Nutritional Composition, Bioactive Components and Antioxidant Activity of Garden Cress (Lepidium sativum L.) Grown Under Deficit Irrigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Water Restriction Treatments
2.3. Determination of Phenolic Compound Amounts of Garden Cress Cultivars
2.4. Determination of Antioxidant Enzymes of Garden Cress Cultivars
2.5. Determination of Anthocyanin Content of Garden Cress Cultivars
2.6. Determination of Sugar Composition of Garden Cress Cultivars
2.7. Determination of Vitamin Content of Garden Cress Cultivars
2.8. Amino Acid Composition of Garden Cress Cultivars
2.9. Statistical Analysis
3. Results
3.1. Phenolic Compounds of Garden Cresses
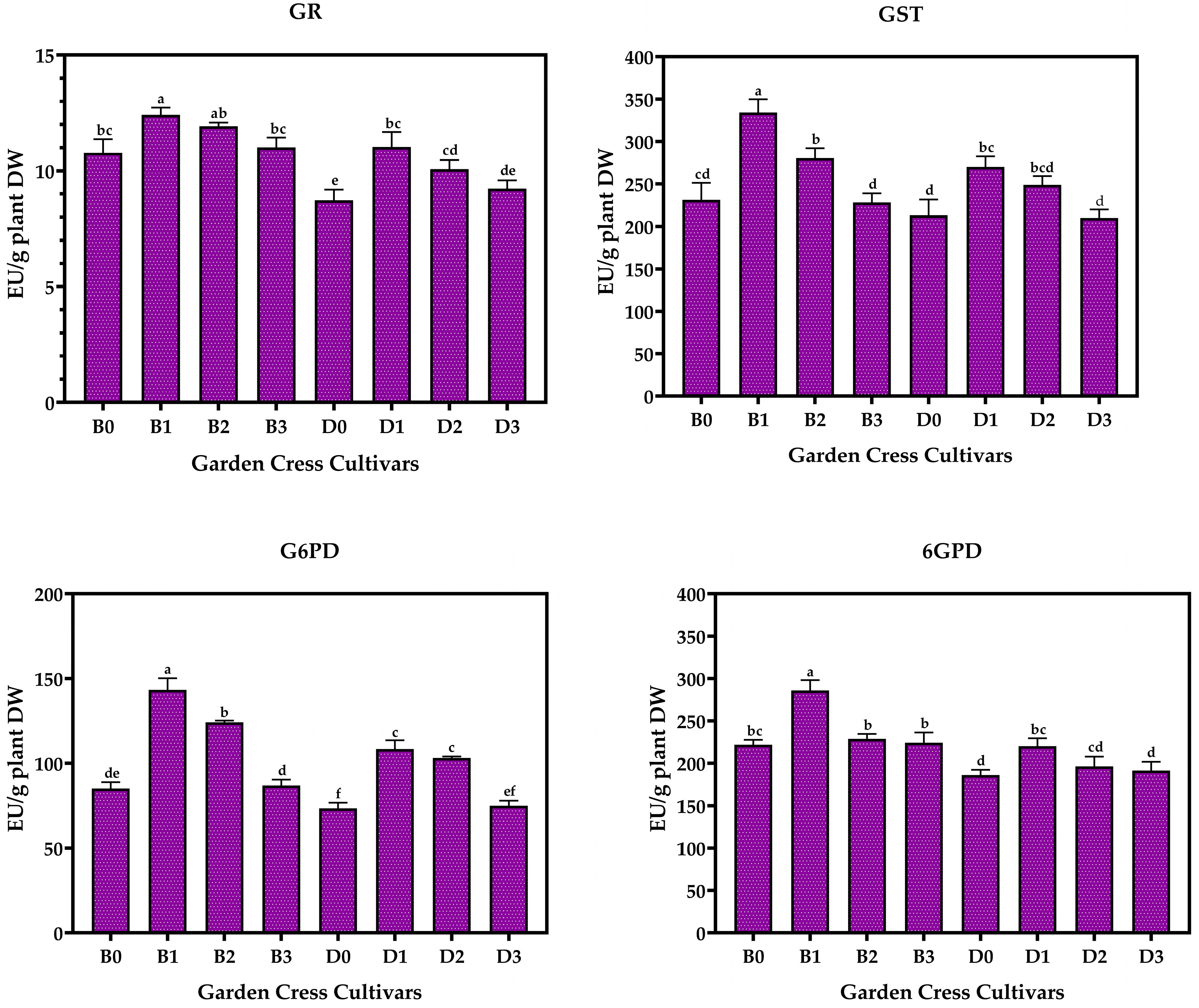
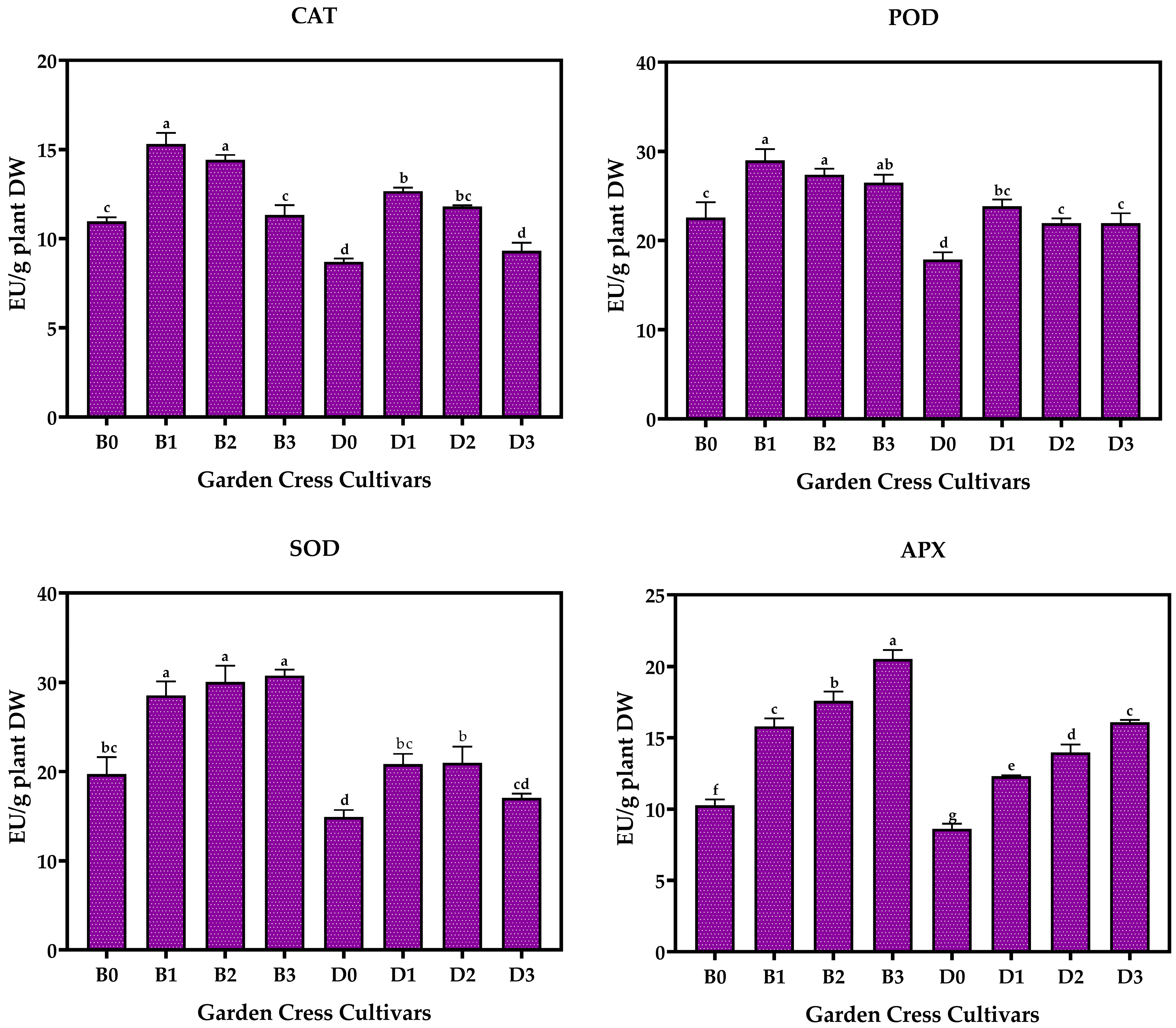
3.2. Antioxidant Enzyme Levels of Garden Cresses
3.3. Anthocyanin Content of Garden Cresses
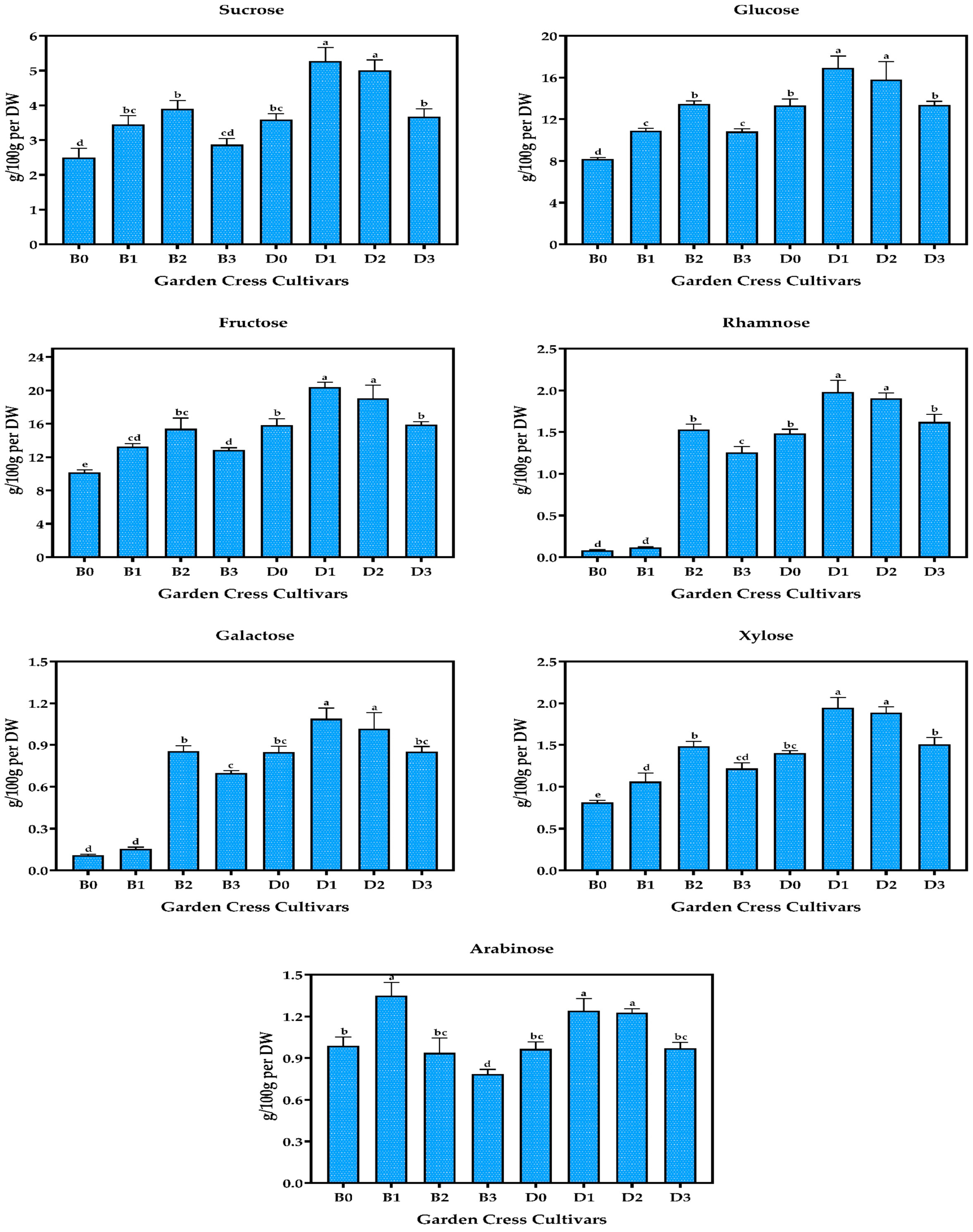
3.4. Sugar Composition of Garden Cresses
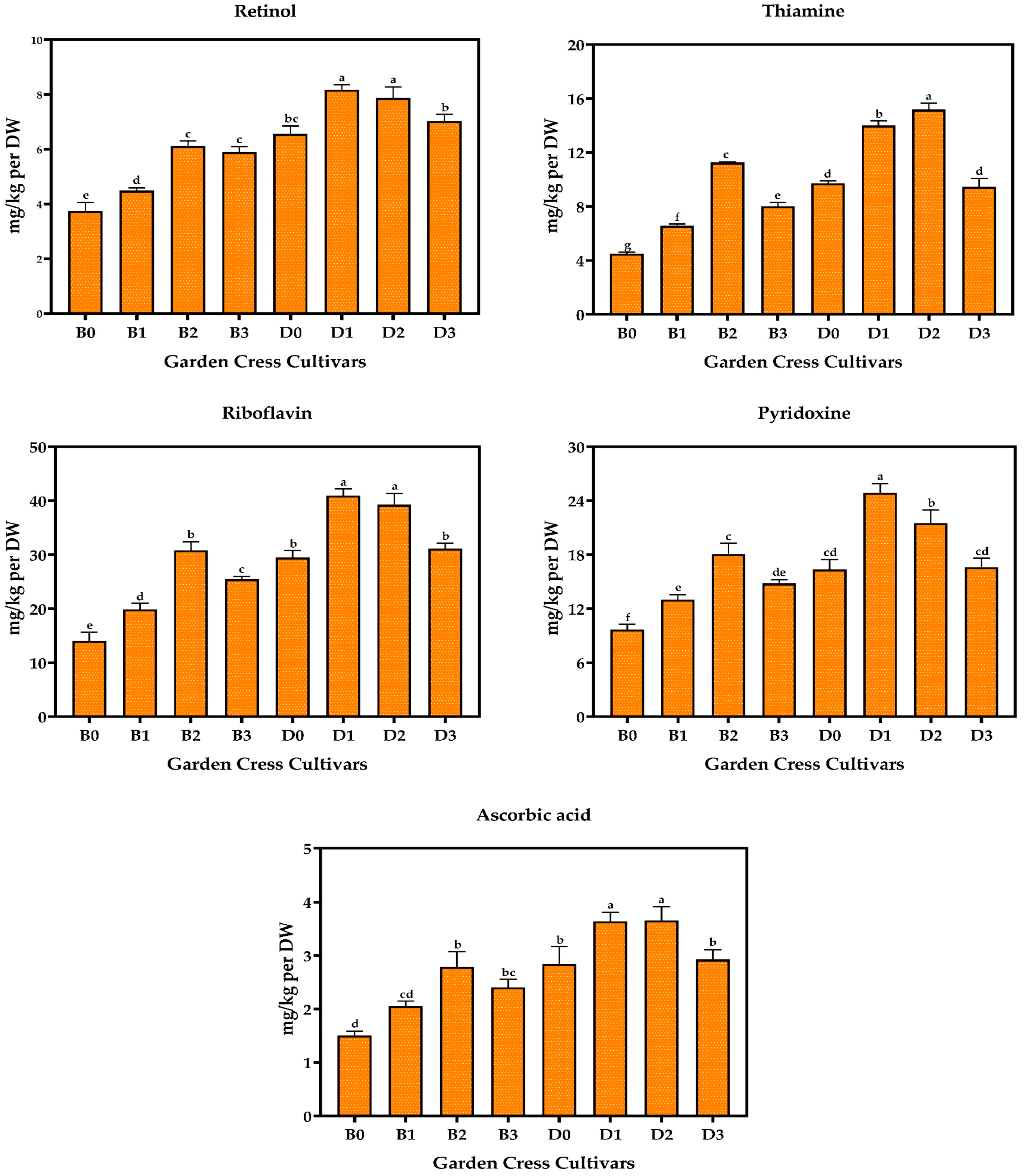
3.5. Vitamin Content of Garden Cresses
3.6. Amino Acid Composition of Garden Cresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Ekinci, M.; Ors, S.; Sahin, U.; Yildirim, E.; Dursun, A. Responses to the irrigation water amount of spinach supplemented with organic amendment in greenhouse conditions. Commun. Soil Sci. Plant Anal. 2015, 46, 327–342. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture, 1st ed.; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Bistgani, Z.E.; Barker, A.V.; Hashemi, M. Physiology of medicinal and aromatic plants under drought stress. Crop J. 2024, 12, 330–339. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Sefidkon, F. Review on ethnobotany, phytochemical, molecular and pharmacological activity of Thymus daenensis Celak. Biocatal. Agric. Biotechnol. 2019, 22, 101400. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Barakat, A.Z.; Mohamed, S.A. Garden cress gum and maltodextrin as microencapsulation coats for entrapment of garden cress phenolic-rich extract: Improved thermal stability, storage stability, antioxidant and antibacterial activities. Food Sci. Biotechnol. 2023, 32, 47–58. [Google Scholar] [CrossRef]
