Ecophysiological Adaptations of Musa haekkinenii to Light Intensity and Water Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
2.2. Experimental Design and Treatment Application
2.3. Morphological Traits
2.4. Physiological Measurements
2.4.1. Chlorophyll Content and Vegetation Indices
2.4.2. Stomatal Conductance and Electron Transport Rate Measurements
2.5. Nutrient Leachate
2.6. Leaf Anatomical Analysis
2.7. Maximum Stomatal Conductance
2.8. Statistical Analysis
3. Results
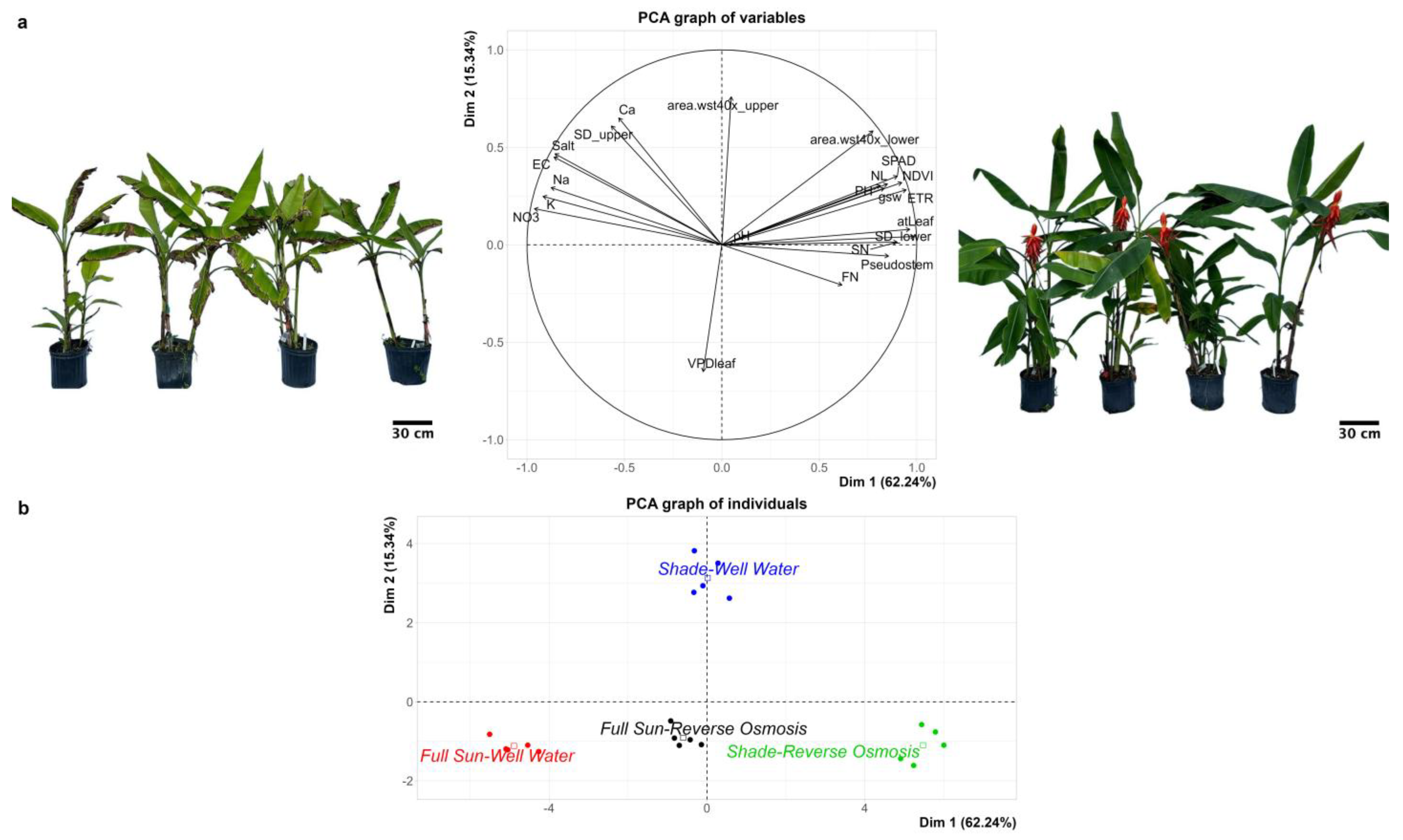
3.1. Morphological Responses to Light Intensity and Water Quality
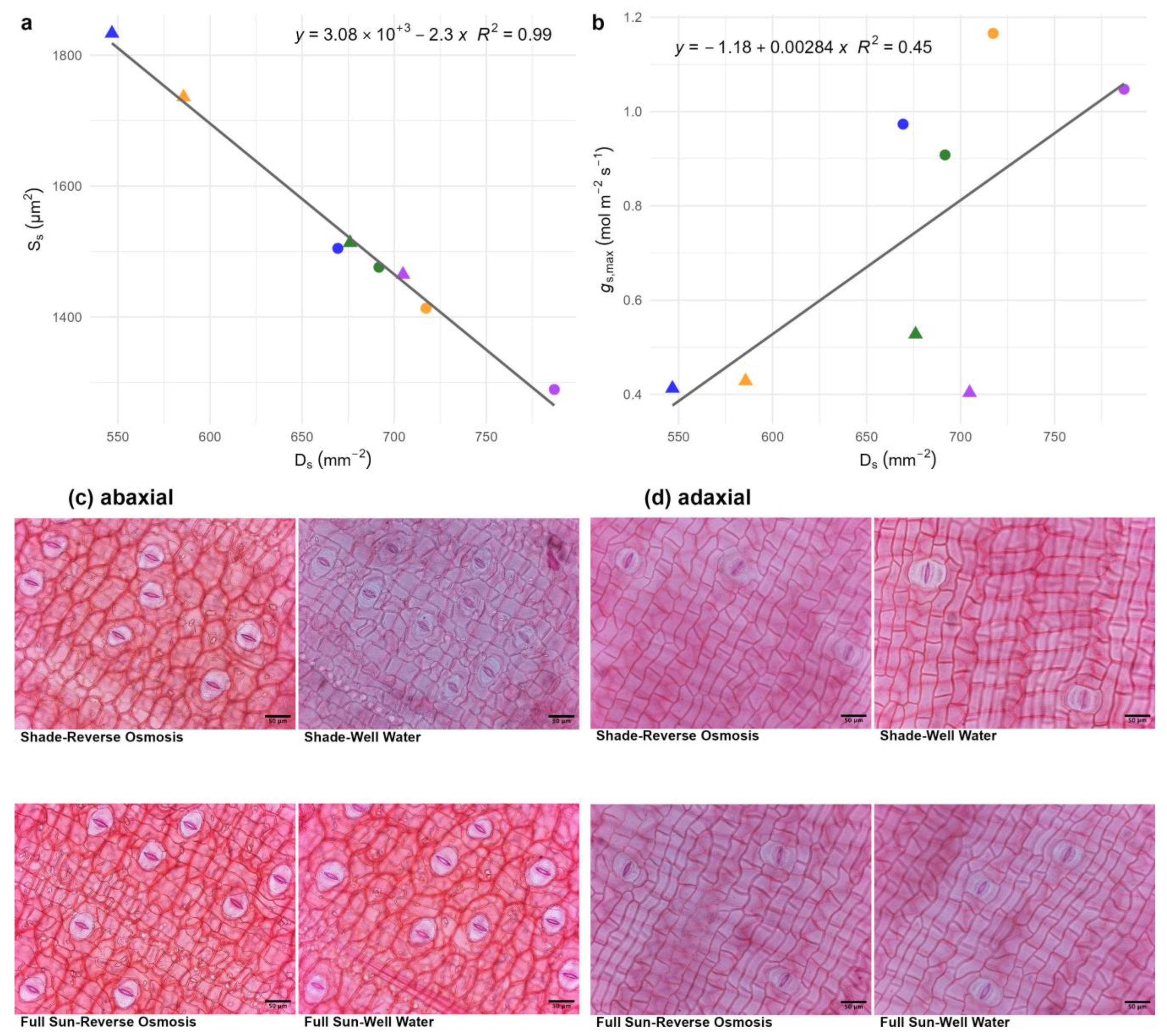
3.2. Chlorophyll Content, Photosynthetic Efficiency, and Gas Exchange Dynamics
3.3. Nutrient Leaching Dynamics and Substrate Salinity in Response to Irrigation Treatments
3.4. Optimizing Environmental Conditions for Large-Scale Cultivation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumalatha, A.; Momin, K.C.; Bhargav, V. Ornamental Horticulture and Landscape Gardening: An Overview and Introduction. In Ornamental Horticulture: Latest Cultivation Practices and Breeding Technologies; Bhargava, B., Kumar, P., Verma, V., Eds.; Springer Nature: Singapore, 2024; pp. 1–14. ISBN 978-981-97-4027-7. [Google Scholar]
- Wani, M.A.; Din, A.; Nazki, I.T.; Rehman, T.U.; Al-Khayri, J.M.; Jain, S.M.; Lone, R.A.; Bhat, Z.A.; Mushtaq, M. Navigating the Future: Exploring Technological Advancements and Emerging Trends in the Sustainable Ornamental Industry. Front. Environ. Sci. 2023, 11, 1188643. [Google Scholar] [CrossRef]
- United States Department of Agriculture, National Agricultural Statistics Service (USDA NASS). Floriculture in 2023; No. 2024-5; USDA: Washington, DC, USA, 2024; p. 2.
- Gabellini, S.; Scaramuzzi, S. Evolving Consumption Trends, Marketing Strategies, and Governance Settings in Ornamental Horticulture: A Grey Literature Review. Horticulturae 2022, 8, 234. [Google Scholar] [CrossRef]
- Thakur Babita Kanwar, R. An Overview of Flowering Pot Plants for Tropical and Subtropical Climate. Int. J. Sci. Res. IJSR 2023, 12, 1274–1280. [Google Scholar] [CrossRef]
