Enhancing Sweet Cherry Quality Through Calcium and Ascophyllum nodosum Foliar Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Trial
2.2. Sweet Cherry Quality Parameters
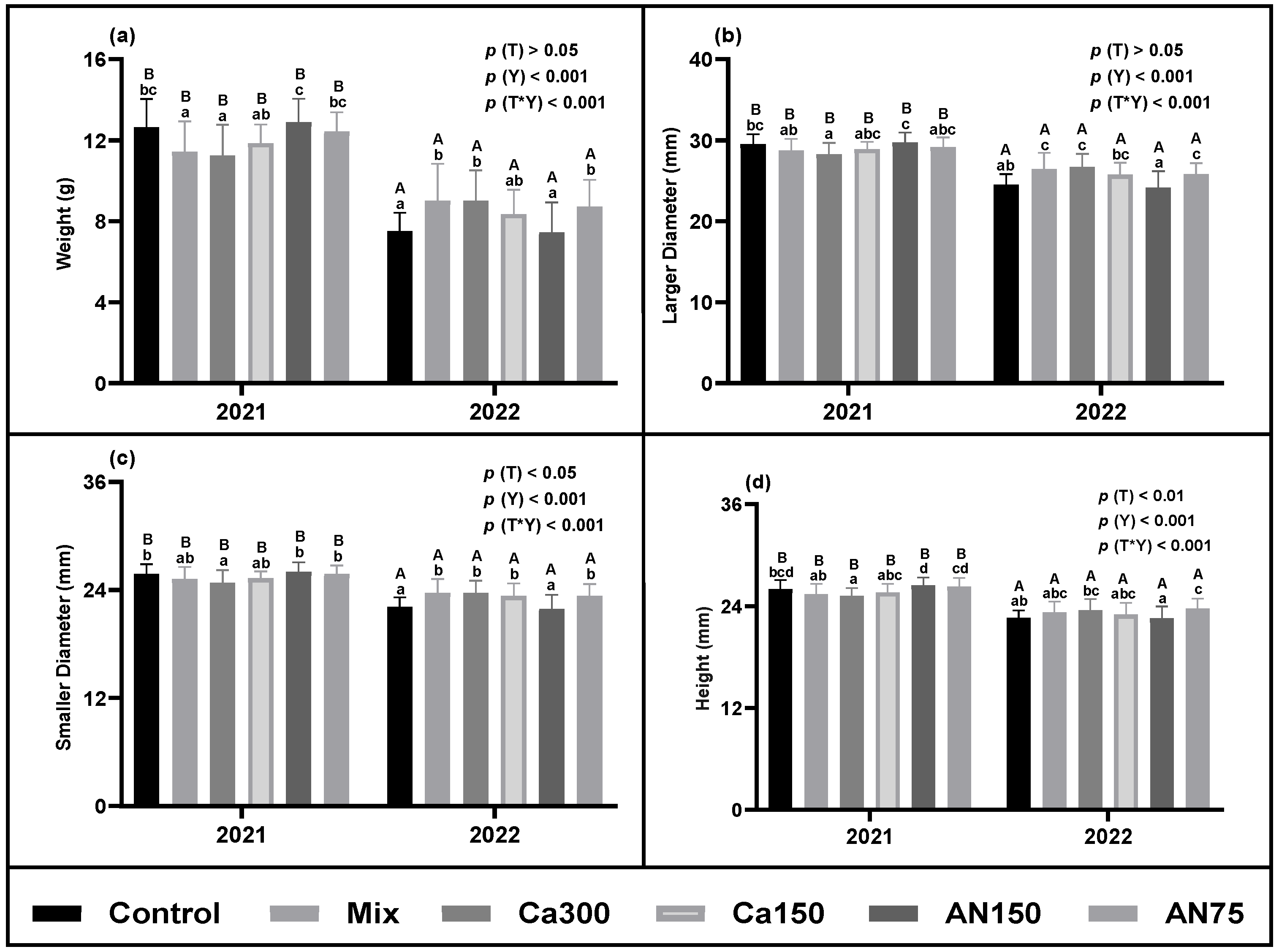
2.2.1. Biometric Parameters
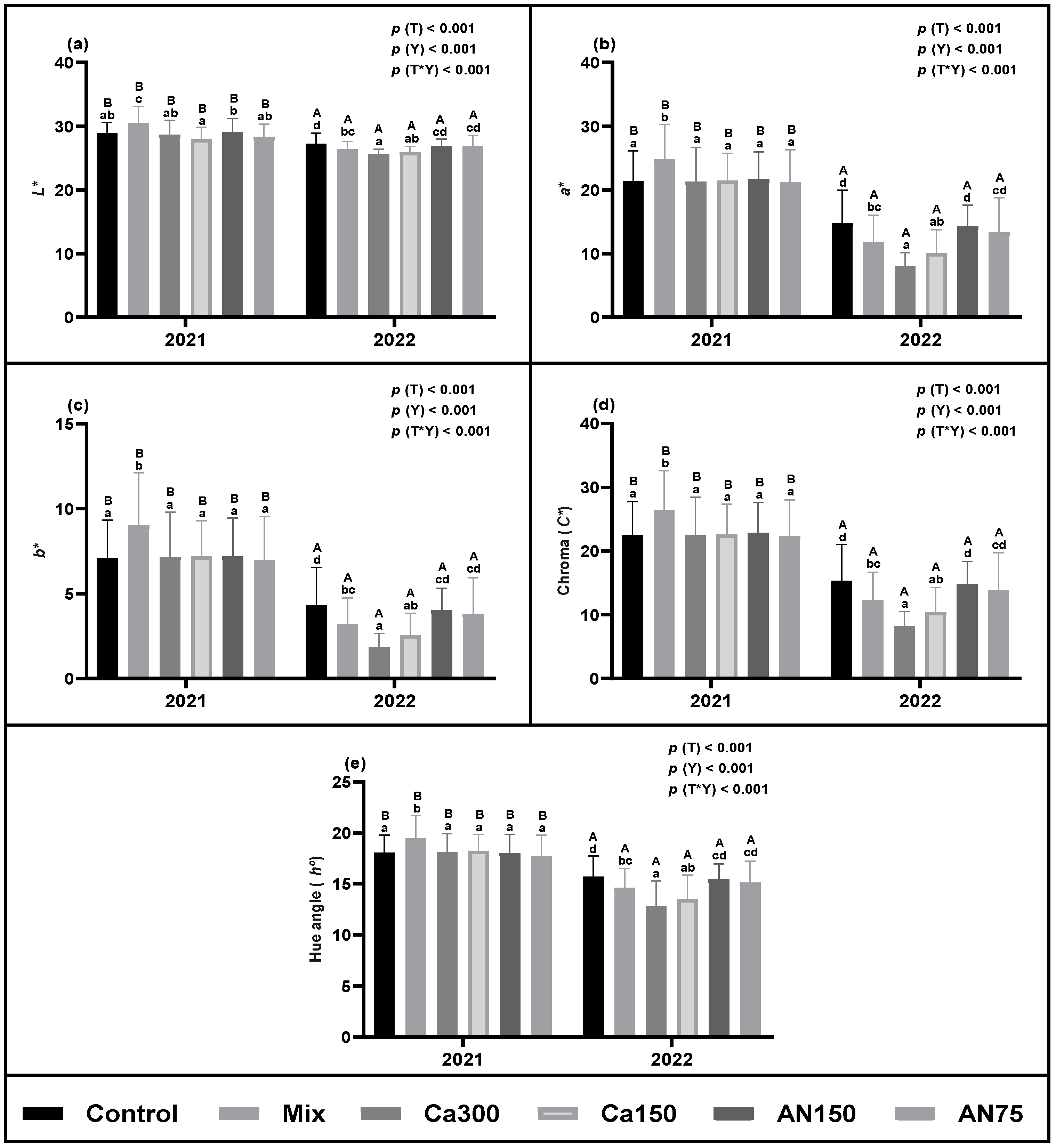
2.2.2. Chromatic Parameters
2.2.3. Texture Parameters
2.2.4. Total Soluble Solids, pH, Titratable Acidity, and Maturity Index
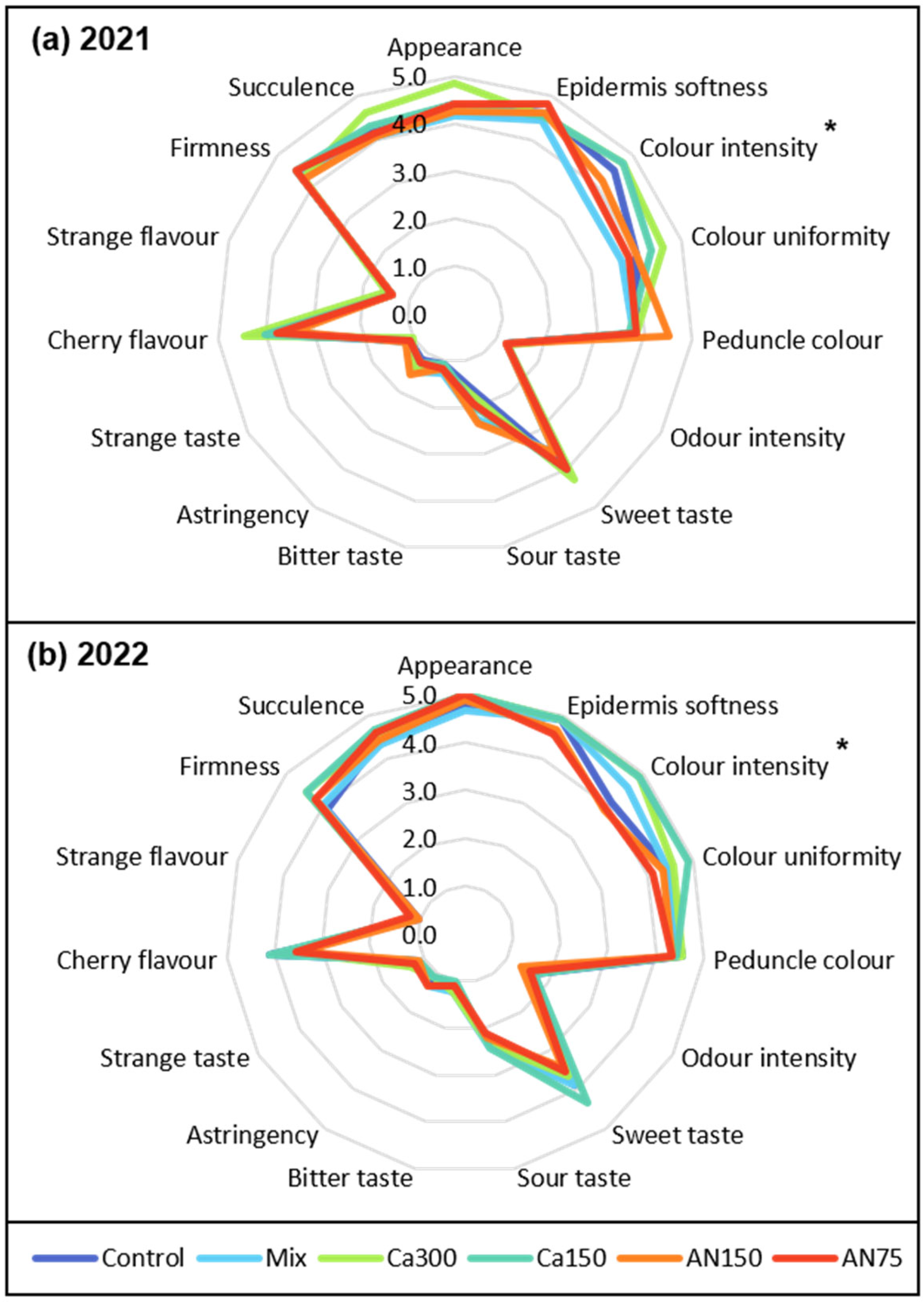
2.3. Sensorial Analysis
2.4. Statistical Analysis
3. Results
3.1. Effect of Calcium and Seaweed-Based Biostimulant on Sweet Cherry Quality Parameters
3.1.1. Biometric Parameters
3.1.2. Chromatic Parameters
3.1.3. Texture Parameters
3.1.4. Total Soluble Solids, pH, Titratable Acidity, and Maturity Index
3.2. Sensorial Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraga, H.; Santos, J.A. Assessment of climate change impacts on chilling and forcing for the main fresh fruit regions in Portugal. Front. Plant Sci. 2021, 12, 689121. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Pereira, S.; Ferreira, H.; Sousa, J.R.; Vilela, A.; Ribeiro, C.; Raimundo, F.; Egea-Cortines, M.; Matos, M.; Gonçalves, B. Optimizing sweet cherry attributes through magnesium and potassium fertilization. Horticulturae 2024, 10, 881. [Google Scholar] [CrossRef]
- Bassi, D.; Cirilli, M.; Rossini, L. Most important fruit crops in Mediterranean Basin (Mb). Milano University Press: Milan, Italy, 2024. [Google Scholar]
- Medda, S.; Fadda, A.; Mulas, M. Influence of climate change on metabolism and biological characteristics in perennial woody fruit crops in the mediterranean environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Silva, B.; Simões, M.; Silva, R.L. Nutrients, bioactive compounds and bioactivity: The health benefits of sweet cherries (Prunus avium L.). Curr. Nutr. Food Sci. 2019, 15, 208–227. [Google Scholar] [CrossRef]
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Chapter 2—Sweet cherry phenolic compounds: Identification, characterization, and health benefits. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 31–78. [Google Scholar] [CrossRef]