- Annunziata, A.; Pascale, P. Consumers’ behaviours and attitudes toward healthy food products: The case of Organic and Functional foods. In Proceedings of the European Association of Agricultural Economists (EAAE) 113th Seminar, Chania, Crete, Greece, 3–6 September 2009. [Google Scholar]
- Kiremit, M.S.; Osman, H.M.; Arslan, H. Response of yield, growth traits, and leaf nutrients of garden cress to deficit saline irrigation waters. J. Plant Nutr. 2023, 46, 1050–1065. [Google Scholar] [CrossRef]
- Tufail, T.; Khan, T.; Bader Ul Ain, H.; Morya, S.; Shah, M.A. Garden cress seeds: A review on nutritional composition, therapeutic potential, and industrial utilization. Food Sci. Nutr. 2024, 12, 3834–3848. [Google Scholar] [CrossRef] [PubMed]
- Azene, M.; Habte, K.; Tkuwab, H. Nutritional, health benefits and toxicity of underutilized garden cress seeds and its functional food products: A review. Food Prod. Process. Nutr. 2022, 4, 33. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, S. Nutritional composition, nutraceutical aspects, and medicinal benefits of garden cress (Lepidium sativum) seeds–A geographical and processing perspective. Trends Food Sci. Technol. 2024, 154, 104791. [Google Scholar]
- Seifarth, J.; Inamine, H.; Buckling, A.; Shea, K. Duration and timing interactions of early-life stress and the potential for recovery. Ecosphere 2021, 12, e03620. [Google Scholar] [CrossRef]
- Xu, X.; Feng, J.; Lü, S.; Lohrey, G.T.; An, H.; Zhou, Y.; Jenks, M.A. Leaf cuticular lipids on the Shandong and Yukon ecotypes of saltwater cress, Eutrema salsugineum, and their response to water deficiency and impact on cuticle permeability. Physiol. Plant. 2014, 151, 446–458. [Google Scholar] [CrossRef]
- El-Sattar, A.; Amira, M.; Tawfik, E.; Ahmed, E.Z. Physiological and genetical responses of Lepidium sativum L. seeds to Ultrasonic pretreatment under heat stress. Egypt. J. Bot. 2024, 64, 257–275. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Ozkan, G.; Yetim, H.; Ekici, L.; Yilmaz, M.T. RP-HPLC–DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chem. 2011, 126, 1749–1758. [Google Scholar] [CrossRef]
