1. Introduction
Hami melons (
Cucumis melo var.
saccharinus) are favored by consumers due to their sweetness, crispness, and nutrition. As a climacteric fruit, Hami melons are highly perishable and should be sold in a short period of time after harvesting [
1]; however, poor markets and a surplus phenomenon that results from the short harvesting period and high harvesting temperatures of Hami melons constitute a serious challenge.
According to European Community standards, fresh-cut fruits are made ready for consumption after washing, peeling, slicing, and packaging. The European and US markets have shown a clear preference for fresh-cut fruits, accounting for more than 10% of total fruit and vegetable sales [
2]. Currently, the demand for fresh-cut fruits in China is also growing rapidly. Fresh-cut processing will cause substantial mechanical injury, biosynthesis of secondary metabolites [
3], and microbe infections [
4]. Various methods are available to retain the quality parameters and prolong the shelf-life of fresh-cut fruits. Disinfecting agents, such as chlorine, organic acids, and ozone, have been used during washing to avoid microbial contamination [
5] but may reduce the fruit flavor and are usually accompanied by hazardous chemical emissions. Active packaging, including edible films, coatings, and modified atmospheres, can extend the shelf-life by inhibiting microbial growth [
6,
7,
8]. For decades, the effectiveness of post-processing treatments, such as pulsed light [
9] and ultrasound processing [
10], on the reduction of microbial growth and counts, and improving quality has been successfully verified. The main difference between fresh-cut and intact produce is food safety [
5]. From an industrial perspective, emerging technology, especially non-thermal processing technology, is very important in maintaining the shelf-stability and safety of fresh-cut fruits.
Cold plasma (CP) is a non-thermal technology with economical, versatile, and environmentally friendly advantages. The technology has gained significant interest for applications in the food industry as an alternative to traditional thermal processing techniques. It shows diverse applications in shelf-life extension, disinfection and sterilization, degradation of pesticide residue, toxin inactivation, and improvement in food safety [
11]. CP has shown effectiveness against various fungi and bacteria by causing morphologic alteration, membrane disruption, intracellular-substance leakage, and DNA damage [
11,
12]. CP for the sterilization of fresh-cut fruits provided up to a 3 log reduction in bacteria [
12,
13,
14,
15]. This technology can achieve microbial decontamination with minimal effects on the quality and nutrition [
13]. It can be used with several fruits, such as melons, bananas, strawberries, etc. [
15,
16,
17]. For fruits and vegetables, this technology can also reduce the activity of enzymes, which can cause browning, cell wall degradation, and antioxidants [
15,
18,
19]. It is necessary to promote the application of this novel technology in enhancing the shelf-stability of fresh-cut fruits. The efficiency of CP depends on the food type, surface area properties, initial microbial load, etc. [
11,
12,
13,
14,
15]. Therefore, the operational parameters of this technology require optimization to achieve desirable results.
Hami melons are mainly consumed fresh and have become a popular fruit for making fresh-cut fruits. There is a need to seek a convenient and effective sterilization technology to ensure quality and safety to, consequently, avoid any potential outbreak due to the consumption of fresh-cut Hami melons. Cold plasma has been proven effective for fresh-cut fruits’ decontamination [
13]; however, the CP voltage used in the previous study was low (about 15 kV), and the treatment time was over 30 min [
15]. Due to the long time and high energy consumption, it is difficult to apply in the food industry. Therefore, this research studied the preservation effect of CP treatment on fresh-cut Hami melons with high voltage and short-term treatment. The quality, enzyme activity, microbiological count, and flavor of fresh-cut Hami melons during storage at 4 °C were investigated after CP treatment at 120 kV and 130 Hz for 150 s.
2. Materials and Methods
2.1. Fruit Material and Treatment
Hami melons (Cucumis melo cv. Jinsenianhua) at commercial mature stage were harvested from a experimental orchard in Turpan, Xinjiang province. After washing, Hami melons were cut into long strips of uniform size with sterilized knives and randomly divided into two groups. Fresh-cut Hami melons were packaged in polypropylene trays with plastic film. One group was treated by a cold plasma generator (CPS-I, YI RUN, Nanjing YI RUN Plasma Technology Co., Ltd., Nanjing, China). The parameters were as follows: voltage 120 kV, frequency 130 Hz, distance 60 mm, treatment time 150 s. The untreated and CP-treated samples were stored at 4 °C and measured at 0, 2, 4, 6, and 8 d. The above experiment was repeated three times.
2.2. Determination of pH
Five grams of the sample was grated into juice with a juice extractor (L12-Energy61 Joyoung, Joyoung Co., Ltd., Shanghai, China) and the pH was measured with a handheld portable pH meter (PHS-25, LEI-CI, Shanghai Yidian Scientific Instrument Co., Ltd., Shanghai, China).
2.3. Colorimeter Measurement
The ends and the middle of the strips of Hami melons were measured by colorimeter measurement (NR110, 3 nh, Guangdong 3 nh Intelligent Technology Co., Ltd., Guangzhou, China) using the D65 illuminant with a 4-millimeter-diameter measuring aperture.