- Vu, T.D.; Vu, D.T.; Janssens, S.B.; De Langhe, E.; Le, L.T.; Kallow, S.; Mertens, A.; Vu, T.T.H.; Nguyen, T.T. The Description, Distribution and Habitat of Wild Banana Species in Northern Viet Nam. Genet. Resour. Crop Evol. 2023, 70, 479–504. [Google Scholar] [CrossRef]
- Lý, N.-S.; Lê, C.-K.; Triệu, T.-D.; Haevermans, A.; Lowry, P.I.P.; Haevermans, T. A Distinctive New Species of Wild Banana (Musa, Musaceae) from Northern Vietnam. Phytotaxa 2012, 75, 33–42. [Google Scholar] [CrossRef]
- Sampaio, J.R.; Oliveira, W.D.D.S.; Junior, L.C.D.S.; Nascimento, F.D.S.; Moreira, R.F.C.; Ramos, A.P.D.S.; Santos-Serejo, J.A.D.; Amorim, E.P.; Jesus, R.D.M.D.; Ferreira, C.F. Diversity of Improved Diploids and Commercial Triploids from Musa Spp. via Molecular Markers. Curr. Issues Mol. Biol. 2024, 46, 11783–11796. [Google Scholar] [CrossRef] [PubMed]
- Drapal, M.; De Carvalho, E.B.; Rouard, M.; Amah, D.; Sardos, J.; Van Den Houwe, I.; Brown, A.; Roux, N.; Swennen, R.; Fraser, P.D. Metabolite Profiling Characterises Chemotypes of Musa Diploids and Triploids at Juvenile and Pre-Flowering Growth Stages. Sci. Rep. 2019, 9, 4657. [Google Scholar] [CrossRef]
- Maseko, K.H.; Regnier, T.; Meiring, B.; Wokadala, O.C.; Anyasi, T.A. Musa Species Variation, Production, and the Application of Its Processed Flour: A Review. Sci. Hortic. 2024, 325, 112688. [Google Scholar] [CrossRef]
- Madail, R.H.; Pio, L.A.S.; Rezende, R.A.L.S.; Pasqual, M.; Silva, S.D.O.E. Banana Leaf Anatomy Characteristics Related to Ploidy Levels. Acta Sci. Agron. 2022, 44, e55709. [Google Scholar] [CrossRef]
- Sankar, C.; Rajangam, J.; Kavino, M.; Auxcilia, J.; Premalakshmi, V.; Rajamanickam, C.; Sundarrajan, R.V.; Naveen, R. Impacts of Polyploidy on Phenotypic Expression in Musa Spp Ssp. Progenies. Discov. Appl. Sci. 2025, 7, 948. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The Role of Light in Regulating Plant Growth, Development and Sugar Metabolism: A Review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Li, H.E.; Gao, C.; Yang, R. Leaf Traits and Photosynthetic Characteristics of Endangered Sinopodophyllum Hexandrum (Royle) Ying under Different Light Regimesin Southeastern Tibet Plateau. Photosynthetica 2019, 57, 548–555. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants Response to Light Stress. J. Genet. Genomics 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2023; ISBN 978-981-99-5735-4. [Google Scholar]
- Zhang, J.J.; Zhu, L.; Zhang, X.; Zhou, J. Photosynthetic Performance and Growth Responses of Liriope Muscari (Decne.) L.H. Bailey (Asparagaceae) Planted within Poplar Forests Having Different Canopy Densities. BMC Ecol. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Théroux-Rancourt, G.; Herrera, J.C.; Voggeneder, K.; De Berardinis, F.; Luijken, N.; Nocker, L.; Savi, T.; Scheffknecht, S.; Schneck, M.; Tholen, D. Analyzing Anatomy over Three Dimensions Unpacks the Differences in Mesophyll Diffusive Area between Sun and Shade Vitis Vinifera Leaves. AoB PLANTS 2023, 15, plad001. [Google Scholar] [CrossRef]
- Bhattacharya, A. Mineral Nutrition of Plants Under Soil Water Deficit Condition: A Review. In Soil Water Deficit and Physiological Issues in Plants; Springer: Singapore, 2021; pp. 287–391. ISBN 978-981-336-275-8. [Google Scholar]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant Salt Stress: Adaptive Responses, Tolerance Mechanism and Bioengineering for Salt Tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Xing, H.; Hershkowitz, J.; Paudel, A.; Sun, Y.; Chen, J.J.; Dai, X.; Chappell, M. Morphological and Physiological Responses of Ornamental Grasses to Saline Water Irrigation. HortScience 2021, 56, 678–686. [Google Scholar] [CrossRef]
- Carillo, P.; Woodrow, P.; Rouphael, Y. An Appraisal of Horticultural Plant Morpho-Physiological and Molecular Responses to Variable Salt Stress Agents. Italus Hortus 2022, 29, 1–17. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Halder, K.; Abdin, M.Z.; Majee, M.; Datta, A. Abiotic Stress Tolerance in Plants: Brassinosteroids Navigate Competently. Int. J. Mol. Sci. 2022, 23, 14577. [Google Scholar] [CrossRef]
