Effect of Volatile Compounds Emitted by an Endophytic Yeast Isolated from the Endemic Plant Echinopsis chiloensis against Botrytis cinerea
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Identification of Endophytic Yeast
2.3. GC-MS/MS Analysis of VOCs Emitted by Endophyte Yeast Ec2
2.4. In Vitro Antifungal Assay of VOCs
2.5. Effect of Volatile Compounds Emitted by Ec2 on B. cinerea
2.6. Mode of Action of VOCs on B. cinerea
2.7. Analysis of the Fungicidal or Fungistatic Effect of VOCs
2.8. Effect of VOCs on B. cinerea Growth In Vivo
2.9. Statistical Analysis
3. Results
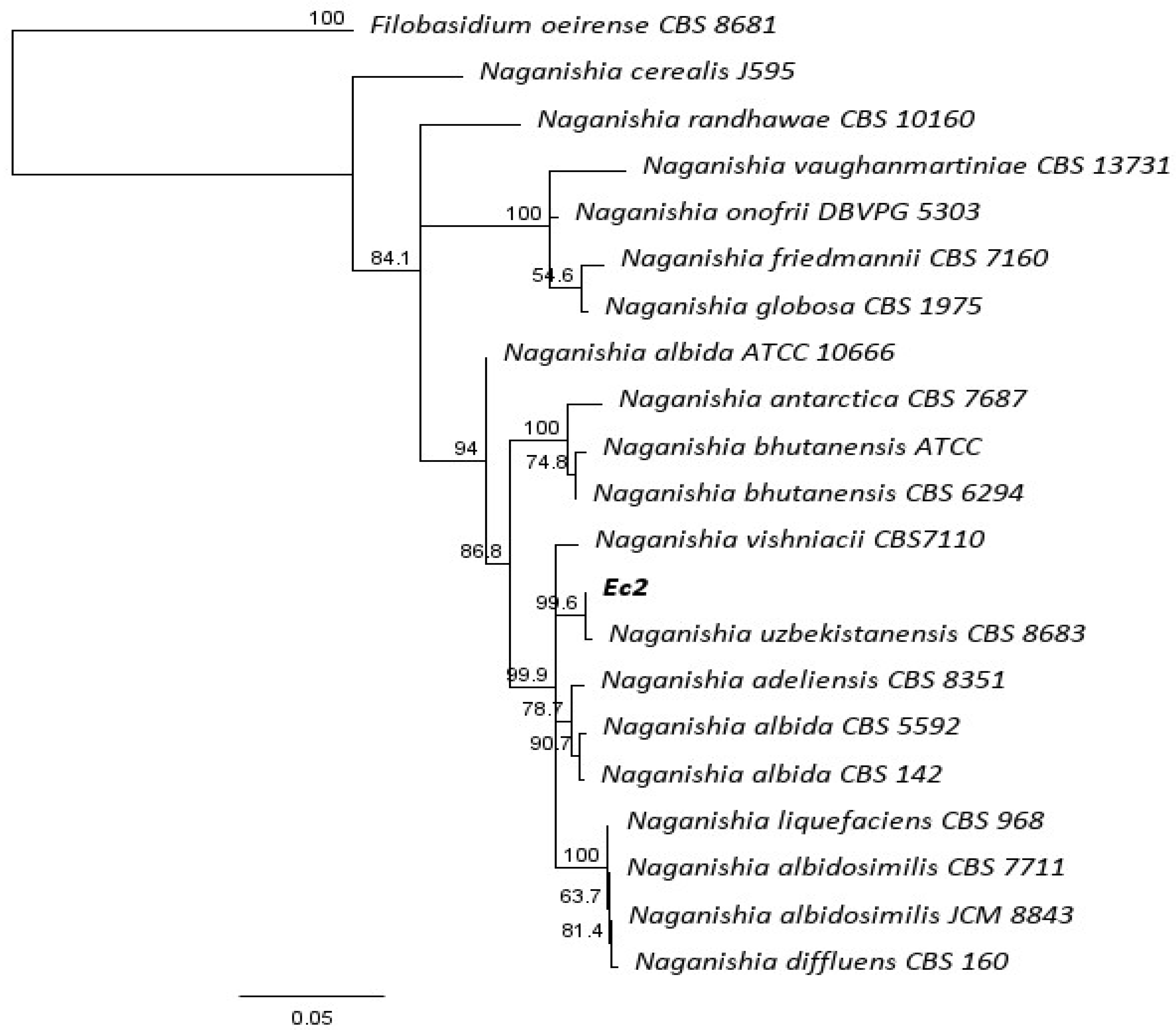
3.1. Molecular Identification of Ec2 Endophyte
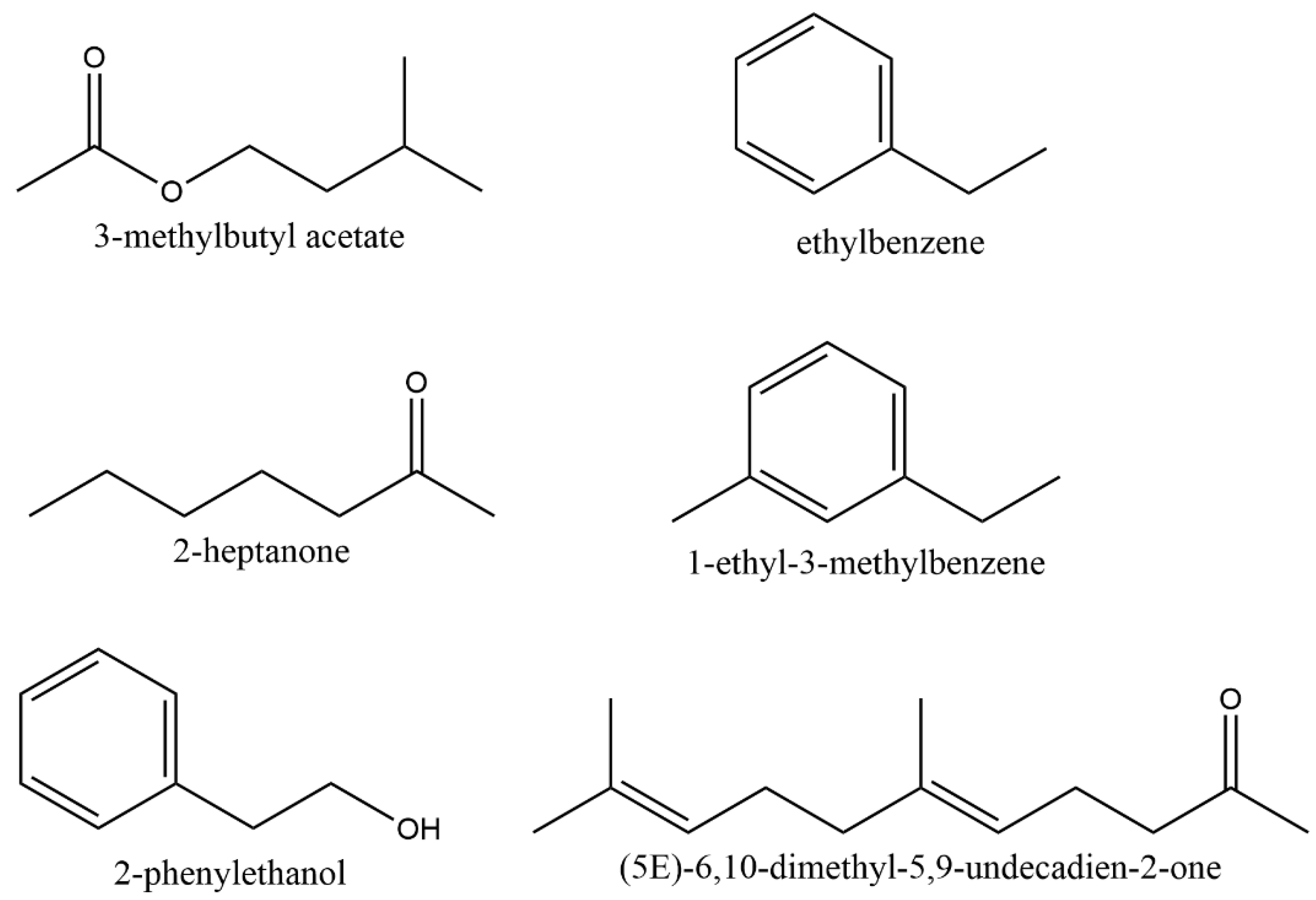
3.2. Identification of VOCs Emitted by Naganishia sp.
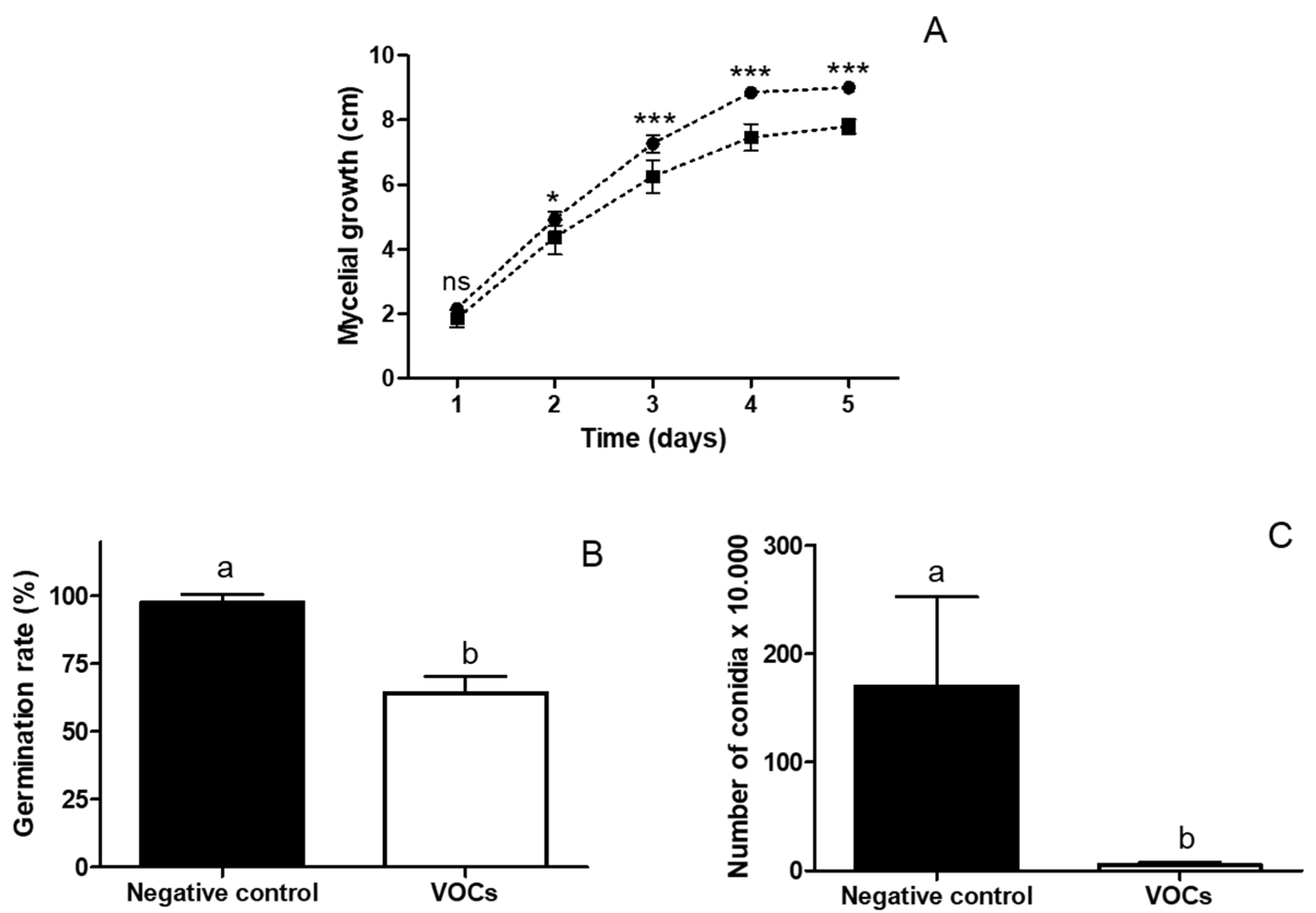
3.3. Effect of VOCs Emitted by Naganishia sp. on B. cinerea
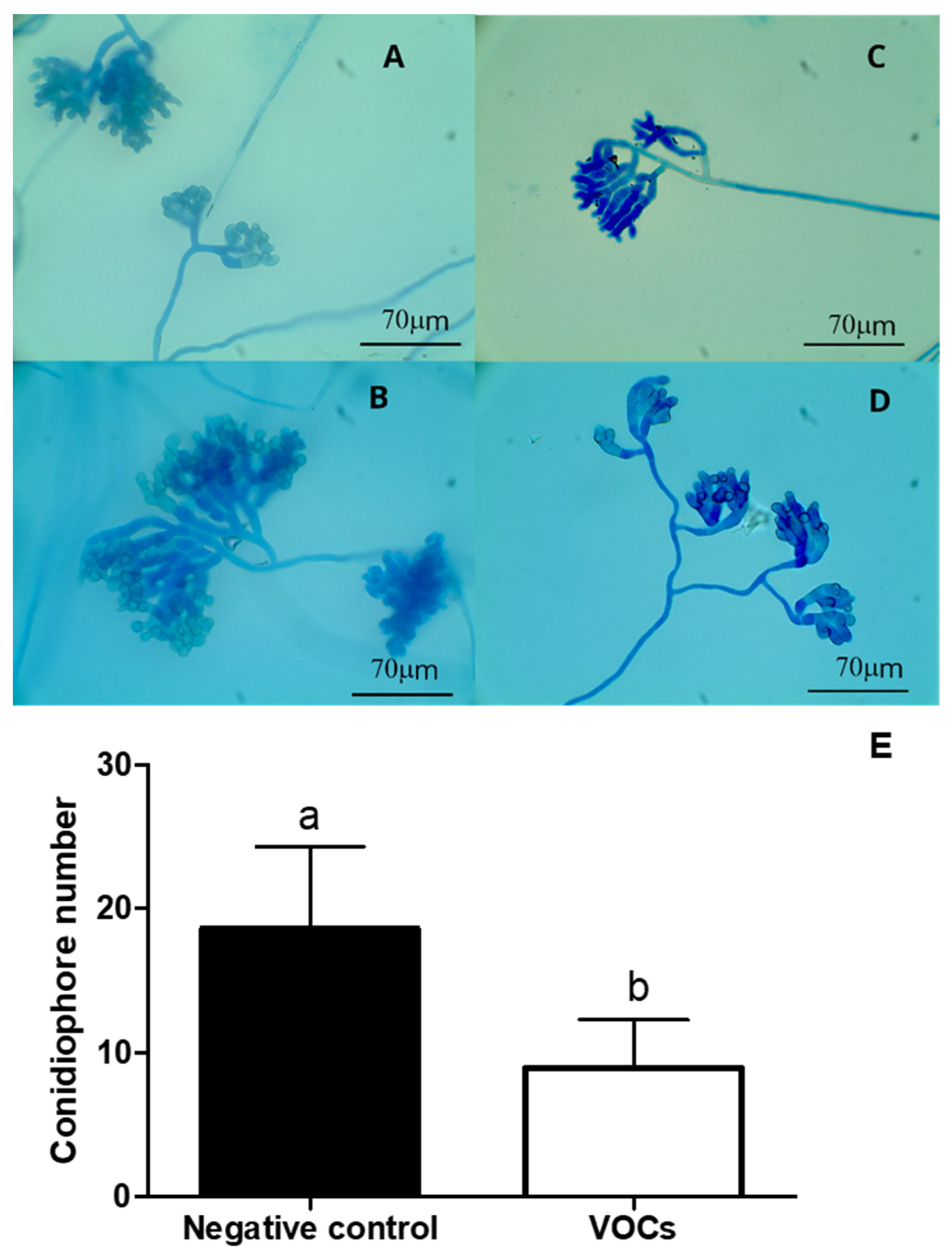
3.4. Conidiophore Morphology of B. cinerea Treated with VOCs Emitted by Naganishia sp.
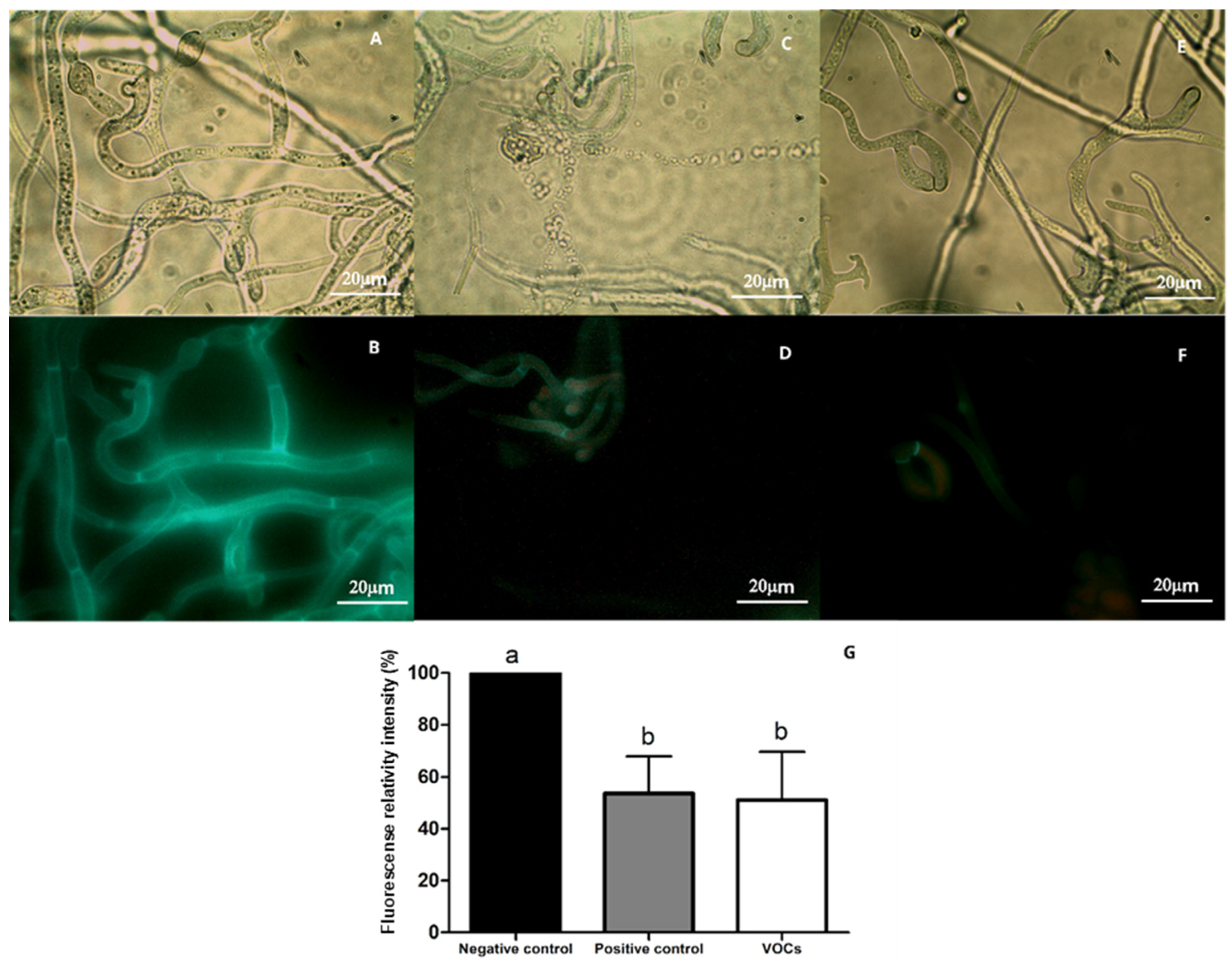
3.5. Effect of VOCs on Cell Wall and Plasma Membrane of B. cinerea
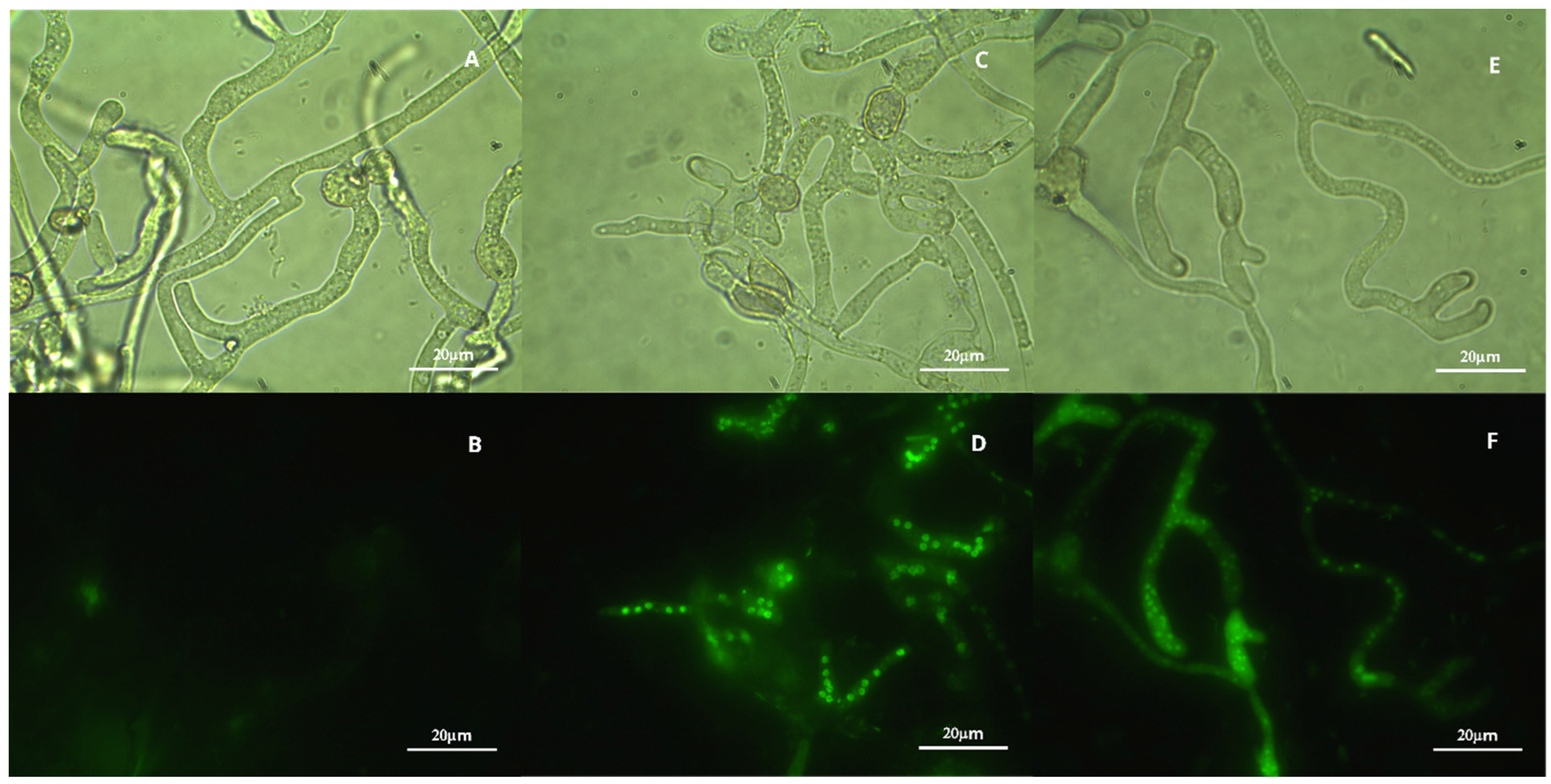
3.6. Oxidative Stress in B. cinerea Treated with VOCs
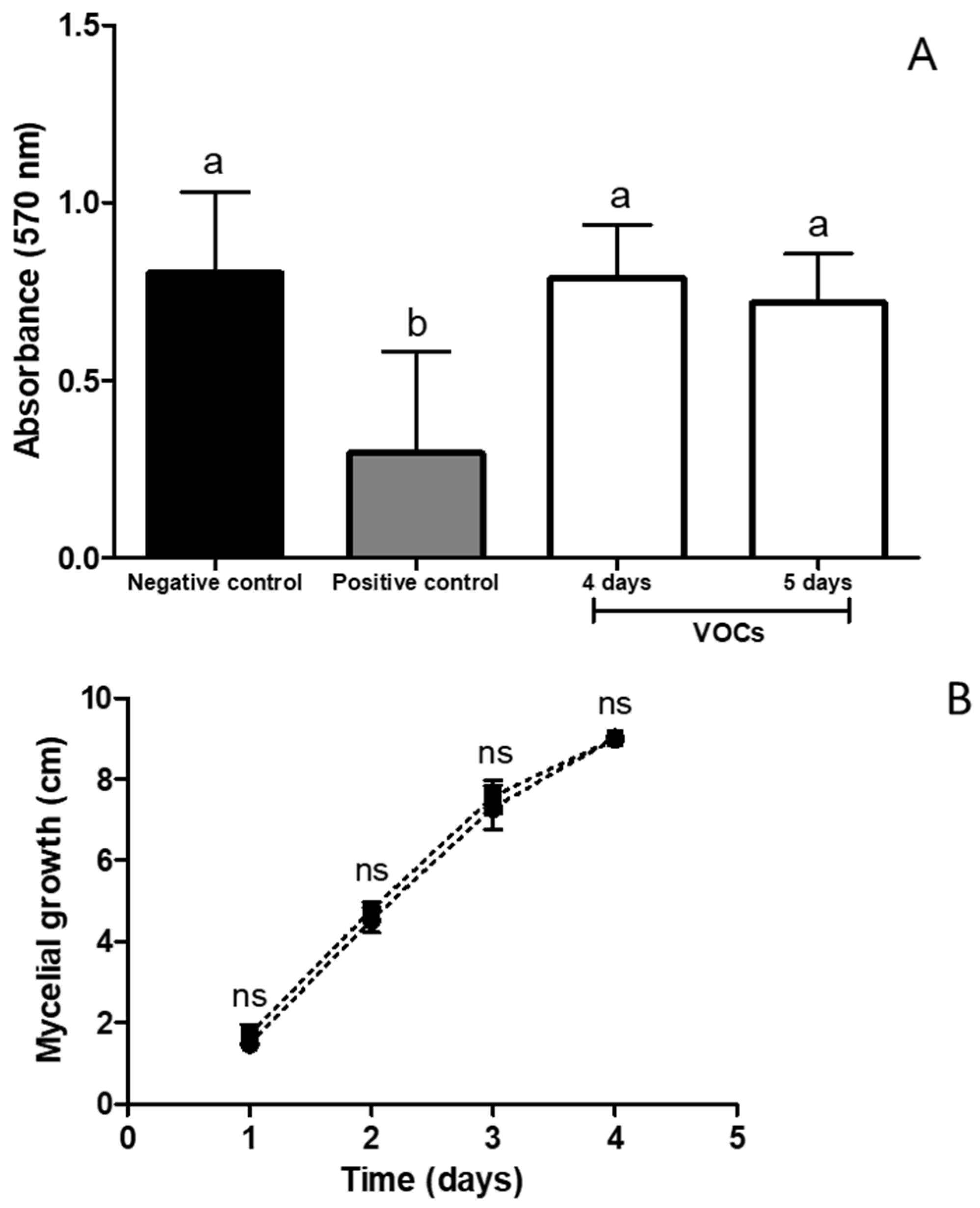
3.7. Fungistatic Effect of VOCs Emitted by Naganishia sp. on B. cinerea
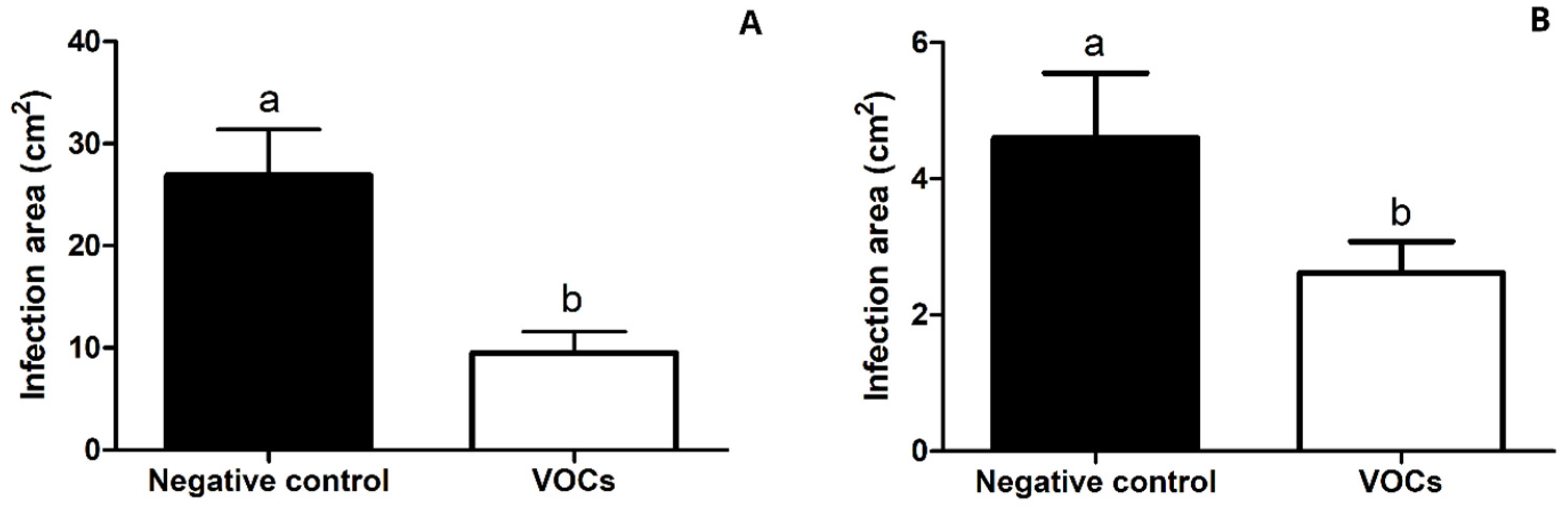
3.8. In Vivo Effect of VOCs Emitted by Naganishia sp. on B. cinerea
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Caseys, C.; Kliebenstein, D.J. Genetic and molecular landscapes of the generalist phytopathogen Botrytis cinerea. Mol. Plant Pathol. 2024, 25, e13404. [Google Scholar] [CrossRef]
- Dean, R.; Kan, J.; Pretorius, Z.; Hammond-Kosack, K.; Pietro, A.; Spanu, P.; Foster, G. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L. Pathogens of autotrophs. In The Fungi, 3rd ed.; Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: London, UK, 2016; pp. 245–292. [Google Scholar]
- Latorre, B.; Elfar, K.; Ferrada, E. Gray mold caused by Botrytis cinerea limits grape production in Chile. Cienc. Investig. Agrar. 2015, 42, 305–330. [Google Scholar] [CrossRef]
