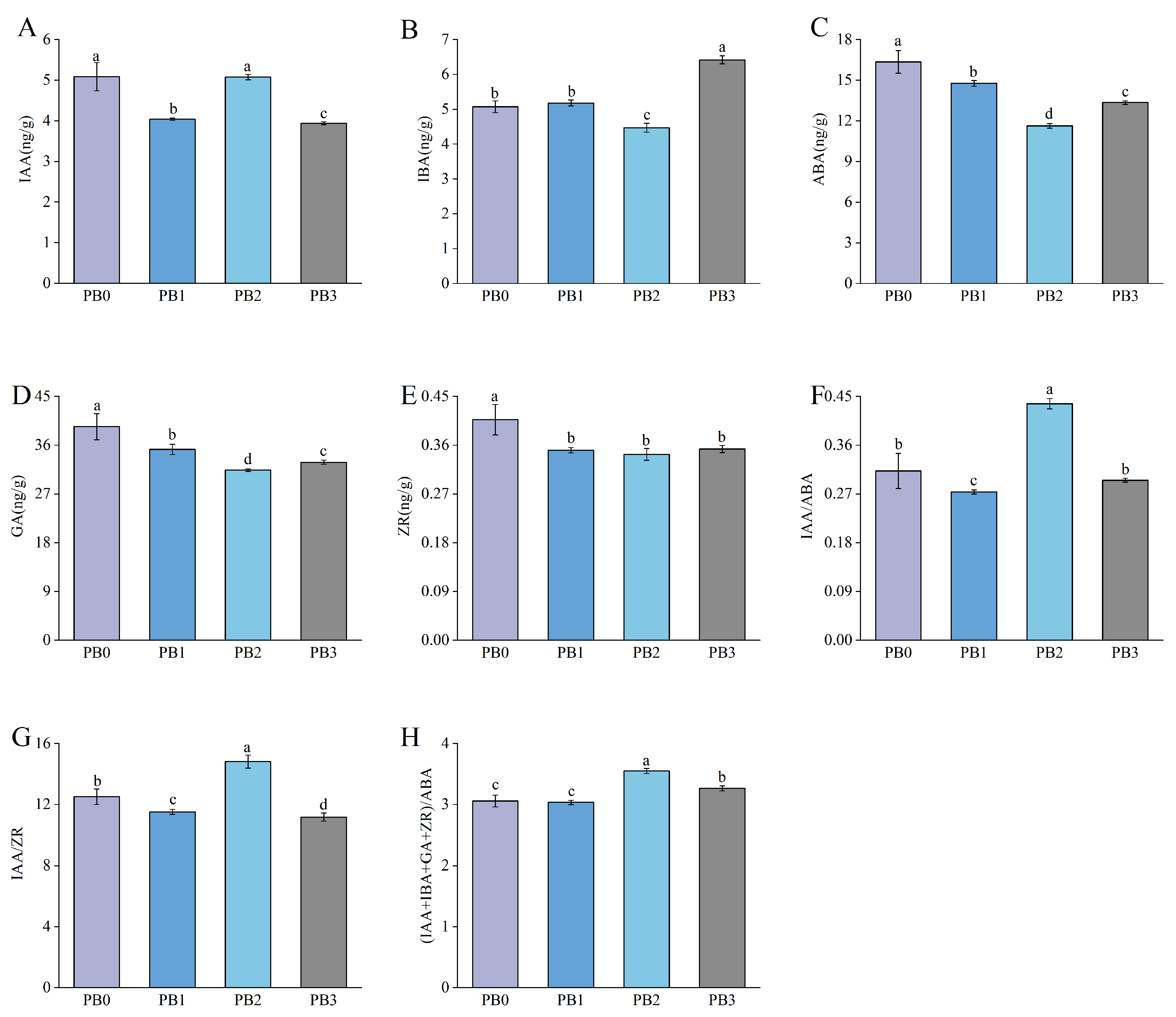
3.3. Changes in Endogenous Hormone Levels During AR Formation
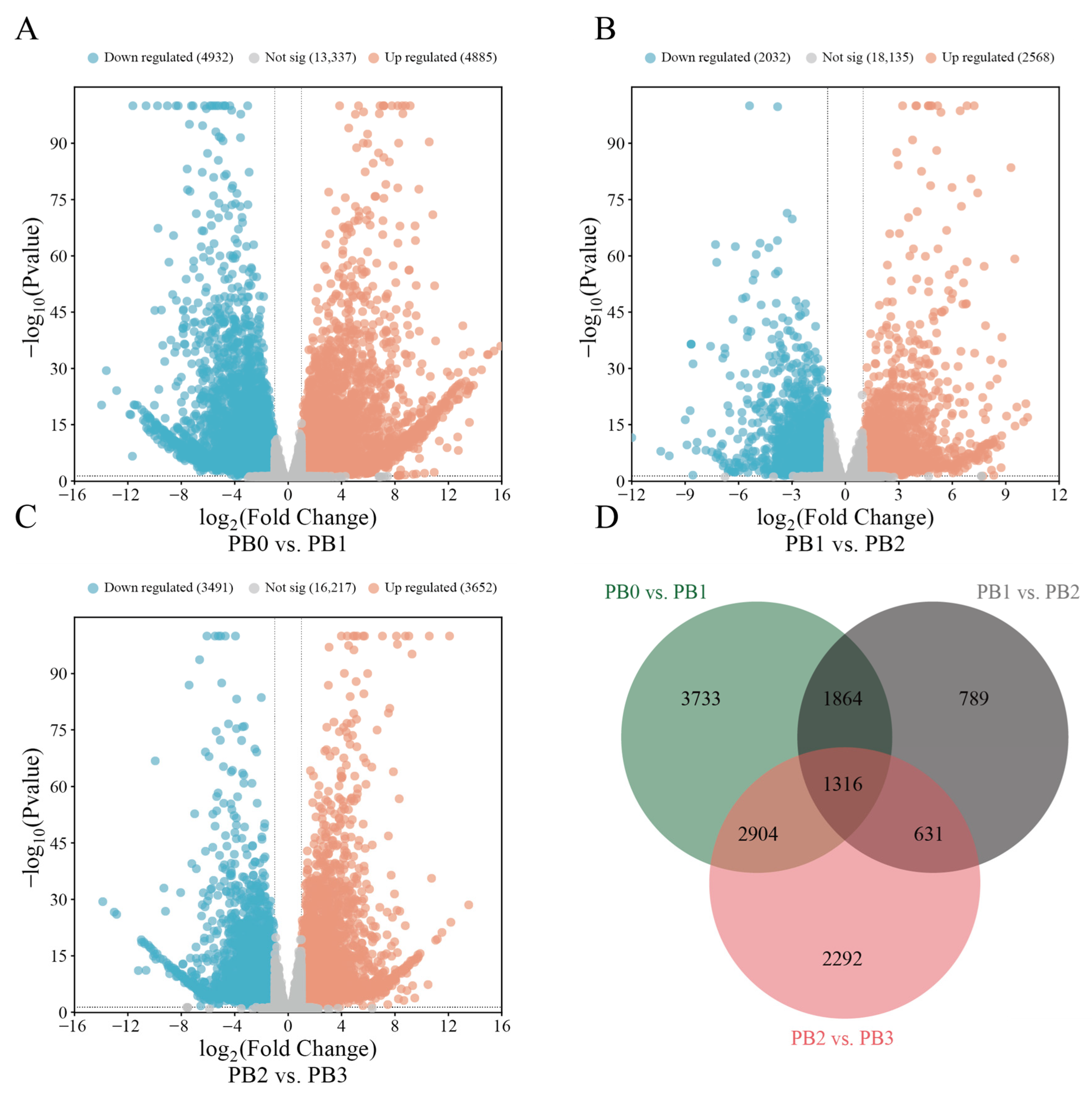
3.4. Transcriptomic Analysis of AR Formation
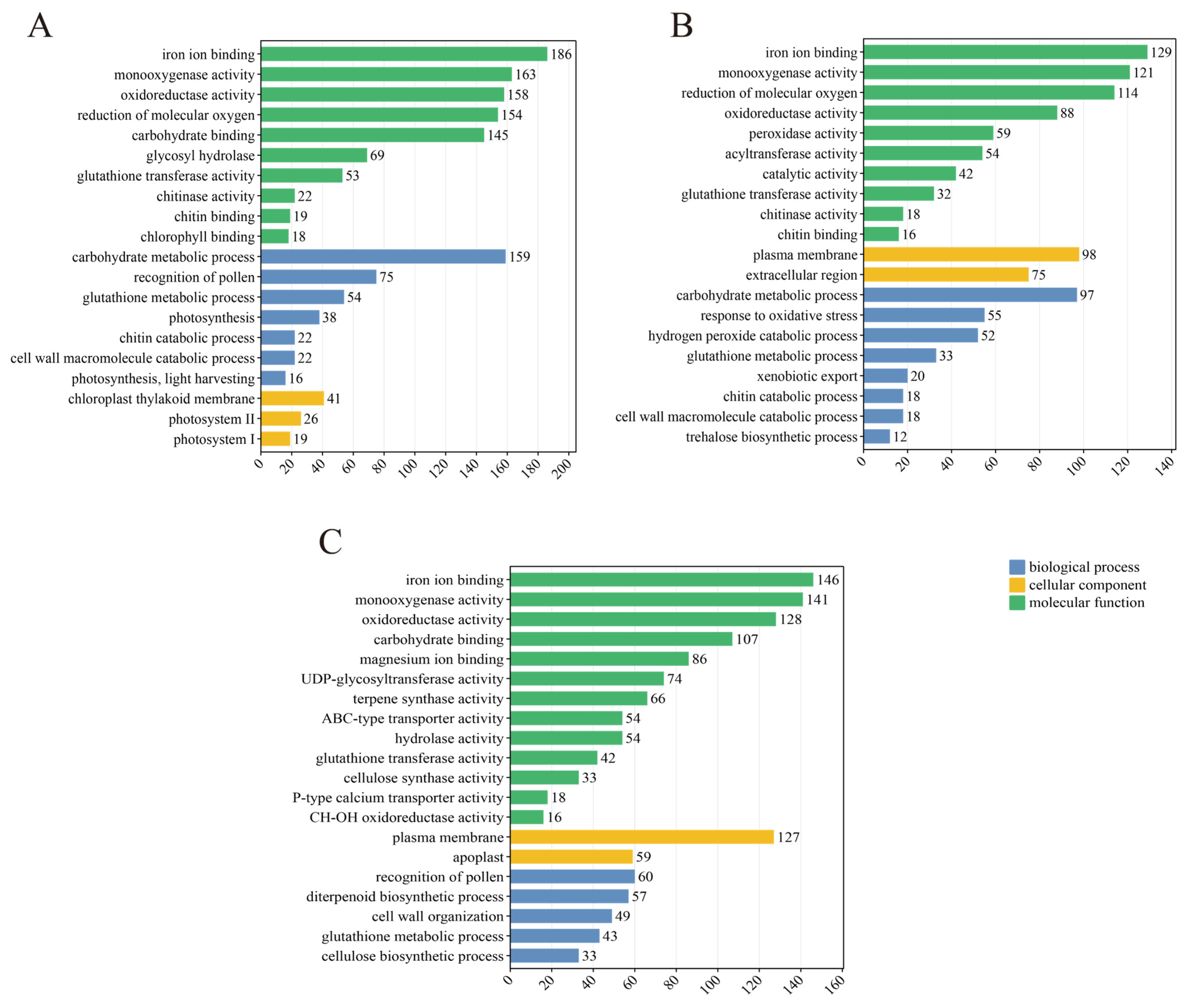
3.5. Functional Annotation and Enrichment Analysis of DEGs
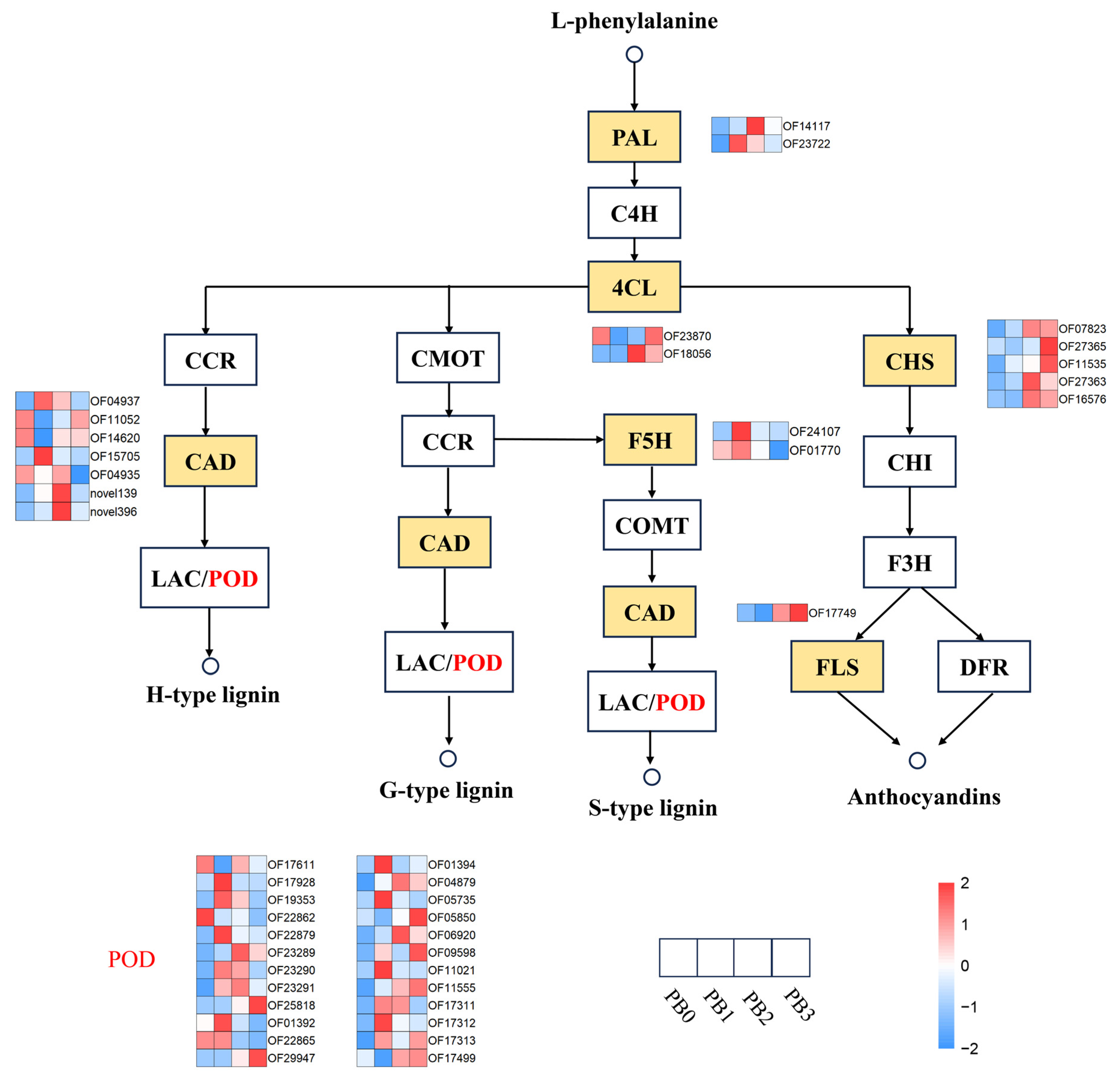
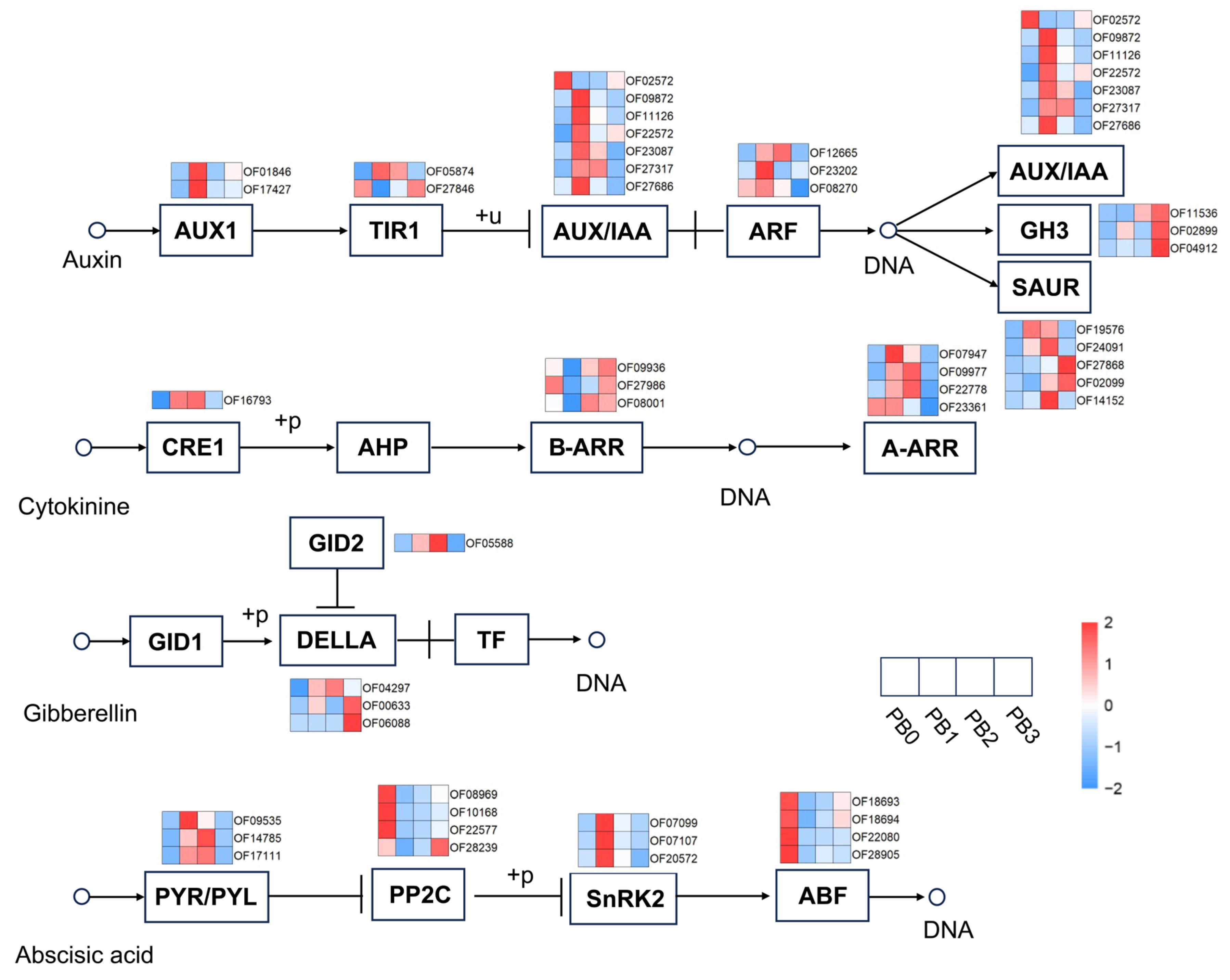
3.6. Phenylpropanoid Biosynthesis and Plant Hormone Signal Transduction in AR Formation
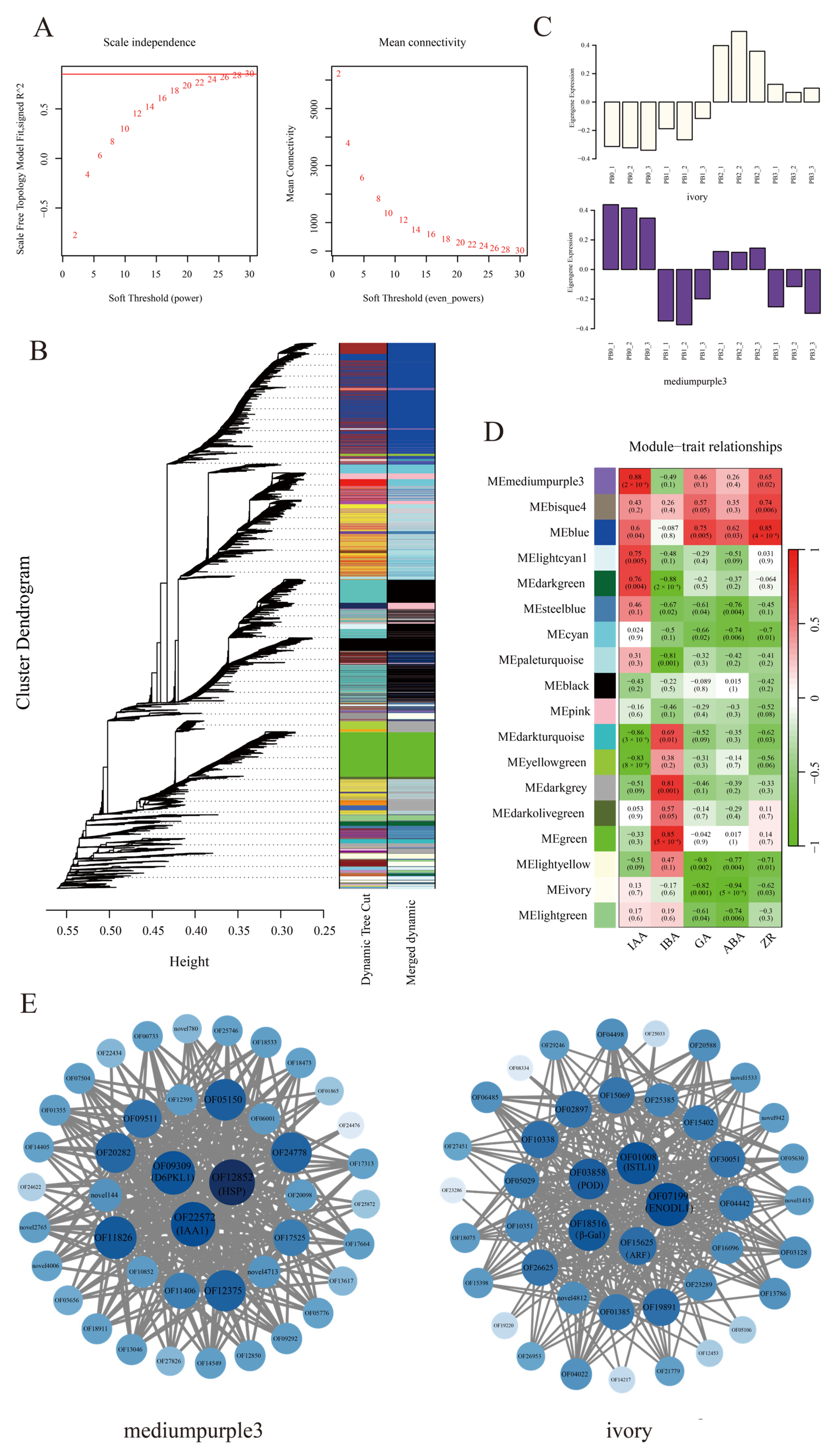
3.7. WGCNA of DEGs and Hormone Traits in AR Formation
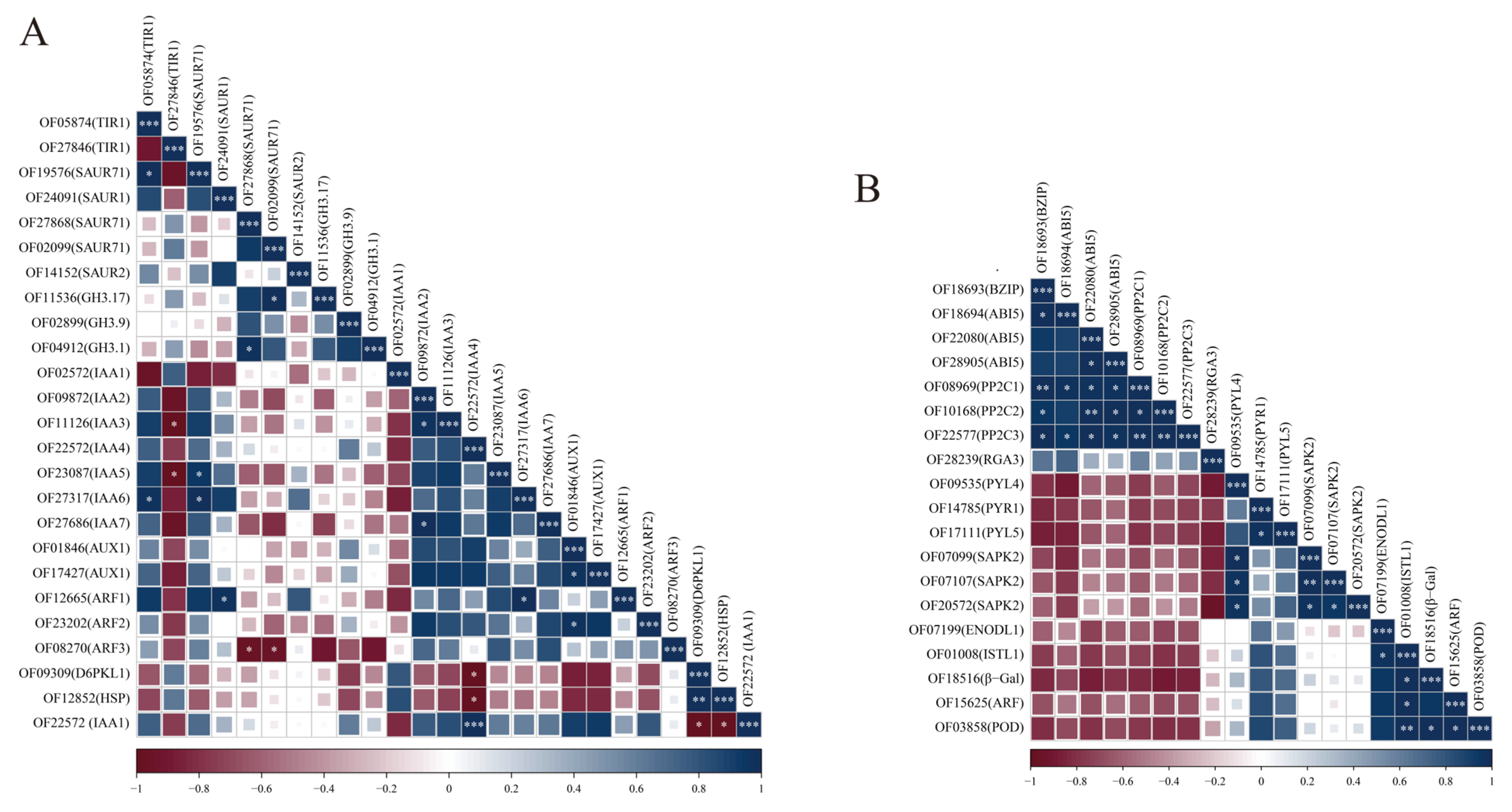
3.8. Correlation Analysis of DEGs and Hub Genes in ABA and IAA Signal Transduction Pathways
4. Discussion
4.1. AR Formation in P. bournei Cuttings
4.2. Physiological Responses During AR Formation
4.3. Regulatory Roles of Endogenous IAA and ABA Signaling
4.4. Dual Regulatory Role of Phenylpropanoid Biosynthesis in AR Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Zhang, J.; Han, S.; Chong, S.L.; Meng, G.; Song, M.; Wang, Y.; Zhou, S.; Liu, C.; Lou, L. The chromosome-scale genome of Phoebe bournei reveals contrasting fates of terpene synthase (TPS)-a and TPS-b subfamilies. Plant Commun. 2022, 3, 100410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Ma, J.; Zhang, X.; Xu, L. Phenotypic variation in Phoebe bournei populations preserved in the primary distribution area. J. For. Res. 2018, 29, 35–44. [Google Scholar] [CrossRef]
- Xu, W.T.; Zhang, M.; Wang, C.; Lou, X.Z.; Han, X.; Zhang, J.L.; Zhang, Y.T.; Tong, Z.K. Somatic embryo induction and Agrobacterium-mediated transformation of embryonic callus tissue in Phoebe bournei, an endangered woody species in Lauraceae. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 572–587. [Google Scholar] [CrossRef]
- Wetzstein, H.Y.; Porter, J.A.; Janick, J.; Ferreira, J.F.S.; Mutui, T.M. Selection and clonal propagation of high artemisinin genotypes of Artemisia annua. Front. Plant Sci. 2018, 9, 358. [Google Scholar] [CrossRef]
- Tate, H.T.; Page, T. Cutting propagation of Santalum austrocaledonicum: The effect of genotype, cutting source, cutting size, propagation medium, IBA and irradiance. New For. 2018, 49, 551–570. [Google Scholar] [CrossRef]