2.4. Texture Measurement
The Hami melons were cut into 2 cm × 2 cm squares. The samples were punctured by a texture analyzer (TLP, Food Technology Corporation, Atlanta, Georgia, USA) with the following parameters: a 5-millimeter-diameter probe, pre-test speed of 60 mm/min, mid-test speed of 60 mm/min, post-test speed of 60 mm/min, and puncture depth of 10 mm. Each sample consisted of three replicates, and the experiment was conducted twice.
2.5. Total Soluble Solids Content (TSS) Measurement
The Hami melons were cut into 5-gram pieces and juiced. The TSS of Hami melon juice was measured with a handheld portable sugar meter (OUSURE, Hengshui OUSURE Trading Co., Ltd., Hengshui, China).
2.6. Determination of Catalase (CAT) Activity
Five grams of the sample was mixed with 5.0 mL of PBS (0.2 M, pH 7.8), centrifuged at 4 °C, 10,000× g for 10 min. Two small test tubes were taken for the sample tube and the blank control tube. The reaction system in the blank control tube included 1.5 mL PBS (0.2 M, pH 7.8), 0.1 mL enzyme extract, 0.2 mL distilled water, 1.0 mL 8% sulfuric acid (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China), and 0.2 mL 0.75% hydrogen peroxide solution (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China). The reaction system in the sample tube was composed of 1.5 mL PBS (0.2 M, pH 7.8), 0.1 mL solution, 0.1 mL enzyme extract, 1.2 mL distilled water. In order to start the reaction, 0.2 mL hydrogen peroxide solution was added into the sample tube. One milliliter of 8% sulphuric acid was added to the sample tube to terminate the reaction after 4 min. The reaction was then adjusted to zero at 240 nm with distilled water and the absorbance was measured in the sample tube and control tube. One unit of CAT activity was defined as the amount of enzyme that produced a decrease In A240 by 0.01 per min.
2.7. Determination of Polyphenol Oxidase (PPO) Activity
Five grams of the sample was added into 5.0 mL of phosphate buffer (PBS, 0.2 M, pH 5.4) and then centrifuged at 4 °C for 10 min at 10,000× g. Three milliliters of the reaction system contained 1.9 mL of 0.2 M pH 6.5 PBS buffer, 1.0 mL of 0.1 M catechol (Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China), and 0.1 mL of the enzyme solution. The reaction system was warmed up at 30 °C for 5 min. The change in absorbance value was measured at 410 nm and recorded every 60 s. One unit of POD activity was defined as the amount of enzyme that produced an increase in A410 by 0.01 per min.
2.8. Determination of Peroxidase (POD) Activity
For POD activity determination, five grams of the sample was added with 5.0 mL of acetate buffer (0.2 M, pH 5.4) and centrifuged at 4 °C, 10,000× g for 10 min. The reaction system contained 2 mL of acetate buffer (0.2 M, pH 5.4), 1 mL of 3% guaiacol (Guangdong Guanghua Sci-Tech Co., Ltd. Guangzhou, China), 0.1 mL of 0.75% H2O2, 0.1 mL of enzyme solution. The absorbance value was measured at 460 nm and recorded every 30 s. One unit of POD activity was defined as the amount of enzyme that produced an increase in A460 by 0.01 per min.
2.9. Measurement of Total Microbial Counts
Ten-gram samples were clipped in 90 mL of sterile saline and mixed with an aseptic homogenizer for 5 min. A series of diluted bacterial suspensions were allocated to plates containing agar medium (PCA) at 30 °C, and the total number of the bacterial colonies was counted after 48 h of incubation.
2.10. Electronic Nose
The flavor substances of Hami melons were measured using an electronic nose (Airsense Pen3, AIRSENSE Analytics GmbH, Schwerin, Germany). The samples were cut into small pieces that weighed 2 g. The samples were incubated for half an hour in a headspace vial at room temperature. Determination of the samples was carried out using an electronic nose with the following parameters: sample interval 5 s, sampling time 150 s, cleaning time 80 s, gas flow 400 mL/min, injection flow 200 mL/min.
2.11. Statistical Analyses
All data are expressed as the mean values ± standard deviations (SD). Statistical analyses were performed using SPSS 25.0 software (Chicago, IL, USA). An independent t-test was used to compare the means of two groups at the same time. A one-way analysis of variance followed by Duncan’s multiple range test was performed to test the difference between different storage times. A p value of < 0.05 was considered statistically significant.
