Evaluation of a Three-Level Cascade Soilless System Under Saline Greenhouse Conditions
Abstract
1. Introduction
- Quantify water and nutrient flow at each of the three-level to assess overall resource-use efficiency under increasing salinity conditions.
- Examine the physiological and agronomic responses of each crop to rising salinity levels, including halophytes with high salt-absorbing capacity.
- Provide data to optimize crop combinations and management strategies for commercial-scale cascade systems, particularly in regions facing water scarcity and high-salinity irrigation sources.
2. Materials and Methods
2.1. Greenhouse Facilities
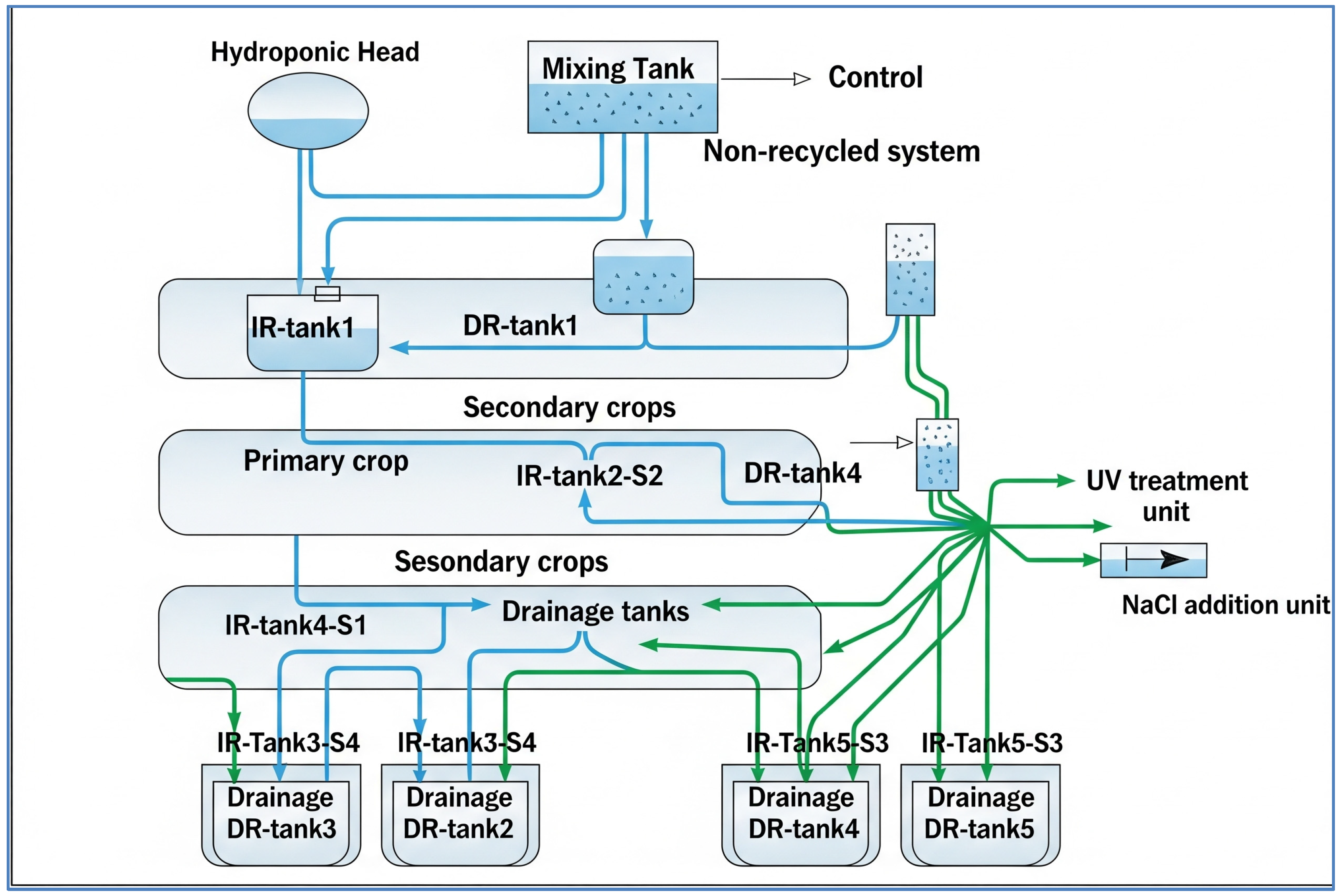
2.2. Experimental Set up and Crop Management
- Primary (donor) Crop: Tomato plants (Solanum lycopersicum L. cultivar Growdena, an F1 hybrid specifically selected for its high yield potential and indeterminate growth habit suitable for hydroponic cultivation in Mediterranean climates), were grown as the primary crop in two greenhouse compartments, totaling 400 m2 (200 m2 each). In each of the compartments, six channels measuring 20 m in length and 0.3 m in width that carried 19 rock wool slabs (Grodan Delta, NL 100 × 15 × 7.5 cm, 0.18 g cm−3, 90% water retention capacity, Roermond, The Netherlands) were installed. Tomato plants were transplanted on 10 March 2020, at a density of 4 plants m−2 (864 plants total, with an initial height of 20 cm). The cultivation period lasted until 10 July 2020, for a total of 122 days.
- Secondary (receiver) Crops: Mint (Mentha piperita L. cultivar ‘Mitcham’, known for its robust growth and essential oil production) and peppermint (Mentha spicata L. cultivar ‘Crispa’, chosen for its distinct flavor profile and adaptability) were cultivated in a separate 200 m2 compartment. The plants were transplanted into rock wool slabs at a density of 4 plants m−2 (216 plants each) on 4 March 2020, and 16 April 2020, respectively, with an initial height of 9 cm. The cultivation cycle for mint lasted until 9 July 2020 (128 days), and for peppermint, until 10 July 2020 (129 days).
- Tertiary (receiver) Crops: Sea-fennel (Crithmum maritimum L.) a native Mediterranean halophyte highly tolerant to saline conditions, and Lemon-balm plants (Melissa officinalis L. cultivar ‘Lemonella’, a popular herb but more sensitive to salt stress), were chosen as tertiary crops and obtained from a local nursery. Plants were transplanted on 5 March 2020, and 27 March 2020, respectively, in rock wool slabs at a density of 4 plants m−2. Initial plant height was approximately 9 cm. The cultivation cycle for the herbs and sea fennel lasted until 7 July and 8 July 2020 (103 and 104 days), respectively.
- Staking (Tomatoes): Staking was performed weekly using plastic clips and strings to support the growing tomato vines and prevent lodging [40].
- Harvesting: All crops were subjected to continuous harvesting. Marketable yield for tomatoes was collected twice a week based on fruit ripeness (red color) [41]. Herbs (mint, peppermint, lemon balm) and sea fennel were harvested selectively as needed, by cutting the top 10–15 cm of the shoots, when plants reached an adequate biomass and visual maturity, ensuring continuous production and optimal plant health.
- Pollination (Tomatoes): Natural pollination for tomatoes was facilitated by commercially purchased bumblebee hives introduced into the greenhouse. No artificial pollination was performed for any crop [42].
- Phytosanitary Management: Phytosanitary management involved regular scouting for pests and diseases. Integrated Pest Management (IPM) principles were strictly followed [43,44]. Biological control agents (e.g., predatory mites for spider mites, parasitic wasps for whiteflies) were applied as a primary strategy, supplemented by targeted organic-approved treatments when necessary to minimize chemical intervention and maintain system integrity [45].
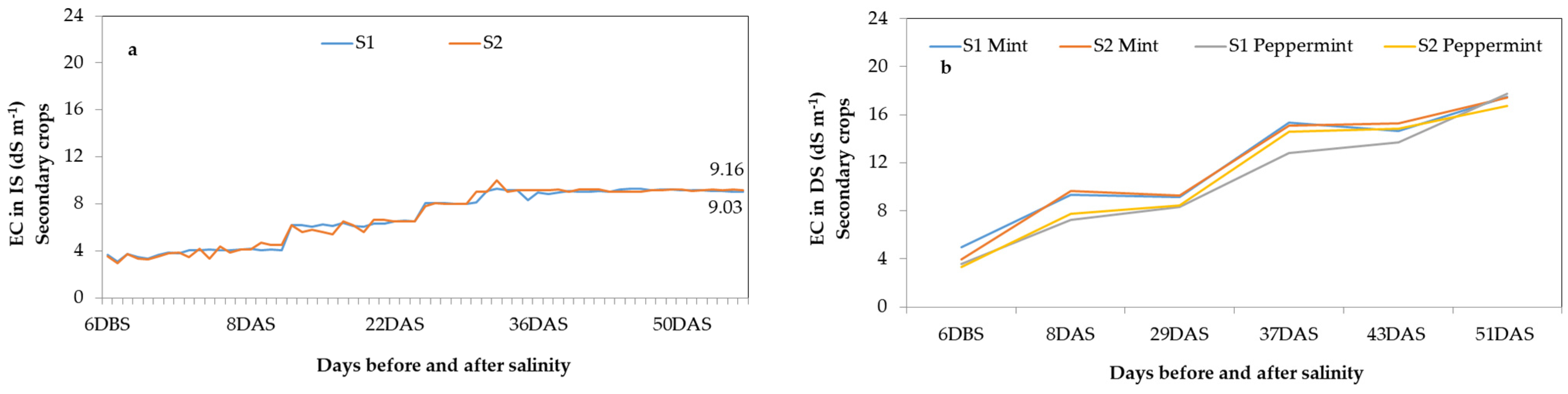
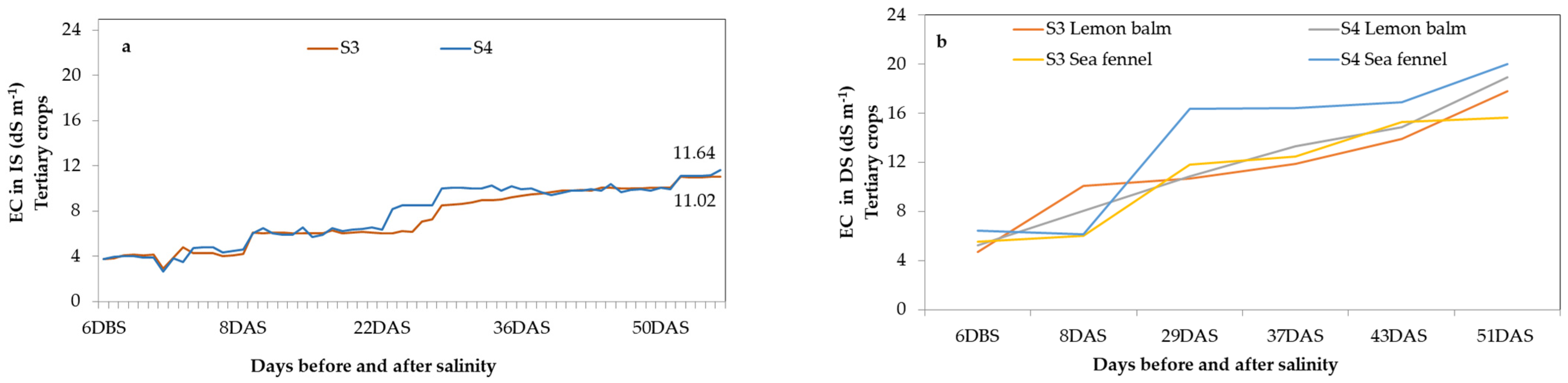
- Saline Stress Management: Saline stress was induced by adding NaCl to the nutrient solution. The electrical conductivity (EC) was monitored daily using a calibrated portable sensor (Combo pH-EC-TDS-Temp, 98,130 Hanna Instruments, Woonsocket, RI, USA). The substrate’s electrical conductivity was monitored daily by analyzing the collected drainage solution.
