Efficacy of Postharvest Application of Aureobasidium pullulans to Control White Haze on Apples and Effect on the Fruit Mycobiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. In Vivo Antagonistic Assay under Storage Conditions
2.3. Statistical Analysis
2.4. Microbiome Sampling, Sequencing, and Bioinformatics
3. Results
3.1. In Vivo Antagonistic Assay under Storage Conditions
3.2. Microbial Diversity and Composition
3.2.1. Alpha Diversity
3.2.2. Beta Diversity
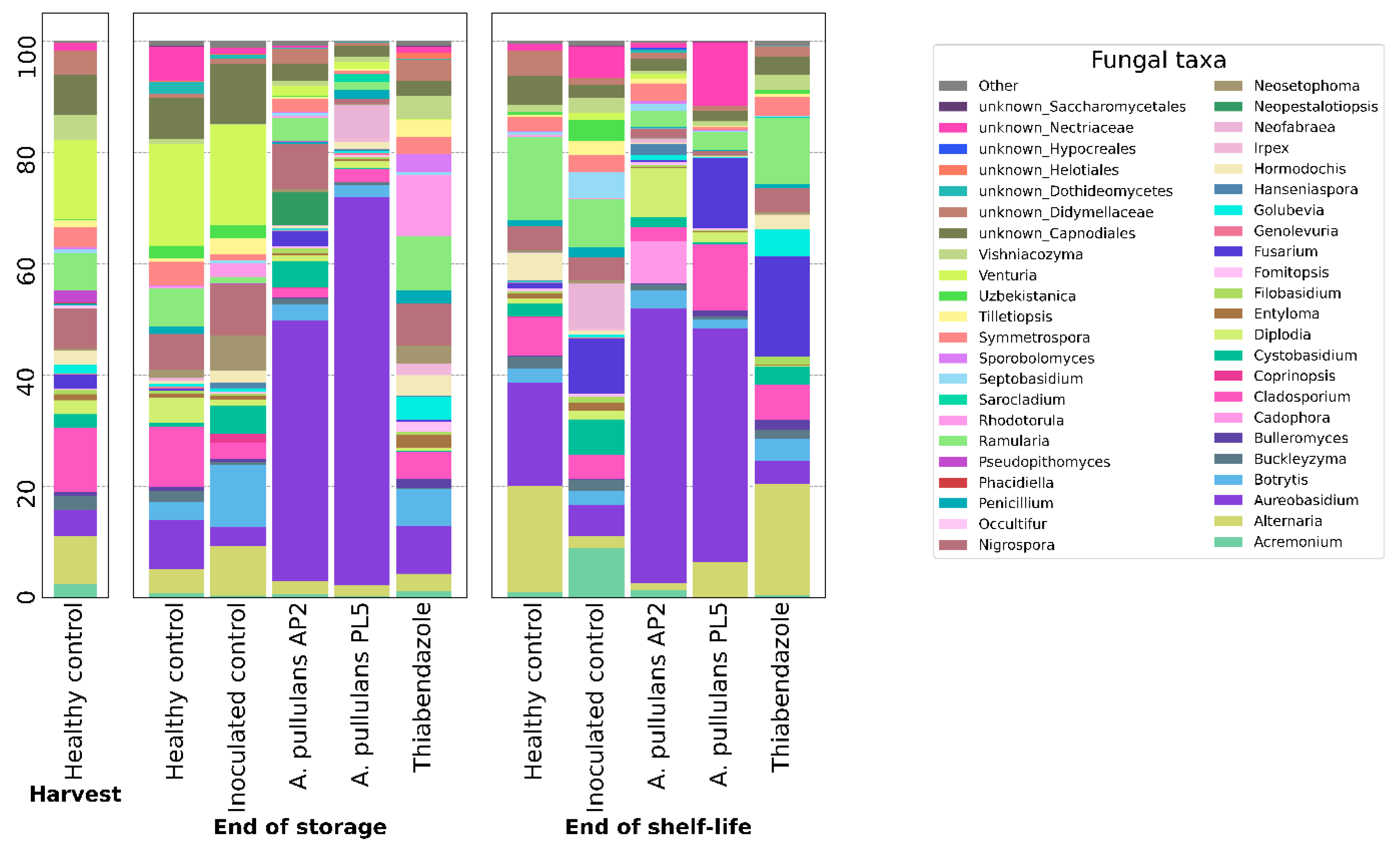
3.2.3. Compositional Analysis of Microbial Population
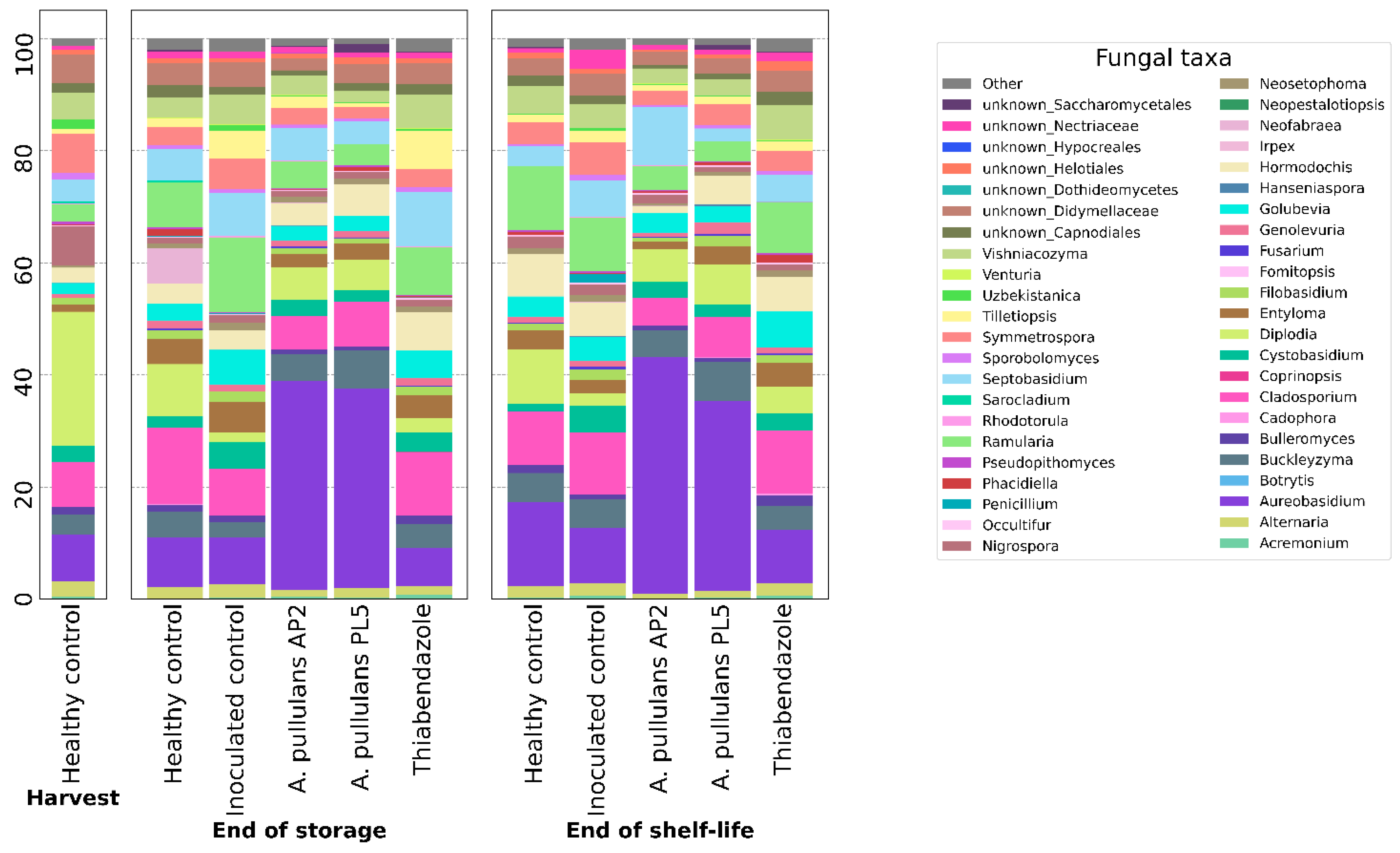
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization. FAOSTAT. Available online: https://www.fao.org/faostat/en/?#data/QCL (accessed on 27 June 2024).
- Garello, M.; Piombo, E.; Prencipe, S.; Schiavon, G.; Berra, L.; Wisniewski, M.; Droby, S.; Spadaro, D. Fruit microbiome: A powerful tool to study the epidemiology of dry lenticel rot and white haze–emerging postharvest diseases of apple. Postharvest Biol. Technol. 2023, 196, 112163. [Google Scholar] [CrossRef]
- Reddy, K.; Spadaro, D.; Lore, A.; Gullino, M.L.; Garibaldi, A. Potential of patulin production by Penicillium expansum strains on various fruits. Mycotoxin Res. 2010, 26, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Remolif, G.M.; Nari, L.; Gualandri, V.; Angeli, D.; Oettl, S.; Dijksterhuis, J.; Boekhout, T.; Spadaro, D. Characterization of fungal species involved in white haze disorder on apples in Northern Italy and description of Golubevia mali sp. nov. and Entyloma mali sp. nov. Postharvest Biol. Technol. 2024, 209, 112678. [Google Scholar] [CrossRef]
- Prencipe, S.; Valente, S.; Nari, L.; Spadaro, D. A quantitative real-time PCR assay for early detection and quantification of Ramularia mali, an emerging pathogen of apple causing dry lenticel rot. Plant Dis. 2023, 107, 1399–1407. [Google Scholar] [CrossRef]
- Primisser, S.; Spadaro, D.; Deltedesco, E.; Oettl, S. Ramularia mali associated with symptoms of dry lenticel rot, an emerging postharvest disease on apples in Italy. Plant Dis. 2024, 108, 2579. [Google Scholar] [CrossRef] [PubMed]
- Boekhout, T.; Gildemacher, P.; Theelen, B.; Müller, W.H.; Heijne, B.; Lutz, M. Extensive colonization of apples by smut anamorphs causes a new postharvest disorder. FEMS Yeast Res. 2006, 6, 63–76. [Google Scholar] [CrossRef]