3. Results
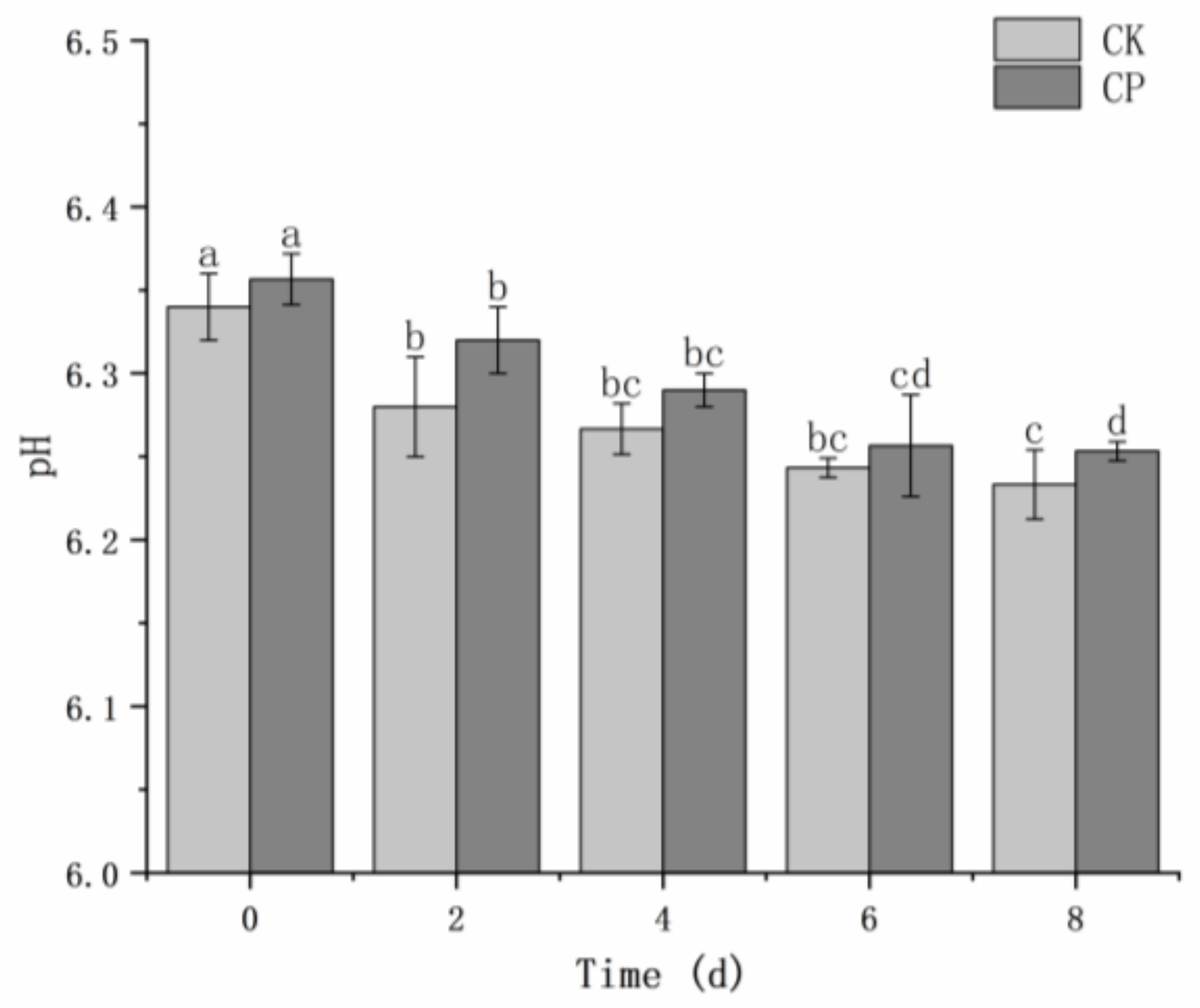
The pH of fresh-cut Hami melons slightly decreased during storage (
Figure 1). The pH value of the CP-treated group was higher than that of the untreated group but there was no statistical difference (
p > 0.05) between the two groups. The average initial pH value of fresh-cut Hami melons was 6.35, and the value approximately decreased by 0.1 after 8 days of storage.
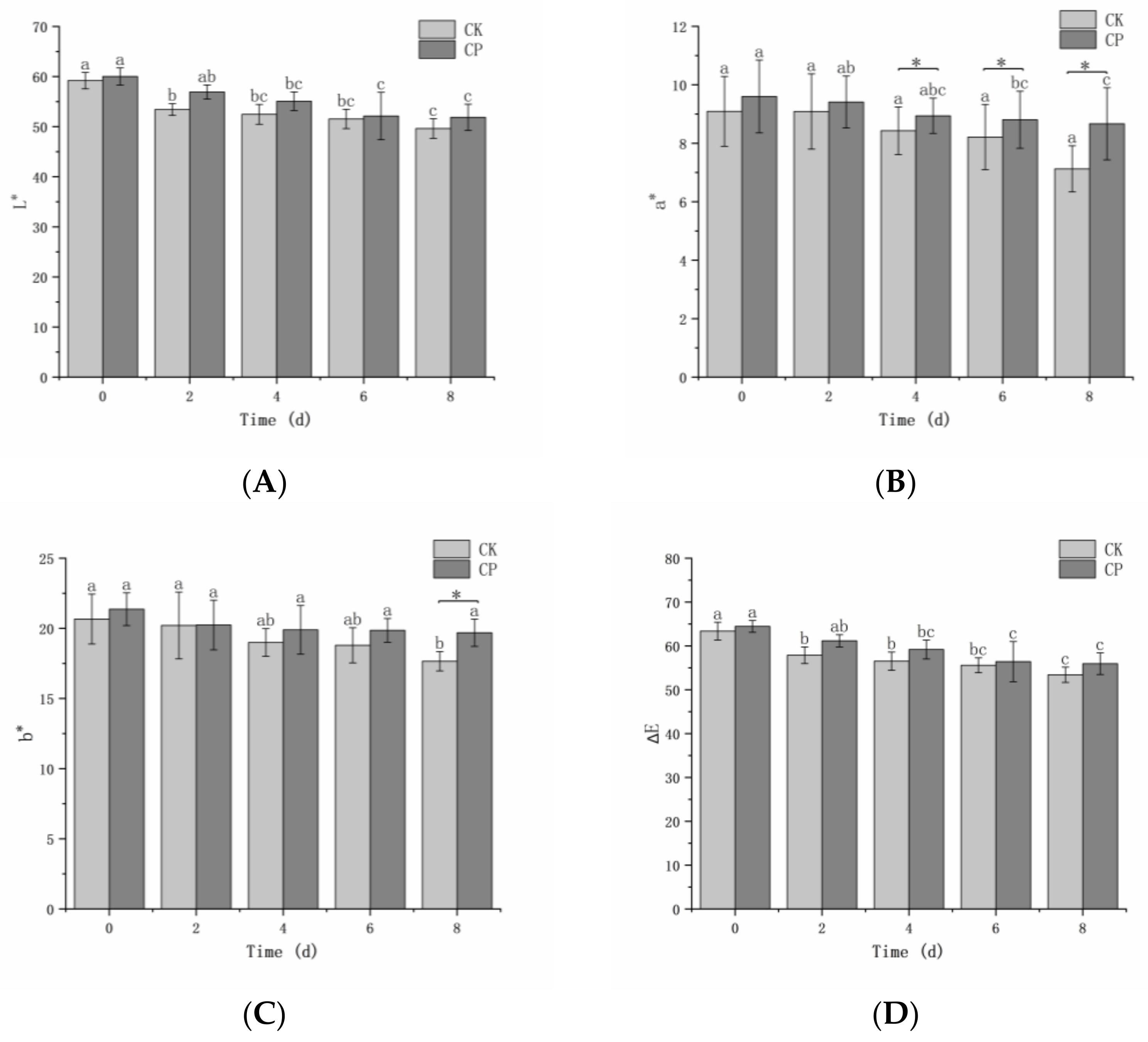
The L*, a*, b*, and ∆E of fresh-cut Hami melons gently decreased (
Figure 2). During storage, the average L*, a*, b*, and ∆E of all samples decreased from 59.60 to 50.75, from 9.35 to 7.90, from 21.02 to 18.67, and from 63.91 to 54.67, respectively. There were no significant differences (