- o
- S1 (Control): Irrigation with a fresh solution (FS) where salinity was gradually increased with fertilizers to an EC of 9 dS m−1.
- o
- S2: Irrigation with 100% drainage solution (DS) from the primary crop, supplemented with NaCl to reach the same target EC of 9 dS m−1.
- o
- S3 (Control): Irrigation with FS where salinity was gradually increased with fertilizers to an EC of 11 dS m−1.
- o
- S4: Irrigation with NS mixed from S2 and S4 treatment DS, with additional NaCl to reach the target EC of 11 dS m−1.
2.3. Measurements
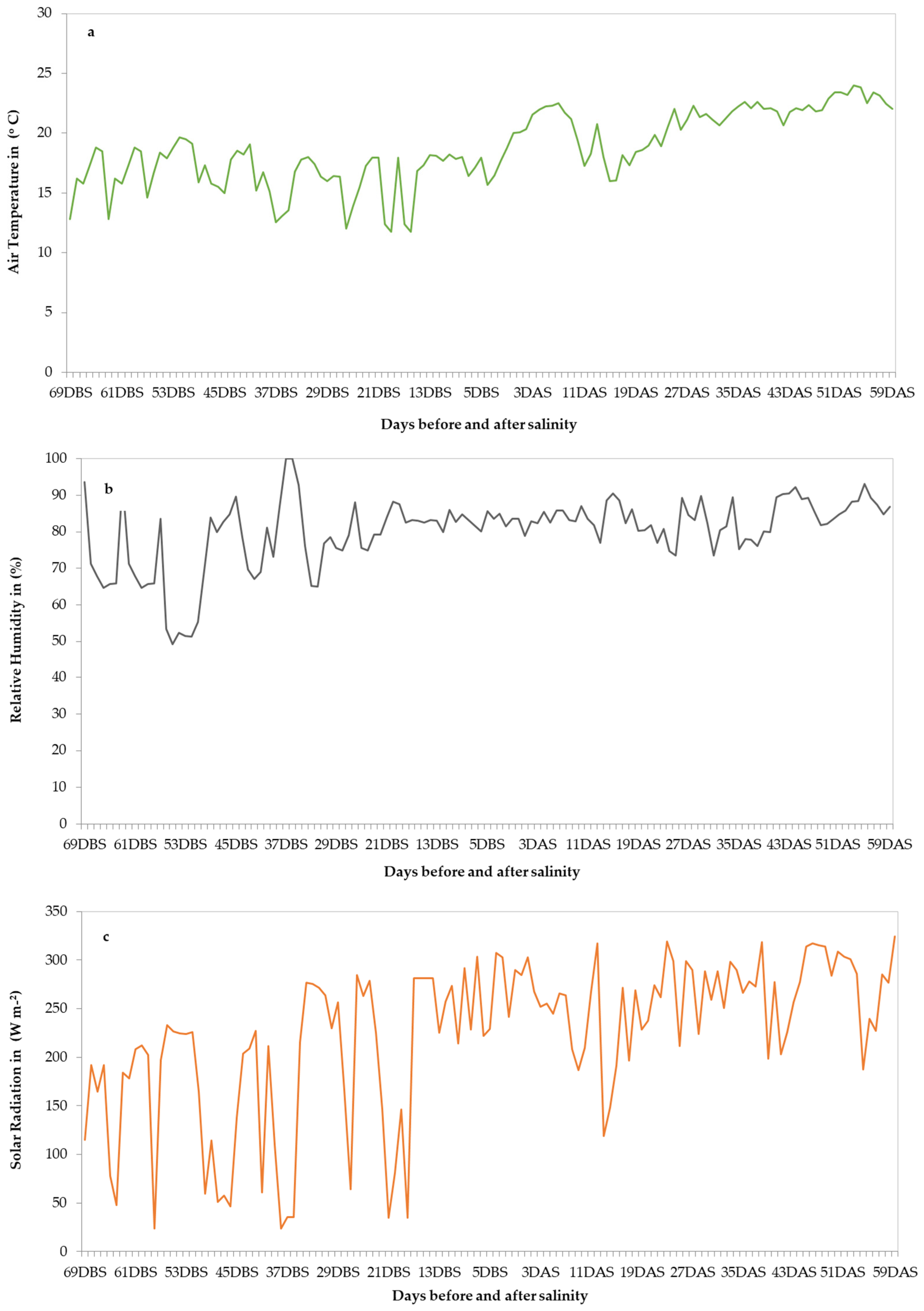
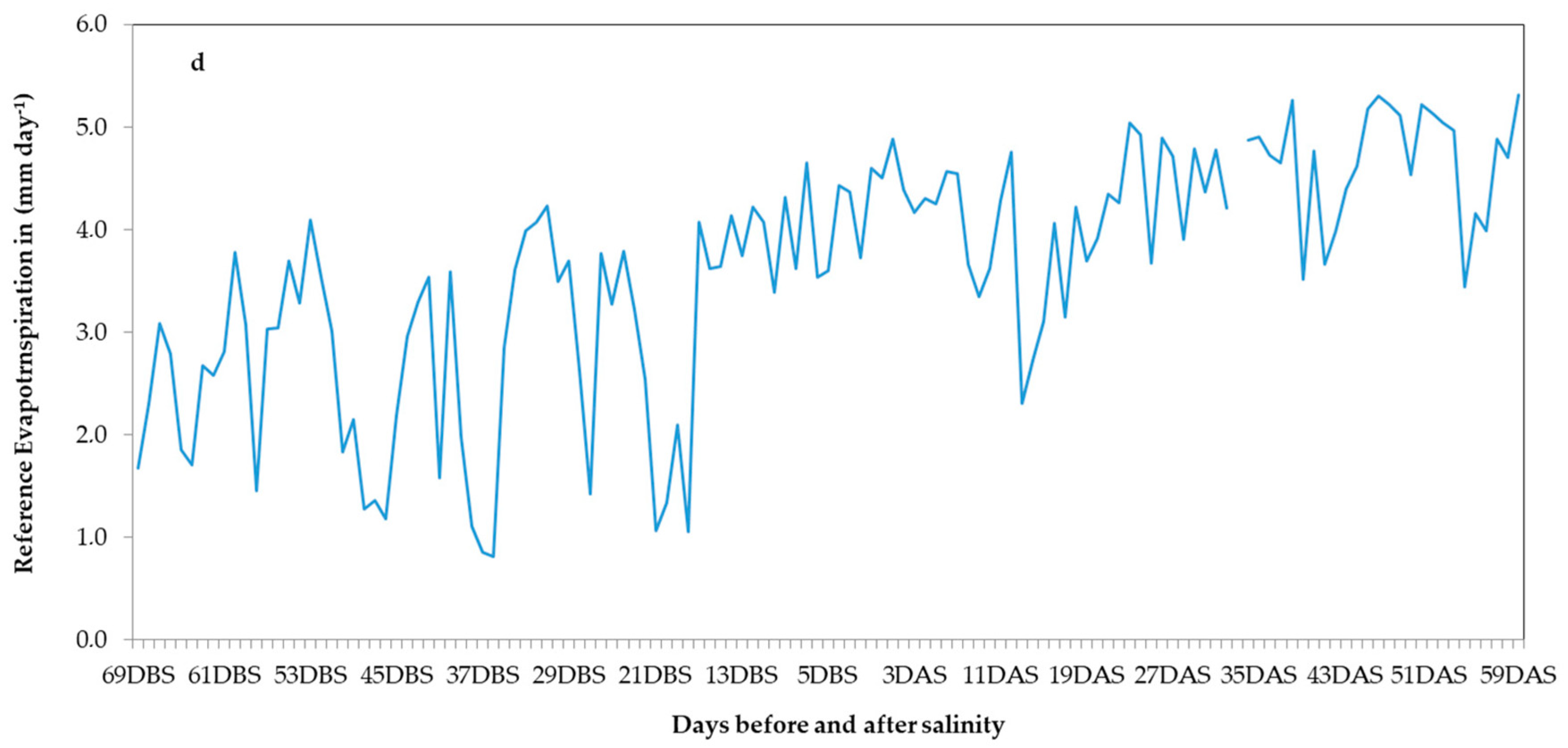
2.3.1. Climate and Nutrient Solution Quality Data
2.3.2. Plant Growth and Nutrient Data
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Quality Assessment of Cascade Solution
3.2. Crop Water Consumption
3.3. Nutrient Concentration
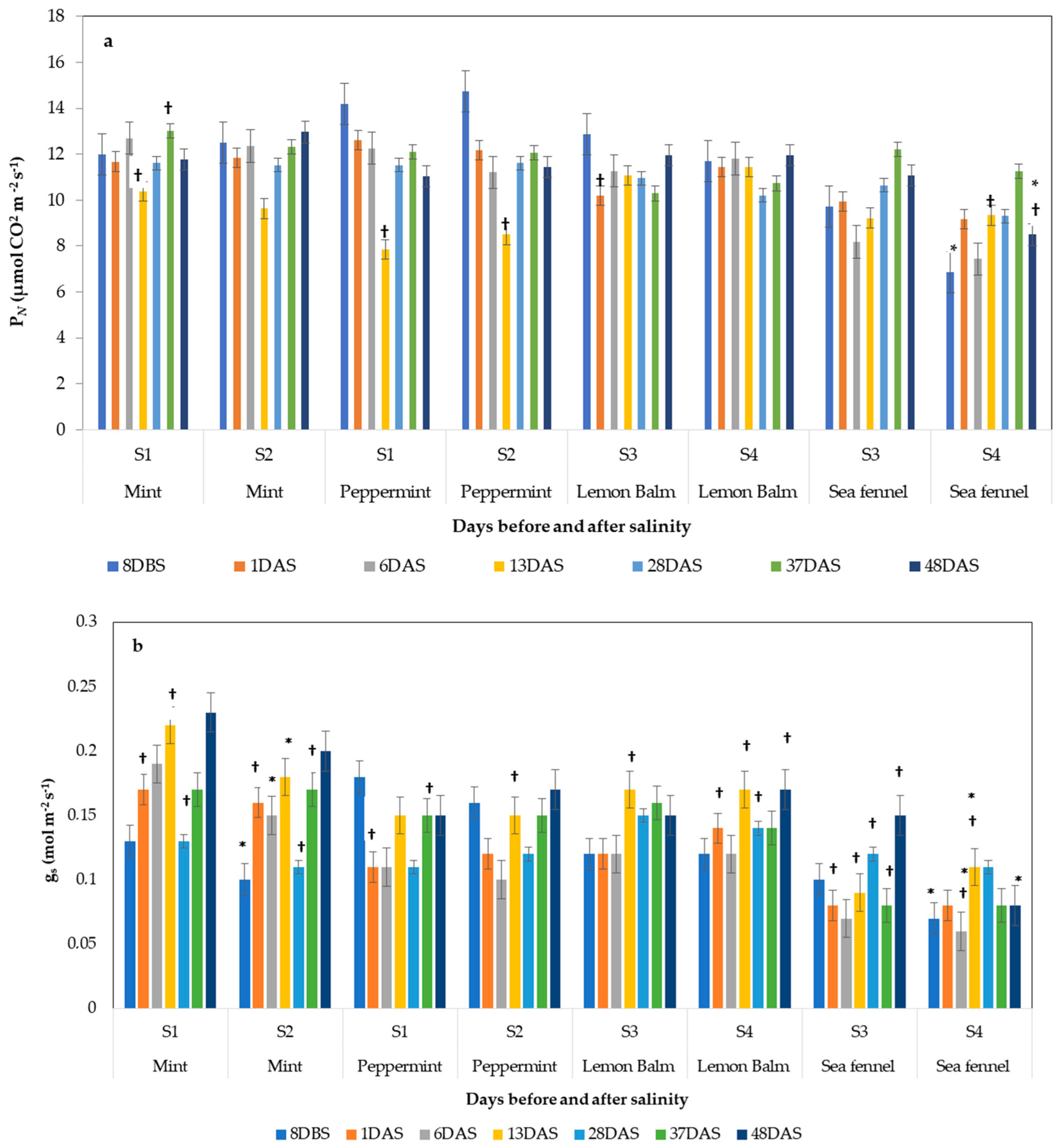
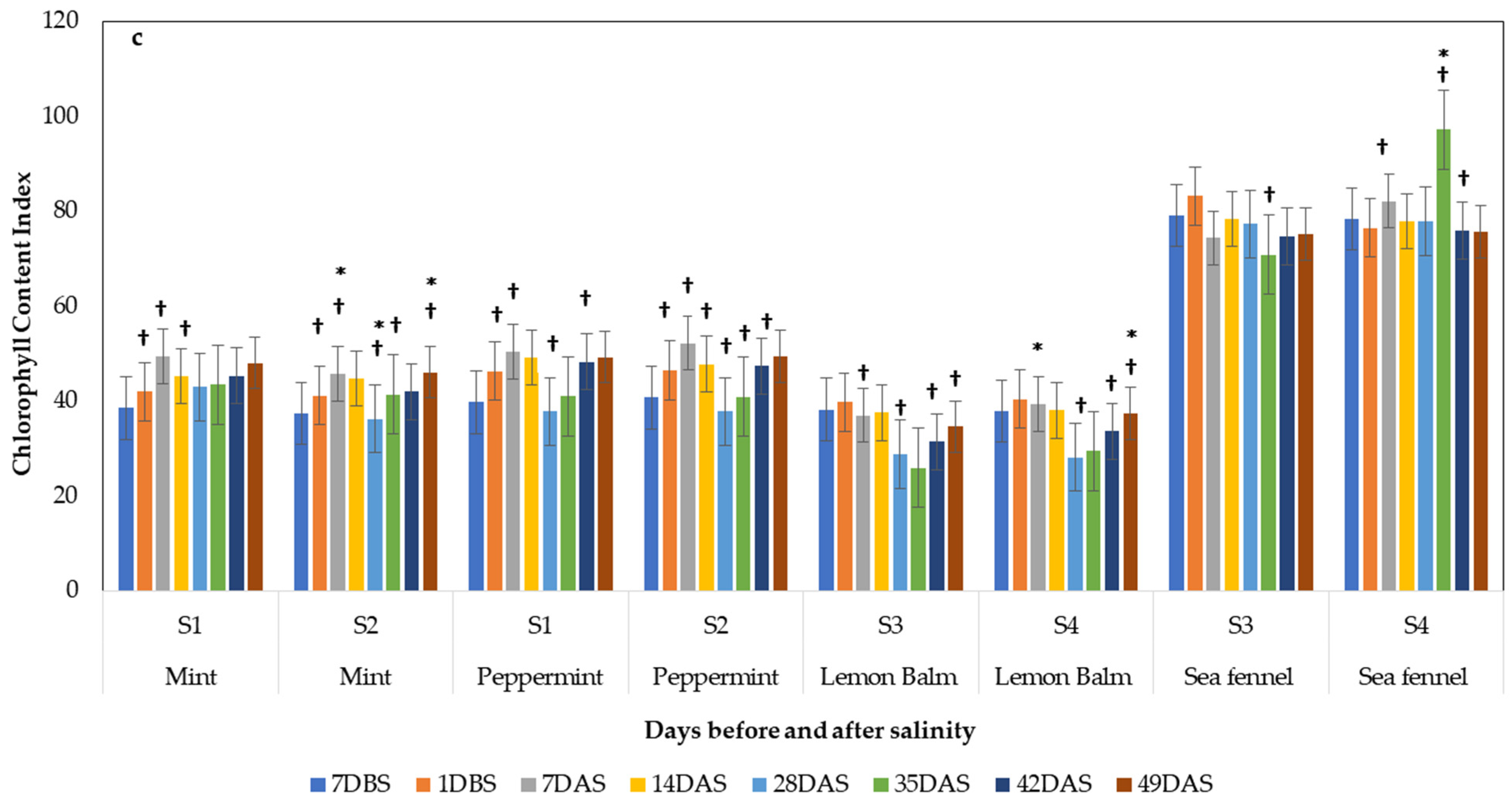
3.4. Effect of Salinity on Plant Physiology
3.4.1. Photosynthetic Performance
3.4.2. Fresh and Dry Matter
3.4.3. Plant Biomass
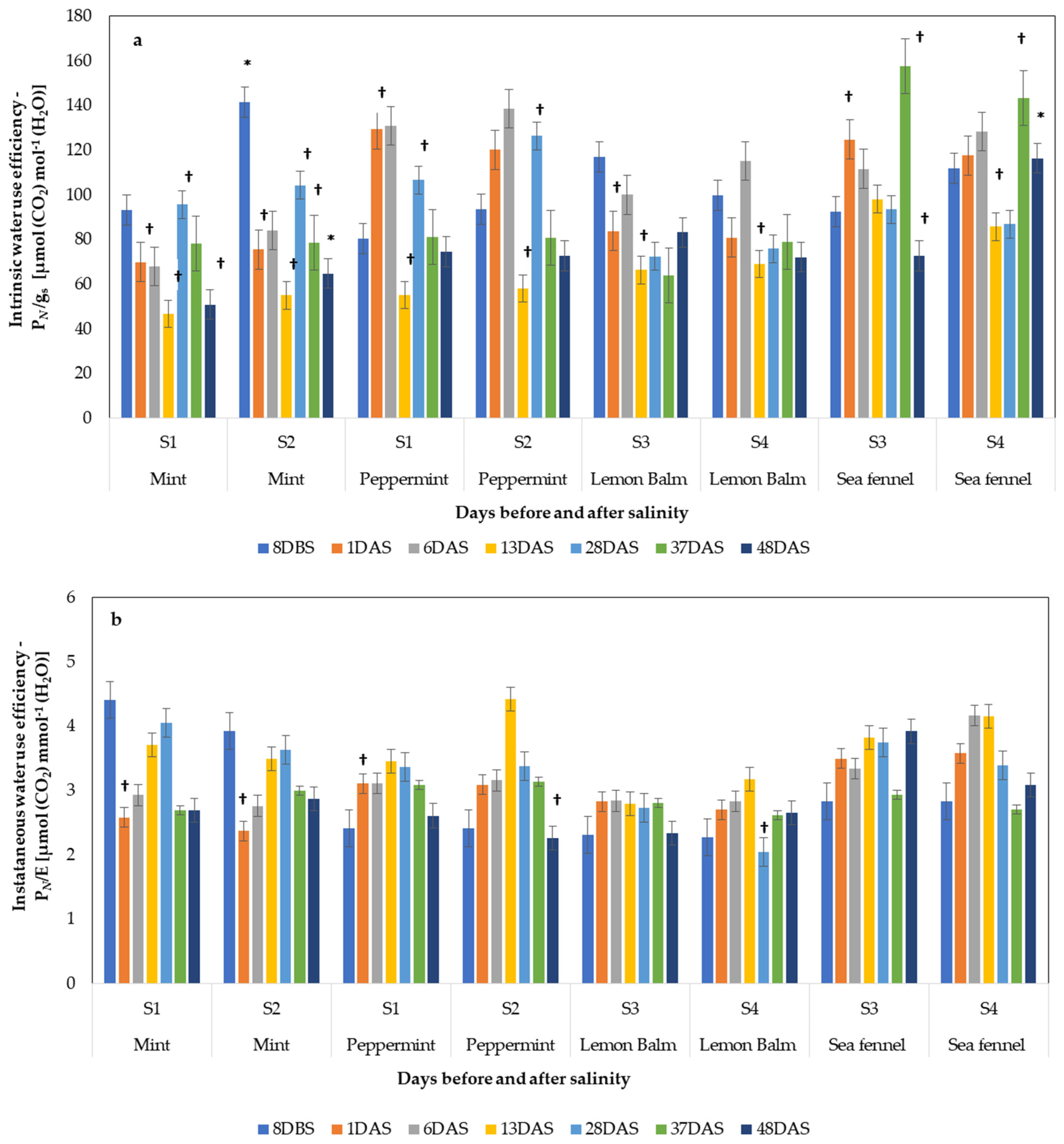
3.5. Water Conservation
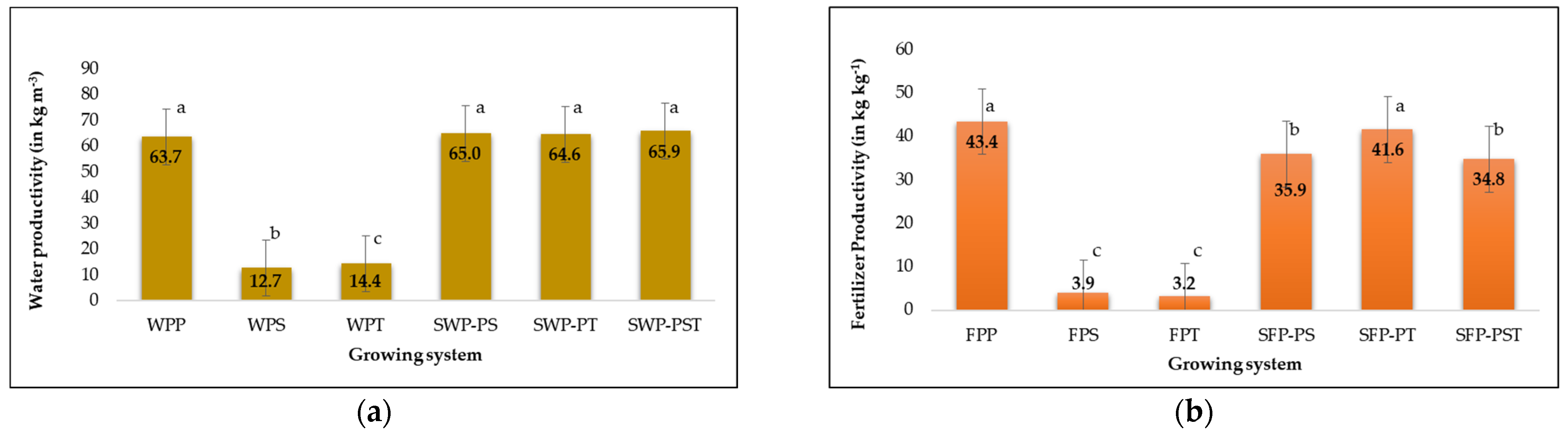
3.6. Water and Fertilize Productivity
4. Discussion
4.1. Evaluation of the System Based on Crops Physiology
4.2. Evaluation of the System Based on Nutrients Concentration
4.3. Evaluation of the System Based on Water and Fertilizer Use Efficiency
4.4. Policy and Market Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Analytical Development Company |
| ANOVA | Analysis of Variance |
| B | Boron |
| Ca | Calcium |
| CCI | Chlorophyll Content Index |
| cm | centimeter |
| CO2 | Carbon Dioxide |
| Cu | Copper |
| °C | degrees Celsius |
| D | Day |
| DAS | Days After Salinity treatment Start |
| DAT | Days After Transplanting |
| DBS | Days Before Salinity treatment Start |
| dS m−1 | deciSiemens per meter |
| DM | Dry Matter |
| DS | Drainage Solution |
| E | Leaf Transpiration |
| EC | Electrical Conductivity |
| ETo | Reference Evapotranspiration |
| FAO | Food and Agriculture Organization |
| Fe | Iron |
| FM | Fresh Matter |
| FP | Fertilizer Productivity |
| FPP | Fertilizer Productivity Primary crop |
| FPS | Fertilizer Productivity Secondary crop |
| FPT | Fertilizer Productivity Tertiary crop |
| FS | Fresh nutrient Solution |
| g cm−3 | grams per cubic centimeter |
| g plant−1 | grams per plant |
| gs | Stomatal Conductance |
| H2O | Water |
| H2SO4 | Sulfuric Acid |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectroscopy |
| ICP–OES | Inductively Coupled Plasma–Optical Emission Spectrometry |
| IS | Irrigation Solution |
| K | Potassium |
| kg | kilogram |
| kg FM m−3 H2O | kilograms Fresh Matter per cubic meter of Water |
| kg FM kg−1 fertilizer | kilograms Fresh Matter per kilogram of fertilizer |
| kg m−2 | kilograms per square meter |
| kg plant−1 m−2 | kilograms per plant per square meter |
| L | Liter |
| L m−2 | Liters per square meter |
| L plant−1 | Liters per plant |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| LSD | Least Significant Difference |
| m2 | square meters |
| m3 | cubic meters |
| Mg | Magnesium |
| Mn | Manganese |
| mmol | millimole |
| mmol L−1 | millimoles per Liter |
| Mo | Molybdenum |
| Na | Sodium |
| Na+ | Sodium ion |
| NaCl | Sodium Chloride |
| NH4+ | Ammonium ion |
| NO3 | Nitrate |
| NO3− | Nitrate ion |
| NO3−-N | Nitrate-Nitrogen |
| NS | Nutrient Solution |
| P | Phosphorus |
| pH | Potential of Hydrogen |
| PN | Photosynthetic Rate |
| PN/E | Instantaneous Water Use Efficiency |
| PN/gs | Intrinsic Water Use Efficiency |
| PO42− | Phosphate ion |
| PO42−-P | Phosphate-Phosphorus |
| ppm | parts per million |
| PPFD | Photosynthetic Photon Flux Density |
| PVC | Polyvinyl Chloride |
| % | percent |
| RH | Relative Humidity |
| RSD% | Reproducibility (as Relative Standard Deviation) |
| S1 | Control (Secondary Crop Treatment) |
| S2 | Recycling Treatment (Secondary Crop) |
| S3 | Control (Tertiary Crop Treatment) |
| S4 | Recycling Treatment (Tertiary Crop) |
| SD | Standard Deviation |
| SDG | Sustainable Development Goals |
| SE | Standard Error |
| SERCOM | SERCOM (climate controller brand) |
| SFP | System Fertilizer Productivity |
| SFP-PS | System Fertilizer Productivity − Primary + Secondary crops |
| SFP-PT | System Fertilizer Productivity − Primary + Tertiary crops |
| SFP-PST | System Fertilizer Productivity − Primary + Secondary + Tertiary crops |
| SO42− | Sulfate ion |
| SPAD | Soil–Plant Analysis Development |
| SPSS | Statistical Product and Service Solutions |
| SR | Solar Radiation |
| SWP | System Water Productivity |
| SWP-PS | System Water Productivity − Primary + Secondary crops |
| SWP-PT | System Water Productivity − Primary + Tertiary crops |
| SWP-PST | System Water Productivity − Primary + Secondary + Tertiary crops |
| Ta | Air Temperature |
| µmol | micromole |
| μmol L−1 | micromoles per Liter |
| WUE | Water Use Efficiency |
| Zn | Zinc |
Appendix A
| Analyte | Method | LOD (µmol L−1) | LOQ (µmol L−1) | Reproducibility (RSD, %) |
|---|---|---|---|---|
| Anions | ||||
| NO3− | Ion Chromatography | ~0.26 1 | ~0.80 1 | <3% 2 |
| PO42− | Ion Chromatography | ~0.01–0.03 3 | ~0.03–0.09 3 | <5% 3 |
| Cations | ||||
| K+, Ca2+, Mg2+ | ICP-AES | ~0.1–2.0 4 | ~0.3–6.0 4 | <5% 5 |