- Baric, S.; Lindner, L.; Marschall, K.; Dalla Via, J. Haplotype diversity of Tilletiopsis spp. causing white haze in apple orchards in Northern Italy. Plant Pathol. 2010, 59, 535–541. [Google Scholar] [CrossRef]
- Weber, R.; Zabel, D. White haze and scarf skin, two little-known cosmetic defects of apples in northern Germany. Eur. J. Hortic. Sci. 2011, 76, 45. [Google Scholar]
- Prencipe, S.; Spadaro, D.; Fruk, G.; Jemric, T. First report of Tilletiopsis pallescens causing white haze on apple in Croatia. Plant Dis. 2016, 100, 225. [Google Scholar] [CrossRef]
- Richter, C.; Yurkov, A.M.; Boekhout, T.; Stadler, M. Diversity of Tilletiopsis-like fungi in Exobasidiomycetes (Ustilaginomycotina) and description of six novel species. Front. Microbiol. 2019, 10, 2544. [Google Scholar] [CrossRef]
- Lindner, L.; Baric, S. White haze: A new disorder of apples caused by fungi colonizing the fruit surface [Malus pumila Mill.; South Tyrol]. Inf. Fitopatol. 2006, 56, 60–63. [Google Scholar]
- Spadaro, D.; Torres, R.; Errampalli, D.; Everett, K.; Ramos, L.; Mari, M. Pome fruits. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019; pp. 55–110. [Google Scholar]
- Angeli, D.; Gualandri, V.; Oliveira Longa, C.M.; Zaffoni, M. Patina bianca, una nuova emergenza in melicoltura. Inf. Agrar. 2021, 77, 50–54. [Google Scholar]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cignola, R.; Zucchinali, S.; Firrao, G.; Di Francesco, A. Aspects of the biocontrol activity of Aureobasidium spp. strain against Penicillium expansum of apple. Ann. Appl. Biol. 2024, 184, 307–313. [Google Scholar] [CrossRef]
- Usall, J.; Teixidó, N.; Fons, E.; Vinas, I. Biological control of blue mould on apple by a strain of Candida sake under several controlled atmosphere conditions. Int. J. Food Microbiol. 2000, 58, 83–92. [Google Scholar] [CrossRef]
- Zhang, D.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Potential biocontrol activity of a strain of Pichia guilliermondii against grey mold of apples and its possible modes of action. Biol. Control 2011, 57, 193–201. [Google Scholar] [CrossRef]
- Tulukoğlu-Kunt, K.S.; Özden, M.; Di Francesco, A. Exploring wild and local fruits as sources of promising biocontrol agents against Alternaria spp. in apples. Horticulturae 2023, 9, 1156. [Google Scholar] [CrossRef]
- Mari, M.; Martini, C.; Spadoni, A.; Rouissi, W.; Bertolini, P. Biocontrol of apple postharvest decay by Aureobasidium pullulans. Postharvest Biol. Technol. 2012, 73, 56–62. [Google Scholar] [CrossRef]