p > 0.05) in L*, b*, and ∆E values between the two groups during 0–6 days of storage. The a* value of the PC-treated group was significantly lower (
p < 0.05) than that of the untreated group after 4 d.
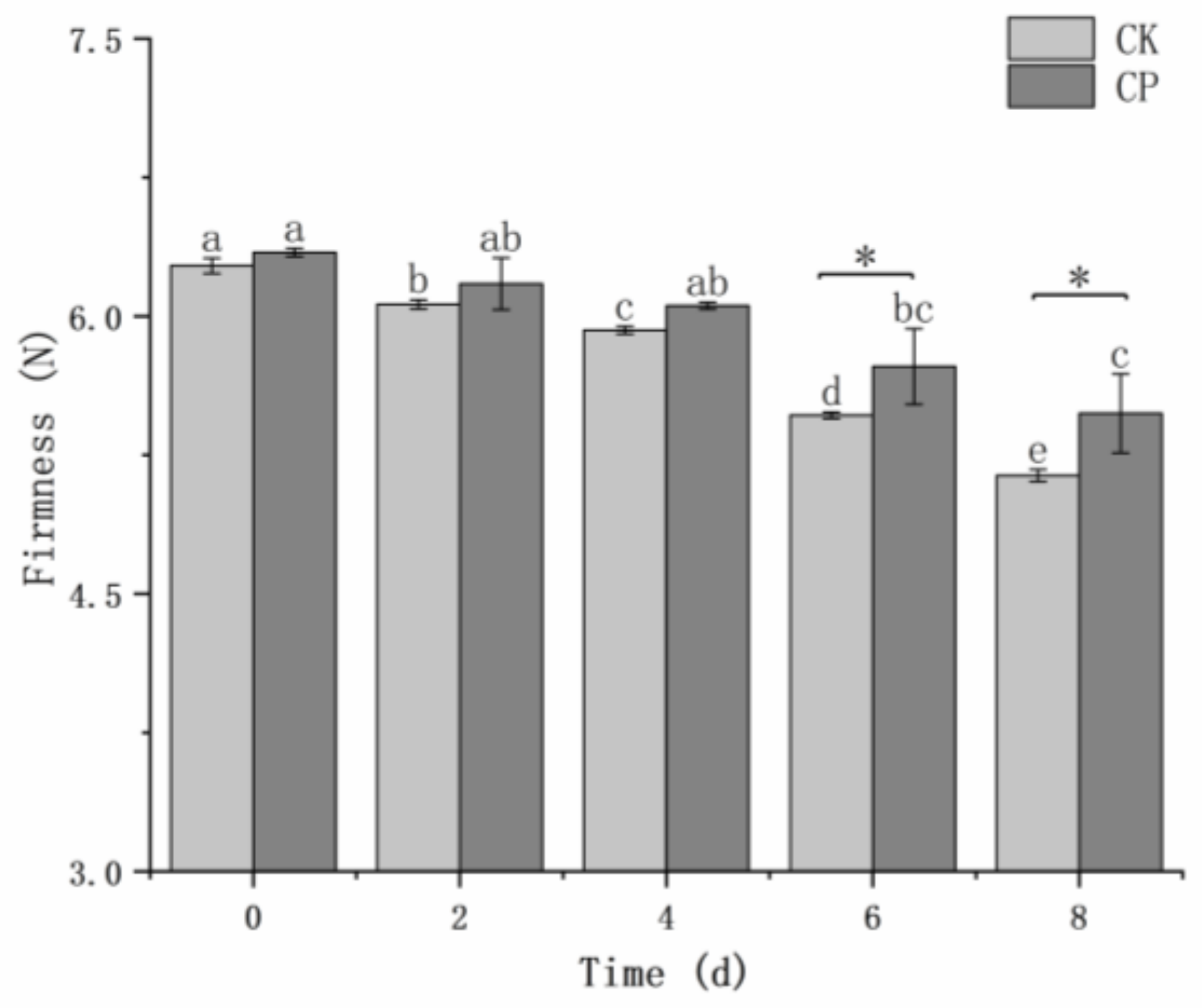
Figure 3 shows the change in firmness of fresh-cut Hami melons during storage. There was no significant difference (
p > 0.05) in the firmness of the two groups until 6 and 8 d. The firmness significantly decreased (
p < 0.05) during storage. The firmness of the untreated and CP-treated samples declined by 1.13 and 0.87, indicating that the change in the CP group is low.