| Na+ | ICP-AES | ~0.5–3.0 4 | ~1.5–9.0 4 | <5% 5 |
| Micronutrients | ||||
| Fe, Zn, Mn, Cu | ICP-OES | ~0.01–0.1 6 | ~0.03–0.3 6 | <5% 7 |
| Total N | Potentiometric (ISO 16634–1:2008) | ~0.07 8 | ~0.21 8 | <2% 9 |
| Mint | N | P | K | Ca | Mg | Fe | Zn | Mn | Cu | |
|---|---|---|---|---|---|---|---|---|---|---|
| Τreatment | % | % | % | % | % | ppm | ppm | ppm | ppm | |
| 1st harvest (15DAS) | S1 | 4.51 ± 0.24 a | 0.39 ± 0.06 a | 2.37 ± 0.37 a | 1.19 ± 0.06 a | 0.86 ± 0.03 a | 113.67 ± 8.02 a | 38.33 ± 3.02 a | 64.44 ± 9.43 a | 18.92 ± 1.74 a |
| S2 | 4.27 a ± 0.23 a | 0.45 ± 0.04 b | 3.86 ± 0.12 b | 1.07 ± 0.02 b | 0.68 ± 0.03 b | 112.44 ± 10.50 a | 41.55 ± 2.83 b | 63.33 ± 8.97 a | 15.37 ± 3.26 b | |
| 2nd harvest (58DAS) | S1 | 3.92 a ± 0.36 a | 0.52 ± 0.05 a | 4.99 ± 0.65 a | 1.03 ± 0.09 a | 0.37 ± 0.03 a | 122.00 ± 36.23 a | 36.00 ± 6.28 a | 60.55 ± 8.62 a | 20.54 ± 5.91 a |
| S2 | 3.20 ± 0.12 a | 0.45 ± 0.05 b | 3.20 ± 0.18 b | 1.06 ± 0.06 a | 0.63 ± 0.04 b | 103.44 ± 9.19 a | 45.33 ± 6.53 b | 78.22 ± 10.99 b | 29.31± 6.47 b | |
| Peppermint | ||||||||||
| 1st harvest (17DAS) | S1 | 3.96 ± 0.30 a | 0.39 ± 0.69 a | 2.63 ± 0.46 a | 1.45 ± 0.10 a | 0.93 ± 0.70 a | 111.33 ± 26.60 a | 29.56 ± 3.04 a | 67.33 ± 16.11 a | 14.42 ± 8.36 a |
| S2 | 3.95 ± 0.16 a | 0.42 ± 0.67 a | 3.11 ± 0.12 a | 1.43 ± 0.79 a | 0.84 ± 0.41 a | 93.78 ± 5.91 a | 32.67 ± 3.08 b | 61.67 ± 15.43 a | 10.94 ± 3.50 a | |
| 2nd harvest (59DAS) | S1 | 3.73 ± 0.20 a | 0.54 ± 0.98 a | 5.37 ± 0.67 a | 1.75 ± 0.35 a | 0.51 ± 0.12 a | 112.11 ± 18.43 a | 31.78 ± 4.60 a | 86.88 ± 17.19 a | 16.08 ± 5.15 a |
| S2 | 3.73 ± 0.23 a | 0.56 ± 0.79 a | 2.69 ± 0.33 b | 1.42 ± 0.26 b | 0.79 ± 0.11 b | 103.55 ± 41.74 a | 38.00 ± 5.92 b | 78.00 ± 22.21 a | 12.21 ± 3.07 a | |
| Lemon balm | ||||||||||
| 1st harvest (22DAS) | S3 | 3.35 ± 0.25 a | 0.37 ± 0.35 a | 3.45 ± 0.17 a | 0.92 ± 0.09 a | 0.58 ± 0.26 a | 63.22 ± 4.26 a | 25.76 ± 2.68 a | 29.11 ± 5.39 a | 7.49 ± 1.52 a |
| S4 | 3.84 ± 0.21 b | 0.42 ± 0.68 b | 3.51 ± 0.73 a | 1.09 ± 0.18 b | 0.66 ± 0.93 b | 69.78 ± 5.65 b | 27.89 ± 2.03 a | 34.22 ± 7.33 a | 9.44 ± 2.07 b | |
| 2nd harvest (59DAS) | S3 | 3.40 ± 0.12 a | 0.51 ± 0.58 a | 5.22 ± 0.79 a | 0.70 ± 0.21 a | 0.41 ± 0.81 a | 79.33 ± 6.63 a | 31.56 ± 7.84 a | 38.00 ± 8.48 a | 7.42 ± 1.60 a |
| S4 | 3.51 ± 0.10 a | 0.38 ± 0.16 b | 3.43 ± 0.12 b | 1.12 ± 0.08 b | 0.61 ± 0.35 b | 81.78 ± 23.36 a | 42.44 ± 4.72 b | 44.67 ± 7.28 a | 8.68 ± 1.46 a | |
| Sea fennel | ||||||||||
| 1st harvest (23 DAS) | S3 | 2.51 ± 0.30 a | 0.55 ± 0.66 a | 4.23 ± 0.75 a | 2.18 ± 0.21 a | 0.33 ± 0.03 a | 39.11 ± 4.88 a | 18.00 ± 3.32 a | 42.33 ± 6.65 a | 15.30 ± 10.06 a |
| S4 | 2.62 ± 0.15 a | 0.49 ± 0.42 b | 4.08 ± 0.49 a | 2.42 ± 0.24 b | 0.37 ± 0.03 b | 42.67 ± 10.65 a | 21.00 ± 2.87 a | 46.44 ± 5.83 a | 6.21 ± 3.94 b | |
| 2nd harvest (56DAS) | S3 | 2.63 ± 0.15 a | 0.65 ± 0.12 a | 3.72 ± 0.58 a | 2.38 ± 0.21 a | 0.31 ± 0.32 a | 49.44 ± 5.98 a | 24.00 ± 3.04 a | 60.00 ± 6.48 a | 9.12 ± 7.73 a |
| S4 | 2.69 ± 0.12 a | 0.53 ± 0.11 b | 2.34 ± 0.40 b | 3.20 ± 0.26 b | 0.43 ± 0.39 b | 46.22 ± 9.16 a | 30.33 ± 4.74 b | 64.33 ± 6.24 a | 9.59 ± 3.96 a |
References
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Sanga, D.L.; Mwamahonje, A.S.; Mahinda, A.J.; Kipanga, E.A. Soil salinization under irrigated farming: A threat to sustainable food security and environment in semi-arid tropics. J. Agric. Sci. Pr. 2024, 9, 32–47. [Google Scholar] [CrossRef]
- Mielcarek, A.; Kłobukowska, K.; Rodziewicz, J.; Janczukowicz, W.; Bryszewski, K.Ł. Water Nutrient Management in Soilless Plant Cultivation versus Sustainability. Sustainability 2024, 16, 152. [Google Scholar] [CrossRef]
- Tola, E.; Al-Gaadi, K.A.; Madugundu, R.; Patil, V.C.; Sygrimis, N. Impact of water salinity levels on the spectral behavior and yield of tomatoes in hydroponics. J. King Saud. Univ. Sci. 2023, 35, 102515. [Google Scholar] [CrossRef]
- Roosta, H.R.; Sharifi Azad, H.; Mirdehghan, S.H. Comparison of the growth, fruit quality and physiological characteristics of cucumber fertigated by three different nutrient solutions in soil culture and soilless culture systems. Sci. Rep. 2025, 15, 203. [Google Scholar] [CrossRef]
- Boneta, A.; Rufí-Salís, M.; Ercilla-Montserrat, M.; Gabarrell, X.; Rieradevall, J. Agronomic and environmental assessment of a polyculture rooftop soilless urban home garden in a mediterranean city. Front. Plant Sci. 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Luo, Z.; Hao, X.; Li, S.; Kang, S. Potential pathways to reduce environmental impact in a greenhouse tomato production: Life cycle assessment for different irrigation and fertilization treatments. Sci. Hortic. 2022, 305, 111411. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Parada, F.; Arcas-Pilz, V.; Petit-Boix, A.; Villalba, G.; Gabarrell, X. Closed-loop crop cascade to optimize nutrient flows and grow low-impact vegetables in cities. Front. Plant Sci. 2020, 11, 596550. [Google Scholar] [CrossRef] [PubMed]
- Atzori, G.; Mancuso, S.; Masi, E. Seawater potential use in soilless culture: A review. Sci. Hortic. 2019, 249, 199–207. [Google Scholar] [CrossRef]
- Caparrotta, S.; Masi, E.; Atzori, G.; Diamanti, I.; Azzarello, E.; Mancuso, S.; Pandolfi, C. Growing spinach (Spinacia oleracea) with different seawater concentrations: Effects on fresh, boiled and steamed leaves. Sci. Hortic. 2019, 256, 108540. [Google Scholar] [CrossRef]