- Janisiewicz, W.; Kurtzman, C.; Buyer, J. Yeasts associated with nectarines and their potential for biological control of brown rot. Yeast 2010, 27, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Calassanzio, M.; Ratti, C.; Mari, M.; Folchi, A.; Baraldi, E. Molecular characterization of the two postharvest biological control agents Aureobasidium pullulans L1 and L8. Biol. Control 2018, 123, 53–59. [Google Scholar] [CrossRef]
- Mari, M.; Martini, C.; Guidarelli, M.; Neri, F. Postharvest biocontrol of Monilinia laxa, Monilinia fructicola and Monilinia fructigena on stone fruit by two Aureobasidium pullulans strains. Biol. Control 2012, 60, 132–140. [Google Scholar] [CrossRef]
- Zhang, D.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Selection and evaluation of new antagonists for their efficacy against postharvest brown rot of peaches. Postharvest Biol. Technol. 2010, 55, 174–181. [Google Scholar] [CrossRef]
- Schena, L.; Nigro, F.; Pentimone, I.; Ligorio, A.; Ippolito, A. Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharvest Biol. Technol. 2003, 30, 209–220. [Google Scholar] [CrossRef]
- EU Pesticides Database. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 28 June 2024).
- Zhang, D.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Efficacy of the antagonist Aureobasidium pullulans PL5 against postharvest pathogens of peach, apple and plum and its modes of action. Biol. Control 2010, 54, 172–180. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 13 June 2024).
- Schiavon, G.; Garello, M.; Prencipe, S.; Meloni, G.R.; Buonsenso, F.; Spadaro, D. Essential oils reduce grey mould rot of apples and modify the fruit microbiome during postharvest storage. J. Fungi 2022, 9, 22. [Google Scholar] [CrossRef]
- Naqib, A.; Poggi, S.; Wang, W.; Hyde, M.; Kunstman, K.; Green, S.J. Making and sequencing heavily multiplexed, high-throughput 16S ribosomal RNA gene amplicon libraries using a flexible, two-stage PCR protocol. In Gene Expression Analysis: Methods and Protocols; Humana Press: New York, NY, USA, 2018; pp. 149–169. [Google Scholar]
- Abdelfattah, A.; Freilich, S.; Bartuv, R.; Zhimo, V.Y.; Kumar, A.; Biasi, A.; Salim, S.; Feygenberg, O.; Burchard, E.; Dardick, C. Global analysis of the apple fruit microbiome: Are all apples the same? Environ. Microbiol. 2021, 23, 6038–6055. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE QIIME Release for Eukaryotes 2. Version 10.05.2021. Available online: https://doi.org/10.15156/BIO/1264861 (accessed on 20 June 2024).