- Choquer, M.; Rascle, C.; Gonçalves, I.R.; Vallée, A.; Ribot, C.; Loisel, E.; Poussereau, N. The infection cushion of Botrytis cinerea: A fungal “weapon” of plant-biomass destruction. Environ. Microbiol. 2021, 23, 2293–2314. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)-prospects and challenges. Biocontrol Sci. Technol. 2018, 29, 207–228. [Google Scholar] [CrossRef]
- Esterio, M.; Osorio-Navarro, C.; Azócar, M.; Copier, C.; Rubilar, M.; Pizarro, L.; Auger, J. Reduced fitness cost and increased aggressiveness in fenhexamid-resistant Botrytis cinerea field isolates from Chile. Phytopathol. Mediterr. 2021, 60, 69–77. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Biol. Chem. 2014, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Emoghene, A.O.; Futughe, A.E. Fungi as an alternative to agrochemicals to control plant diseases. In Fungal Applications in Sustainable Environmental Biotechnology; Purchase, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 43–62. [Google Scholar]
- Jia, M.; Chen, L.; Xin, H.; Zheng, C.; Rahman, K.; Han, T.; Qin, L. A friendly relationship between endophytic fungi and medicinal plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef]
- Barra-Bucarei, L.; France Iglesias, A.; Gerding González, M.; Silva Aguayo, G.; Carrasco-Fernández, J.; Castro, J.F.; Ortiz Campos, J. Antifungal activity of Beauveria bassiana endophyte against Botrytis cinerea in two Solanaceae crops. Microorganisms 2019, 8, 65. [Google Scholar] [CrossRef]
- Castro, P.; Parada, R.; Corrial, C.; Mendoza, L.; Cotoras, M. Endophytic fungi Isolated from Baccharis linearis and Echinopsis chiloensis with antifungal activity against Botrytis cinerea. J. Fungi 2022, 8, 197. [Google Scholar] [CrossRef]
- Hormazabal, E.; Piontelli, E. Endophytic fungi from Chilean native gymnosperms: Antimicrobial activity against human and phytopathogenic fungi. World J. Microbiol. Biotechnol. 2009, 25, 813–819. [Google Scholar] [CrossRef]