- Tsukagoshi, S.; Shinohara, Y. Nutrition and nutrient uptake in soilless culture systems. In Plant Factory; Academic Press: Salt Lake City, UT, USA, 2020; pp. 221–229. [Google Scholar] [CrossRef]
- Zhou, J.; Fan, P.; Zhou, S.; Pan, Y.; Ping, J. Machine learning-assisted implantable plant electrophysiology microneedle sensor for plant stress monitoring. Biosens. Bioelectron. 2025, 271, 117062. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.; Ahn, T.I.; Son, J.E. Forecasting root-zone electrical conductivity of nutrient solutions in closed-loop soilless cultures via a recurrent neural network using environmental and cultivation information. Front. Plant Sci. 2018, 9, 859. [Google Scholar] [CrossRef]
- Puccinelli, M.; Carmassi, G.; Pardossi, A.; Incrocci, L. Wild edible plant species grown hydroponically with crop drainage water in a Mediterranean climate: Crop yield, leaf quality, and use of water and nutrients. Agric. Water Manag. 2023, 282, 108275. [Google Scholar] [CrossRef]
- Ullah, I.; Mao, H.; Rasool, G.; Gao, H.; Javed, Q.; Sarwar, A.; Khan, M.I. Effect of deficit irrigation and reduced N fertilization on plant growth, root morphology and water use efficiency of tomato grown in soilless culture. Agronomy 2021, 11, 228. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, A.; Tran, L.S.P. Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Khan, W.U.D.; Tanveer, M.; Shaukat, R.; Ali, M.; Pirdad, F. An overview of salinity tolerance mechanism in plants. In Salt and Drought Stress Tolerance in Plants: Signaling Networks and Adaptive Mechanisms; Springer: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. New Phytol. 2015, 205, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Glenn, E.P.; Brown, J.J.; Khan, M.J. Mechanisms of Salt Tolerance in Higher Plants. In Mechanisms of Environmental Stress Resistance in Plants; Rouledge: London, UK, 2022; pp. 83–110. [Google Scholar]
- García-Caparrós, P.; Llanderal, A.; Velasquez, C.; Lao, M.T. Water and Nutrient Balance in an Ornamental Cascade Cropping System. Agronomy 2021, 11, 1251. [Google Scholar] [CrossRef]
- Giménez, A.; Gallegos-Cedillo, V.M.; Benaissa, R.R.; Egea-Gilabert, C.; Signore, A.; Ochoa, J.; Gruda, N.S.; Arnao, M.; Fernández, J.A. Enhancing the cultivation of Salicornia fruticosa with agroindustrial compost leachates in a cascade cropping system: Evaluating the impact of melatonin application. Front. Plant Sci. 2024, 15, 1441884. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Petit-Boix, A.; Villalba, G.; Sanjuan-Delmás, D.; Parada, F.; Ercilla Montserrat, M.; Arcas, V.; Muñoz Liesa, J.; Rieradevall, J.; Gabarrell Durany, X. Recirculating water and nutrients in urban agriculture: An opportunity towards environmental sustainability and water use efficiency? J. Clean. Prod. 2020, 261, 121213. [Google Scholar] [CrossRef]
- Blomsma, F.; Brennan, G. The emergence of the circular economy: A new framing around prolonging resource productivity. J. Ind. Ecol. 2017, 21, 603–614. [Google Scholar] [CrossRef]
- Campbell-Johnston, K.; Vermeulen, W.J.V.; Reike, D.; Brullot, S. The circular economy and cascading: Towards a framework. Resour. Conserv. Recycl. X 2020, 7, 100038. [Google Scholar] [CrossRef]
- Rehberger, M.; Hiete, M. Allocation of environmental impacts in circular and cascade use of resources—Incentive-driven allocation as a prerequisite for cascade persistence. Sustainability 2020, 12, 4366. [Google Scholar] [CrossRef]
- Dyśko, J.; Szczech, M.; Kaniszewski, S.; Kowalczyk, W. Parameters of drainage waters collected during soilless tomato cultivation in mineral and organic substrates. Agronomy 2020, 10, 9. [Google Scholar] [CrossRef]
- Grigas, A.; Savickas, D.; Steponavičius, D.; Niekis, Ž.; Balčiūnas, J. The Influence of Different Irrigation Scenarios on the Yield and Sustainability of Wheat Fodder under Hydroponic Conditions. Agronomy 2023, 13, 860. [Google Scholar] [CrossRef]
- Olsson, O.; Roos, A.; Guisson, R.; Bruce, L.; Lamers, P.; Hektor, B.; Thrän, D.; Hartley, D.; Ponitka, J.; Hildebrandt, J. Time to tear down the pyramids? A critique of cascading hierarchies as a policy tool. Wiley Interdiscip. Rev. Energy Environ. 2018, 7, e279. [Google Scholar] [CrossRef]
- Teuber, L.; Osburg, V.S.; Toporowski, W.; Militz, H.; Krause, A. Wood polymer composites and their contribution to cascading utilisation. J. Clean. Prod. 2016, 110, 9–15. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; El-Tarawy, A.; Maksimovic, I.; Lao, M.T. Crop and Irrigation Management Systems under Greenhouse Conditions. Water 2018, 10, 62. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Maksimovic, I.; Lao, M.T. Cascade Cropping System with Horticultural and Ornamental Plants under Greenhouse Conditions. Water 2018, 10, 125. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; Contreras, J.I.; Baeza, R.; Segura, M.L.; Lao, M.T. Integral Management of Irrigation Water in Intensive Horticultural Systems of Almería. Sustainability 2017, 9, 2271. [Google Scholar] [CrossRef]
- Plaza, B.M.; Paniagua, F.; Ruiz, M.R.; Jiménez-Becker, S.; Lao, M.T. Nutritional responses of Cordyline fruticosa var. ‘Red Edge’ to fertigation with leachates vs. conventional fertigation: Sodium, potassium, calcium and magnesium. Sci. Hortic. 2017, 15, 157–163. [Google Scholar] [CrossRef]