- Li, T.; Min, X. Dormancy characteristics and germination requirements of Phoebe bournei seed. Sci. Hortic. 2020, 260, 108903. [Google Scholar] [CrossRef]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What makes adventitious roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, J.; Liao, T.; Wang, Y.; Guo, L.; Yao, Y.; Cao, J. Histological dissection of cutting-inducible adventitious rooting in Platycladus orientalis reveals developmental endogenous hormonal homeostasis. Ind. Crops Prod. 2021, 170, 113817. [Google Scholar] [CrossRef]
- Wang, S.T.; Sun, G.D.; Luo, Y.; Qian, W.J.; Fan, K.; Ding, Z.T.; Hu, J.H. Role of IAA and Primary Metabolites in Two Rounds of Adventitious Root Formation in Softwood Cuttings of Camellia sinensis (L.). Agronomy 2022, 12, 2486. [Google Scholar] [CrossRef]
- Ma, W.Y.; Li, J.J.; Qu, B.Y.; He, X.; Zhao, X.Q.; Li, B.; Fu, X.D.; Tong, Y.P. Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J. 2014, 78, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Habets, M.E.J.; Offringa, R. PIN-driven polar auxin transport in plant developmental plasticity: A key target for environmental and endogenous signals. New Phytol. 2014, 203, 362–377. [Google Scholar] [CrossRef]
- Ugartechea-Chirino, Y.; Swarup, R.; Swarup, K.; Péret, B.; Whitworth, M.; Bennett, M.; Bougourd, S. The AUX1 LAX family of auxin influx carriers is required for the establishment of embryonic root cell organization in Arabidopsis thaliana. Ann. Bot. 2009, 105, 277–289. [Google Scholar] [CrossRef]
- Tahir, M.M.; Mao, J.P.; Li, S.H.; Li, K.; Liu, Y.; Shao, Y.; Zhang, D.; Zhang, X.Y. Insights into factors controlling adventitious root formation in apples. Horticulturae 2022, 8, 276. [Google Scholar] [CrossRef]
- Meng, X.Y.; Wang, Z.; He, S.L.; Shi, L.Y.; Song, Y.L.; Lou, X.Y.; He, D. Endogenous hormone levels and activities of IAA-modifying enzymes during adventitious rooting of tree peony cuttings and grafted scions. Hortic. Environ. Biotechnol. 2019, 60, 187–197. [Google Scholar] [CrossRef]
