Pseudomonas sp. UW4 Enhances Drought Resistance in Garlic by Modulating Growth and Physiological Parameters
Abstract
1. Introduction
2. Material and Methods
2.1. Plant and Biological Materials
2.2. Plant Growth Conditions and Treatments
2.3. Determination of Plant Growth Parameters
2.4. Determination of the Chlorophyll a, b, and Total Contents
2.5. Determination of the Osmolyte Contents
2.6. Determination of Malondialdehyde (MDA) and Relative Electric Conductivity
2.7. Determination of Antioxidant Enzyme Activity
2.8. Statistical Analysis
3. Results
3.1. Effects of UW4 Inoculation on Garlic Plant Growth Parameters Under Drought Stress
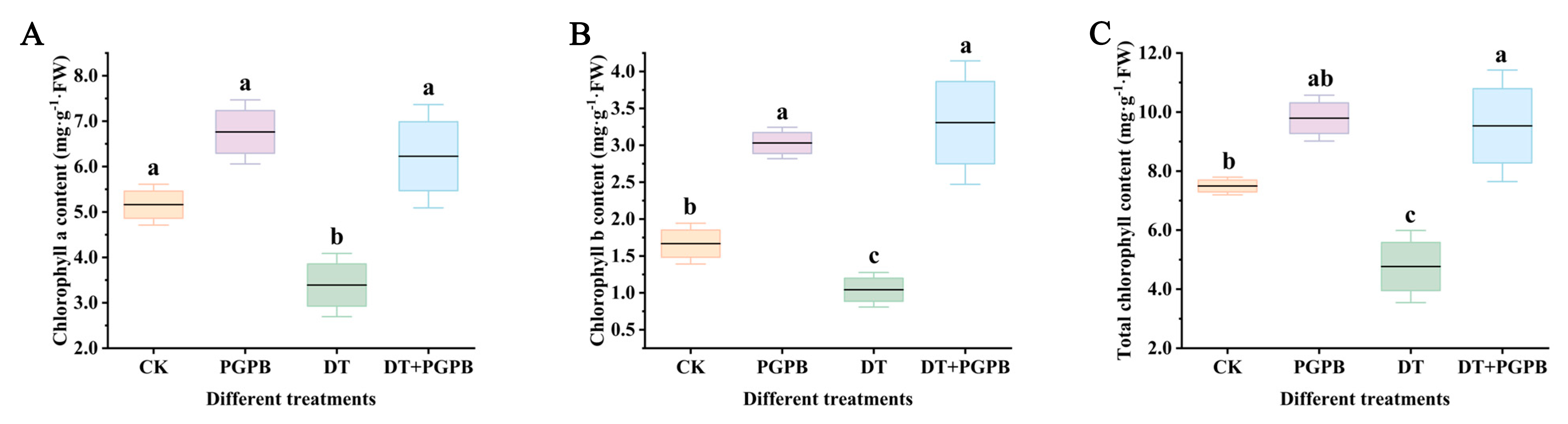
3.2. Effect of Strain UW4 Inoculation on Chlorophyll Content of Garlic Under Drought Stress
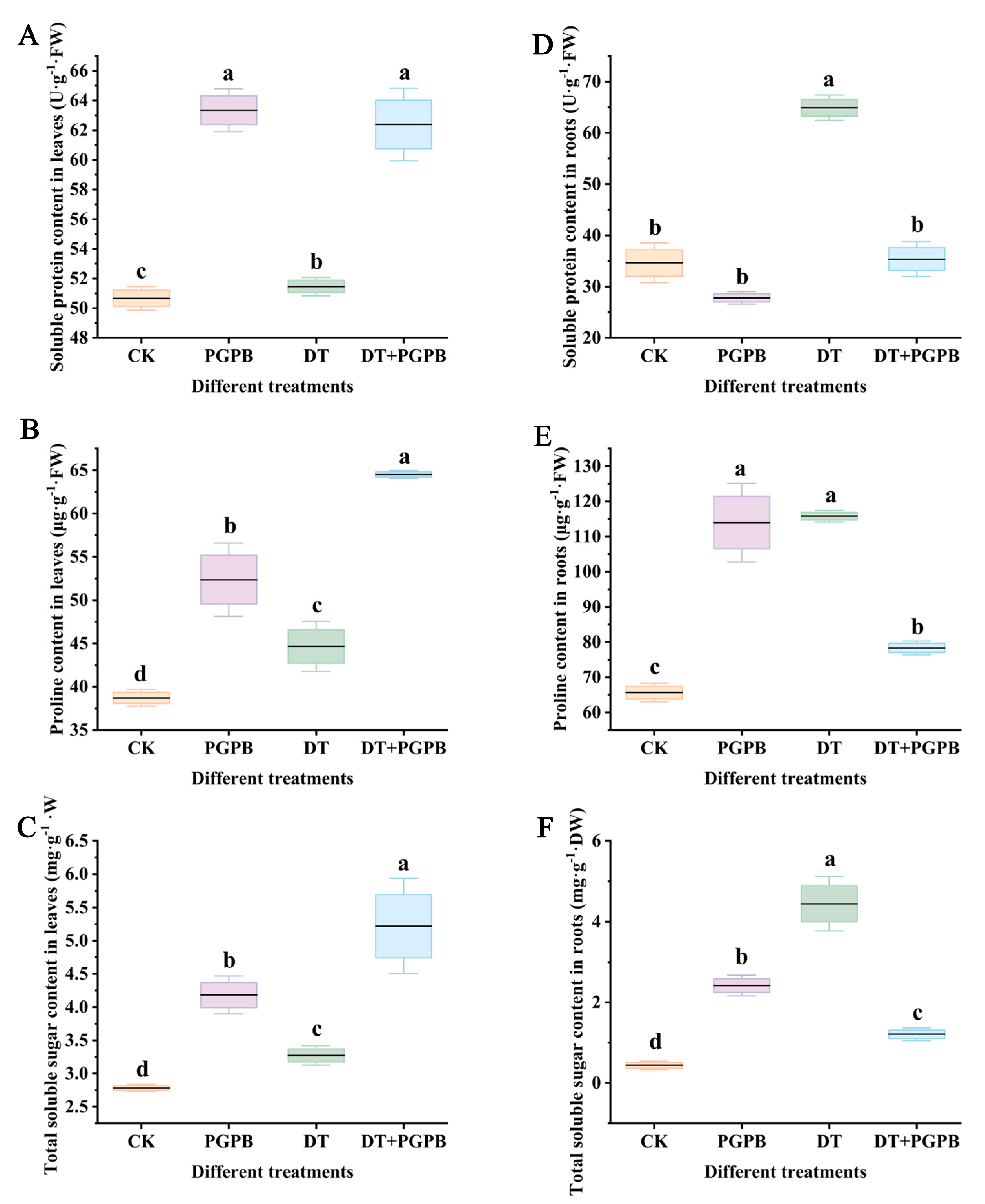
3.3. Effects of Strain UW4 Inoculation on Osmolyte Content in Garlic Plants Under Drought Stress
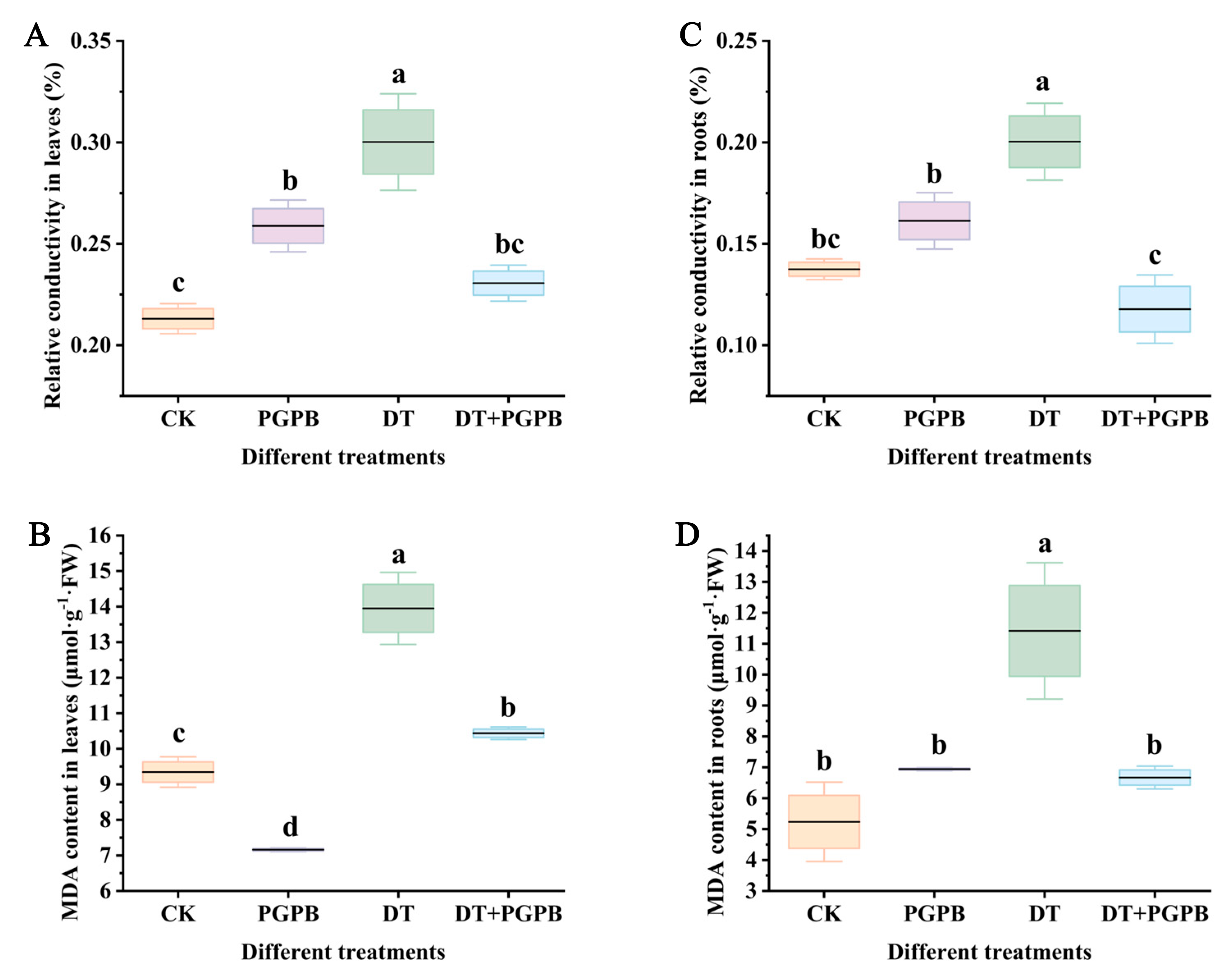
3.4. Effects of Strain UW4 Inoculation on the Relative Electrical Conductivity and MDA Content in Garlic Plants Under Drought Stress
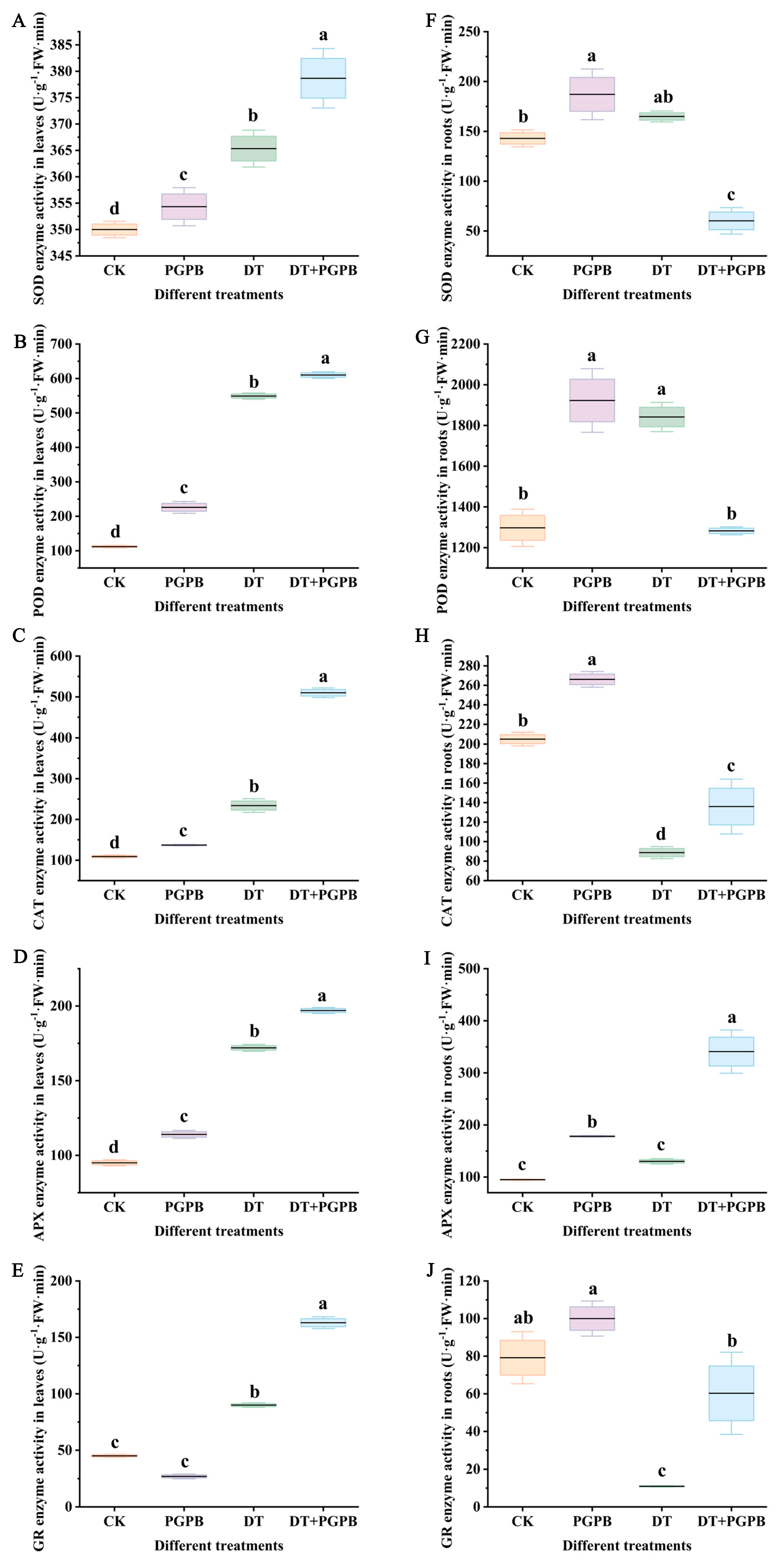
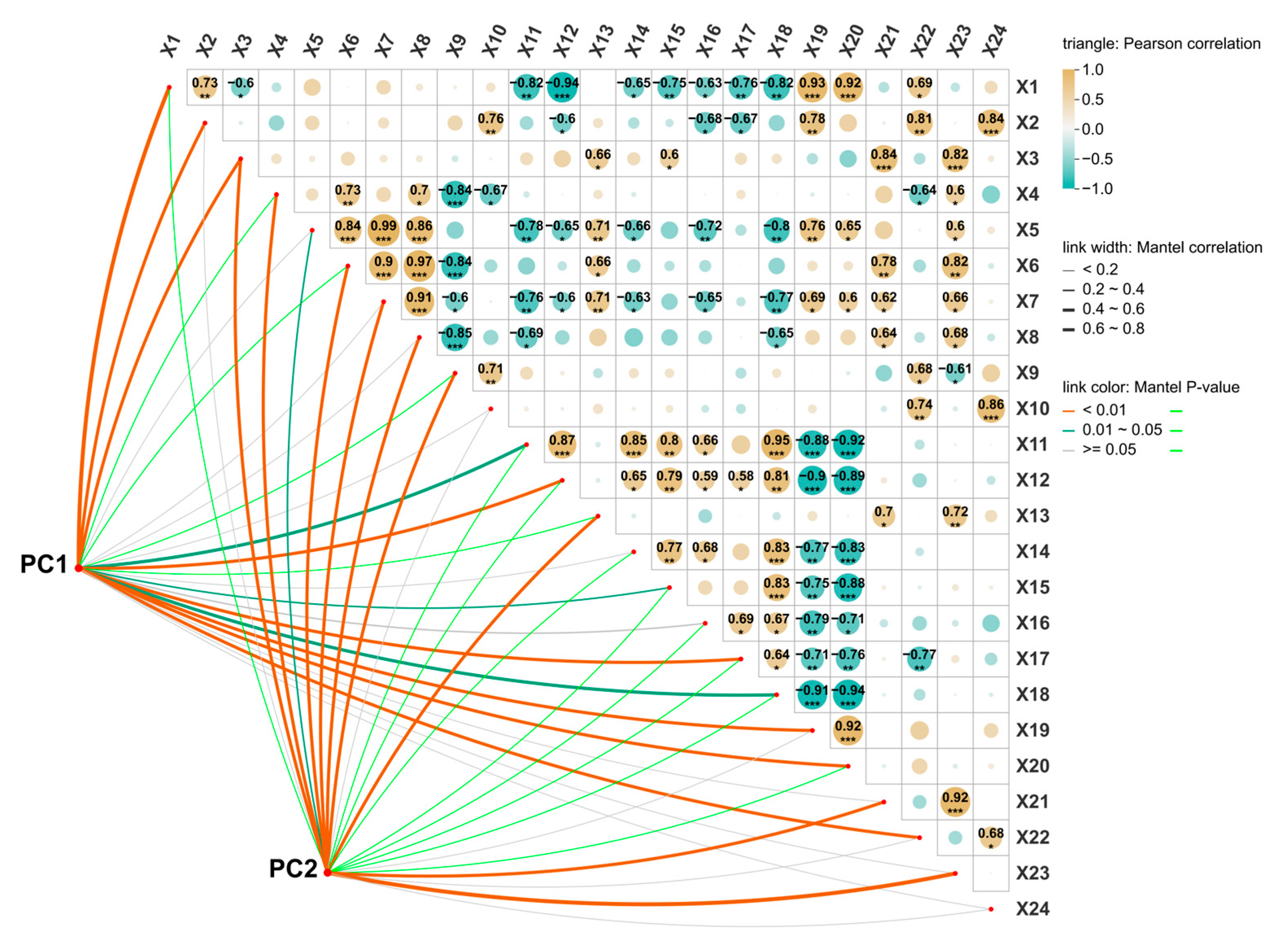
3.5. Effects of Strain UW4 Inoculation on Garlic Antioxidant Enzyme Activity Under Drought Stress
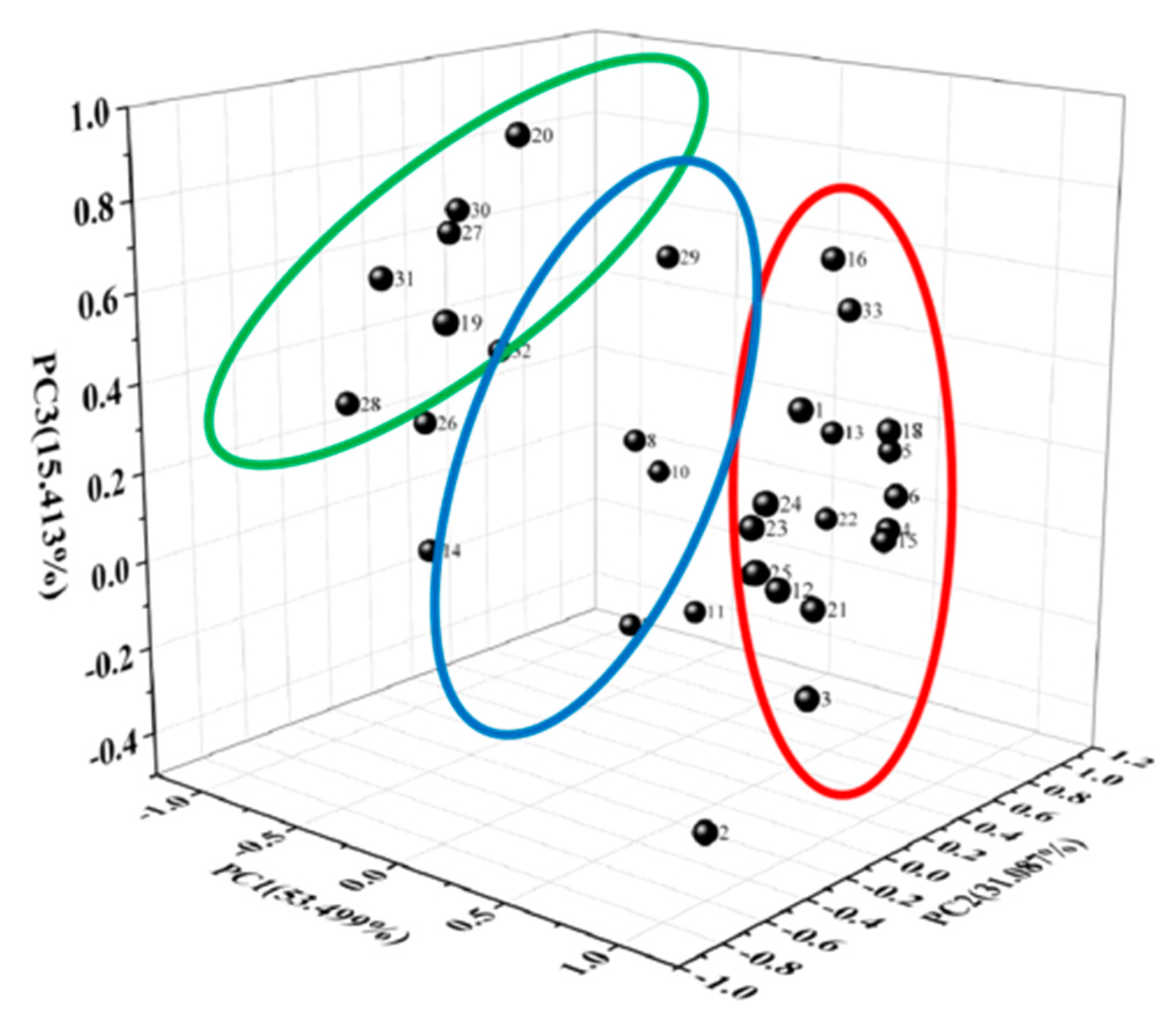
3.6. Principal Component Analysis (PCA)
3.7. Comprehensive Evaluation of Drought Resistance of Garlic Under Different Treatments
3.8. Analysis of Heat Map Clustering
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayaz, E.; Alpsoy, H.C. Garlic (Allium sativum) and traditional medicine. Turk. Parazitolojii Derg. 2007, 31, 145–149. [Google Scholar]