- Hernández-Chover, V.; Castellet-Viciano, L.; Fuentes, R.; Hernández-Sancho, F. Circular economy and efficiency to ensure the sustainability in the wastewater treatment plants. J. Clean. Prod. 2023, 384, 135563. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Espino, C.V.; Lao, M.T. Comparative behavior of Dracaena marginata plants integrated into a cascade cropping system with the addition of hydrogen peroxide. Agronomy 2021, 11, 218. [Google Scholar] [CrossRef]
- Muñoz, P.; Paranjpe, A.; Montero, J.I.; Antón, A. Cascade crops: An alternative solution for increasing sustainability of greenhouse tomato crops in Mediterranean zone. Acta Hortic. 2012, 927, 801–805. [Google Scholar] [CrossRef]
- Fathidarehnijeh, E.; Nadeem, M.; Cheema, M.; Thomas, R.; Krishnapillai, M.; Galagedara, L. Current perspective on nutrient solution management strategies to improve the nutrient and water use efficiency in hydroponic systems. Can. J. Plant Sci. 2023, 104, 88–102. [Google Scholar] [CrossRef]
- Lhamo, T.; Gyalmo, T.; Pem, T.; Bajgai, Y. Effect of different pruning systems on yield and quality of tomato grown under greenhouse. Bhutanese J. Agric. 2022, 5, 71–82. [Google Scholar] [CrossRef]
- Maboko, M.M.; Du Plooy, C.P. Response of field-grown indeterminate tomato to plant density and stem pruning on yield. Int. J. Veg. Sci. 2018, 24, 612–621. [Google Scholar] [CrossRef]
- Alam, M.S.; Islam, N.; Ahmad, S.; Hossen, M.I.; Islam, M.R. Effect of different staking methods and stem pruning on yield and quality of summer tomato. Bangladesh J. Agric. Res. 2016, 41, 419–432. [Google Scholar] [CrossRef]
- Xiao-yi, H.; Hai-ying, W.; Xiao-ke, J.; Qin-ru, S.; Ting, C.; Zhi-rong, C. Effects of harvest ripeness on storage quality of tomato fruits. Emir. J. Food Agric. 2023, 35, 978–987. [Google Scholar] [CrossRef]
- Velthuis, H.H.; Van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest. Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef]
- Haque, M.T.; Howlader, N.C.; Miah, M.H.; Roy, T.K.; Antu, U.B.; Hasan, T.; Hamim, K.A.B.; Shumon, M.S.I.; Ali, M.J.; Ahmed, S.; et al. Response of Integrated Pest Management Framework to Insect Pest Infestations of Tomato. Iraqi J. Ind. Res. 2025, 12, 118–133. [Google Scholar] [CrossRef]
- Bouri, M.; Arslan, K.S.; Şahin, F. Climate-Smart Pest Management in Sustainable Agriculture: Promises and Challenges. Sustainability 2023, 15, 4592. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Niu, X.X.; Chen, X.W.; Su, H.; Eneji, A.E.; Guo, Y.H.; Dong, X.H. Changes of secondary metabolites and trace elements in Gentiana macrophylla flowers: A potential medicinal plant part. Chin. Herb. Med. 2014, 6, 145–151. [Google Scholar] [CrossRef]
- Al-Raddadi, T.M.; Al-Khateeb, L.A.; Sadaka, M.W.; Bahaffi, S.O. Trace Element Speciation and Nutrient Distribution in Boerhavia elegans: Evaluation and Toxic Metal Concentration Across Plant Tissues. Toxics 2025, 13, 14. [Google Scholar] [CrossRef]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf nitrogen determination using non-destructive techniques—A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- ISO 16634–1:2008; Food Products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content—Part 1: Oilseeds and Animal Feeding Stuffs. International Organization for Standardization: Geneva, Switzerland, 2008.
- Marie, N.; Verdier, C.; Le Bot, B.; Burgot, G. Analysis of sodium and potassium in total parenteral nutrition bags by ICP-MS and ICP-AES: Critical influence of the ingredients. Am. J. Anal. Chem. 2011, 2, 573–581. [Google Scholar] [CrossRef]
- EN-13805:2002; Foodstuffs—Determination of Trace Elements. European Committee for Standardization (CEN): Brussels, Belgium, 2002.
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.; Chen, Z.; Cao, B.; Xu, K. Comparative transcriptome analysis of two contrasting Chinese cabbage (Brassica rapa L.) genotypes reveals that ion homeostasis is a crucial biological pathway involved in the rapid adaptive response to salt stress. Front. Plant Sci. 2021, 12, 683891. [Google Scholar] [CrossRef]
- Assaf, M.; Korkmaz, A.; Karaman, Ş.; Kulak, M. Effect of plant growth regulators and salt stress on secondary metabolite composition in Lamiaceae species. South Afr. J. Bot. 2022, 144, 480–493. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, S.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Colak Esetlili, B.; Yildiz Aktas, L.; Esetlili, M.T.; Oztekin, T.; Kılıc, C.C.; Kurucu, Y. Salinity Tolerance Mechanism of Crithmum maritimum L.: Implications for Sustainable Agriculture in Saline Soils. Sustainability 2024, 16, 8165. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Shabala, S.; Mackay, H. Ion transport in halophytes: Understanding and exploiting salt tolerance in plants. Plant Physiol. 2011, 155, 643–654. [Google Scholar]
- Hamdani, F.; Derridj, A.; Roger, H.J. Diverse salinity responses in Crithmum maritimum tissues at different salinities over time. J. Soil. Sci. Plant Nutr. 2017, 17, 716–734. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 934–953. [Google Scholar] [CrossRef]