- Kaya, O.; Ates, F.; Kara, Z.; Turan, M.; Gutiérrez-Gamboa, G. Study of primary and secondary metabolites of stenospermocarpic, parthenocarpic and seeded raisin varieties. Horticulturae 2022, 8, 1030. [Google Scholar] [CrossRef]
- Keskin, N.; Kaya, O.; Ates, F.; Turan, M.; Gutiérrez-Gamboa, G. Drying grapes after the application of different dipping solutions: Effects on hormones, minerals, vitamins, and antioxidant enzymes in Gök Üzüm (Vitis vinifera L.) raisins. Plants 2022, 11, 529. [Google Scholar] [CrossRef]
- Fang, Z.-Z.; Lin-Wang, K.; Zhou, D.-R.; Lin, Y.-J.; Jiang, C.-C.; Pan, S.-L.; Espley, R.V.; Andre, C.M.; Ye, X.F. Activation of PsMYB10.2 transcription causes anthocyanin accumulation in flesh of the red-fleshed mutant of ‘Sanyueli’ (Prunus salicina Lindl.). Front. Plant Sci. 2021, 12, 680469. [Google Scholar] [CrossRef] [PubMed]
- Nikolidaki, E.K.; Chiou, A.; Christea, M.; Gkegka, A.P.; Karvelas, M.; Karathanos, V.T. Sun dried Corinthian currant (Vitis Vinifera L., var. Apyrena) simple sugar profile and macronutrient characterization. Food Chem. 2017, 221, 365–372. [Google Scholar] [CrossRef]
- Mohagheghian, B.; Saeidi, G.; Arzani, A. Phenolic compounds, antioxidant enzymes, and oxidative stress in barley (Hordeum vulgare L.) genotypes under field drought-stress conditions. BMC Plant Biol. 2025, 25, 709. [Google Scholar] [CrossRef]
- Yang, F.; Hu, J.; Li, J.; Wu, X.; Qian, Y. Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul. 2009, 58, 131–136. [Google Scholar] [CrossRef]
- Nasirzadeh, L.; Sorkhilaleloo, B.; Majidi Hervan, E.; Fatehi, F. Changes in antioxidant enzyme activities and gene expression profiles under drought stress in tolerant, intermediate, and susceptible wheat genotypes. Cereal Res. Commun. 2021, 49, 83–89. [Google Scholar] [CrossRef]