- Badal, D.S.; Dwivedi, A.K.; Kumar, V.; Singh, S.; Prakash, A.; Verma, S.; Kumar, J. Effect of organic manures and inorganic fertilizers on growth, yield and its attributing traits in garlic (Allium sativum L.). J. Pharmacogn. Phytochem. 2019, 8, 587–590. [Google Scholar]
- Rahman, K. Historical perspective on garlic and cardiovascular disease. J. Nutr. 2001, 131, 977S–979S. [Google Scholar] [CrossRef]
- Heim, R.R. A review of twentieth-century drought indices used in the United States. Bull. Am. Meteorol. Soc. 2002, 83, 1149–1165. [Google Scholar] [CrossRef]
- Ashraf, M.; Wu, L. Breeding for Salinity Tolerance in Plants. Crit. Rev. Plant Sci. 1994, 13, 17–42. [Google Scholar] [CrossRef]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Control of Drought Stress in Wheat Using Plant-Growth-Promoting Bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.; Caputo, L.; Sakr, S.; De Feo, V. Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. J. Biol. Res.—Boll. Soc. Ital. Biol. Sper. 2019, 92, 39–45. [Google Scholar] [CrossRef]
- Bano, Q.; Ilyas, N.; Bano, A.; Zafar, N.; Akram, A.; Ul Hassan, F. Effect of azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak. J. Bot. 2013, 45, 13–20. [Google Scholar]
- Glick, B.R.; Nascimento, F.X. Pseudomonas 1-Aminocyclopropane-1-carboxylate (ACC) Deaminase and Its Role in Beneficial Plant-Microbe Interactions. Microorganisms 2021, 9, 2467. [Google Scholar] [CrossRef]
- Glick, B.R.; Gamalero, E. Recent Developments in the Study of Plant Microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef]