- Liu, H.; Todd, J.L.; Luo, H. Turfgrass Salinity Stress and Tolerance—A Review. Plants 2023, 12, 925. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Ullah, M.; Batool, M.; El-Badri, A.M.; Ikram, M.; Zheng, L.; Khalid, B.; El Khalek Abd El Mola Mohamed, I.A.; Chang, L.; Wang, B.; Kuai, J.; et al. A Comprehensive Report on Glucosinolate Involvement in Abiotic Stress Responses in Brassicaceae Family. Hortic. Plant J. 2025, in press. [Google Scholar] [CrossRef]
- Darre, N.C.; Toor, G.S. Desalination of Water: A Review. Curr. Pollut. Rep. 2018, 4, 104–111. [Google Scholar] [CrossRef]
- Robinson, J.C.; Galán Saúco, V. Morphological Characteristics and Plant Development. In Bananas and Plantains; Robinson, J.C., Galán Saúco, V., Eds.; CABI: Wallingford, UK, 2010; pp. 51–66. ISBN 978-1-84593-658-7. [Google Scholar]
- Altman, N.; Krzywinski, M. Split Plot Design. Nat. Methods 2015, 12, 165–166. [Google Scholar] [CrossRef]
- Piepho, H.-P.; Gabriel, D.; Hartung, J.; Büchse, A.; Grosse, M.; Kurz, S.; Laidig, F.; Michel, V.; Proctor, I.; Sedlmeier, J.E.; et al. One, Two, Three: Portable Sample Size in Agricultural Research. J. Agric. Sci. 2022, 160, 459–482. [Google Scholar] [CrossRef]
- Hunt, J.R.; Kirkegaard, J.A.; Harris, F.A.; Porker, K.D.; Rattey, A.R.; Collins, M.J.; Celestina, C.; Cann, D.J.; Hochman, Z.; Lilley, J.M.; et al. Exploiting Genotype × Management Interactions to Increase Rainfed Crop Production: A Case Study from South-Eastern Australia. J. Exp. Bot. 2021, 72, 5189–5207. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lara, D.A.; Polanco-Díaz, E.; Deaquiz-Oyola, Y.A.; Betancourt-Vásquez, M.; Rodríguez-Yzquierdo, G.A. Evaluation of the Effect of Biostimulant Substances on the Growth and Physiological Response of Dominico-Hartón Plantain (Musa AAB). Rev. Colomb. Cienc. Hortícolas 2024, 18. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Farfan-Vignolo, E.R.; Gutierrez, R.; Blas, R.; Awais, K. Harvest Index Is a Key Trait for Screening Drought-Tolerant Potato Genotypes (Solanum Tuberosum). J. Crop Sci. Biotechnol. 2024, 27, 91–103. [Google Scholar] [CrossRef]