- Espadas, J.L.; Castaño, E.; Marina, M.L.; Rodríguez, L.C.; Plaza, M. Phenolic compounds increase their concentration in Carica papaya leaves under drought stress. Acta Physiol. Plant. 2019, 41, 180. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress enhances the synthesis of secondary plant products: The impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Nikzad, S.; Mirmohammady Maibody, S.A.M.; Ehtemam, M.H.; Golkar, P.; Mohammadi, S.A. Response of seed yield and biochemical traits of Eruca sativa Mill. to drought stress in a collection study. Sci. Rep. 2023, 13, 11157. [Google Scholar] [CrossRef]
- Hou, P.; Wang, F.; Luo, B.; Li, A.; Wang, C.; Shabala, L.; Ahmed, H.A.I.; Deng, S.; Zhang, H.; Song, P.; et al. Antioxidant enzymatic activity and osmotic adjustment as components of the drought tolerance mechanism in Carex duriuscula. Plants 2021, 10, 436. [Google Scholar] [CrossRef]
- DaCosta, M.; Huang, B. Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. J. Am. Soc. Hortic. Sci. 2007, 132, 319–326. [Google Scholar] [CrossRef]
- Šerbo, A.; Fazlić, D.; Hadžić, A.; Sinanović, Ć.Z.; Murtić, S. Positive effects of drought on the quality of aromatic plants: Improvement of total phenolic and flavonoid contents and total antioxidant capacity. Int. J. Agric. Nat. Sci. 2024, 17, 304–312. [Google Scholar]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Miao, Y.; Ayyaz, A.; Huang, Q.; Hannan, F.; Zou, H.-X.; Zhang, K.; Yan, X.; Farooq, M.A.; Zhou, W. Anthocyanin accumulation enhances drought tolerance in purple-leaf Brassica napus: Transcriptomic, metabolomic, and physiological evidence. Ind. Crops Prod. 2025, 223, 120149. [Google Scholar] [CrossRef]
- Ünal, N.; Okatan, V. Effects of drought stress treatment on phytochemical contents of strawberry varieties. Sci. Hortic. 2023, 316, 112013. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Impact of drought stress on vitamin C and anthocyanin content in cultivated lettuces (Lactuca sativa L.) and wild relatives (Lactuca spp.). Front. Plant Sci. 2024, 15, 1369658. [Google Scholar] [CrossRef]