- Rosa-Rodríguez, R.; Lara-Herrera, A.; Trejo-Téllez, L.I.; Padilla-Bernal, L.; Solis-Sánchez, L.; Ortiz-Rodriguez, J.M. Water and fertilizers use efficiency in two hydroponic systems for tomato production. Hortic. Bras. 2020, 38, 47–52. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal. 2019. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 1 July 2025).
- United Nations. Sustainable Development Goals. 2015. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 1 July 2025).
- FAO. The State of Food and Agriculture 2020: Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2020. [Google Scholar]
- Majkowska-Gadomska, J.; Kaliniewicz, Z.; Mikulewicz, E.; Francke, A.; Jadwisieńczak, K.K.; Marks, M.; Choszcz, D.J.; Kozłowski, W. Effect of Different Sustainable Cultivation Methods on the Biometric Parameters and Yield of Mint. Sustainability 2024, 16, 7126. [Google Scholar] [CrossRef]
- Jagadish, R.; Shanmugaselvan, V.A. Quantification of Inorganic Anions in Tea (Camellia sinensis (L) O. Kuntze) Tissues and Soil Using Ion Chromatography Coupled with Conductivity Detector. Commun. Soil. Sci. Plant Anal. 2018, 49, 875–888. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T.; E Furze, M.; Kuhlman, I.; Schmid, S.; Wiesenbauer, J.; Wild, B.; Gleixner, G.; Hartmann, H.; et al. Standardized Protocols and Procedures can Precisely and Accurately Quantify Non-Structural Carbohydrates. In Proceedings of the 2018 ESA Annual Meeting, New Orleans, LA, USA, 5–10 August 2018. [Google Scholar] [CrossRef]
- Wieczorek, D.; Żyszka-Haberecht, B.; Kafka, A.; Lipok, J. Determination of phosphorus compounds in plant tissues: From colourimetry to advanced instrumental analytical chemistry. Plant Methods 2022, 18, 22. [Google Scholar] [CrossRef]
- Wang, Z.; Kuo, H.F.; Chiou, T.J. Intracellular phosphate sensing and regulation of phosphate transport systems in plants. Plant Physiol. 2021, 187, 2043–2055. [Google Scholar] [CrossRef]
- Höhner, R.; Tabatabaei, S.; Kunz, H.H.; Fittschen, U. A rapid total reflection X-ray fluorescence protocol for micro analyses of ion profiles in Arabidopsis thaliana. Spectrochim. Acta Part B At. Spectrosc. 2016, 125, 159–167. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Frignani, E.; Marchetti, A.; Pigani, L.; Rivi, M.; Roncaglia, F. Long-Term Variability in the Content of Some Metals and Metalloids in Aesculus Flowers: A Four-Year Study Using ICP OES and PCA Analysis. Molecules 2025, 30, 908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Aarts, M.G.; van der Ent, A. A simple and low-cost method for fluoride analysis of plant materials using alkali extraction and ion-selective electrode. Plant Methods 2025, 21, 98. [Google Scholar] [CrossRef] [PubMed]
- Isildak, Ö.; Özbek, O. Application of potentiometric sensors in real samples. Crit. Rev. Anal. Chem. 2021, 51, 218–231. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Unit | Primary Crop | Primary Crop | Primary Crop | Secondary Crop | Tertiary Crop |
|---|---|---|---|---|---|---|
| Vegetative Stage | Flowering Stage | Fruiting Stage | Vegetative Stage | Vegetative Stage | ||
| pH | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 | |
| EC | (dS m−1) | 2.2 | 2.6 | 2.0 | 2.4 | 2.8 |
| NO3 | (mmol L−1) | 17.4 | 15.6 | 11.9 | 12.5 | 11.4 |
| P | (mmol L−1) | 0.8 | 1.0 | 0.8 | 0.3 | 0.4 |
| K | (mmol L−1) | 6.0 | 6.0 | 5.4 | 2.4 | 5.4 |
| Ca | (mmol L−1) | 4.9 | 5.6 | 4.3 | 4.3 | 3.2 |
| Na | (mmolL−1) | 1.2 | 2.9 | 1.3 | 9.3 | 11.5 |
| Mg | (mmol L−1) | 2.5 | 2.9 | 2.5 | 2.1 | 2.3 |
| NH4 | (mmol L−1) | 1.2 | 1.2 | 1.2 | 1.0 | 0.2 |
| Fe | (μmol L−1) | 18.1 | 24.7 | 16.5 | 10.4 | 29.1 |
| Zn | (μmol L−1) | 8.7 | 5.7 | 4 | 2.4 | 3.5 |
| Mn | (μmol L−1) | 13.0 | 10.6 | 10.2 | 2.1 | 3.2 |
| Cu | (μmol L−1) | 0.7 | 1 | 0.7 | 1.3 | 1.1 |
| B | (μmol L−1) | 35.0 | 30.0 | 30.0 | 20.0 | 50.0 |
| Mo | (μmol L−1) | 0.5 | 0.5 | 0.5 | 1 | 0.1 |
| Crop Species | Volume of NS Applied | Volume of NS Drained | Water Uptake | Percetage Drained (%) | |
|---|---|---|---|---|---|
| (L m−2) | (L m−2) | ||||
| Tomato | 409 | 160.5 | 248.5 | 39.2 | |
| Volume of NS applied (L m−2) | Volume of NS drained (L m−2) | Water uptake (L m−2) | |||
| Mint | Water | DS | |||
| S1 | 92 | 0 | 29 | 63 | 31.5 |
| S2 | 0 | 86 | 29 | 57 | 33.7 |
| Peppermint | |||||
| S1 | 75 | 0 | 32 | 43 | 42.7 |
| S2 | 0 | 70 | 32 | 38 | 45.7 |
| Lemon balm | |||||
| S3 | 59 | 0 | 18 | 41 | 30.5 |
| S4 | 0 | 54 | 18 | 36 | 33.3 |
| Sea fennel | |||||
| S3 | 48 | 0 | 19 | 29 | 39.6 |
| S4 | 0 | 40 | 15 | 25 | 37.5 |
| IRRIGATION | NO3 | P | K | Ca | Na | Mg | Fe | Zn | Mn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| SOLUTION (IS) | mmol L−1 | mmol L−1 | mmol L−1 | mmol L−1 | mmol L−1 | mmol L−1 | μmol L−1 | μmol L−1 | μmol L−1 | μmol L−1 |
| 6DBS | ||||||||||
| Mint_Peppermint_S1 | 12.46 | 0.34 | 2.36 | 4.32 | 9.27 | 2.10 | 10.44 | 2.37 | 2.10 | 1.29 |
| Mint_Peppermint_S2 | 15.36 | 0.46 | 4.30 | 4.62 | 4.83 | 3.09 | 24.48 | 6.26 | 3.36 | 3.72 |
| Lemon balm_Sea fennel_S3 | 11.37 | 0.42 | 5.44 | 3.20 | 11.48 | 2.25 | 29.09 | 3.46 | 3.21 | 1.13 |
| Lemon balm_Sea fennel_S4 | 16.68 | 0.58 | 6.09 | 4.98 | 4.43 | 3.42 | 23.91 | 6.17 | 3.69 | 2.73 |
| 8DAS | ||||||||||
| Tomato NS | 16.83 | 1.13 | 6.85 | 5.53 | 1.49 | 2.75 | 24.46 | 6.05 | 12.80 | 0.70 |
| Mint_Peppermint_S1 | 12.64 | 0.54 | 3.23 | 6.05 | 14.03 | 2.18 | 10.23 | 2.24 | 2.30 | 0.70 |
| Mint_Peppermint_S2 | 19.30 | 0.79 | 7.44 | 6.24 | 8.22 | 3.99 | 32.34 | 6.71 | 7.20 | 2.26 |