- Beule, L.; Karlovsky, P. Improved normalization of species count data in ecology by scaling with ranked subsampling (SRS): Application to microbial communities. Peer J. 2020, 8, e9593. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Guillaume Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.5–3. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 June 2024).
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Halko, N.; Martinsson, P.-G.; Shkolnisky, Y.; Tygert, M. An algorithm for the principal component analysis of large data sets. SIAM J. Sci. Comput. 2011, 33, 2580–2594. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Ippolito, A.; El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol. Technol. 2000, 19, 265–272. [Google Scholar] [CrossRef]
- Schena, L.; Ippolito, A.; Zahavi, T.; Cohen, L.; Nigro, F.; Droby, S. Genetic diversity and biocontrol activity of Aureobasidium pullulans isolates against postharvest rots. Postharvest Biol. Technol. 1999, 17, 189–199. [Google Scholar] [CrossRef]
- Bencheqroun, S.K.; Bajji, M.; Massart, S.; Labhilili, M.; El Jaafari, S.; Jijakli, M.H. In vitro and in situ study of postharvest apple blue mold biocontrol by Aureobasidium pullulans: Evidence for the involvement of competition for nutrients. Postharvest Biol. Technol. 2007, 46, 128–135. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; D’Aquino, S.; Pagnotta, E.; Mari, M. Biocontrol of Monilinia laxa by Aureobasidium pullulans strains: Insights on competition for nutrients and space. Int. J. Food Microbiol. 2017, 248, 32–38. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; González, M.B.; Garat, M.F.; Wisniewski, M. Aureobasidium pullulans as a biocontrol agent of postharvest pathogens of apples in Uruguay. Biocontrol Sci. Technol. 2009, 19, 1033–1049. [Google Scholar] [CrossRef]