- Cisse, E.H.M.; Zhang, J.; Li, D.-D.; Miao, L.F.; Yin, L.Y.; Yang, F. Exogenous ABA and IAA modulate physiological and hormonal adaptation strategies in Cleistocalyx operculatus and Syzygium jambos under long-term waterlogging conditions. BMC Plant Biol. 2022, 22, 523. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Liang, J.; Qiao, Y.; Yan, Y.; Li, L.; Dai, Y. Involvement of G1-to-S transition and AhAUX-dependent auxin transport in abscisic acid-induced inhibition of lateral root primodia initiation in Arachis hypogaea L. J. Plant Physiol. 2012, 169, 1102–1111. [Google Scholar] [CrossRef]
- Tartoura, K.A.H. Effect of abscisic acid on endogenous IAA, auxin protector levels and peroxidase activity during adventitious root initiation in Vigna radiata cuttings. Acta Physiol. Plant. 2001, 23, 149–156. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Farag, N.B.B.; Massoud, H.Y.; Kasem, M.M. Exogenous IBA stimulated adventitious root formation of Zanthoxylum beecheyanum K. Koch stem cutting: Histo-physiological and phytohormonal investigation. Plant Physiol. Biochem. 2023, 197, 107639. [Google Scholar] [CrossRef]
- Kuroha, T.; Kato, H.; Asami, T.; Yoshida, S.; Kamada, H.; Satoh, S. A trans-zeatin riboside in root xylem sap negatively regulates adventitious root formation on cucumber hypocotyls. J. Exp. Bot. 2002, 53, 2193–2200. [Google Scholar] [CrossRef]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhry, S.; Kepinski, S. Auxin in root development. Cold Spring Harb. Perspect. Biol. 2022, 14, 039933. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Xu, Q.Q.; Liao, W.B.; Ma, Z.J.; Xu, X.T.; Wang, M.; Ren, P.J.; Niu, L.J.; Jin, X.; Zhu, Y.C. Hydrogen peroxide is involved in abscisic acid-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J. Plant Biol. 2016, 59, 536–548. [Google Scholar] [CrossRef]
- Li, S.W.; Shi, R.F.; Leng, Y.; Zhou, Y. Transcriptomic analysis reveals the gene expression profile that specifically responds to IBA during adventitious rooting in mung bean seedlings. BMC Genom. 2016, 17, 43. [Google Scholar] [CrossRef]