- Chukwuneme, C.F.; Babalola, O.O.; Kutu, F.R.; Ojuederie, O.B. Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J. Plant Interact. 2020, 15, 93–105. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, e05106. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Drought-Tolerant Pseudomonas spp. Improve the Growth Performance of Finger Millet (Eleusine coracana (L.) Gaertn.) Under Non-Stressed and Drought-Stressed Conditions. Pedosphere 2018, 28, 227–240. [Google Scholar] [CrossRef]
- Choi, S.M.; Kang, B.R.; Kim, Y.C. Transcriptome Analysis of Induced Systemic Drought Tolerance Elicited by Pseudomonas chlororaphis O6 in Arabidopsis thaliana. Plant Pathol. J. 2013, 29, 209–220. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Chai, R.; Li, R.; Li, Y.F.; Li, T.; Li, Y.A.; Gao, Y.Q.; Qiu, L.Y. Chemotactic rhizocompetence is strengthened by efficient adaptational methylation modification of the 1-aminocyclopropane-1-carboxylic acid chemoreceptor in Pseudomonas sp. UW4. Plant Soil 2024, 494, 589–601. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Shen, C.H.; Li, H.R.; Qiu, L.Y. 1-Aminocyclopropane-1-Carboxylate: A Novel and Strong Chemoattractant for the Plant Beneficial Rhizobacterium Pseudomonas putida UW4. Mol. Plant-Microbe Interact. 2019, 32, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Nordstedt, N.P.; Jones, M.L. Pseudomonas putida UW4 increases horticulture crop quality and stress tolerance during severe drought stress. In Proceedings of the IV International Symposium on Horticulture in Europe-SHE2021 1327, Stuttgart, Germany, 8–12 March 2021; pp. 541–548. [Google Scholar]
