Transcriptomic and Metabolomic Analyses Provide New Insights into the Response of Strawberry (Fragaria × ananassa Duch.) to Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurement of Physiological Indexes
2.3. Transcriptome Sequencing and Analysis
2.3.1. Extraction and Detection of RNA Samples
2.3.2. RNA Library Construction
2.3.3. Analysis of Differentially Expressed Genes (DEGs)
2.4. Targeted Metabolomic Analysis
2.4.1. Preparation of the Metabolomic Standards
2.4.2. Metabolite Extraction
2.4.3. Chromatographic and Mass Spectrometry Detection
2.4.4. Metabolic Data Analysis
3. Results
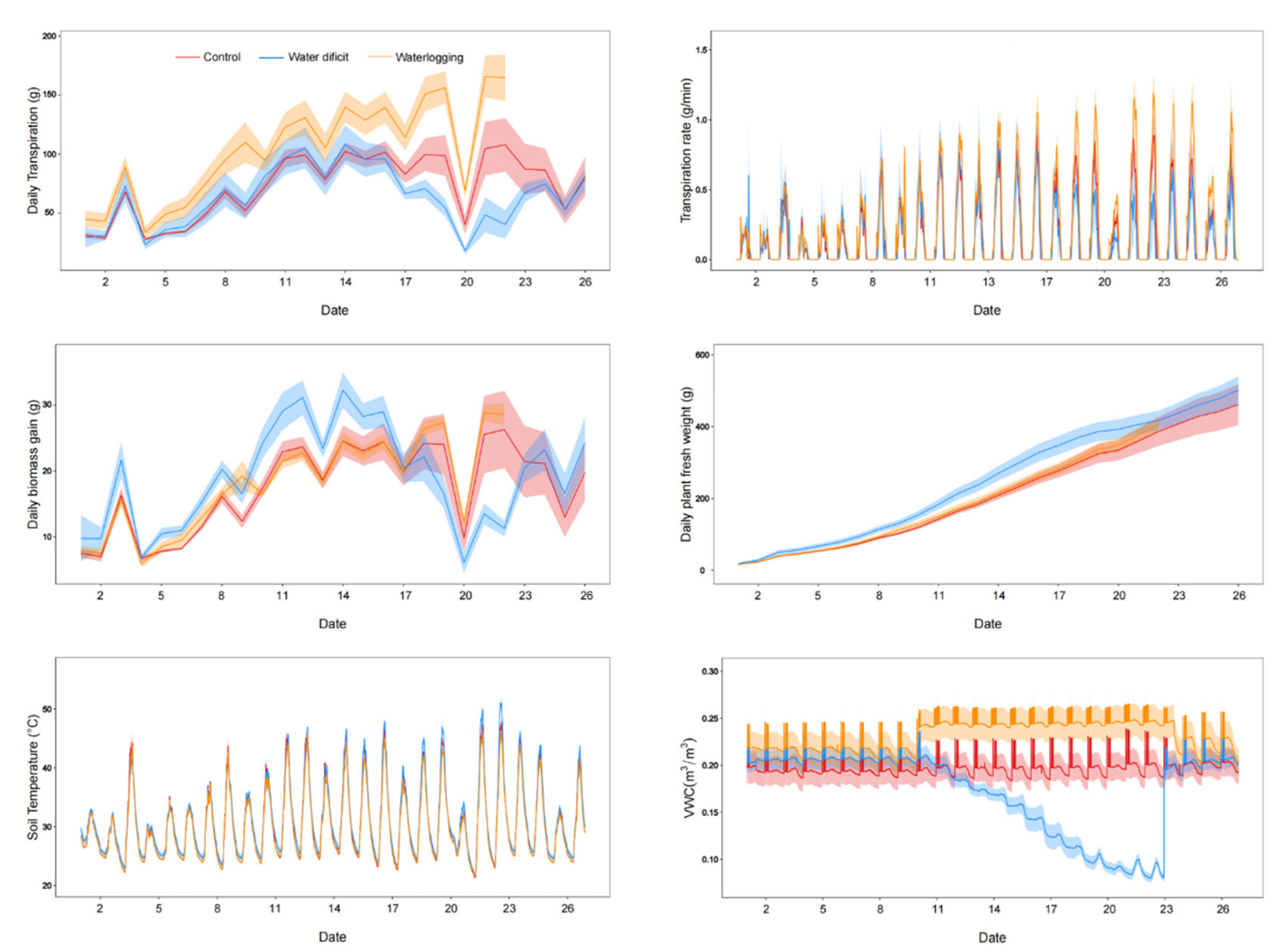
3.1. Effects of Drought on the Physiology of Strawberry
3.2. Transcriptome Analysis
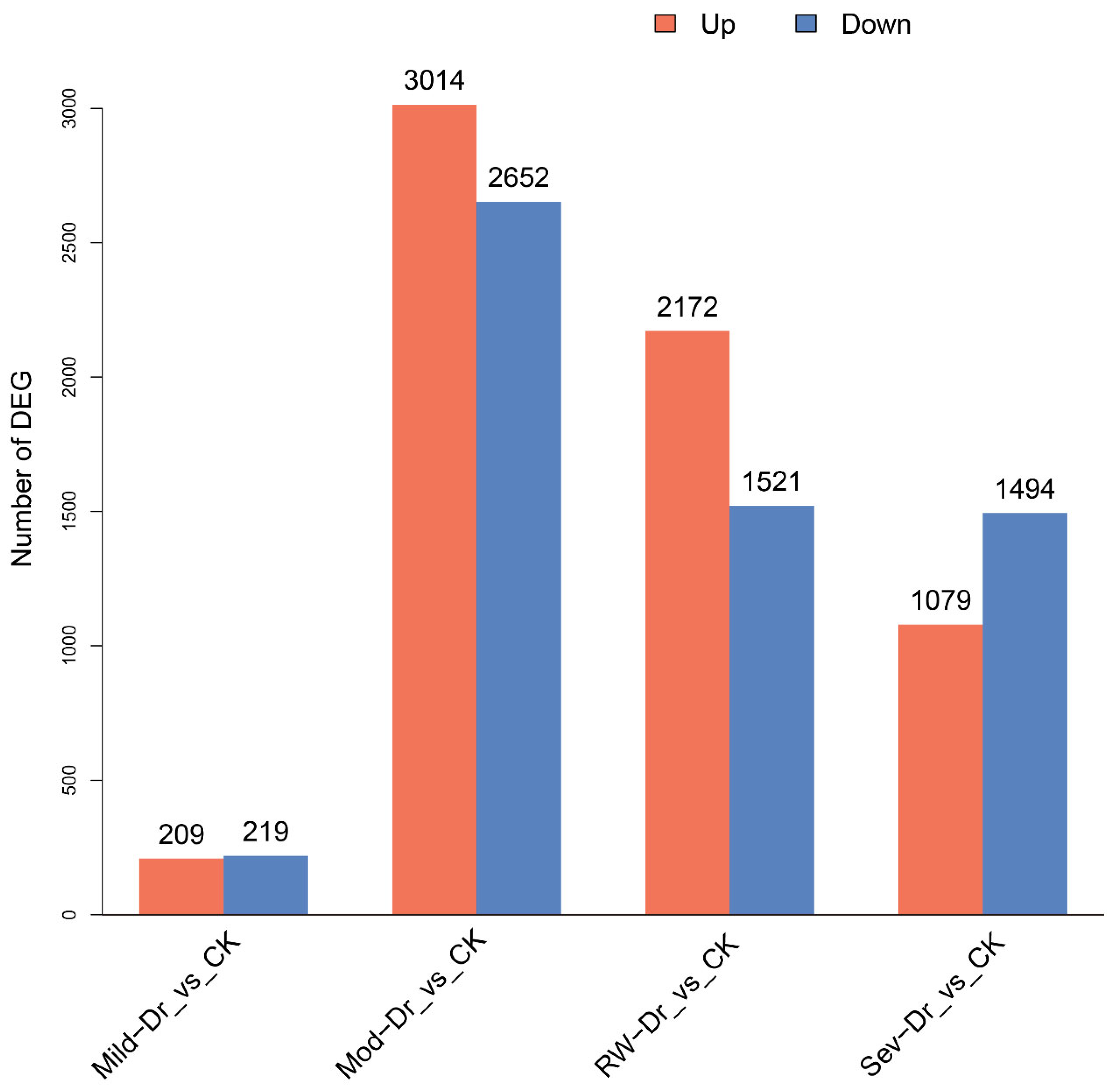
3.2.1. Transcriptome Sequencing and Identification of DEGs
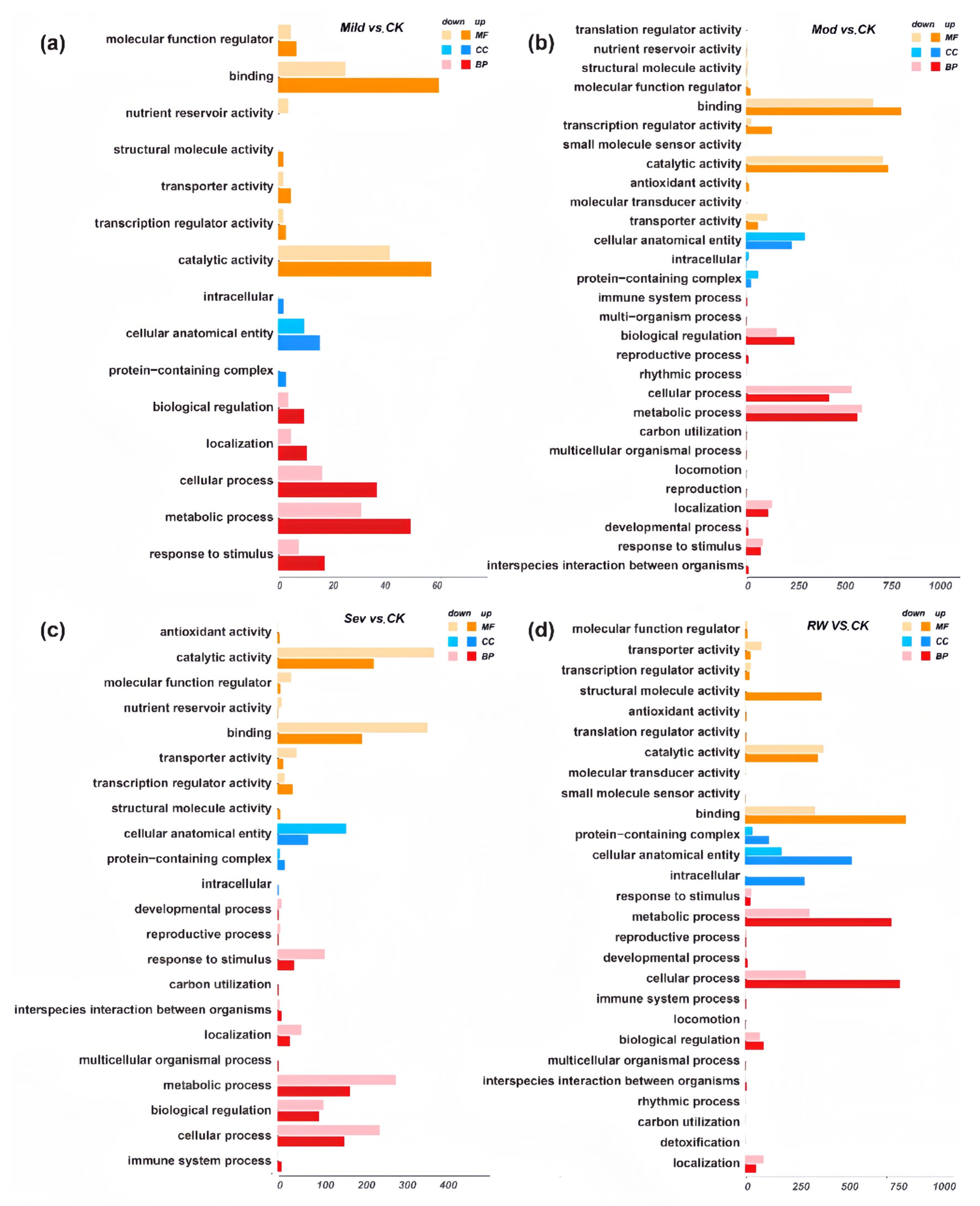
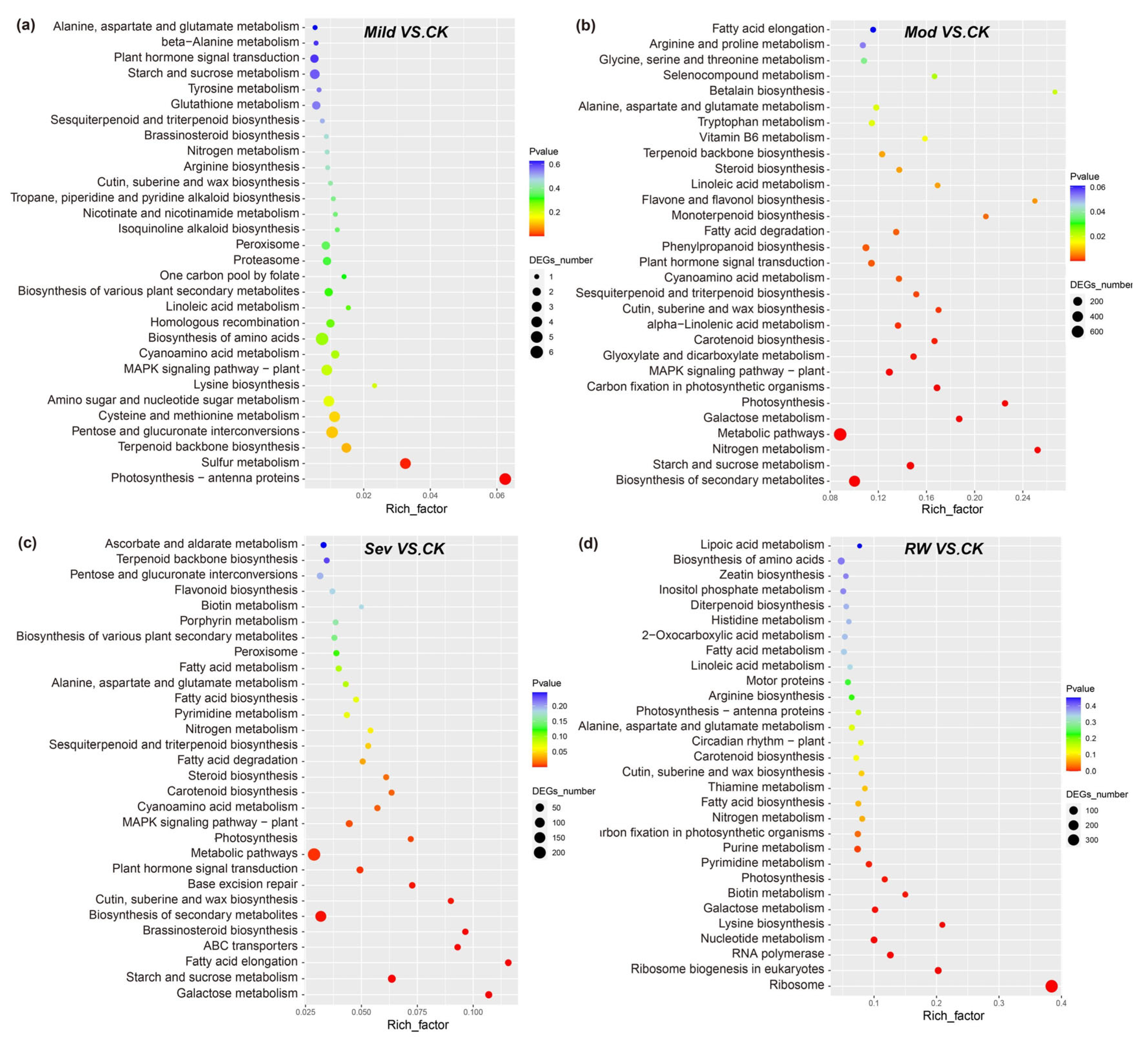
3.2.2. GO and KEGG Pathway Enrichment Analyses
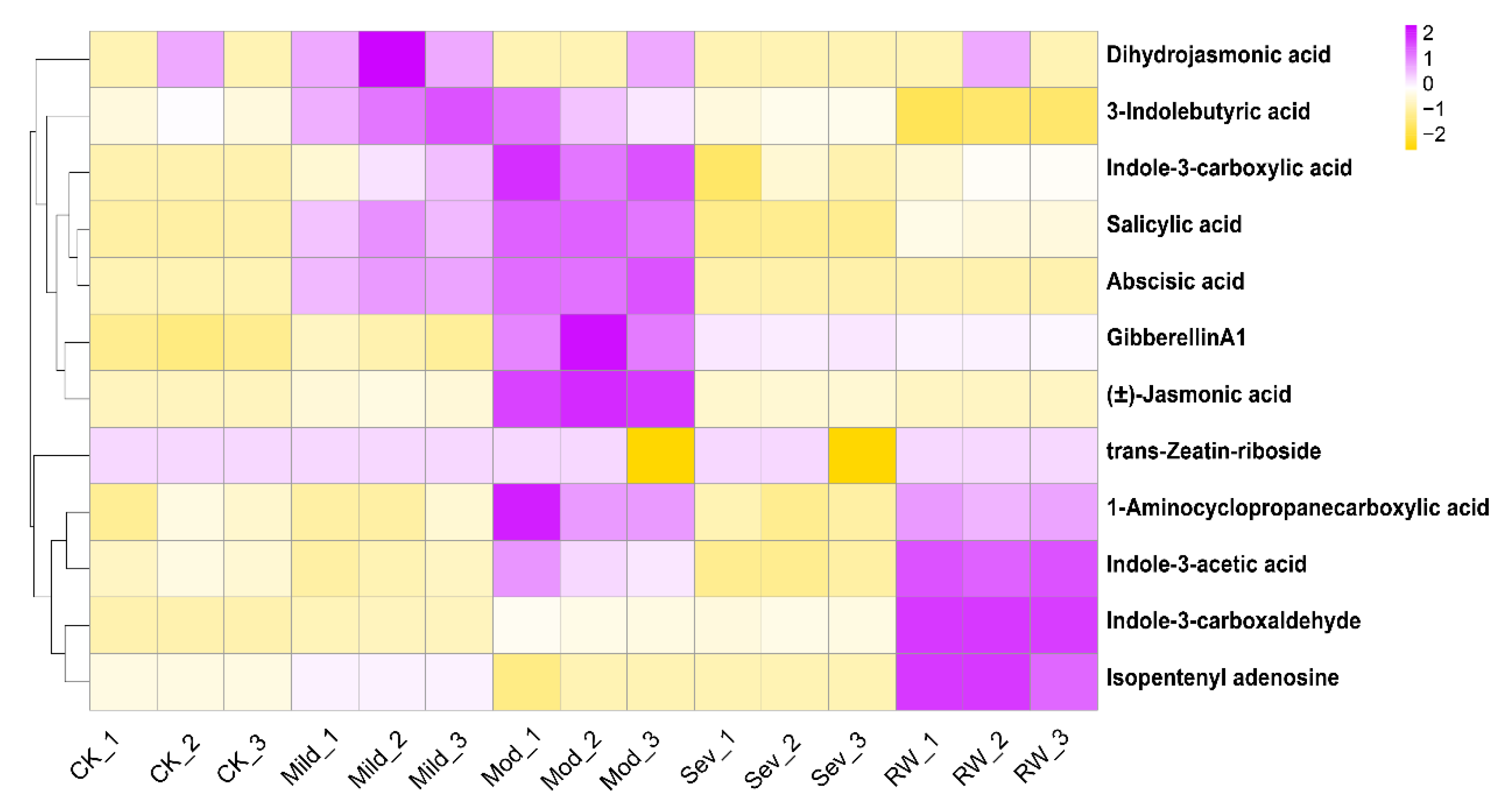
3.3. Metabolomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, C.C.; Bo, W.H.; El-Kassaby, Y.A.; Li, W. Transcriptome profiles reveal response mechanisms and key role of PsNAC1 in Pinus sylvestris