- Nogueira Souza Costa, B.; Khoddamzadeh, A.A. Data-Driven Nitrogen Application for Satinleaf: Leveraging Optical Sensors in Urban Landscape Management. Front. Plant Sci. 2025, 16, 1522662. [Google Scholar] [CrossRef]
- Zsebő, S.; Bede, L.; Kukorelli, G.; Kulmány, I.M.; Milics, G.; Stencinger, D.; Teschner, G.; Varga, Z.; Vona, V.; Kovács, A.J. Yield Prediction Using NDVI Values from GreenSeeker and MicaSense Cameras at Different Stages of Winter Wheat Phenology. Drones 2024, 8, 88. [Google Scholar] [CrossRef]
- Khoddamzadeh, A.A.; Souza Costa, B.N. Best Nitrogen Management Practices Using Sensor-Based Smart Agriculture in Nursery Production of Cacao. Horticulturae 2023, 9, 454. [Google Scholar] [CrossRef]
- Jiang, G.-F.; Li, S.-Y.; Dinnage, R.; Cao, K.-F.; Simonin, K.A.; Roddy, A.B. Diverse Mangroves Deviate from Other Angiosperms in Their Genome Size, Leaf Cell Size and Cell Packing Density Relationships. Ann. Bot. 2023, 131, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. Maximum Leaf Conductance Driven by CO2 Effects on Stomatal Size and Density over Geologic Time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef]
- De Boer, H.J.; Price, C.A.; Wagner-Cremer, F.; Dekker, S.C.; Franks, P.J.; Veneklaas, E.J. Optimal Allocation of Leaf Epidermal Area for Gas Exchange. New Phytol. 2016, 210, 1219–1228. [Google Scholar] [CrossRef]
- Tanaka, E.; Hui, F.K.C. Symbolic Formulae for Linear Mixed Models. In Statistics and Data Science; Nguyen, H., Ed.; Communications in Computer and Information Science; Springer: Singapore, 2019; Volume 1150, pp. 3–21. ISBN 978-981-15-1959-8. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Lenth, R.V. Estimated Marginal Means, Aka Least-Squares Means [R Package Emmeans Version 1.11.2-8]. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 28 September 2025).