- Gao, X.Y.; Li, T.; Liu, W.L.; Zhang, Y.; Shang, D.; Gao, Y.A.; Qi, Y.C.; Qiu, L.Y. Enhancing the 1-Aminocyclopropane-1-Carboxylate Metabolic Rate of Pseudomonas sp. UW4 Intensifies Chemotactic Rhizocompetence. Microorganisms 2020, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Q.; Hale, L.; Crowley, D. Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol. Fertil. Soils 2016, 52, 515–522. [Google Scholar] [CrossRef]
- Srinivasan, R.; Subramanian, P.; Tirumani, S.; Gothandam, K.M.; Ramya, M. Ectopic expression of bacterial 1-aminocyclopropane 1-carboxylate deaminase in Chlamydomonas reinhardtii enhances algal biomass and lipid content under nitrogen deficit condition. Bioresour. Technol. 2021, 341, 125830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Zhang, G.; Wen, Y.M.; Li, T.; Gao, Y.Q.; Meng, F.M.; Qiu, L.Y.; Ai, Y.C. Pseudomonas sp. UW4 acdS gene promotes primordium initiation and fruiting body development of Agaricus bisporus. World J. Microbiol. Biotechnol. 2019, 35, 163. [Google Scholar] [CrossRef]
- Albano, L.J.; Macfie, S.M. Investigating the ability of Pseudomonas fluorescens UW4 to reduce cadmium stress in Lactuca sativa via an intervention in the ethylene biosynthetic pathway. Can. J. Microbiol. 2016, 62, 1057–1062. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Gupta, V.; Franco, C.M.M.; Paasricha, N.; Saifi, S.K.; Tuteja, N.; Sharma, A.K. Field performance of bacterial inoculants to alleviate water stress effects in wheat (Triticum aestivum L.). Plant Soil 2019, 441, 261–281. [Google Scholar] [CrossRef]