- Hinojosa-Gómez, J.; San Martín-Hernández, C.; Heredia, J.B.; León-Félix, J.; Osuna-Enciso, T.; Muy-Rangel, M.D. Anthocyanin induction by drought stress in the calyx of roselle cultivars. Molecules 2020, 25, 1555. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Isayenkov, S.V. The role of anthocyanins in plant tolerance to drought and salt stresses. Plants 2023, 12, 2558. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, X.; Qu, Z.; Wang, X. Effects of drought stress at different stages on soluble sugar content of soybeans. Plant Soil Environ. 2023, 69, 500–511. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 2019, 141, 164–171. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhang, Y.; Wang, R.; Guo, Z.; Xie, Z. Impacts of drought stress on the morphology, physiology, and sugar content of Lanzhou lily (Lilium davidii var. unicolor). Acta Physiol. Plant. 2020, 42, 127. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of drought stress during soybean R2–R6 growth stages on sucrose metabolism in leaf and seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Aziz, A.; Akram, N.A.; Ashraf, M. Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem. 2018, 123, 192–203. [Google Scholar] [CrossRef]
- Šola, I.; Stić, P.; Rusak, G. Effect of flooding and drought on the content of phenolics, sugars, photosynthetic pigments and vitamin C, and antioxidant potential of young Chinese cabbage. Eur. Food Res. Technol. 2021, 247, 1913–1920. [Google Scholar] [CrossRef]
- Hu, H.; Fei, X.; He, B.; Chen, X.; Ma, L.; Han, P.; Luo, Y.; Liu, Y.; Wei, A. UPLC-MS/MS profile combined with RNA-Seq reveals the amino acid metabolism in Zanthoxylum bungeanum leaves under drought stress. Front. Nutr. 2022, 9, 921742. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arab. Book Am. Soc. Plant Biol. 2010, 8, e0140. [Google Scholar] [CrossRef]
- Haghighi, T.M.; Saharkhiz, M.J.; Kavoosi, G.; Jowkar, A. Monitoring amino acid profile and protein quality of Licorice (Glycyrrhiza glabra L.) under drought stress, silicon nutrition and mycorrhiza inoculation. Sci. Hortic. 2022, 295, 110808. [Google Scholar] [CrossRef]
- Qu, X.; Wang, H.; Chen, M.; Liao, J.; Yuan, J.; Niu, G. Drought stress–induced physiological and metabolic changes in leaves of two oil tea cultivars. J. Am. Soc. Hortic. Sci. 2019, 144, 439–447. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Dong, S. Biochemical characterization and metabolic reprogramming of amino acids in Soybean roots under drought stress. Physiol. Plant. 2024, 176, e14319. [Google Scholar] [CrossRef] [PubMed]