- Vidal, A.; Parada, R.; Mendoza, L.; Cotoras, M. Endophytic Fungi isolated from plants growing in Central Andean Precordillera of Chile with antifungal activity against Botrytis cinerea. J. Fungi 2020, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Cobian, G.M.; Egan, C.P.; Amend, A.S. Plant-microbe specificity varies as a function of elevation. ISME J. 2019, 13, 2778–2788. [Google Scholar] [CrossRef]
- Jain, R.; Bhardwaj, P.; Pandey, S.S.; Kumar, S. Arnebia euchroma, a plant species of cold desert in the Himalayas, harbors beneficial cultivable endophytes in roots and leaves. Front. Microbiol. 2021, 12, 696667. [Google Scholar] [CrossRef]
- Oses, R.; Hernández, V.; Campos, L.; Becerra, J.; Irribarren-Riquelme, D.; Lavín, P.; Rodríguez, J. Advances in research on biodiversity and bioprospecting of endophytic fungi in Chile. In Neotropical Endophytic Fungi; Rosa, L., Ed.; Springer: Cham, Switzerland, 2021; pp. 53–91. [Google Scholar]
- Armesto, J.; Kalin, M.; Hinojosa, F. The Mediterranean Environment of Central Chile. In The Physical Geography of South America; Veblen, T.T., Young, K.R., Orme, A.R., Eds.; Oxford Academic Press: New York, NY, USA, 2007; pp. 184–199. [Google Scholar]
- Cares, R.; Medel, R.; Botto-Mahan, C. Frugivory in Echinopsis chiloensis (Caryophyllales: Cactaceae). Rev. Chil. Hist. Nat. 2013, 86, 489–491. [Google Scholar] [CrossRef][Green Version]
- Muñoz, G.; Hinrichsen, P.; Brygoo, Y.; Giraud, T. Genetic characterisation of Botrytis cinerea populations in Chile. Mycol. Res. 2002, 106, 594–601. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; New York Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Urbina, K.; Villarreal, P.; Nespolo, R.F.; Salazar, R.; Santander, R.; Cubillos, F.A. Volatile compound screening using HS-SPME-GC/MS on Saccharomyces eubayanus strains under low-temperature pilsner wort fermentation. Microorganisms 2020, 8, 755. [Google Scholar] [CrossRef]