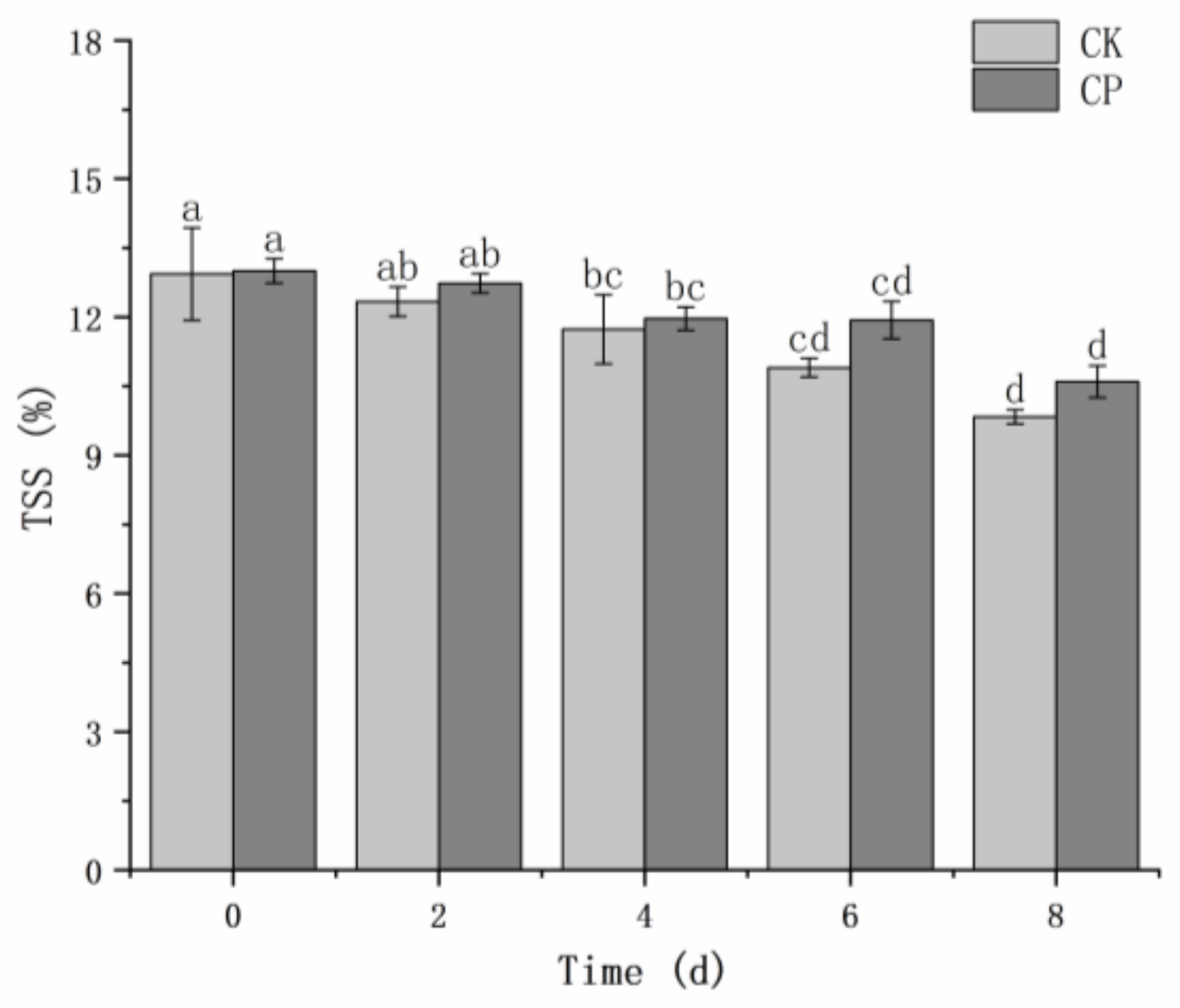
No significant difference (
p > 0.05) was observed in the soluble solids content between the two groups (
Figure 4). The soluble solids content decreased across storage time. The initial soluble solids content (0 d) was 12.97% on average. After 8 days of storage, the soluble solids content of the untreated and CP-treated samples reduced by 3.10% and 2.41%, respectively.