| GCT | GA | VA | TCA | T-p-CA | FA | CA | C | EC | Q | R | M | T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B0 | 3.73 ± 0.20 f | 4.94 ± 0.11 f | 4.14 ± 0.20 e | 4.88 ± 0.17 e,f | 8.42 ± 0.19 c | 5.40 ± 0.45 d | 5.11 ± 0.03 e | 2.82 ± 0.19 c | 9.32 ± 0.25 b,c | 3.56 ± 0.16 e | 1.14 ± 0.02 d | 5.02 ± 0.21 e |
| B1 | 6.35 ± 0.07 c | 7.44 ± 0.38 c | 6.15 ± 0.08 c | 6.27 ± 0.18 b,c | 12.67 ± 0.44 a | 8.65 ± 0.28 b | 9.24 ± 0.18 b | 3.93 ± 0.09 b | 12.84 ± 0.26 a | 5.42 ± 0.30 b | 2.51 ± 0.25 c | 8.64 ± 0.51 c |
| B2 | 6.16 ± 0.20 c | 6.71 ± 0.21 d | 5.90 ± 0.23 c | 5.96 ± 0.45 c d | 10.55 ± 0.78 b | 8.34 ± 0.26 b | 8.14 ± 0.32 b,c | 3.64 ± 0.40 b | 10.26 ± 0.44 b | 5.29 ± 0.13 b | 2.93 ± 0.31 b,c | 8.56 ± 0.27 c |
| B3 | 4.10 ± 0.13 f | 5.21 ± 0.19 f | 4.23 ± 0.10 e | 4.76 ± 0.16 f | 7.87 ± 0.51 c | 5.71 ± 0.29 d | 5.47 ± 0.16 d,e | 2.92 ± 0.09 c | 8.36 ± 0.56 c | 3.92 ± 0.24 d,e | 1.49 ± 0.04 d | 5.43 ± 0.23 d,e |
| D0 | 4.68 ± 0.26 e | 5.62 ± 0.08 e,f | 4.82 ± 0.24 d | 5.25 ± 0.26 e f | 8.08 ± 0.18 c | 6.33 ± 0.10 c d | 6.83 ± 0.28 c d | 3.48 ± 0.24 b,c | 8.33 ± 0.23 c | 4.40 ± 0.19 c d | 1.74 ± 0.03 d | 6.16 ± 0.59 d |
| D1 | 7.82 ± 0.12 a | 9.58 ± 0.18 a | 8.07 ± 0.19 a | 8.24 ± 0.24 a | 12.04 ± 0.42 a | 10.58 ± 0.34 a | 11.70 ± 0.87 a | 5.11 ± 0.37 a | 13.50 ± 0.29 a | 6.44 ± 0.09 a | 3.71 ± 0.27 a | 11.56 ± 0.34 a |
| D2 | 7.30 ± 0.05 b | 8.14 ± 0.38 b | 7.41 ± 0.26 b | 6.90 ± 0.15 b | 9.87 ± 0.72 b | 9.84 ± 0.64 a | 11.27 ± 1.10 a | 4.99 ± 0.13 a | 9.83 ± 0.50 b | 6.29 ± 0.28 a | 3.49 ± 0.38 a,b | 10.33 ± 0.19 b |
| D3 | 5.23 ± 0.15 d | 6.01 ± 0.21 e | 4.94 ± 0.12 d | 5.49 ± 0.19 d,e | 7.29 ± 0.47 c | 6.81 ± 0.35 c | 6.34 ± 0.19 d,e | 3.63 ± 0.12 b | 7.27 ± 0.17 d | 4.64 ± 0.29 c | 1.78 ± 0.05 d | 6.26 ± 0.27 d |
| Garden Cress Types | D3G | C3G | P3G | PEO3G | M3G | PEO3GA | M3GA | M3GpC |
|---|---|---|---|---|---|---|---|---|