- Comprehensive R Archive Network (CRAN) Multivariate Exploratory Data Analysis and Data Mining [R Package FactoMineR Version 2.12]. Available online: https://cran.r-project.org/web/packages/FactoMineR/FactoMineR.pdf (accessed on 28 September 2025).
- Lozano-Isla, F. Tools and Statistical Procedures in Plant Science [R Package Inti Version 0.6.8]. Available online: https://cran.r-project.org/web/packages/inti/index.html (accessed on 28 September 2025). [CrossRef]
- Sembada, A.A.; Faizal, A.; Sulistyawati, E. Photosynthesis Efficiency as Key Factor in Decision-Making for Forest Design and Redesign: A Systematic Literature Review. Ecol. Front. 2024, 44, 1128–1139. [Google Scholar] [CrossRef]
- Roddy, A.B.; Théroux-Rancourt, G.; Abbo, T.; Benedetti, J.W.; Brodersen, C.R.; Castro, M.; Castro, S.; Gilbride, A.B.; Jensen, B.; Jiang, G.-F.; et al. The Scaling of Genome Size and Cell Size Limits Maximum Rates of Photosynthesis with Implications for Ecological Strategies. Int. J. Plant Sci. 2020, 181, 75–87. [Google Scholar] [CrossRef]
- Mielcarek, A.; Kłobukowska, K.; Rodziewicz, J.; Janczukowicz, W.; Bryszewski, K.Ł. Water Nutrient Management in Soilless Plant Cultivation versus Sustainability. Sustainability 2023, 16, 152. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A Meta-analysis of Plant Responses to Light Intensity for 70 Traits Ranging from Molecules to Whole Plant Performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Divya, D.B.S.; Padmalatha, T.; Rao, A.D.; Subbaramamma, P. Morphological Characterization of Ornamental Banana Accessions for Vegetative Traits in Godavari Zone of Andhra Pradesh. Int. J. Res. Agron. 2024, 7, 450–455. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Chang, L.; Li, X.; Xie, L.; Deng, J.; Zhou, W.; Hu, Y.; Yan, Q.; Peng, H.; et al. Contrasting Light Capture Strategies between Shade-Tolerant and -Intolerant Tree Seedlings Responding to Solar Canopy Spectral Composition. Environ. Exp. Bot. 2024, 225, 105857. [Google Scholar] [CrossRef]
- Adamiec, M.; Pietrowska-Borek, M.; Luciński, R. Editorial: The Influence of Environmental Conditions on Chloroplast Functioning and Development. Front. Plant Sci. 2024, 15, 1517094. [Google Scholar] [CrossRef] [PubMed]
- Suvendran, S.; Johnson, D.; Acevedo, M.; Smithers, B.; Xu, P. Effect of Irrigation Water Quality and Soil Compost Treatment on Salinity Management to Improve Soil Health and Plant Yield. Water 2024, 16, 1391. [Google Scholar] [CrossRef]
- Nagial, K.S.; Kumar, N.; Poddar, A. A Comprehensive Analysis of Institutional Wastewater for Irrigating Ornamental Plants. Water Supply 2024, 24, 2830–2843. [Google Scholar] [CrossRef]
- Altıntaş, S.; Yasemin, S.; Çatkın, S.; İnal, B. Effectiveness of Manganese Foliar Spraying to Mitigate Salt Stress in Ornamental Cabbage: Insights into Morphological, Physiological Biochemical Adaptations and mTERF Gene Responses. South Afr. J. Bot. 2024, 168, 462–475. [Google Scholar] [CrossRef]