Figure 5 shows the activity changes of three oxidation enzymes. Although the change in enzyme activity of the untreated group was similar to that of the CP-treated group, there is a significant difference (
p < 0.05) between the two groups at the same storage times. CP treatment induced a remarkable increase in CAT activity (
Figure 5A) but decreased the POD activity (
Figure 5C). The CAT and PPO activities dramatically increased (
p < 0.05) during the first 6 days of storage, and then significantly decreased (
p < 0.05) at 8 d, while the POD activity gradually increased during the 8 days of storage. Compared to the untreated group, the CAT activity in the CP-treated group was 17% higher on average. The POD activity of the CP group showed a significantly lower (
p < 0.05) level (about 11% lower on average) than that of the CK group during the 8 days of storage, while the PPO activity of the CP-treated group was significantly lower (
p < 0.05) within 4–8 days of storage.
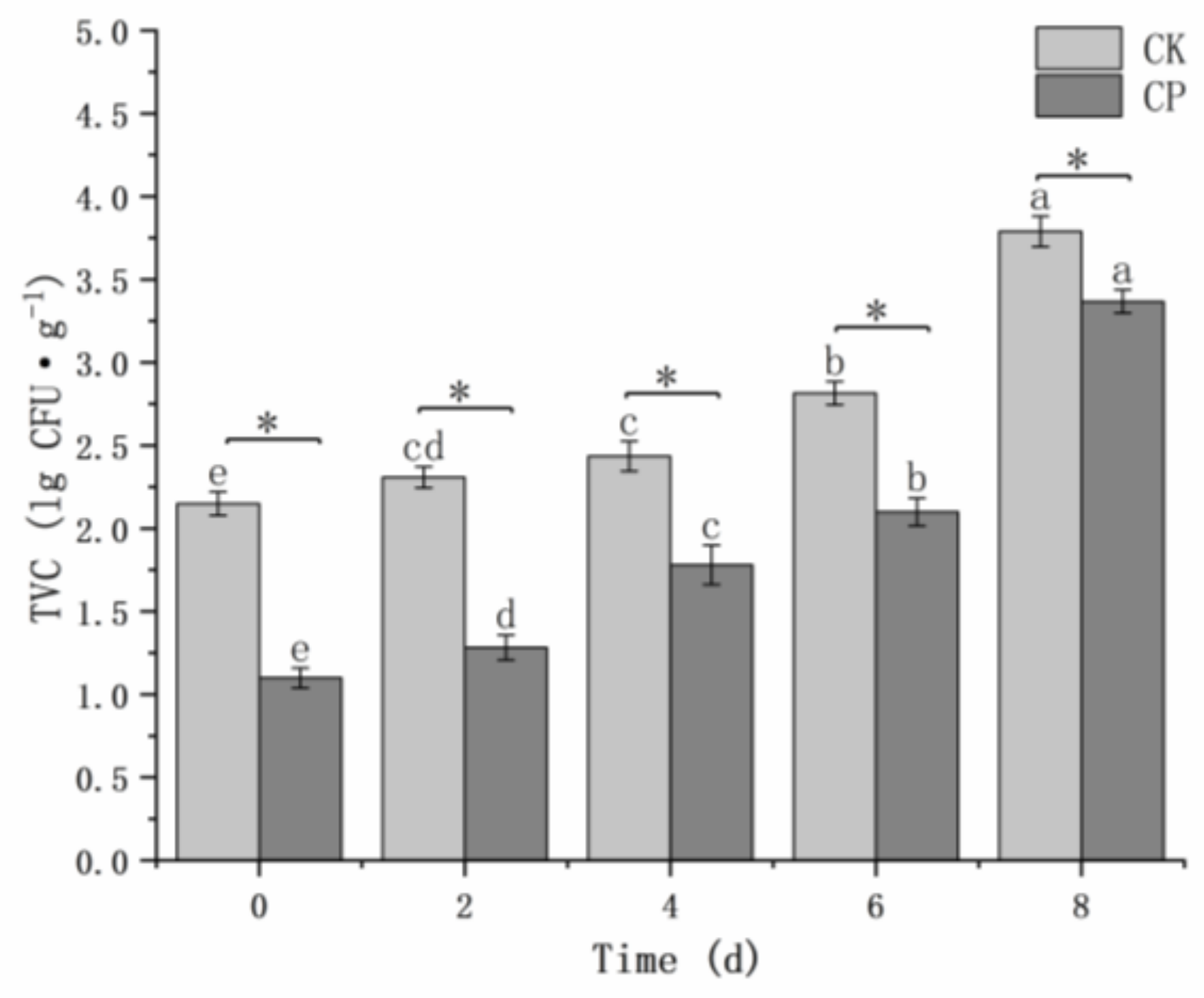
The TVC of the CP-treated group was significantly (
p < 0.05) lower than that of the untreated group (
Figure 6). For the CP-treated sample, TVC values were 1.06 log lower than the untreated sample at 0 d, indicating that the CP treatment conditions used can effectively reduce microbial contamination. The bacterial levels of all fresh-cut Hami melons increased gradually with storage time. The difference between the two groups was 0.42 log after 8 days of storage.