- Chang, E.M.; Guo, W.; Xie, Y.H.; Jiang, Z.P.; Dong, Y.; Jia, Z.R.; Zhao, X.L.; Liu, J.F.; Zhang, J. Changes of lignified-callus and wound-induced adventitious rooting in ancient Platycladus orientalis cuttings as affected by tree age. Ind. Crops Prod. 2023, 203, 117183. [Google Scholar] [CrossRef]
- Palama, T.L.; Menard, P.; Fock, I.; Choi, Y.H.; Bourdon, E.; Govinden-Soulange, J.; Bahut, M.; Payet, B.; Verpoorte, R.; Kodja, H. Shoot differentiation from protocorm callus cultures of Vanilla planifolia (Orchidaceae): Proteomic and metabolic responses at early stage. BMC Plant Biol. 2010, 10, 82. [Google Scholar] [CrossRef]
- Aumond, M.L., Jr.; de Araujo, A.T., Jr.; de Oliveira Junkes, C.F.; de Almeida, M.R.; Matsuura, H.N.; de Costa, F.; Fett-Neto, A.G. Events associated with early age-related decline in adventitious rooting competence of Eucalyptus globulus Labill. Front. Plant Sci. 2017, 8, 1734. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Wen, S.S.; Miao, D.P.; Cui, H.Y.; Li, S.H.; Gu, Y.N.; Jia, R.R.; Leng, Y.F. Physiology and transcriptomic analysis of endogenous hormones regulating in vitro adventitious root formation in tree peony. Sci. Hortic. 2023, 318, 112122. [Google Scholar] [CrossRef]
- Yu, W.W.; Li, Y.H.; Zhang, Y.T.; Luo, C.L.; Zheng, Y.J.; Zhang, T.; Fu, C.; Liu, X.L. Integrated Physiological and Transcriptomic Analyses Reveal Mechanisms Regulating Endogenous Phytohormones in Adventitious Root Formation During Cinnamomum bodinieri Cutting Propagation. Forests 2025, 16, 509. [Google Scholar] [CrossRef]
- Moreno-Sanz, P.; D’Amato, E.; Nebish, A.; Costantini, L.; Grando, M. An optimized histological proceeding to study the female gametophyte development in grapevine. Plant Methods 2020, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Ji, Z.Y.; Han, Y.Y.; Sun, Y. The mitigation effects of exogenous dopamine treatment on continuous cropping obstacles in watermelon. J. Soil Sci. Plant Nutr. 2023, 23, 4233–4249. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Feng, Y.Q.; Zhou, Q.; Jiang, T. Chamaecyparis lawsoniana with different cutting mediums and growth regulators. J. West China For. Sci. 2017, 46, 32–37. (In Chinese) [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cheng, L.B.; Zhao, C.; Zhao, M.R.; Han, Y.Y.; Li, S.Y. Lignin Synthesis, Affected by Sucrose in Lotus (Nelumbo nucifera) Seedlings, Was Involved in Regulation of Root Formation in the Arabidopsis thanliana. Int. J. Mol. Sci. 2022, 23, 2250. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Nitric oxide induced modulations in adventitious root growth, lignin content and lignin synthesizing enzymes in the hypocotyls of Vigna radiata. Plant Physiol. Biochem. 2019, 141, 225–230. [Google Scholar] [CrossRef]
- Luo, C.L.; Zhang, T.; Dai, X.Y.; Zhang, Y.T.; Zheng, Y.J.; Liu, X.L.; Zhang, X.H. Physiological characteristics and transcriptomic analysis of young stems differentiation in adventitious bud and root formation in Cinnamomum parthenoxylon. Forests 2025, 16, 1049. [Google Scholar] [CrossRef]
- Luo, C.L.; Liu, X.L.; Zheng, Y.J.; Dai, X.Y.; Tang, X.L.; Zhang, T.; Zhang, X.H. Physiological and transcriptomic analysis reveal the regulation of adventitious root formation in Cinnamomum parthenoxylon cuttings. BMC Plant Biol. 2024, 24, 1217. [Google Scholar] [CrossRef]
- Bao, S.H.; Li, J.N.; Wang, J.Q.; Lan, T.; Wei, M.Y.; Sun, X.Y.; Fang, Y.L.; Ma, T.T. How Does Nature Create the Painting “Gradient Coloration of ‘Manicure Finger’ Grape”? Integrated Omics Unveil the Pigments Basis and Metabolism Networks of Its Formation. Food Front. 2025, 6, 921–939. [Google Scholar]
- Ni, L.J.; Wang, J.F.; Zhou, F.W.; Chen, Z.M. Integrated multi-omics reveals Li-miR828z-LiMYB114 regulatory module controlling anthocyanin biosynthesis during flower color development in Lagerstroemia indica. Ind. Crops Prod. 2025, 234, 121524. [Google Scholar]
- Martins, M.; Gomes, A.F.G.; da Silva, É.M.; da Silva, D.F.; Peche, P.M.; Magalhães, T.A.; Pio, R. Effects of anatomical structures and phenolic compound deposition on the rooting of olive cuttings. Rhizosphere 2022, 23, 100557. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar]
- Zhang, W.X.; Fan, J.J.; Tan, Q.Q.; Zhao, M.M.; Zhou, T.; Cao, F.L. The effects of exogenous hormones on rooting process and the activities of key enzymes of Malus hupehensis stem cuttings. PLoS ONE 2017, 12, e0172320. [Google Scholar]