- Bian, H.Y.; Zhou, Q.Y.; Du, Z.P.; Zhang, G.N.; Han, R.; Chen, L.S.; Tian, J.; Li, Y. Integrated Transcriptomics and Metabolomics Analysis of the Fructan Metabolism Response to Low-Temperature Stress in Garlic. Genes 2023, 14, 1290. [Google Scholar] [CrossRef]
- Li, H.; Li, H. Principles and Techniques of Plant Physiological Biochemical Experimental; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts—Polyphenoloxidase in beta-vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, J.L.; García-Molina, F.; García-Ruiz, P.A.; Arribas, E.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J.N. Enzymatic and chemical oxidation of trihydroxylated phenols. Food Chem. 2009, 113, 435–444. [Google Scholar] [CrossRef]
- Quintanilla-Guerrero, F.; Duarte-Vázquez, M.A.; García-Almendarez, B.E.; Tinoco, R.; Vazquez-Duhalt, R.; Regalado, C. Polyethylene glycol improves phenol removal by immobilized turnip peroxidase. Bioresour. Technol. 2008, 99, 8605–8611. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, Z.X.; Zhang, L.; Trush, M.A.; Li, Y.B. Glutathione and glutathione-linked enzymes in normal human aortic smooth muscle cells: Chemical inducibility and protection against reactive oxygen and nitrogen species-induced injury. Mol. Cell. Biochem. 2007, 301, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193, 70–84. [Google Scholar] [CrossRef]
- Vurukonda, S.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016, 18, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.; Glick, B.R. The Recent Use of Plant-Growth-Promoting Bacteria to Promote the Growth of Agricultural Food Crops. Agriculture 2023, 13, 1089. [Google Scholar] [CrossRef]