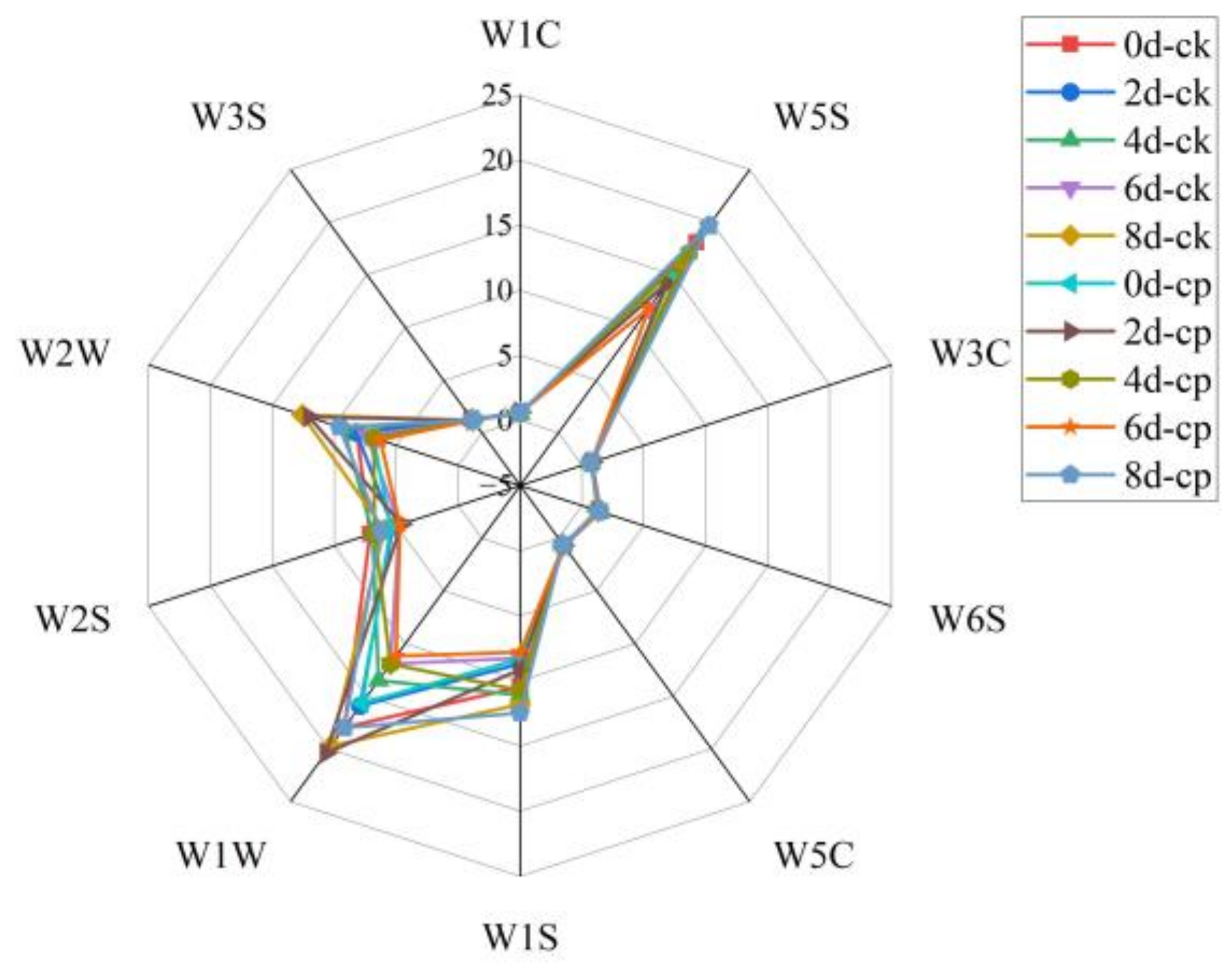
The electronic nose responses to the fresh-cut Hami melons are shown in
Figure 7. The radar map showed that response values of electronic nose probes can be divided into three levels: greater than 10, between 5 and 10, and less than 5. The response values of W1W (sensitive to sulphur) and W5S (sensitive to ketones and terpenes) exceeded 10. The signals of the sensors W1S, W2S, and W2W (sensitive towards polar and broad-range compounds, alcohols, and aromatic and sulphur organic compounds, respectively) almost ranged from 5 to 10. On the contrary, the sensors sensitive to aromatic compounds, methane, hydrogen, aldehydes, ketones, and less-polar compounds (W1C, W3S, W6S, W3C, and W5C) had a low response value (below 1.5). After CP treatment (0 d), the only values of W1W, W5S, W1S, and W2W increased, indicating that CP treatment had an impact on flavor substances, especially for substances with high content. At the end of storage (8 d), only the values of W1W and W2W in the CP-treated group were lower than those in the untreated group, suggesting the adverse odor caused by CP treatment was limited. The signal trend in the two groups was similar. During storage, the signal intensity of W1W and W5S showed the largest change followed by sensors W1S and W2W but other sensors changed little.
4. Discussion
In practice, there are four types of CP based on the source of generation, including dielectric-barrier discharge plasma, corona discharge plasma, radiofrequency discharge plasma, and microwave discharge plasma. A piece of equipment with a dielectric-barrier discharge plasma source was used in the present study. This equipment has application prospects in the food industry due to its relative simplicity and ability to be applied to different types of gases [
18,
20].