var. mongolica to drought stress. BMC Plant Biol. 2024, 24, 343. [Google Scholar] [CrossRef] [PubMed]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.K. Chlorophyll fluorescence imaging for early detection of drought and heat stress in strawberry plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Gaecía-López, J.V.; Redondo-Gómez, S.; Flores-Duarte, N.J.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Mateos-Naranjo, E. PGPR-based biofertilizer modulates strawberry photosynthetic apparatus tolerance responses by severe drought, soil salinization and short extreme heat event. Plant Stress 2024, 12, 100448. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.L.; Shan, C.J. Effects of selenomethionine on the antioxidative enzymes, water physiology and fruit quality of strawberry plants under drought stress. Hortic. Sci. 2022, 49, 10–18. [Google Scholar] [CrossRef]
- Űnal, N.; Okatan, V. Effects of drought stress treatment on phytochemical contents of strawberry varieties. Sci. Hortic. 2023, 316, 112013. [Google Scholar] [CrossRef]
- Guo, L.L.; Lu, S.X.; Liu, T.; Nai, G.J.; Ren, J.X.; Gou, H.M.; Chen, B.H.; Mao, J. Genome-wide identification and abiotic stress response analysis of PP2C gene family in woodland and pineapple strawberries. Int. Mol. Sci. 2023, 24, 4049. [Google Scholar] [CrossRef] [PubMed]