- Zajc, J.; Cernosa, A.; Di Francesco, A.; Casteria, R.; De Curtis, F.; Lima, G.; Badri, H.; Jijakli, H.; Ippolito, A.; Gostincar, C. Characterization of Aureobasidium pullulans isolates selected as biocontrol agents against fruit decay pathogens. Fungal Genom. Biol. 2020, 10, 163. [Google Scholar]
- Liu, X.; Wang, J.; Gou, P.; Mao, C.; Zhu, Z.-R.; Li, H. In vitro inhibition of postharvest pathogens of fruit and control of gray mold of strawberry and green mold of citrus by aureobasidin A. Int. J. Food Microbiol. 2007, 119, 223–229. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Foggia, M.; Corbetta, M.; Baldo, D.; Ratti, C.; Baraldi, E. Biocontrol activity and plant growth promotion exerted by Aureobasidium pullulans strains. J. Plant Growth Regul. 2021, 40, 1233–1244. [Google Scholar] [CrossRef]
- Castoria, R.; De Curtis, F.; Lima, G.; Caputo, L.; Pacifico, S.; De Cicco, V. Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: Study on its modes of action. Postharvest Biol. Technol. 2001, 22, 7–17. [Google Scholar] [CrossRef]
- Banani, H.; Spadaro, D.; Zhang, D.; Matic, S.; Garibaldi, A.; Gullino, M.L. Biocontrol activity of an alkaline serine protease from Aureobasidium pullulans expressed in Pichia pastoris against four postharvest pathogens on apple. Int. J. Food Microbiol. 2014, 182, 1–8. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Zhang, D.; Spadaro, D.; Valente, S.; Garibaldi, A.; Gullino, M.L. Cloning, characterization, expression and antifungal activity of an alkaline serine protease of Aureobasidium pullulans PL5 involved in the biological control of postharvest pathogens. Int. J. Food Microbiol. 2012, 153, 453–464. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Droby, S. Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst. Appl. Microbiol. 2001, 24, 395–399. [Google Scholar] [CrossRef]