- Zheng, X.; Yue, C.; Gallardo, K.; McCracken, V.; Luby, J.; McFerson, J. What attributes are consumers looking for in sweet cherries? evidence from choice experiments. Agric. Resour. Econ. Rev. 2016, 45, 124–142. [Google Scholar] [CrossRef]
- Gonçalves, B.; Aires, A.; Oliveira, I.; Baltazar, M.; Cosme, F.; Afonso, S.; Pinto, T.; Anjos, M.R.; Inês, A.; Morais, M.C.; et al. From orchard to wellness: Unveiling the health effects of sweet cherry nutrients. Nutrients 2024, 16, 3660. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors affecting quality and health promoting compounds during growth and postharvest life of sweet cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Bujdosó, G.; Hrotko, K.; Feldmane, D.; Giovannini, D.; Demirsoy, H.; Tao, R.; Ercisli, S.; Ertek, N.; Malchev, S. What kind of sweet cherries do the final consumers prefer? South West. J. Hortic. Biol. Environ. 2020, 11, 37–48. [Google Scholar]
- Benedicta Adewoyin, O. Pre-harvest and postharvest factors affecting quality and shelf life of harvested produce. In New Advances in Postharvest Technology; Kahramanoglu, I., Ed.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Dichi, D. Review on factors affecting postharvest quality of fruits. J. Plant Sci. Res. 2018, 5, 180. [Google Scholar]
- Bacelar, E.; Pinto, T.; Anjos, R.; Morais, M.C.; Oliveira, I.; Vilela, A.; Cosme, F. Impacts of climate change and mitigation strategies for some abiotic and biotic constraints influencing fruit growth and quality. Plants 2024, 13, 1942. [Google Scholar] [CrossRef] [PubMed]
- Predieri, S.; Dris, R.; Sekse, L.; Rapparini, F. Influence of environmental factors and orchard management on yield and quality of sweet cherry. Food Agric. Environ. 2003, 1, 263–266. [Google Scholar]
- Thokar, N.; Kattel, D.; Subedi, S. Effect of pre-harvest factors on postharvest quality of horticultural products. Food Agri Econ. Rev. 2022, 2, 92–95. [Google Scholar] [CrossRef]
- Salama, A.-M.; Ezzat, A.; El-Ramady, H.; Alam-Eldein, S.M.; Okba, S.K.; Elmenofy, H.M.; Hassan, I.F.; Illés, A.; Holb, I.J. Temperate fruit trees under climate change: Challenges for dormancy and chilling requirements in warm winter regions. Horticulturae 2021, 7, 86. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Warang, O.; Das, S. Impact of climate change on fruit crops—A review. Curr. World Environ. 2022, 17, 319–330. [Google Scholar] [CrossRef]