- Han, J.X.; Li, X.G.; Li, W.H.; Yang, Q.; Li, Z.H.; Cheng, Z.; Lv, L.; Zhang, L.H.; Han, D.G. Isolation and preliminary functional analysis of FvlCE1, involved in cold and drought tolerance in Fragaria vesca through overexpression and CRISPR/Cas9 technologies. Plant Physiol. Biochem. 2023, 196, 270–280. [Google Scholar] [CrossRef]
- Yu, Z.P.; Duan, X.B.; Luo, L.; Dai, S.J.; Ding, Z.J.; Xia, G.M. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Cao, Q.; Huang, L.; Li, J.M.; Qu, P.; Tao, P.; Crabbe, M.; James, C.; Zhang, T.C.; Qiao, Q. Integrated transcriptome and methylome analyses reveal the molecular regulation of drought stress in wild strawberry (Fragaria nilgerrensis). BMC Plant Biol. 2022, 22, 613. [Google Scholar] [CrossRef] [PubMed]
- Halperin, O.; Gebremedhin, A.; Wallach, R.; Moshelion, M. High-throughput physiological phenotyping and screening system for the characterization of plant-environment interactions. Plant J. Cell 2017, 89, 839–850. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, J.X.; Ding, L.; Liu, X.R. Visualized analysis of the research progress and trend of lysimeter in the measurement of evapotranspiration based on CiteSpace. Tech. Superv. Water Resour. 2023, 241–248. [Google Scholar] [CrossRef]
- Wei, S.W.; Zhang, L.; Huo, G.T.; Ge, G.J.; Luo, L.J.; Yang, Q.C.; Yang, X.; Long, P. Comparative transcriptomics and metabolomics analyses provide insights into thermal resistance in lettuce (Lactuca sativa L.). Sci. Hortic. 2021, 289, 110423. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.Y.; Chen, G.P.; Lu, X.; Wei, R.N.; Xu, L.; Feng, X.J.; Yang, X.M.; Chai, Q.; Zhang, X.C.; et al. New insights into the occurrence of continuous cropping obstacles in pea (Pisum sativum L.) from soil bacterial communities, root metabolism and gene transcription. BMC Plant Biol. 2023, 23, 226. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Jia, X.L.; Wang, W.L.; Jin, Y.M.; Liu, W.Z.; Wang, D.M.; Mao, Y.X.; Xie, C.T.; Liu, T. Floridean Starch and Floridoside Metabolic Pathways of Neoporphyra haitanensis and Their Regulatory Mechanism under Continuous Darkness. Mar. Drugs 2021, 19, 664. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, S.W.; Liu, B.; Guo, D.D.; Zheng, B.X.; Feng, L.; Liu, Y.M.; Francisco, A.T.B.; Luo, L.J.; Huang, D.F. A novel integrated non-targeted metabolomic analysis reveals significant metabolite variations between different lettuce (Lactuca sativa L) varieties. Hortic. Res. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J.G. MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Galli, V.; Rafael, D.S.M.; Perin, E.C.; Borowski, J.M.; Bamberg, A.L.; Rombaldi, C.V. Mild salt stress improves strawberry fruit quality. LWT-Food Sci. Technol. 2016, 73, 693–699. [Google Scholar] [CrossRef]
- Dong, C.; Xi, Y.; Chen, X.; Cheng, Z.M. Genome-wide identification of AP2/EREBP in Fragaria vesca and expression pattern analysis of the FvDREB subfamily under drought stress. BMC Plant Biol. 2021, 21, 295. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Khan, M.Q.N.; Sevgin, N.; Rizwana, H.; Arif, N. Exogenous melatonin mitigates the adverse effects of drought stress in strawberry by upregulating the antioxidant defense system. S. Afr. J. Bot. 2023, 162, 658–666. [Google Scholar] [CrossRef]
- Anderson, J.T.; Mitchell-Olds, T. Ecological genetics and genomics of plant defenses: Evidence and approaches. Funct. Ecol. 2011, 25, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant metabolomics in biotic and abiotic stress: A critical overview. Phytochem. Rev. 2021, 21, 503–524. [Google Scholar] [CrossRef]