- de Oliveira da Costa, L.; Ribeiro Castro, C.R.; Kenji Taniguchi, C.A.; Bezerra, M.A. Assessment of Ornamental Grass Accessions under Varying Salinity Levels. Ornam. Hortic. 2024, 30, e242812. [Google Scholar] [CrossRef]
- Hernández, J.A. Salinity Tolerance in Plants: Trends and Perspectives. Int. J. Mol. Sci. 2019, 20, 2408. [Google Scholar] [CrossRef]
- Gonzalez, P.D.; Tucker, D.A.; Nageswara-Rao, M.; Griffith, M.P.; Balaji Baskar, M.S.; Ross, M.; Khoddamzadeh, A.A. Enhancing Cabbage Palm Resilience to Saltwater Stress through Silicon Applications. HortScience 2025, 60, 1547–1554. [Google Scholar] [CrossRef]
- Kalaivani, J.; Jegadeeswari, V.; Vijayalatha, K.R.; Arulmozhiyan, R.; Meena, S.; Selvarajan, R.; Ravi, I.; Jeyabaskaran, K.J. Impact of Salinity in Tropical Fruit Crops—A Review. Commun. Soil Sci. Plant Anal. 2024, 55, 3286–3306. [Google Scholar] [CrossRef]
- Wei, J.; Liu, D.; Liu, Y.; Wei, S. Physiological Analysis and Transcriptome Sequencing Reveal the Effects of Salt Stress on Banana (Musa Acuminata Cv. BD) Leaf. Front. Plant Sci. 2022, 13, 822838. [Google Scholar] [CrossRef]
- Dikayani; Anas; Nuraini, A.; Qosim, W.A. Effect of Salinity Stress on Shoot Musa Acuminata L. Barangan Cultivar in Vitro Culture. Pak. J. Biol. Sci. 2019, 22, 201–205. [Google Scholar] [CrossRef]
- Cowan, I.; Farquhar, G. Stomatal Function in Relation to Leaf Metabolism and Environment. Symp. Soc. Exp. Biol. 1977, 31, 471–505. [Google Scholar]
- Giordano, M.; Petropoulos, S.A.; Cirillo, C.; Rouphael, Y. Biochemical, Physiological, and Molecular Aspects of Ornamental Plants Adaptation to Deficit Irrigation. Horticulturae 2021, 7, 107. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Ferrante, A. Molecular Responses of Vegetable, Ornamental Crops, and Model Plants to Salinity Stress. Int. J. Mol. Sci. 2023, 24, 3190. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.; Romero, R.; Cedeño, Á.; Verdosoto, Á.; Peñafiel, M.; Canchignia, H. Stomatal Density, Chlorophyll Content and Phylogenetic Relationship in 17 Cultivars of Musa spp. Sci. Agropecu. 2019, 10, 47–54. [Google Scholar] [CrossRef]
- Ekanayake, I.J.; Ortiz, R.; Vuylsteke, D.R. Leaf Stomatal Conductance and Stomatal Morphology of Musa Germplasm. Euphytica 1998, 99, 221–229. [Google Scholar] [CrossRef]
- Razi Ismai, M.; Yusoff, M.K.; Marziah, M. Growth, Water Relations, Stomatal Conductance and Proline Concentration in Water Stressed Banana (Musa Spp.) Plants. Asian J. Plant Sci. 2004, 3, 709–713. [Google Scholar] [CrossRef]
- Turner, D.W.; Fortescue, J.A.; Thomas, D.S. Environmental Physiology of the Bananas (Musa Spp.). Braz. J. Plant Physiol. 2007, 19, 463–484. [Google Scholar] [CrossRef]