- Bouri, M.; Arslan, K.S.; Şahin, F. Climate-smart pest management in sustainable agriculture: Promises and challenges. Sustainability 2023, 15, 4592. [Google Scholar] [CrossRef]
- Blakeney, M. Agricultural innovation and sustainable development. Sustainability 2022, 14, 2698. [Google Scholar] [CrossRef]
- Dong, L. Toward resilient agriculture value chains: Challenges and opportunities. Prod. Oper. Manag. 2021, 30, 666–675. [Google Scholar] [CrossRef]
- Matteo, M.; Zoffoli, J.P.; Ayala, M. Calcium sprays and crop load reduction increase fruit quality and postharvest storage in sweet cherry (Prunus avium L.). Agronomy 2022, 12, 829. [Google Scholar] [CrossRef]
- Jaime-Guerrero, M.; Alvarez-Herrera, J.; Fischer, G. Effect of calcium on fruit quality: A review. Agron. Colomb. 2024, 42, e112026. [Google Scholar] [CrossRef]
- Correia, S.; Queirós, F.; Ribeiro, C.; Vilela, A.; Aires, A.; Barros, A.I.; Schouten, R.; Silva, A.P.; Gonçalves, B. Effects of calcium and growth regulators on sweet cherry (Prunus avium L.) quality and sensory attributes at harvest. Sci. Hortic. 2019, 248, 231–240. [Google Scholar] [CrossRef]
- Erbaş, D.; Koyuncu, M.A. Effect of preharvest calcium chloride treatment on some quality characteristics and bioactive compounds of sweet cherry cultivars. J. Agric. Sci. 2022, 28, 481–489. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Correia, S.; Santos, M.; Glińska, S.; Gapińska, M.; Matos, M.; Carnide, V.; Schouten, R.; Silva, A.P.; Gonçalves, B. Effects of exogenous compound sprays on cherry cracking: Skin properties and gene expression. J. Sci. Food Agric. 2020, 100, 2911–2921. [Google Scholar] [CrossRef]
- Varaldo, A.; Giacalone, G. Enhancing cracking resistance and post-harvest quality of sweet cherries (Prunus avium L.) through calcium and potassium-based foliar treatments. Horticulturae 2025, 11, 30. [Google Scholar] [CrossRef]