| B0 | 4.11 ± 0.44 d | 3.64 ± 0.05 d | 7.27 ± 0.17 d | 91.50 ± 2.14 c,d | 51.12 ± 0.73 c | 54.56 ± 2.19 e | 19.17 ± 0.88 e | 2.57 ± 0.13 d |
| B1 | 6.69 ± 0.35 b | 5.35 ± 0.18 b | 10.09 ± 1.04 b | 125.56 ± 9.96 a | 96.61 ± 5.22 b | 91.36 ± 3.14 c | 19.48 ± 2.02 d | 3.20 ± 0.18 c,d |
| B2 | 6.99 ± 0.07 b | 4.77 ± 0.18 c | 10.04 ± 0.43 b | 98.82 ± 1.68 b,c | 96.04 ± 8.19 b | 86.79 ± 4.77 c | 29.83 ± 1.87 c,d | 4.03 ± 0.26 b |
| B3 | 4.34 ± 0.26 d | 3.86 ± 0.19 d | 7.79 ± 0.23 c,d | 85.46 ± 3.18 c,d | 54.75 ± 2.63 c | 58.73 ± 4.90 d,e | 29.63 ± 1.32 d | 3.39 ± 0.23 b,c |
| D0 | 4.89 ± 0.30 c,d | 4.50 ± 0.07 c | 8.57 ± 0.22 b,c,d | 87.92 ± 1.82 c,d | 64.52 ± 0.93 c | 63.26 ± 2.82 d,e | 30.97 ± 2.55 c,d | 3.06 ± 0.24 c,d |
| D1 | 7.84 ± 0.24 a | 6.48 ± 0.29 a | 13.56 ± 0.52 a | 119.48 ± 9.38 a,b | 116.61 ± 10.81 a | 119.92 ± 4.12 a | 40.55 ± 1.17 a | 5.06 ± 0.28 a |
| D2 | 7.28 ± 0.19 a,b | 5.73 ± 0.13 b | 12.81 ± 1.10 a | 102.17 ± 10.89 b,c | 116.59 ± 10.08 a | 103.68 ± 5.70 b | 38.00 ± 0.70 a,b | 4.03 ± 0.27 b |
| D3 | 5.19 ± 0.30 c | 4.60 ± 0.23 c | 9.12 ± 0.27 b,c | 79.39 ± 3.19 d | 66.69 ± 3.21 c | 67.62 ± 5.64 d | 34.16 ± 1.34 b,c | 3.02 ± 0.20 c,d |
| GCT | Phenylalanine | Valine | Tryptophan | Isoleucine | Methionine | Histidine | Leucine | Lysine | Asparagine | Tyrosine | Proline |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B0 | 54.09 ± 2.25 f | 31.89 ± 1.32 e | 46.32 ± 1.93 d | 46.65 ± 1.45 d | 40.96 ± 1.70 c | 41.24 ± 1.71 e | 47.38 ± 1.97 e | 32.55 ± 1.36 e | 32.67 ± 1.36 d | 32.55 ± 1.35 f | 35.70 ± 1.48 f |
| B1 | 131.01 ± 4.93 b | 77.25 ± 2.91 b | 113.18 ± 2.54 b | 112.19 ± 4.22 b | 102.52 ± 2.19 a | 99.89 ± 3.76 a,b | 114.74 ± 4.32 b,c | 79.83 ± 1.30 b | 79.11 ± 2.98 b | 78.83 ± 2.97 a,b | 86.48 ± 3.25 b,c |
| B2 | 102.76 ± 2.57 e | 59.99 ± 1.98 d | 89.95 ± 0.77 c | 88.95 ± 2.50 c | 79.41 ± 3.11 b | 79.93 ± 3.07 d | 90.82 ± 2.44 d | 61.15 ± 2.10 d | 71.36 ± 2.12 c | 61.15 ± 2.10 e | 66.76 ± 2.72 e |
| B3 | 140.54 ± 7.14 a,b | 80.90 ± 2.62 a,b | 114.15 ± 1.80 b | 124.16 ± 9.98 a,b | 107.21 ± 9.13 a | 101.28 ± 6.89 a,b | 126.83 ± 10.16 a,b | 79.22 ± 4.39 b | 90.19 ± 3.42 a | 75.89 ± 3.28 b,c | 87.23 ± 5.29 b |