- Kim, E.A.; Shin, E.J.; Sunwoo, Y.; Lee, J.H.; Nam, S.Y.; Ahn, J.J. Shading Treatments Affect the Growth Characteristics, Ornamental Value, and Photosynthetic Activities of Various Peperomia Species and Cultivars. Glas. Future 2024, 7, 1–19. [Google Scholar] [CrossRef]
- Lee, J.H.; You, N.H.; Nam, S.Y. Chlorophyll Fluorescence and Growth Evaluation of Ornamental Foliage Plants in Response to Light Intensity Levels under Continuous Lighting Conditions. Flower Res. J. 2021, 29, 320–322. [Google Scholar] [CrossRef]
- Mahanty, D.S. Physiology of Shade Loving Plants: A Comparative Analysis with Shade Avoiding Plants. Indian J. Appl. Pure Biol. 2023, 38, 536–546. [Google Scholar]
- Zeng, J.; Liu, F.; Zhu, Y.; Li, J.; Ruan, Y.; Quan, X.; Wang, C.; Wang, X. Degree of Shade Tolerance Shapes Seasonality of Chlorophyll, Nitrogen and Phosphorus Levels of Trees and Herbs in a Temperate Deciduous Forest. J. For. Res. 2024, 35, 72. [Google Scholar] [CrossRef]
- Haq, I.; Soomro, F.; Parveen, N.; Dahot, M.U.; Mirbahar, A.A. Certain Growth Related Attributes of Micropropagated Banana under Different Salinity Levels. Pak. J. Bot. 2011, 43, 1655–1658. [Google Scholar]
- Rodrigues, A.J.O.; Silva, C.D.F.B.D.; Sousa, A.B.O.D.; Bezerra, M.A.; Araújo, B.D.A.; Rodrigues, A.M.G.; Vieira, J.Q.; Sousa, J.S.D. Acclimatization of Banana Plantlets Inoculated with Bacillus Sp. and Irrigated with Low-Salinity Water. Rev. Bras. Eng. Agríc. E Ambient. 2023, 27, 407–414. [Google Scholar] [CrossRef]
- Aldorfová, A.; Dostálek, T.; Münzbergová, Z. Effects of Soil Conditioning, Root and Shoot Litter Addition Interact to Determine the Intensity of Plant–Soil Feedback. Oikos 2022, 2022, e09025. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munoz-Salas, M.N.; Roddy, A.B.; Dastpak, A.; Nogueira Souza Costa, B.; Khoddamzadeh, A.A. Ecophysiological Adaptations of Musa haekkinenii to Light Intensity and Water Quality. Horticulturae 2025, 11, 1188. https://doi.org/10.3390/horticulturae11101188
Munoz-Salas MN, Roddy AB, Dastpak A, Nogueira Souza Costa B, Khoddamzadeh AA. Ecophysiological Adaptations of Musa haekkinenii to Light Intensity and Water Quality. Horticulturae. 2025; 11(10):1188. https://doi.org/10.3390/horticulturae11101188
Chicago/Turabian StyleMunoz-Salas, Milagros Ninoska, Adam B. Roddy, Arezoo Dastpak, Bárbara Nogueira Souza Costa, and Amir Ali Khoddamzadeh. 2025. "Ecophysiological Adaptations of Musa haekkinenii to Light Intensity and Water Quality" Horticulturae 11, no. 10: 1188. https://doi.org/10.3390/horticulturae11101188
APA StyleMunoz-Salas, M. N., Roddy, A. B., Dastpak, A., Nogueira Souza Costa, B., & Khoddamzadeh, A. A. (2025). Ecophysiological Adaptations of Musa haekkinenii to Light Intensity and Water Quality. Horticulturae, 11(10), 1188. https://doi.org/10.3390/horticulturae11101188