- Winkler, A.; Knoche, M. Calcium uptake through skins of sweet cherry fruit: Effects of different calcium salts and surfactants. Sci. Hortic. 2021, 276, 109761. [Google Scholar] [CrossRef]
- Choulot, M.; Le Guillard, C.; Bourgougnon, N.; Michalak, I. Chapter 13—Seaweed-based fertilizing products. In Algae and Aquatic Macrophytes in Cities; Pandey, V.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 271–313. [Google Scholar] [CrossRef]
- Kaur, I. Seaweeds: Soil health boosters for sustainable agriculture. In Soil Health; Giri, B., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 163–182. [Google Scholar] [CrossRef]
- Raghunandan, B.L.; Vyas, R.V.; Patel, H.K.; Jhala, Y.K. Perspectives of Seaweed as Organic Fertilizer in Agriculture. In Soil Fertility Management for Sustainable Development; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2019; pp. 267–289. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Meyer, A.S.; Gonçalves, B. Biostimulants to improved tree physiology and fruit quality: A review with special focus on sweet cherry. Agronomy 2022, 12, 659. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Mughunth, R.J.; Velmurugan, S.; Mohanalakshmi, M.; Vanitha, K. A review of seaweed extract’s potential as a biostimulant to enhance growth and mitigate stress in horticulture crops. Sci. Hortic. 2024, 334, 113312. [Google Scholar] [CrossRef]
- Lopes, T.; Silva, A.P.; Ribeiro, C.; Carvalho, R.; Aires, A.; Vicente, A.A.; Gonçalves, B. Ecklonia maxima and glycine–betaine-based biostimulants improve blueberry yield and quality. Horticulturae 2024, 10, 920. [Google Scholar] [CrossRef]
- Monteiro, E.; Baltazar, M.; Pereira, S.; Correia, S.; Ferreira, H.; Alves, F.; Cortez, I.; Castro, I.; Gonçalves, B. Ascophyllum nodosum extract and glycine betaine preharvest application in grapevine: Enhancement of berry quality, phytochemical content and antioxidant properties. Antioxidants 2023, 12, 1835. [Google Scholar] [CrossRef]
- Yasmeen, A.R.; Maharajan, T.; Rameshkumar, R.; Sindhamani, S.; Banumathi, B.; Prabakaran, M.; Atchaya, S.; Rathinapriya, P. Role of seaweeds for improving soil fertility and crop development to address global food insecurity. Crops 2025, 5, 29. [Google Scholar] [CrossRef]