| D0 | 115.27 ± 3.73 c,d | 65.40 ± 2.28 c,d | 96.47 ± 0.84 c | 96.80 ± 0.50 c | 85.36 ± 0.66 b | 83.59 ± 4.63 c,d | 98.86 ± 0.62 d | 65.33 ± 2.08 c,d | 66.23 ± 1.99 c | 66.67 ± 2.44 d,e | 72.81 ± 3.24 d,e |
| D1 | 117.20 ± 0.99 c | 68.50 ± 1.22 c | 96.64 ± 3.23 c | 99.64 ± 1.54 c | 90.34 ± 2.84 b | 90.94 ± 2.79 b,c | 103.47 ± 1.61 c,d | 69.83 ± 1.36 c | 70.08 ± 1.38 c | 69.83 ± 1.35 c,d | 76.96 ± 0.98 c,d |
| D2 | 104.83 ± 3.76 d,e | 61.81 ± 2.21 c,d | 97.76 ± 0.38 c | 96.43 ± 2.58 c | 86.03 ± 2.95 b | 79.93 ± 2.86 d | 91.81 ± 3.29 d | 63.08 ± 2.26 c,d | 67.30 ± 1.22 c | 63.07 ± 2.25 e | 69.20 ± 2.47 d,e |
| D3 | 144.61 ± 2.53 a | 86.46 ± 3.51 a | 125.91 ± 3.93 a | 125.24 ± 4.56 a | 109.58 ± 2.08 a | 110.42 ± 2.20 a | 128.32 ± 5.05 a | 88.37 ± 3.82 a | 85.38 ± 1.99 a | 85.03 ± 2.05 a | 97.59 ± 5.30 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, E.; Ekinci, M.; Turan, M.; Goktas, H.; Budak, D.N.; Sagdic, O. Nutritional Composition, Bioactive Components and Antioxidant Activity of Garden Cress (Lepidium sativum L.) Grown Under Deficit Irrigation. Horticulturae 2025, 11, 1239. https://doi.org/10.3390/horticulturae11101239
Yildirim E, Ekinci M, Turan M, Goktas H, Budak DN, Sagdic O. Nutritional Composition, Bioactive Components and Antioxidant Activity of Garden Cress (Lepidium sativum L.) Grown Under Deficit Irrigation. Horticulturae. 2025; 11(10):1239. https://doi.org/10.3390/horticulturae11101239
Chicago/Turabian StyleYildirim, Ertan, Melek Ekinci, Metin Turan, Hamza Goktas, Derya Nil Budak, and Osman Sagdic. 2025. "Nutritional Composition, Bioactive Components and Antioxidant Activity of Garden Cress (Lepidium sativum L.) Grown Under Deficit Irrigation" Horticulturae 11, no. 10: 1239. https://doi.org/10.3390/horticulturae11101239
APA StyleYildirim, E., Ekinci, M., Turan, M., Goktas, H., Budak, D. N., & Sagdic, O. (2025). Nutritional Composition, Bioactive Components and Antioxidant Activity of Garden Cress (Lepidium sativum L.) Grown Under Deficit Irrigation. Horticulturae, 11(10), 1239. https://doi.org/10.3390/horticulturae11101239