- Roussos, P.A. Adventitious root formation in plants: The implication of hydrogen peroxide and nitric oxide. Antioxidants 2023, 12, 862. [Google Scholar] [CrossRef]
- Kora, D.; Bhattacharjee, S. Redox gateway associated with adventitious root formation under stress and hormonal signalling in plants. Curr. Sci. 2020, 119, 462–472. [Google Scholar] [CrossRef]
- Ilczuk, A.; Jacygrad, E. The effect of IBA on anatomical changes and antioxidant enzyme activity during the in vitro rooting of smoke tree (Cotinus coggygria Scop.). Sci. Hortic. 2016, 210, 268–276. [Google Scholar] [CrossRef]
- Ueda, Y.; Uehara, N.; Sasaki, H.; Kobayashi, K.; Yamakawa, T. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol. Biochem. 2013, 70, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Montagu, M.v.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar]
- Druege, U.; Hilo, A.; Pérez-Pérez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.; Franken, P.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of plant hormone response pathways. Annu. Rev. Plant Biol. 2020, 71, 327–353. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, Y. Adaptive growth: Shaping auxin-mediated root system architecture. Trends Plant Sci. 2020, 25, 121–123. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, W.; Wang, Q.W.; Cao, Y.Y.; Xu, F.Y.; Dodd, I.C.; Xu, W.F. ABA regulation of root growth during soil drying and recovery can involve auxin response. Plant Cell Environ. 2022, 45, 871–883. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef]
- Spartz, A.K.; Lee, S.H.; Wenger, J.P.; Gonzalez, N.; Itoh, H.; Inzé, D.; Peer, W.A.; Murphy, A.S.; Overvoorde, P.J.; Gray, W.M. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012, 70, 978–990. [Google Scholar] [CrossRef]