- Bons, H.K.; Sharma, A. Impact of foliar sprays of potassium, calcium, and boron on fruit setting behavior, yield, and quality attributes in fruit crops: A review. J. Plant Nutr. 2023, 46, 3232–3246. [Google Scholar] [CrossRef]
- Micheli, E.; Schád, P.; Spaargaren, O.; Dent, D.; Nachtergaele, F.; Wrb, I. World Reference Base for Soil Resources 2006: A Framework for International Classification, Correlation and Communication; FAO: Rome, Italy, 2006. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Chauvin, M.A.; Whiting, M.; Ross, C.F. The influence of harvest time on sensory properties and consumer acceptance of sweet cherries. HortTechnology 2009, 19, 748–754. [Google Scholar] [CrossRef]
- Santos, M.; Maia, C.; Meireles, I.; Pereira, S.; Egea-Cortines, M.; Sousa, J.R.; Raimundo, F.; Matos, M.; Gonçalves, B. Effects of calcium- and seaweed-based biostimulants on sweet cherry profitability and quality. Biol. Life Sci. Forum 2024, 27, 45. [Google Scholar] [CrossRef]
- Correia, S.; Oliveira, I.; Queirós, F.; Ribeiro, C.; Ferreira, L.; Luzio, A.; Silva, A.P.; Gonçalves, B. Preharvest application of seaweed based biostimulant reduced cherry (Prunus avium L.) cracking. Procedia Environ. Sci. 2015, 29, 251–252. [Google Scholar] [CrossRef]
- Gonçalves, B.; Morais, M.C.; Sequeira, A.; Ribeiro, C.; Guedes, F.; Silva, A.P.; Aires, A. Quality preservation of sweet cherry cv. ‘Staccato’ by using glycine-betaine or Ascophyllum nodosum. Food Chem. 2020, 322, 126713. [Google Scholar] [CrossRef]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-based biostimulant as sustainable alternative to synthetic growth regulators in two sweet cherry cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Liu, Z.; Wang, L.; Yang, M.; Liu, M. The underlying molecular mechanisms of hormonal regulation of fruit color in fruit-bearing plants. Plant Mol. Biol. 2024, 114, 104. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Ribeiro, C.; Vilela, A.; Meyer, A.S.; Gonçalves, B. Exploring the role of biostimulants in sweet cherry (Prunus avium L.) fruit quality traits. Agriculture 2024, 14, 1521. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Michailidis, M.; Polychroniadou, C.; Kosmidou, M.-A.; Petraki-Katsoulaki, D.; Karagiannis, E.; Molassiotis, A.; Tanou, G. An early calcium loading during cherry tree dormancy improves fruit quality features at harvest. Horticulturae 2021, 7, 135. [Google Scholar] [CrossRef]