- Gujar, A.; Anderson, T.; Cavagnino, D.; Patel, A. Comparative analysis of mass spectral matching for confident compound identification using the advanced electron ionization source for GC-MS. Thermoscientific 2018, 10598, 1–7. [Google Scholar]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 2020, 10, 4498. [Google Scholar] [CrossRef]
- Rizal, L.M.; Hereward, J.P.; Brookes, D.R.; Furlong, M.J.; Walter, G.H. Hidden diversity within Beauveria and Metarhizium–comparing morphology, barcoding, multilocus phylogenies and whole-genome sequences. Fungal Ecol. 2024, 67, 101304. [Google Scholar] [CrossRef]
- Morales, J.; Mendoza, L.; Cotoras, M. Alteration of oxidative phosphorylation as a possible mechanism of the antifungal action of p-coumaric acid against Botrytis cinerea. J. Appl. Microbiol. 2017, 123, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Pagès, M.; Kleiber, D.; Violleau, F. Ozonation of three different fungal conidia associated with apple disease: Importance of spore surface and membrane phospholipid oxidation. Food Sci. Nutr. 2020, 8, 5292–5297. [Google Scholar] [CrossRef]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R. Improving the thiobarbituric acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bijlani, S.; Parker, C.; Singh, N.K.; Sierra, M.A.; Foox, J.; Wang, C.C.C.; Mason, C.E.; Venkateswaran, K. Genomic characterization of the titan-like Cell producing Naganishia tulchinskyi, the first novel eukaryote isolated from the international space station. J. Fungi 2022, 8, 165. [Google Scholar] [CrossRef]
- Herth, W.; Schnepf, E. The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 1980, 105, 129–133. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Kachalkin, A.; Glushakova, A.; Venzhik, A. Presence of clinically significant endophytic yeasts in agricultural crops: Monitoring and ecological safety assessment. IOP Conf. Ser. Environ. Earth Sci. 2021, 723, 042005. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, X.; Yang, J.; Liu, J.; Zhao, L.; He, X. Fungal endophytic community and diversity associated with desert shrubs driven by plant identity and organ differentiation in extremely arid desert ecosystem. J. Fungi 2021, 7, 578. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial volatile organic compounds: Antifungal mechanisms, applications, and challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; De Stradis, A.; Lo Cantore, P.; Iacobellis, N.S. Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 2015, 6, 1056. [Google Scholar] [CrossRef]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The destructive fungal pathogen Botrytis cinerea-Insights from genes studied with mutant analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef]
- Siegmund, U.; Heller, J.; van Kan, J.A.; Tudzynski, P. The NADPH oxidase complexes in Botrytis cinerea: Evidence for a close association with the ER and the tetraspanin Pls1. PLoS ONE 2013, 8, e55879. [Google Scholar]
- Singh, Y.; Nair, A.M.; Verma, P.K. Surviving the odds: From perception to survival of fungal phytopathogens under host-generated oxidative burst. Plant Commun. 2021, 2, 100142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Li, B.; Chen, T.; Tian, S. Reactive oxygen species: A generalist in regulating development and pathogenicity of phytopathogenic fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Chen, T.; Ma, D.; Liu, J.; Xu, Y.; Tian, S. Inhibitory effects of methyl thujate on mycelial growth of Botrytis cinerea and possible mechanisms. Postharvest Biol. Technol. 2018, 142, 46–54. [Google Scholar] [CrossRef]
- Chung, K.R. Reactive oxygen species in the citrus fungal pathogen Alternaria alternata: The roles of NADPH-dependent oxidase. Physiol. Mol. Plant Pathol. 2014, 88, 10–17. [Google Scholar] [CrossRef]
- Wei, W.; Zhu, W.; Cheng, J.; Xie, J.; Jiang, D.; Li, G.; Chen, W.; Fu, Y. Nox Complex signal and MAPK cascade pathway are cross-linked and essential for pathogenicity and conidiation of mycoparasite Coniothyrium minitans. Sci. Rep. 2016, 6, 24325. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Garrido, C.; Collado, I.G. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phytochem. Rev. 2019, 19, 721–740. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Cai, J.; Liu, X.; Huang, G. Antifungal activity of volatile compounds generated by endophytic fungi Sarocladium brachiariae HND5 against Fusarium oxysporum f. sp. cubense. PLoS ONE 2021, 16, e0260747. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Kimura, M.; Yabe, Y.; Tsukamoto, D.; Sakamoto, M.; Horibe, I.; Okuno, Y. Use of solid phase microextraction (SPME) for profiling the volatile metabolites produced by Glomerella cingulata. J. Oleo Sci. 2008, 57, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Gotor-Vila, A.; Teixidó, N.; Di Francesco, A.; Usall, J.; Ugolini, L.; Torres, R.; Mari, M. Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 2017, 64, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Kowalska, J.; Drożdżyński, D.; Remlein-Starosta, D.; Sas-Paszt, L.; Malusá, E. Use of Cryptococcus albidus for controlling grey mould in the production and storage of organically grown strawberries. J. Plant Dis. Prot 2012, 119, 174–178. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Göker, M.; Groenewald, M.; Kachalkin, A.V.; Lumbsch, H.T.; Millanes, A.M.; Wedin, M.; Yurkov, A.M.; Boekhout, T.; et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [CrossRef]
- Tian, S.P.; Qin, G.Z.; Xu, Y.; Wang, Y.S. Survival of antagonistic yeasts under field conditions and their biocontrol ability against postharvest diseases of sweet cherry. Postharvest Biol. Technol. 2004, 33, 327–331. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Yu, T. Biological control of postharvest diseases of peach with Cryptococcus laurentii. Food Control 2007, 18, 287–291. [Google Scholar] [CrossRef]
- Alves, Z.; Melo, A.; Figueiredo, A.R.; Coimbra, M.A.; Gomes, A.C.; Rocha, S.M. Exploring the Saccharomyces cerevisiae Volatile metabolome: Indigenous versus commercial strains. PLoS ONE 2015, 10, e0143641. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Vrhovsek, U. Early fermentation volatile metabolite profile of non- Saccharomyces yeasts in red and white grape must: A targeted approach. Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Zhan, P.; Shao, X. ROS stress and cell membrane disruption are the main antifungal mechanisms of 2-phenylethanol against Botrytis cinerea. J. Agric. Food Chem. 2022, 70, 14468–14479. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.; Córdoba, M.; Casquete, R.; Serradilla, M.; Martín, A. Selection and application of antifungal VOCs-producing yeasts as biocontrol agents of grey mould in fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Q.; Wu, C.; Yuan, Y.; Ni, X.; Wu, T.; Yuan, Y.; Ni, X.; Wu, T.; Chang, R.; et al. Volatile organic compounds produced by Metschnikowia pulcherrima yeast T-2 inhibited the growth of Botrytis cinerea in postharvest blueberry fruits. Horticult. Plant J. 2024. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. Microbiologyopen 2019, 8, e00813. [Google Scholar] [CrossRef]
- Kalogiannis, S.; Tjamos, S.; Stergiou, A.; Antoniou, P.; Ziogas, B.; Tjamos, E. Selection and evaluation of phyllosphere yeasts as biocontrol agents against grey mould of tomato. Eur. J. Plant Pathol. 2006, 116, 69–76. [Google Scholar] [CrossRef]
- Santos, A.; Sánchez, A.; Marquina, D. Yeasts as biological agents to control Botrytis cinerea. Microbiol. Res. 2004, 159, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance material review on phenylethyl alcohol. Food Chem. Toxicol. 2012, 50, S224–S239. [Google Scholar] [CrossRef]
- Nizovoy, P.; Bellora, N.; Haridas, S.; Sun, H.; Daum, C.; Barry, K.; Grigoriev, I.V.; Libkind, D.; Connell, L.B.; Moliné, M. Unique genomic traits for cold adaptation in Naganishia vishniacii, a polyextremophile yeast isolated from Antarctica. FEMS Yeast Res. 2021, 21, foaa056. [Google Scholar] [CrossRef]




| Amount (µL) | Double Petri Dish Volume (mL) | Concentration (µL mL−1) |
|---|---|---|
| 0 | 160 | 0.00 |
| 5 | 0.03 | |
| 15 | 0.09 | |
| 30 | 0.19 | |
| 50 | 0.31 |
| RT | RSI | Identified Compound | m/z | Molecular Formula | CAS Registration Number |
|---|---|---|---|---|---|
| 14.31 | 935 | ethylbenzene | 106.1 | C8H10 | 100-41-4 |