- Karabulut, O.; Tezcan, H.; Daus, A.; Cohen, L.; Wiess, B.; Droby, S. Control of preharvest and postharvest fruit rot in strawberry by Metschnikowia fructicola. Biocontrol Sci. Technol. 2004, 14, 513–521. [Google Scholar] [CrossRef]
- Spadaro, D.; Lorè, A.; Garibaldi, A.; Gullino, M.L. A new strain of Metschnikowia fructicola for postharvest control of Penicillium expansum and patulin accumulation on four cultivars of apple. Postharvest Biol. Technol. 2013, 75, 1–8. [Google Scholar] [CrossRef]
- Piombo, E.; Sela, N.; Wisniewski, M.; Hoffmann, M.; Gullino, M.L.; Allard, M.W.; Levin, E.; Spadaro, D.; Droby, S. Genome sequence, assembly and characterization of two Metschnikowia fructicola strains used as biocontrol agents of postharvest diseases. Front. Microbiol. 2018, 9, 593. [Google Scholar] [CrossRef]
- Macarisin, D.; Droby, S.; Bauchan, G.; Wisniewski, M. Superoxide anion and hydrogen peroxide in the yeast antagonist–fruit interaction: A new role for reactive oxygen species in postharvest biocontrol? Postharvest Biol. Technol. 2010, 58, 194–202. [Google Scholar] [CrossRef]
- Banani, H.; Spadaro, D.; Zhang, D.; Matic, S.; Garibaldi, A.; Gullino, M.L. Postharvest application of a novel chitinase cloned from Metschnikowia fructicola and overexpressed in Pichia pastoris to control brown rot of peaches. Int. J. Food Microbiol. 2015, 199, 54–61. [Google Scholar] [CrossRef]
- Angeli, D.; Turrini, L.; Zeni, F.; Longa, C.; Gualandri, V.; Roman, T. Controlling white haze disease under in vitro controlled conditions. In Proceedings of the VI International Symposium on Postharvest Pathology: Innovation and Advanced Technologies for Managing Postharvest Pathogens 1363, Limassol, Cyprus, 29 May–2 June 2022; pp. 95–100. [Google Scholar]