- Stanislas, T.; Hüser, A.; Barbosa, I.C.R.; Kiefer, C.S.; Brackmann, K.; Pietra, S.; Gustavsson, A.; Zourelidou, M.; Schwechheimer, C.; Grebe, M. Arabidopsis D6PK is a lipid domain-dependent mediator of root epidermal planar polarity. Nat. Plants 2015, 1, 15162. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Kumar, R.; Min, D.; Jagadish, S.V.K. Genetic and molecular mechanisms underlying root architecture and function under heat stress—A hidden story. Plant Cell Environ. 2022, 45, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Yuan, W.; Wang, Y.; Garcia-Maquilon, I.; Dang, X.L.; Li, Y.; Zhang, J.H.; Zhu, Y.Y.; Rodriguez, P.L.; Xu, W.F. Low ABA concentration promotes root growth and hydrotropism through relief of ABA INSENSITIVE 1-mediated inhibition of plasma membrane H+-ATPase 2. Sci. Adv. 2021, 7, 12. [Google Scholar] [CrossRef]
- Zeng, Y.; Verstraeten, I.; Trinh, H.K.; Heugebaert, T.; Stevens, C.V.; Garcia-Maquilon, I.; Rodriguez, P.L.; Vanneste, S.; Geelen, D. Arabidopsis hypocotyl adventitious root formation is suppressed by ABA signaling. Genes 2021, 12, 1141. [Google Scholar] [CrossRef]
- Fidler, J.; Graska, J.; Gietler, M.; Nykiel, M.; Prabucka, B.; Rybarczyk-Płońska, A.; Muszyńska, E.; Morkunas, I.; Labudda, M. PYR/PYL/RCAR receptors play a vital role in the abscisic-acid-dependent responses of plants to external or internal stimuli. Cells 2022, 11, 1352. [Google Scholar] [CrossRef]
- Buono, R.A.; Paez-Valencia, J.; Miller, N.D.; Goodman, K.; Spitzer, C.; Spalding, E.P.; Otegui, M.S. Role of SKD1 regulators LIP5 and IST1-LIKE1 in endosomal sorting and plant development. Plant Physiol. 2016, 171, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Ntui, V.O.; Tripathi, J.N.; Shah, T.; Tripathi, L. Targeted knockout of early nodulin-like 3 (MusaENODL3) gene in banana reveals its function in resistance to Xanthomonas wilt disease. Plant Biotechnol. J. 2024, 22, 1101–1112. [Google Scholar] [CrossRef]
- Geshi, N.; Johansen, J.N.; Dilokpimol, A.; Rolland, A.; Belcram, K.; Verger, S.; Kotake, T.; Tsumuraya, Y.; Kaneko, S.; Tryfona, T. A galactosyltransferase acting on arabinogalactan protein glycans is essential for embryo development in Arabidopsis. Plant J. 2013, 76, 128–137. [Google Scholar] [CrossRef]
- Dubey, S.M.; Han, S.; Stutzman, N.; Prigge, M.J.; Medvecká, E.; Platre, M.P.; Busch, W.; Fendrych, M.; Estelle, M. The AFB1 auxin receptor controls the cytoplasmic auxin response pathway in Arabidopsis thaliana. Mol. Plant 2023, 16, 1120–1130. [Google Scholar] [CrossRef]