- Winkler, A.; Fiedler, B.; Knoche, M. Calcium physiology of sweet cherry fruits. Trees 2020, 34, 1157–1167. [Google Scholar] [CrossRef]
- Winkler, A.; Knoche, M. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 2019, 245, 107–115. [Google Scholar] [CrossRef]
- Erogul, D. Effect of preharvest calcium treatments on sweet cherry fruit quality. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 150–153. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Karamanoli, K.; Lazaridou, A.; Matsi, T.; Molassiotis, A. Metabolomic and physico-chemical approach unravel dynamic regulation of calcium in sweet cherry fruit physiology. Plant Physiol. Biochem. 2017, 116, 68–79. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Wang, X.; Yan, L.; Hu, X.; Lian, M. Effect of foliar calcium fertilization on fruit quality, cell wall enzyme activity and expression of key genes in chinese cherry. Int. J. Fruit Sci. 2023, 23, 200–216. [Google Scholar] [CrossRef]
- Ricardo-Rodrigues, S.; Laranjo, M.; Agulheiro-Santos, A.C. Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 2023, 103, 463–478. [Google Scholar] [CrossRef]
- Varaldo, A.; Alchera, F.; Brigante, L.; Giacalone, G. Foliar applications of calcium and potassium increase cracking resistance and enhance fruit quality in sweet cherries. Italus Hortus 2023, 30, 25–36. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Liu, H.P.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Synergistic roles of leaf boron and calcium during the growing season in affecting sugar and starch accumulation in ripening apple fruit. Acta Physiol. Plant. 2013, 35, 2483–2492. [Google Scholar] [CrossRef]
- Gao, Q.; Xiong, T.; Li, X.; Chen, W.; Zhu, X. Calcium and calcium sensors in fruit development and ripening. Sci. Hortic. 2019, 253, 412–421. [Google Scholar] [CrossRef]