- Rajapakse, N.C.; Banks, N.H.; Hewett, E.W.; Cleland, D.J. Development of oxygen concentration gradients in flesh tissues of bulky plant organs. J. Am. Soc. Hortic. Sci. 1990, 115, 793–797. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Viskelis, J.; Viskelis, P.; Liaudanskas, M.; Janulis, V. Changes in the biochemical composition and physicochemical properties of apples stored in controlled atmosphere conditions. Appl. Sci. 2021, 11, 6215. [Google Scholar] [CrossRef]
- Olimi, E.; Kusstatscher, P.; Wicaksono, W.A.; Abdelfattah, A.; Cernava, T.; Berg, G. Insights into the microbiome assembly during different growth stages and storage of strawberry plants. Environ. Microbiome 2022, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, C.; Fang, D.; Che, J.; Wu, W.; Lyu, L.; Li, W. The impact of storage temperature on the development of microbial communities on the surface of blueberry fruit. Foods 2023, 12, 1611. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Kumar, A.; Biasi, A.; Abdelfattah, A.; Sharma, V.K.; Salim, S.; Feygenberg, O.; Bartuv, R.; Freilich, S.; Whitehead, S.R. Assembly and dynamics of the apple carposphere microbiome during fruit development and storage. Front. Microbiol. 2022, 13, 928888. [Google Scholar] [CrossRef]
- Biasi, A.; Zhimo, V.Y.; Kumar, A.; Abdelfattah, A.; Salim, S.; Feygenberg, O.; Wisniewski, M.; Droby, S. Changes in the fungal community assembly of apple fruit following postharvest application of the yeast biocontrol agent Metschnikowia fructicola. Horticulturae 2021, 7, 360. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Ngea, G.L.N.; Zhang, X.; Yang, Q.; Zhang, Q.; Xu, X.; Zhang, H. Changes of the microbial community in kiwifruit during storage after postharvest application of Wickerhamomyces anomalus. Food Chem. 2023, 404, 134593. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, L.; Hatami Rad, S.; Etebarian, H.R. Apple endophytic fungi and their antagonism against apple scab disease. Front. Microbiol. 2022, 13, 1024001. [Google Scholar] [CrossRef] [PubMed]