In this study, pH, color, and firmness indicators showed that CP treatment can effectively maintain the natural quality of fresh-cut Hami melons. The pH is related to chemical changes during fruit ripening, and with the growth of acid-producing bacteria during storage, etc. Previous studies reported that the effect of CP on pH varied among fruits. For example, CP treatment significantly reduced the pH of bananas [
16] but did not affect strawberries [
17] or mandarins [
21]. Misra et al. [
22] reported that the increment effect of CP on pH was inversely related to the treatment time; in this study, pH was not affected by CP treatment, possibly due to the short treatment time (150 s).
The inhibition effects in softening and nutritional loss are essential to extending the shelf-life of fruit. The firmness reduction indicates the fruit tissue is suffering from softening deformation due to the damage to plant cell walls. The reduction in soluble solids reflects the loss of nutrients in the fruit, which is related to the aging processes of respiratory climacteric fruits. The present study showed that the CP treatment conditions used do not result in apparent cell damage but the inhibition effects in softening and nutritional loss were limited. A previous study showed that the effects of CP in inhibiting softening and maintaining nutrition were associated with a lowering of respiration rates, inactivation of cell-wall-degrading enzymes, and a reduction in pathogens that attack plant cell walls [
23]. As a climacteric fruit, Hami melons need to inhibit softening and delay the aging process to enhance postharvest preservation. Further research can investigate the effects of CP treatments on physiological aspects of fruit ripening.
Color is related to pigments with antioxidant capacity, and its change is mainly due to enzymatic browning [
24]. CP treatment can effectively maintain the color of fresh-cut Hami melons, which may be attributed to the denaturation and inactivation of enzymes that play an important role in fruit browning [
25,
26]. CAT, PPO, and POD, as antioxidant enzymes in the defense system, are considered to be associated with free radical scavenging and postharvest browning [
27]. High levels of antioxidant enzymes have been shown to be responsible for chilling injury, cell senescence, and membrane damage in fruits [
28]. The impact of CP treatment on CAT, PPO, and POD was different due to the fact that the inactivation effect of CP on oxidase activity is related to the spatial conformation of the enzyme [
29]. The initial activities of CAT and POD of the Hami melons were enhanced in agreement with previous studies, which proved that the CAT activity in blueberry was enhanced by CP treatment [
19]. During storage, the increment of three enzymes in PC-treated Hami melons was inhibited, suggesting that CP treatment substantially improved the antioxidant capacities of fresh-cut Hami melons. The reduction effect on enzyme activity was attributed to the alteration of protein structure and conformation by the UV photons and reactive oxygen species (ROS) produced by gas plasma [
30].
The microbial contamination of fresh-cut fruits can occur during cutting, shredding, slicing, and storage [
13]. In this experiment, polythene bags were used to prevent the attachment of environmental microorganisms during storage. Therefore, the microorganisms in the fruit flesh were mainly due to the transfer of microflora from the peel surface and cutting tools. In the present study, the preservation effect of CP treatment on fresh-cut Hami melons occurred mainly by the reduction in TVC. The high-voltage and short-time CP treatment can effectively reduce the initial bacterial count on the surface of fresh-cut fruits but the reduction was lower than the literature data, which found 2 log reductions after CP treatment at 15 kV and 12.5 kHz for 1 h [
15].
Thermal processing technology is common in the food industry but it is difficult to apply in fruit processing due to the fact that fruits, such as melon, produce off-odors when cooked [
31]. An electronic nose was used to monitor the freshness and quality of fresh-cut Hami melons due to its advantages of fast response, high sensitivity, and low cost. Electronic nose signals, which respond to aroma intensity, have a potential correlation with the fruit maturity stages [
32,
33]. In this experiment, Hami melons at 80% maturity were used, which are commonly used in commercial transportation. This study confirms that CP treatment can affect flavor, due to the fact that the food was in contact with the plasma discharge, which enhanced the interactions between food and reactive species [
14]. The results showed that only four electronic nose response values changed, indicating that CP treatment affected less flavor substances than thermal processes. The change in the aroma pattern of fruit is related to oxygen levels and fermentative metabolites [
34]. After 8 days of storage, CP treatment exerted a better retention of Hami melon flavor, suggesting CP inhibited the generation of fermentation products.
5. Conclusions
Disinfecting agents and thermal processes make it difficult to achieve the preservation of fresh-cut fruits due to their adverse effects on flavor and quality. CP is a novel non-thermal technology that can be used for sterilization; however, the voltage of CP used in previous research was too low and the sterilization time was too long. In this study, the preservation effect of high-voltage and short-time CP treatment on fresh-cut Hami melons was evaluated based on physicochemical properties, enzyme activity, microbial population, and flavor. This study shows that the CP treatment had no significant effect on the physicochemical quality and electronic nose responses, indicating that it is useful in sensitive fruits. This technology showed a positive effect on the inhibition of oxidation and the reduction in microbial contamination of fresh-cut Hami melons during storage. Thus, CP has the potential to prolong shelf-life and does not affect the natural quality characteristics of fresh-cut fruits. Moreover, the CP processing time studied in this article is very short, only 150 s, which is beneficial for application in the food industry. The present study is helpful for the development and application of non-thermal sterilization technology in the preservation of thermosensitive fruits.