- Booker, F.L.; Miller, J.E. Phenylpropanoid metabolism and phenolic composition of soybean [Glycine max (L.) Merr.] leaves following exposure to ozone. J. Exp. Bot. 1998, 49, 1191–1202. [Google Scholar] [CrossRef]
- Stevens, M.E.; Woeste, K.E.; Pijut, P.M. Localized gene expression changes during adventitious root formation in black walnut (Juglans nigra L.). Tree Physiol. 2018, 38, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Z.; Xu, P.P.; He, S.L.; da Silva, J.A.T.; Tanaka, M. Dynamic changes in enzyme activities and phenolic content during in vitro rooting of tree peony (Paeonia suffruticosa Andr.) plantlets. Maejo Int. J. Sci. Technol. 2011, 5, 252. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, H.; Zheng, Y.; Luo, C.; Zhang, Y.; Liu, X.; Wu, Y. Integration of Physiological and Transcriptomic Analyses Provides Insights into the Regulatory Mechanisms of Adventitious Root Formation in Phoebe bournei Cuttings. Horticulturae 2025, 11, 1238. https://doi.org/10.3390/horticulturae11101238
Li Y, Xu H, Zheng Y, Luo C, Zhang Y, Liu X, Wu Y. Integration of Physiological and Transcriptomic Analyses Provides Insights into the Regulatory Mechanisms of Adventitious Root Formation in Phoebe bournei Cuttings. Horticulturae. 2025; 11(10):1238. https://doi.org/10.3390/horticulturae11101238
Chicago/Turabian StyleLi, Yuhua, Haining Xu, Yongjie Zheng, Chenglin Luo, Yueting Zhang, Xinliang Liu, and Yanfang Wu. 2025. "Integration of Physiological and Transcriptomic Analyses Provides Insights into the Regulatory Mechanisms of Adventitious Root Formation in Phoebe bournei Cuttings" Horticulturae 11, no. 10: 1238. https://doi.org/10.3390/horticulturae11101238
APA StyleLi, Y., Xu, H., Zheng, Y., Luo, C., Zhang, Y., Liu, X., & Wu, Y. (2025). Integration of Physiological and Transcriptomic Analyses Provides Insights into the Regulatory Mechanisms of Adventitious Root Formation in Phoebe bournei Cuttings. Horticulturae, 11(10), 1238. https://doi.org/10.3390/horticulturae11101238