- Ansari, W.A.; Krishna, R.; Kashyap, S.P.; Al-Anazi, K.M.; Abul Farah, M.; Jaiswal, D.K.; Yadav, A.; Zeyad, M.T.; Verma, J.P. Relevance of plant growth-promoting bacteria in reducing the severity of tomato wilt caused by Fusarium oxysporum f. sp. lycopersici by altering metabolites and related genes. Front. Microbiol. 2025, 15, 1534761. [Google Scholar] [CrossRef] [PubMed]
- Mugani, R.; El Khalloufi, F.; Redouane, E.; Haida, M.; Zerrifi, S.E.; Campos, A.; Kasada, M.; Woodhouse, J.; Grossart, H.P.; Vasconcelos, V.; et al. Bacterioplankton Associated with Toxic Cyanobacteria Promote Pisum sativum (Pea) Growth and Nutritional Value through Positive Interactions. Microorganisms 2022, 10, 1511. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Yang, B.; Wen, H.W.; Wang, S.S.; Zhang, J.H.; Wang, Y.Z.; Zhang, T.; Yuan, K.; Lu, L.H.; Liu, Y.T.; Xue, Q.H.; et al. Enhancing Drought Resistance and Yield of Wheat through Inoculation with Streptomyces pactum Act12 in Drought Field Environments. Agronomy 2024, 14, 692. [Google Scholar] [CrossRef]
- Mun, B.G.; Hussain, A.; Park, Y.G.; Kang, S.M.; Lee, I.J.; Yun, B.W. The PGPR Bacillus aryabhattai promotes soybean growth via nutrient and chlorophyll maintenance and the production of butanoic acid. Front. Plant Sci. 2024, 15, 1341993. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, B.; Hu, T.X.; Chen, H.; Li, H.; Zhang, W.; Zhong, Y.; Hu, H.L. Combined action of an antioxidant defence system and osmolytes on drought tolerance and post-drought recovery of Phoebe zhennan S. Lee saplings. Acta Physiol. Plant. 