| Lemon balm_Sea fennel_S3 | 11.23 | 0.53 | 6.57 | 3.68 | 14.50 | 2.63 | 32.43 | 2.51 | 5.31 | 1.10 |
| Lemon balm_Sea fennel_S4 | 21.87 | 0.58 | 7.50 | 7.21 | 11.57 | 4.59 | 35.18 | 5.23 | 6.30 | 2.45 |
| 29DAS | ||||||||||
| Tomato NS | 11.90 | 0.77 | 5.36 | 4.33 | 1.35 | 2.48 | 16.50 | 3.98 | 10.23 | 0.66 |
| Mint_Peppermint_S1 | 21.35 | 0.18 | 1.65 | 9.42 | 27.81 | 2.42 | 15.61 | 1.45 | 2.19 | 0.75 |
| Mint_Peppermint_S2 | 20.79 | 0.43 | 4.31 | 7.19 | 26.70 | 4.41 | 29.46 | 5.56 | 6.94 | 2.59 |
| Lemon balm_Sea fennel_S3 | 13.18 | 1.07 | 10.24 | 4.79 | 38.22 | 2.38 | 143.20 | 4.03 | 2.89 | 1.52 |
| Lemon balm_Sea fennel_S4 | 23.54 | 0.33 | 5.24 | 9.43 | 32.57 | 6.05 | 41.68 | 3.93 | 3.84 | 3.05 |
| 37DAS | ||||||||||
| Tomato NS | 13.80 | 0.80 | 5.41 | 4.59 | 1.66 | 2.39 | 16.81 | 5.40 | 9.91 | 0.74 |
| Mint_Peppermint_S1 | 20.81 | 0.56 | 3.05 | 9.16 | 37.14 | 2.11 | 10.04 | 3.39 | 1.98 | 0.86 |
| Mint_Peppermint_S2 | 16.42 | 0.34 | 2.85 | 6.88 | 34.62 | 4.87 | 26.29 | 5.02 | 4.60 | 1.46 |
| Lemon balm_Sea fennel_S3 | 12.70 | 0.96 | 7.74 | 4.31 | 40.34 | 2.26 | 149.38 | 2.64 | 2.61 | 1.22 |
| Lemon balm_Sea fennel_S4 | 17.72 | 0.32 | 2.98 | 7.14 | 51.43 | 4.83 | 24.39 | 3.65 | 5.19 | 1.10 |
| 43DAS | ||||||||||
| Tomato NS | 14.13 | 0.72 | 5.29 | 4.97 | 1.28 | 2.38 | 28.79 | 5.24 | 11.71 | 0.74 |
| Mint_Peppermint_S1 | 41.34 | 3.86 | 19.38 | 17.96 | 12.00 | 3.50 | 26.50 | 5.16 | 1.56 | 4.73 |
| Mint_Peppermint_S2 | 21.21 | 1.19 | 7.38 | 8.46 | 27.89 | 4.91 | 45.98 | 6.09 | 2.37 | 2.25 |
| Lemon balm_Sea fennel_S3 | 16.65 | 7.56 | 40.23 | 7.13 | 16.98 | 4.89 | 153.45 | 11.83 | 3.45 | 10.34 |
| Lemon balm_Sea fennel_S4 | 26.46 | 0.60 | 5.89 | 12.87 | 55.96 | 7.74 | 49.27 | 4.48 | 2.43 | 3.40 |
| 51DAS | ||||||||||
| Tomato NS | 14.79 | 0.77 | 6.42 | 4.30 | 1.97 | 2.52 | 37.32 | 6.32 | 12.52 | 0.95 |
| Mint_Peppermint_S1 | 41.99 | 4.12 | 23.41 | 18.13 | 15.58 | 3.95 | 30.13 | 12.64 | 0.84 | 3.92 |
| Mint_Peppermint_S2 | 25.39 | 1.36 | 8.91 | 9.54 | 44.65 | 4.73 | 54.59 | 7.08 | 2.42 | 2.66 |
| Lemon balm_Sea fennel_S3 | 23.67 | 7.71 | 50.41 | 8.09 | 14.63 | 5.37 | 252.86 | 13.43 | 3.68 | 7.90 |
| Lemon balm_Sea fennel_S4 | 31.94 | 0.54 | 6.97 | 14.15 | 58.26 | 8.75 | 81.54 | 3.77 | 3.56 | 3.86 |
| DRAINAGE | NO3 | P | K | Ca | Na | Mg | Fe | Zn | Mn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| SOLUTION (DS) | mmol L−1 | mmol L−1 | mmol L−1 | mmol L−1 | mmol L−1 | mmol L−1 | μmol L−1 | μmol L−1 | μmol L−1 | μmol L−1 |
| 6DBS | ||||||||||
| Tomato NS | 20.20 | 0.40 | 5.51 | 5.91 | 2.62 | 3.51 | 23.64 | 8.61 | 4.41 | 2.89 |
| Mint S1 | 15.10 | 0.09 | 2.06 | 8.13 | 18.40 | 4.24 | 17.05 | 0.75 | 0.28 | 5.22 |
| Mint S2 | 19.80 | 0.12 | 5.60 | 7.08 | 6.81 | 4.47 | 33.58 | 0.77 | 0.21 | 5.45 |
| Peppermint S1 | 12.20 | 0.10 | 1.97 | 5.25 | 12.12 | 2.71 | 10.34 | 2.49 | 0.33 | 3.08 |
| Peppermint S2 | 14.98 | 0.25 | 4.58 | 5.39 | 4.99 | 3.38 | 27.11 | 2.27 | 0.21 | 4.99 |
| Lemon Balm S3 | 15.97 | 0.18 | 7.07 | 4.82 | 14.36 | 3.36 | 28.58 | 2.08 | 0.21 | 2.49 |
| Lemon Balm S4 | 24.14 | 0.44 | 10.18 | 6.88 | 10.12 | 4.69 | 24.54 | 3.64 | 1.78 | 3.37 |
| Sea fennel S3 | 21.43 | 0.21 | 8.93 | 5.52 | 19.05 | 4.70 | 35.08 | 3.99 | 0.77 | 3.63 |
| Sea fennel S4 | 32.12 | 0.24 | 12.05 | 8.24 | 12.79 | 6.55 | 33.90 | 3.20 | 1.57 | 4.08 |
| 8DAS | ||||||||||
| Tomato NS | 28.91 | 0.59 | 7.97 | 8.78 | 4.26 | 6.15 | 45.86 | 5.94 | 6.98 | 4.34 |
| Mint S1 | 26.61 | 0.03 | 0.29 | 21.96 | 51.57 | 8.29 | 34.78 | 1.78 | 0.31 | 9.86 |
| Mint S2 | 55.05 | 0.05 | 5.41 | 25.48 | 34.03 | 18.89 | 152.42 | 3.80 | 0.21 | 14.69 |
| Peppermint S1 | 16.70 | 0.02 | 0.33 | 13.94 | 34.82 | 4.35 | 21.58 | 2.29 | 0.46 | 5.52 |
| Peppermint S2 | 35.99 | 0.06 | 5.58 | 17.08 | 24.75 | 10.86 | 88.32 | 3.74 | 0.21 | 8.69 |
| Lemon Balm S3 | 23.42 | 0.06 | 11.89 | 11.03 | 70.23 | 7.97 | 132.93 | 8.26 | 0.38 | 6.43 |
| Lemon Balm S4 | 34.68 | 0.14 | 7.71 | 16.23 | 32.83 | 10.21 | 64.20 | 2.64 | 1.14 | 7.31 |
| Sea fennel S3 | 21.43 | 0.21 | 8.93 | 5.52 | 19.05 | 4.70 | 35.08 | 3.99 | 0.77 | 3.63 |
| Sea fennel S4 | 31.15 | 0.25 | 8.35 | 9.89 | 19.60 | 7.20 | 39.52 | 2.65 | 1.42 | 4.35 |
| 29DAS | ||||||||||
| Tomato NS | 19.03 | 0.68 | 5.81 | 6.48 | 3.62 | 4.21 | 26.54 | 5.50 | 11.51 | 0.90 |
| Mint S1 | 33.23 | 0.05 | 2.72 | 16.68 | 52.38 | 4.22 | 16.60 | 4.00 | 0.21 | 4.01 |
| Mint S2 | 34.14 | 0.12 | 7.05 | 13.07 | 41.19 | 7.70 | 41.76 | 2.67 | 0.21 | 4.20 |
| Peppermint S1 | 28.93 | 0.04 | 2.73 | 13.03 | 42.94 | 3.34 | 14.77 | 4.97 | 0.21 | 2.41 |
| Peppermint S2 | 31.98 | 0.09 | 6.72 | 11.10 | 40.52 | 6.86 | 38.23 | 1.75 | 0.21 | 3.46 |
| Lemon Balm S3 | 16.95 | 0.04 | 8.72 | 7.20 | 74.54 | 3.56 | 134.26 | 4.78 | 0.21 | 1.11 |
| Lemon Balm S4 | 42.97 | 0.04 | 8.11 | 18.75 | 52.09 | 11.80 | 85.71 | 2.10 | 0.21 | 6.28 |
| Sea fennel S3 | 37.94 | 0.11 | 8.72 | 14.12 | 89.75 | 10.54 | 157.19 | 6.95 | 0.21 | 5.12 |
| Sea fennel S4 | 55.47 | 0.12 | 9.40 | 20.61 | 85.71 | 15.33 | 157.12 | 9.44 | 0.21 | 7.65 |
| 37DAS | ||||||||||
| Tomato NS | 15.12 | 0.24 | 2.23 | 5.90 | 3.76 | 4.30 | 19.75 | 2.34 | <0.01 | 2.42 |
| Mint S1 | 52.81 | 0.09 | 4.30 | 31.13 | 100.57 | 10.44 | 53.76 | 3.91 | 0.56 | 7.39 |
| Mint S2 | 46.18 | 0.05 | 4.42 | 26.34 | 101.52 | 12.50 | 65.66 | 3.18 | 0.21 | 7.87 |
| Peppermint S1 | 45.92 | 0.14 | 5.08 | 25.37 | 74.64 | 5.53 | 28.74 | 6.62 | 6.42 | 5.17 |