- Wei, T.L.; Wang, Y.; Xie, Z.Z.; Guo, D.Y.; Chen, C.W.; Fan, Q.J.; Deng, X.D.; Liu, J.H. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol. J. 2018, 17, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.Q.; Xia, Y.H.; Ma, C.; Zhu, G.X.; Wang, Z.C.; Tu, Q.; Chen, X.B.; Wu, J.S.; Su, Y.R. Response of organic carbon mineralization of nitrogen addition in micro-aerobic and anaerobic layers of paddy soil. Huan Jing Ke Xue 2023, 44, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Plant Stress Responses 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Carmen, J.M.; Andrea, M.M.P.; Pablo, M.G.; Manuel, R.; Antonio, H.J.; Gregorio, B.E.; Pedro, D.V.; José, M.G.P. Comprehensive study of the hormonal, enzymatic and osmoregulatory response to drought in Prunus species. Sci. Hortic. 2024, 326, 112786. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Sun, Y.; Wang, W.M.; Xie, Z.Z.; Zhan, C.H.; Jin, L.; Huang, J.L. OsJAZ10 negatively modulates the drought tolerance by integrating hormone signaling with systemic electrical activity in rice. Plant Physiol. Biochem. 2024, 211, 108683. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Song, R.; Wang, X.; Wang, J.; Wu, C. Transcriptomic and Metabolomic Analyses Provide New Insights into the Response of Strawberry (Fragaria × ananassa Duch.) to Drought Stress. Horticulturae 2024, 10, 734. https://doi.org/10.3390/horticulturae10070734
Jiang L, Song R, Wang X, Wang J, Wu C. Transcriptomic and Metabolomic Analyses Provide New Insights into the Response of Strawberry (Fragaria × ananassa Duch.) to Drought Stress. Horticulturae. 2024; 10(7):734. https://doi.org/10.3390/horticulturae10070734
Chicago/Turabian StyleJiang, Lili, Ruimin Song, Xiaofang Wang, Jie Wang, and Chong Wu. 2024. "Transcriptomic and Metabolomic Analyses Provide New Insights into the Response of Strawberry (Fragaria × ananassa Duch.) to Drought Stress" Horticulturae 10, no. 7: 734. https://doi.org/10.3390/horticulturae10070734
APA StyleJiang, L., Song, R., Wang, X., Wang, J., & Wu, C. (2024). Transcriptomic and Metabolomic Analyses Provide New Insights into the Response of Strawberry (Fragaria × ananassa Duch.) to Drought Stress. Horticulturae, 10(7), 734. https://doi.org/10.3390/horticulturae10070734