2015, 37, 84. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct. Plant Biol. 2008, 35, 141–151. [Google Scholar] [CrossRef]
- Shintu, P.V.; Jayaram, K.M. Phosphate solubilising bacteria (Bacillus polymyxa)—An effective approach to mitigate drought in tomato (Lycopersicon esculentum Mill.). Trop. Plant Res. 2015, 2, 17–22. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Mostafa Heidari, A.G. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef]
- Gusain, D.Y.; Singh, U.; Sharma, A. Bacterial Mediated Amelioration of Drought Stress in Drought Tolerant and Susceptible Cultivars of Rice (Oryza sativa L.). Afr. J. Biotechnol. 2015, 14, 764–773. [Google Scholar] [CrossRef]


| Group | Plant Height (cm) | Leaf Width (cm) | Shoot Dry Weight (g) | Total Root Length (cm) | Total Root Surface Area (cm2) | Total Root Volume (cm3) | Average Root Diameter (mm) | Number of Root Tips | Root Dry Weight (g) | Root Projection Area (cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| CK | 55.00 ± 0.42 b | 1.97 ± 0.24 a | 6.24 ± 1.0 a | 634.24 ± 35.01 c | 185.17 ± 3.96 b | 6.91 ± 0.01 d | 0.84 ± 0.03 b | 121.00 ± 15.56 ab | 0.53 ± 0.02 a | 58.94 ± 1.26 b |
| PGPB | 62.00 ± 1.27 a | 1.83 ± 0.08 ab | 6.31 ± 2.0 a | 794.84 ± 18.71 a | 293.70 ± 2.22 a | 13.94 ± 2.03 a | 1.17 ± 0.09 a | 146.00 ± 5.35 a | 0.61 ± 0.03 a | 93.49 ± 7.97 a |
| DT | 50.30 ± 2.12 c | 1.57 ± 0.12 c | 4.68 ± 0.56 b | 433.64 ± 16.51 d | 145.16 ± 10.83 c | 8.28 ± 1.91 c | 1.14 ± 0.05 a | 83.00 ± 22.63 c | 0.30 ± 0.01 c | 46.06 ± 3.79 c |