- Romano, G.S.; Cittadini, E.D.; Pugh, B.; Schouten, R. Sweet cherry quality in the horticultural production chain. Stewart Postharvest Rev. 2006, 6, 1–9. [Google Scholar] [CrossRef]
- Karageorgiadou, M.; Rodovitou, M.; Nasiopoulou, E.; Titeli, V.S.; Michailidis, M. Sweet cherry fruit firmness evaluation using compression distance methods. Horticulturae 2024, 10, 435. [Google Scholar] [CrossRef]
- Zhi, H.; Dong, Y. Seaweed-based biostimulants improves quality traits, postharvest disorders, and antioxidant properties of sweet cherry fruit and in response to gibberellic acid treatment. Sci. Hortic. 2024, 336, 113454. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle Its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Haokip, S.W.; Shankar, K.; Lalrinngheta, J. Climate change and its impact on fruit crops. J. Pharmacogn. Phytochem. 2020, 9, 435–438. [Google Scholar]
- Li, X.; Xu, C.; Korban, S.S.; Chen, K. Regulatory mechanisms of textural changes in ripening fruits. Crit. Rev. Plant Sci. 2010, 29, 222–243. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.; Ferreira, H.; Sousa, J.R.; Vilela, A.; Ribeiro, C.; Egea-Cortines, M.; Matos, M.; Gonçalves, B. Enhancing Sweet Cherry Quality Through Calcium and Ascophyllum nodosum Foliar Applications. Horticulturae 2025, 11, 1171. https://doi.org/10.3390/horticulturae11101171
Santos M, Ferreira H, Sousa JR, Vilela A, Ribeiro C, Egea-Cortines M, Matos M, Gonçalves B. Enhancing Sweet Cherry Quality Through Calcium and Ascophyllum nodosum Foliar Applications. Horticulturae. 2025; 11(10):1171. https://doi.org/10.3390/horticulturae11101171
Chicago/Turabian StyleSantos, Marlene, Helena Ferreira, João Ricardo Sousa, Alice Vilela, Carlos Ribeiro, Marcos Egea-Cortines, Manuela Matos, and Berta Gonçalves. 2025. "Enhancing Sweet Cherry Quality Through Calcium and Ascophyllum nodosum Foliar Applications" Horticulturae 11, no. 10: 1171. https://doi.org/10.3390/horticulturae11101171
APA StyleSantos, M., Ferreira, H., Sousa, J. R., Vilela, A., Ribeiro, C., Egea-Cortines, M., Matos, M., & Gonçalves, B. (2025). Enhancing Sweet Cherry Quality Through Calcium and Ascophyllum nodosum Foliar Applications. Horticulturae, 11(10), 1171. https://doi.org/10.3390/horticulturae11101171