- Van Kan, J.A.; Shaw, M.W.; Grant-Downton, R.T. Botrytis species: Relentless necrotrophic thugs or endophytes gone rogue? Mol. Plant Pathol. 2014, 15, 957–961. [Google Scholar] [CrossRef] [PubMed]

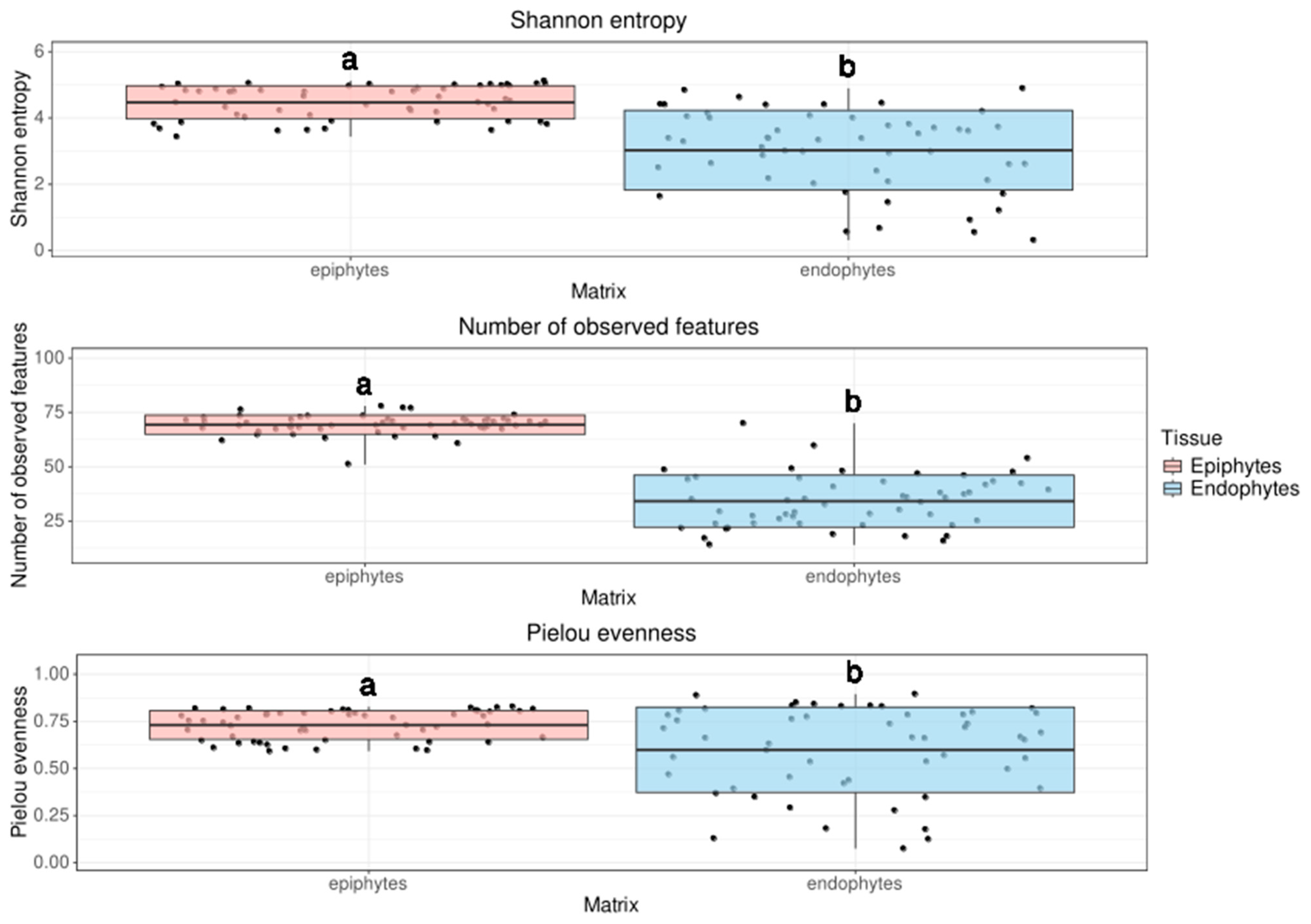
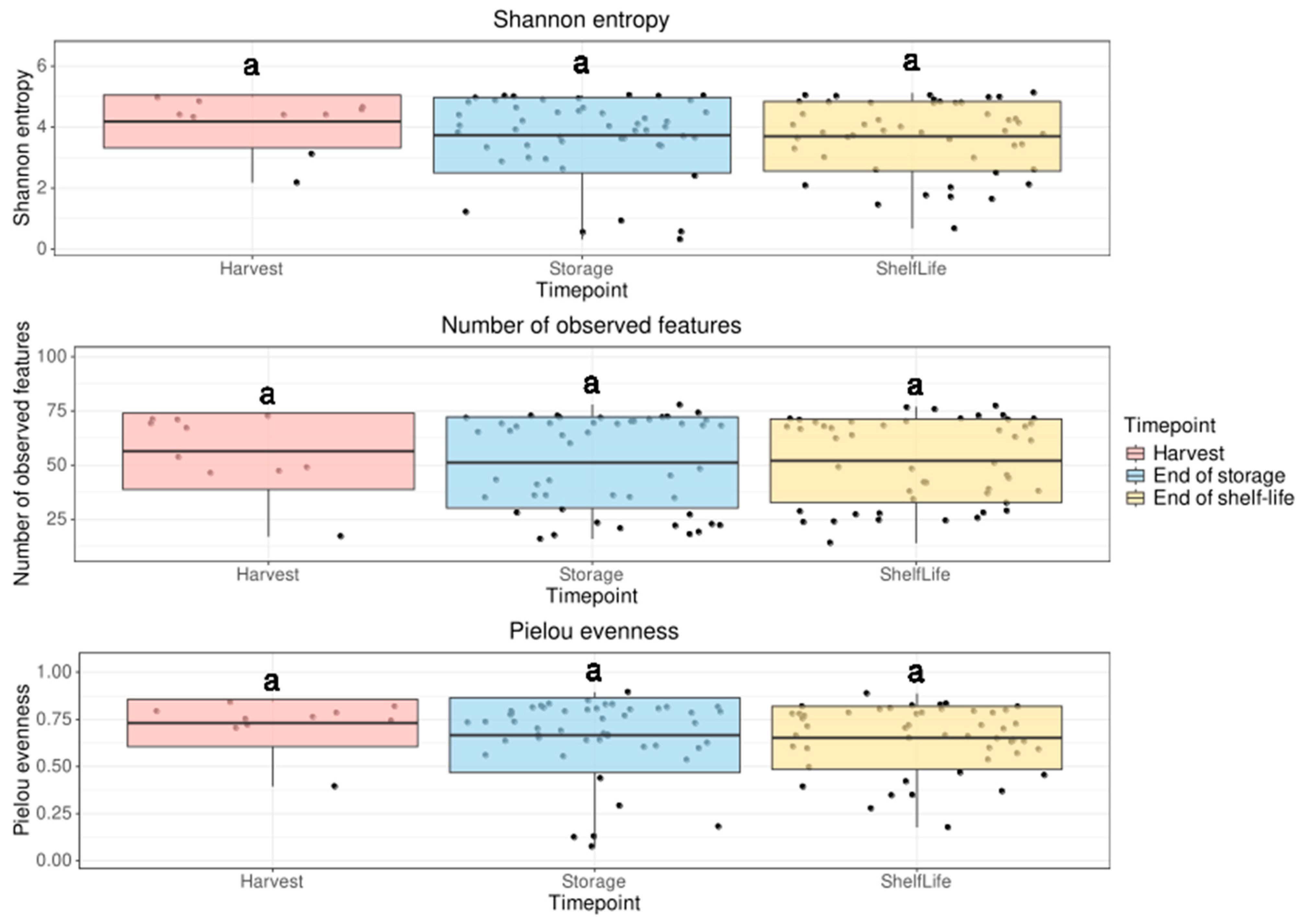
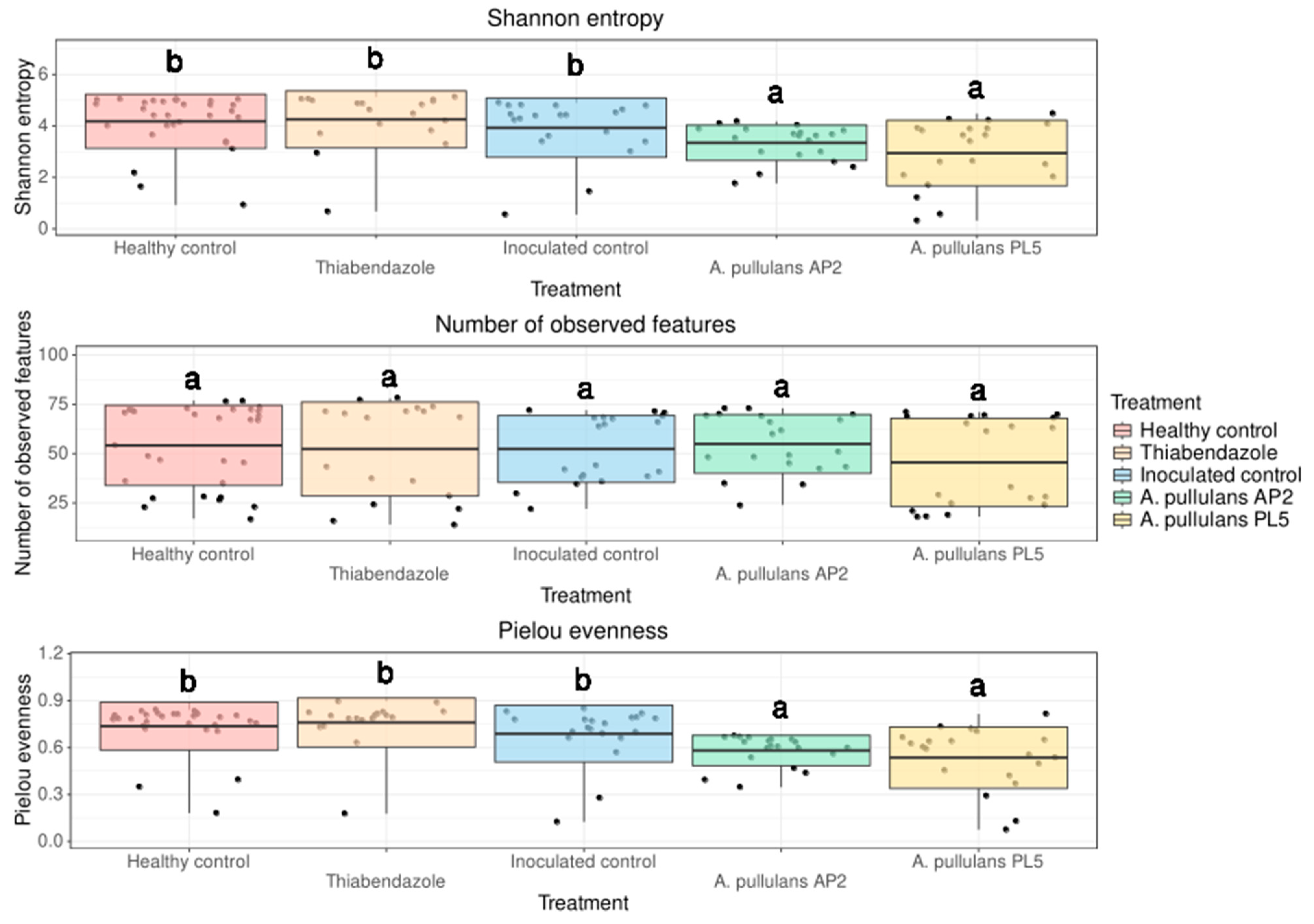
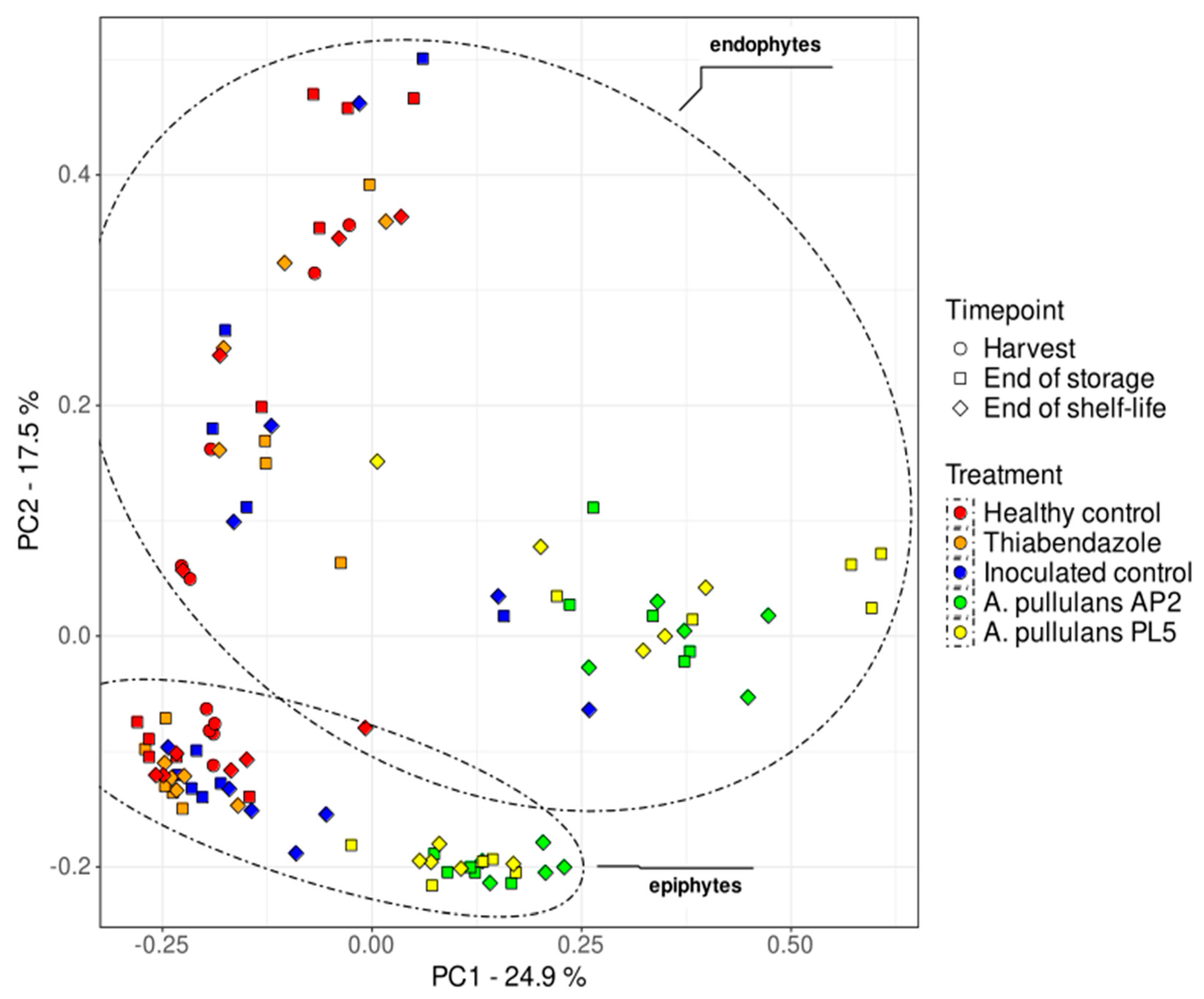
| Parameter | F.Model | R2 | Pr(>F) |
|---|---|---|---|
| Treatment | 9.75 | 22.5% | 0.001 |
| Matrix | 25.17 | 15% | 0.001 |
| Timepoint | 1.75 | 2.5% | 0.012 |
| Treatment × Matrix | 1.04 | 2% | 0.391 |
| Treatment × Timepoint | 1.25 | 3% | 0.066 |
| Matrix × Timepoint | 1.73 | 2.5% | 0.017 |
| Treatment × Matrix × Timepoint | 1.12 | 3% | 0.158 |
| Residuals | NA | 49.5% | NA |
| Total | NA | 100% | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remolif, G.; Schiavon, G.; Garello, M.; Spadaro, D. Efficacy of Postharvest Application of Aureobasidium pullulans to Control White Haze on Apples and Effect on the Fruit Mycobiome. Horticulturae 2024, 10, 927. https://doi.org/10.3390/horticulturae10090927
Remolif G, Schiavon G, Garello M, Spadaro D. Efficacy of Postharvest Application of Aureobasidium pullulans to Control White Haze on Apples and Effect on the Fruit Mycobiome. Horticulturae. 2024; 10(9):927. https://doi.org/10.3390/horticulturae10090927
Chicago/Turabian StyleRemolif, Giulia, Giada Schiavon, Marco Garello, and Davide Spadaro. 2024. "Efficacy of Postharvest Application of Aureobasidium pullulans to Control White Haze on Apples and Effect on the Fruit Mycobiome" Horticulturae 10, no. 9: 927. https://doi.org/10.3390/horticulturae10090927
APA StyleRemolif, G., Schiavon, G., Garello, M., & Spadaro, D. (2024). Efficacy of Postharvest Application of Aureobasidium pullulans to Control White Haze on Apples and Effect on the Fruit Mycobiome. Horticulturae, 10(9), 927. https://doi.org/10.3390/horticulturae10090927