| DT + PGPB | 54.23 ± 2.41 b | 1.73 ± 0.12 b | 6.31 ± 0.43 a | 729.19 ± 14.80 b | 271.53 ± 25.06 a | 11.10 ± 1.57 b | 1.25 ± 0.03 a | 101.33 ± 4.11 b | 0.40 ± 0.02 b | 86.43 ± 3.45 a |
| Treatments | CI1 | CI2 | CI3 | U2 | U3 | D Value |
|---|---|---|---|---|---|---|
| PGPB | 0.939 | −0.155 | 1.160 | 0.445 | 0.913 | 0.726 |
| DT + PGPB | 0.081 | 1.403 | −0.525 | 1.000 | 0.313 | 0.642 |
| CK | 0.382 | −0.963 | −1.085 | 0.157 | 0.113 | 0.406 |
| DT | −1.402 | −0.285 | 0.450 | 0.398 | 0.660 | 0.226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Guo, C.; Glick, B.R.; Tian, J. Pseudomonas sp. UW4 Enhances Drought Resistance in Garlic by Modulating Growth and Physiological Parameters. Horticulturae 2025, 11, 1170. https://doi.org/10.3390/horticulturae11101170
Yan Y, Guo C, Glick BR, Tian J. Pseudomonas sp. UW4 Enhances Drought Resistance in Garlic by Modulating Growth and Physiological Parameters. Horticulturae. 2025; 11(10):1170. https://doi.org/10.3390/horticulturae11101170
Chicago/Turabian StyleYan, Yiwei, Chunqian Guo, Bernard R. Glick, and Jie Tian. 2025. "Pseudomonas sp. UW4 Enhances Drought Resistance in Garlic by Modulating Growth and Physiological Parameters" Horticulturae 11, no. 10: 1170. https://doi.org/10.3390/horticulturae11101170
APA StyleYan, Y., Guo, C., Glick, B. R., & Tian, J. (2025). Pseudomonas sp. UW4 Enhances Drought Resistance in Garlic by Modulating Growth and Physiological Parameters. Horticulturae, 11(10), 1170. https://doi.org/10.3390/horticulturae11101170