| Peppermint S2 | 36.33 | 0.03 | 6.20 | 18.91 | 88.77 | 12.61 | 72.44 | 2.34 | 0.21 | 6.12 |
| Lemon Balm S3 | 23.48 | 0.59 | 19.20 | 9.23 | 101.14 | 5.63 | 270.00 | 5.64 | 0.70 | 3.36 |
| Lemon Balm S4 | 40.03 | 0.03 | 7.66 | 18.99 | 102.29 | 11.18 | 72.29 | 2.82 | 1.43 | 5.71 |
| Sea fennel S3 | 28.71 | 0.62 | 17.14 | 10.74 | 119.39 | 7.20 | 293.93 | 13.91 | 5.09 | 3.77 |
| Sea fennel S4 | 74.46 | 0.05 | 9.27 | 32.92 | 150.87 | 23.35 | 168.64 | 5.92 | 4.23 | 11.23 |
| 43DAS | ||||||||||
| Tomato NS | 16.94 | 0.32 | 3.60 | 6.80 | 5.07 | 4.32 | 29.29 | 3.48 | 2.08 | 1.42 |
| Mint S1 | 104.42 | 0.17 | 2.58 | 52.91 | 72.35 | 7.17 | 47.09 | 6.71 | 4.19 | 9.14 |
| Mint S2 | 38.44 | 0.03 | 2.27 | 21.83 | 95.30 | 13.78 | 76.39 | 2.79 | 0.21 | 4.83 |
| Peppermint S1 | 83.61 | 0.19 | 3.98 | 40.70 | 49.49 | 5.08 | 38.32 | 13.14 | 12.21 | 5.66 |
| Peppermint S2 | 37.20 | 0.02 | 4.10 | 19.18 | 89.78 | 11.88 | 73.82 | 2.11 | 0.21 | 4.72 |
| Lemon Balm S3 | 26.27 | 0.53 | 16.63 | 11.06 | 75.54 | 4.89 | 338.69 | 5.69 | 0.41 | 3.26 |
| Lemon Balm S4 | 39.94 | 0.07 | 5.23 | 19.25 | 84.23 | 10.29 | 70.92 | 3.18 | 1.76 | 4.41 |
| Sea fennel S3 | 29.83 | 1.22 | 18.29 | 10.93 | 79.91 | 6.20 | 351.13 | 9.85 | 4.97 | 4.28 |
| Sea fennel S4 | 55.83 | 0.09 | 6.47 | 23.48 | 107.81 | 14.90 | 96.54 | 4.82 | 3.49 | 6.36 |
| 51DAS | ||||||||||
| Tomato NS | 11.04 | 0.47 | 4.26 | 3.65 | 4.29 | 2.83 | 38.30 | 4.16 | 4.50 | 1.52 |
| Mint S1 | 133.51 | 9.34 | 60.64 | 45.53 | 48.51 | 15.64 | 88.26 | 19.86 | 7.35 | 24.28 |
| Mint S2 | 47.62 | 0.27 | 7.41 | 22.13 | 121.74 | 13.06 | 109.83 | 2.87 | 0.21 | 4.75 |
| Peppermint S1 | 97.47 | 8.51 | 52.93 | 36.26 | 37.29 | 11.93 | 68.71 | 17.79 | 6.47 | 14.72 |
| Peppermint S2 | 50.46 | 0.37 | 11.34 | 20.56 | 115.93 | 11.73 | 109.21 | 1.82 | 0.21 | 4.70 |
| Lemon Balm S3 | 48.14 | 11.44 | 99.32 | 15.07 | 49.28 | 14.34 | 514.46 | 13.61 | 2.12 | 22.18 |
| Lemon Balm S4 | 56.99 | 0.25 | 7.82 | 24.64 | 113.74 | 15.44 | 117.52 | 2.92 | 1.32 | 6.38 |
| Sea fennel S3 | 39.24 | 11.75 | 71.15 | 13.68 | 40.17 | 10.45 | 370.77 | 19.54 | 11.18 | 15.31 |
| Sea fennel S4 | 57.58 | 0.46 | 9.00 | 22.22 | 99.78 | 14.66 | 113.36 | 4.97 | 4.23 | 6.56 |
| Mint_1st Harvest (15DAS) | FM g Plant−1 | DM g Plant−1 | FM/DM |
|---|---|---|---|
| S1 | 441.78 a ± 98.05 | 51.94 a ± 10.64 | 8.53 a ± 0.90 |
| S2 | 543.67 b ± 96.56 | 71.67 b ± 12.12 | 7.65 b ± 1.00 |
| 2nd harvest (58DAS) | |||
| S1 | 736.89 a ± 161.05 | 117.67 a ± 25.75 | 6.37 a ± 1.10 |
| S2 | 596.76 b ± 82.51 | 120.33 a ± 12.48 | 4.96 b ± 0.31 |
| Peppermint_1st harvest (17DAS) | |||
| S1 | 426.39 a ± 92.05 | 89.83 a ± 18.08 | 4.74 a ± 0.19 |
| S2 | 388.05 a ± 65.58 | 80.28 a ± 14.70 | 4.86 a ± 0.46 |
| 2nd harvest (59DAS) | |||
| S1 | 441.17 a ± 133.32 | 102.61 a ± 27.59 | 4.27 a ± 0.25 |
| S2 | 392.05 a ± 81.89 | 92.89 a ± 18.93 | 4.23 a ± 0.32 |
| Lemon balm_1st harvest (22DAS) | |||
| S3 | 237.17 a ± 51.81 | 50.39 a ± 12.97 | 4.79 a ± 0.54 |
| S4 | 264.28 a ± 69.51 | 49.89 b ± 14.65 | 5.34 b ± 0.26 |
| 2nd harvest (57DAS) | |||
| S3 | 387.55 a ± 87.90 | 87.89 a ± 19.02 | 4.43 a ± 0.30 |
| S4 | 284.05 b ± 56.80 | 70.61 b ± 16.74 | 4.06 b ± 0.31 |
| Sea fennel_1st harvest (23DAS) | |||
| S3 | 339.50 a ± 72.49 | 41.05 a ± 9.52 | 8.32 a ± 0.41 |
| S4 | 328.28 a ± 56.44 | 38.00 a ± 7.47 | 8.82 a ± 1.40 |
| 2nd harvest (56DAS) | |||
| S3 | 888.00 a ± 231.83 | 133.39 a ± 31.98 | 6.65 a ± 0.44 |
| S4 | 792.78 a ± 179.26 | 128.11 a ± 30.43 | 6.21 b ± 0.42 |
| S1 (kg) | S2 (kg) | S1 (kg m−2) | S2 (kg m−2) | |
| Mint 1st harvest (15 DAS) | 51.3 | 55.9 | 1.0 a | 1.1 b |
| Mint 2nd harvest (58DAS) | 75.0 | 62.0 | 1.5 a | 1.2 b |
| Mint: Total biomass crop−1 treatment−1 | 126.2 | 117.9 | 2.5 | 2.4 |
| Peppermint 1st harvest (17DAS) | 37.5 | 41.3 | 0.8 a | 0.8 a |
| Peppermint 2nd harvest (59DAS) | 48.5 | 47.8 | 1.0 a | 1.0 a |
| Peppermint: Total biomass crop−1 treatment−1 | 86.0 | 89.0 | 1.7 | 1.8 |
| S3 (kg) | S4 (kg) | S3 (kg m−2) | S4 (kg m−2) | |
| Lemon balm 1st harvest (22DAS) | 22.1 | 21.8 | 0.4 a | 0.4 a |
| Lemon balm 2nd harvest (57DAS) | 37.3 | 27.3 | 0.7 a | 0.5 b |
| Lemon balm: Total biomass crop−1 treatment−1 | 59.4 | 49.1 | 1.2 | 1.0 |
| Sea fennel 1st harvest (23DAS) | 36.7 | 35.5 | 0.7 a | 0.7 a |
| Sea fennel 2nd harvest (56DAS) | 62.5 | 59.4 | 1.2 a | 1.2 a |
| Sea fennel: Total biomass crop−1 treatment−1 | 99.1 | 94.9 | 2.0 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatsivou, E.; Elvanidi, A.; Katsoulas, N. Evaluation of a Three-Level Cascade Soilless System Under Saline Greenhouse Conditions. Horticulturae 2025, 11, 1168. https://doi.org/10.3390/horticulturae11101168
Karatsivou E, Elvanidi A, Katsoulas N. Evaluation of a Three-Level Cascade Soilless System Under Saline Greenhouse Conditions. Horticulturae. 2025; 11(10):1168. https://doi.org/10.3390/horticulturae11101168
Chicago/Turabian StyleKaratsivou, Eleni, Angeliki Elvanidi, and Nikolaos Katsoulas. 2025. "Evaluation of a Three-Level Cascade Soilless System Under Saline Greenhouse Conditions" Horticulturae 11, no. 10: 1168. https://doi.org/10.3390/horticulturae11101168
APA StyleKaratsivou, E., Elvanidi, A., & Katsoulas, N. (2025). Evaluation of a Three-Level Cascade Soilless System Under Saline Greenhouse Conditions. Horticulturae, 11(10), 1168. https://doi.org/10.3390/horticulturae11101168