| 14.82 | 906 | 3-methylbutyl acetate | 130.2 | C7H14O2 | 123-92-2 |
| 15.40 | 862 | 2-heptanone | 114.2 | C7H14O | 110-43-0 |
| 18.41 | 905 | 1-ethyl-3-methylbenzene | 120.2 | C9H12 | 620-14-4 |
| 24.07 | 946 | 2-phenylethanol | 122.2 | C8H10O | 60-12-8 |
| 34.36 | 930 | (5E)-6,10-dimethyl-5,9-undecadien-2-one | 194.3 | C13H22O | 3796-70-1 |
| Abundance Relative Peak Area (%) | ||||||
|---|---|---|---|---|---|---|
| Day | 3-Methylbutyl Acetate | Ethylbenzene | 2-Heptanone | 1-Ethyl-3-Methylbenzene | 2-Phenylethanol | (5E)-6,10-Dimethyl-5,9-Undecadien-2-One |
| 3 | 3.0 ± 0.9 | ND | ND | ND | 5.5 ± 2.5 | ND |
| 7 | 3.9 ± 2.7 | ND | 0.1 ± 0.0 | 0.9 ± 0.2 | 9.0 ± 3.1 | ND |
| 12 | 3.3 ± 0.7 | 2.0 ± 0.5 | 0.2 ± 0.0 | ND | 12.3 ± 2.7 | 4.9 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, A.; Castro, P.; Navarro, F.; Parada, R.; Mendoza, L.; Cotoras, M. Effect of Volatile Compounds Emitted by an Endophytic Yeast Isolated from the Endemic Plant Echinopsis chiloensis against Botrytis cinerea. Horticulturae 2024, 10, 1005. https://doi.org/10.3390/horticulturae10091005
Vidal A, Castro P, Navarro F, Parada R, Mendoza L, Cotoras M. Effect of Volatile Compounds Emitted by an Endophytic Yeast Isolated from the Endemic Plant Echinopsis chiloensis against Botrytis cinerea. Horticulturae. 2024; 10(9):1005. https://doi.org/10.3390/horticulturae10091005
Chicago/Turabian StyleVidal, Araceli, Paulo Castro, Freddy Navarro, Rodolfo Parada, Leonora Mendoza, and Milena Cotoras. 2024. "Effect of Volatile Compounds Emitted by an Endophytic Yeast Isolated from the Endemic Plant Echinopsis chiloensis against Botrytis cinerea" Horticulturae 10, no. 9: 1005. https://doi.org/10.3390/horticulturae10091005
APA StyleVidal, A., Castro, P., Navarro, F., Parada, R., Mendoza, L., & Cotoras, M. (2024). Effect of Volatile Compounds Emitted by an Endophytic Yeast Isolated from the Endemic Plant Echinopsis chiloensis against Botrytis cinerea. Horticulturae, 10(9), 1005. https://doi.org/10.3390/horticulturae10091005






