1. Introduction
The most reliable way to extend the shelf life of vegetable products is preservation by drying when the concentration of water is reduced to a limit that creates unfavorable conditions for the growth, development, or reproduction of microorganisms. Therefore, dried products can be stored until the new season’s harvest or even longer [
1,
2]. Also, dried food products have the advantage of having a low volume and weight compared to raw materials [
3].
The conventional drying of fruits and vegetables is still the most commonly used drying method in the food industry; however, during this process, the quality of the vegetable raw materials decreases [
4]. According to researchers, the total phenolic and flavonoid content and the total antioxidant activity reduce after the drying process compared with fresh fruits and vegetables [
5,
6,
7,
8]. During conventional drying, the evaporation of capillary moisture causes the contraction of superficial tissues, which limits the smooth removal of water from deeper tissues. The level of drying temperature influences the physical properties of the products and their characteristics. According to Guldiken et al. [
5], the total phenolic content of red beetroot decreased by 65%, the total antioxidant activity decreased by 72–76%, and the betanin content decreased by 75% after a convection-drying process in comparison with fresh samples. Researchers have claimed that the high level of reduction in the betanin content in dried red beetroot could be linked to the low thermal stability of betalains [
5,
9].
Vacuum freeze-drying is one of the best water-removal methods for producing the highest-quality final products. Furthermore, the solid state of water during freeze-drying protects the primary structure and the shape of the material. As the process involves the direct transition from a solid to a gaseous state, without exposing the product to high temperatures, structural changes and shrinkage are largely avoided; therefore, the product structure remains porous, the lower temperatures in the process allow for maximal nutrient and bioactive compound retention, and the flavor, color, and appearance of the products are largely preserved [
10,
11,
12,
13]. The results from a study by Hung and Duy [
7] show that the total phenolic and flavonoid contents in red beetroot were significantly higher using the freeze-drying method than the conventional drying method. However, the increase in the drying temperature of up to 60 °C during the freeze-drying process might cause a decrease in the total phenolic content and the antioxidant activity in some vegetables [
13].
On the other hand, freeze-drying is a high energy-consuming process, with high capital costs, which limits its industrial application [
14]. According to a review by Nowak and Jakubczyk [
13], there are a lot of research results that have shown the effect of the process conditions on the physical properties of freeze-dried foods. This is important for food product color changes, indicating the degradation of pigments and some bioactive compounds [
13,
14]. Dhiman et al. [
15] stated that freezing as a pre-treatment process has been found to be the most effective method for reducing the drying time, which can affect the qualitative changes in food products.
One more drying method that is applied for the treatment of vegetable raw materials is spray-drying. Spray-drying is a process that is considered valuable in producing powders out of fluids [
16]. However, when using food material containing valuable oxidative compounds, spray-drying at high temperatures, using enormous amounts of air, is not as effective as freeze-drying. A significant increase in oxidation compounds and losses of product quality, by reducing the amount of nutrient compounds, is possible with this method. To improve the product quality obtained by the spray-drying process, while preserving bioactive compounds and reducing yield losses due to decomposition and stickiness, supplemental additives are recommended [
12]. According to Coy-Barrera [
17], spray-drying resulted in good yields of encapsulated betalains (90–98%, from different sources).
Beetroot (
Beta vulgaris L.) is a crop belonging to the
Chenopodiaceae family and is grown in many countries worldwide. It is a plant that is usually cultivated for its dark red and thick roots [
18]. Beetroot is a source of valuable betalain pigments, which consist of red-purple betacyanins and yellow-orange betaxanthins [
19,
20,
21]. The results of scientific studies have shown that environmental factors aid in the accumulation of betalain pigments and other bioactive components in beetroot. The level of sunlight, soil conditions, high temperatures, and the use of conventional, organic, or integrated farming systems could influence betalain biosynthesis, which has an impact on the health benefits of beetroot [
21,
22]. The benefits of betalains for humans are immeasurable, because this compound acts as an antioxidant that destroys disease-causing free radicals in the body, suppresses inflammation, and has a detoxifying effect [
17,
21,
23]. As Wiczkowski et al. [
24] indicated, the decrease in the amount of betalains may be affected and triggered by the aging process of red beetroots during storage.
Beetroot can be used raw, boiled, roasted, and pickled, as well as for juice extraction [
5,
25,
26]. Nowadays, the increasing popularity of products processed using beetroot can be observed in the different products available, such as crunchy beetroot slices sold as convenience food, or ready-to-eat beetroot produced by means of microwave vacuum drying [
24]. However, it is essential for the food industry to extract pigments from beetroot that can be used as natural food colorings and that allow for the production of food products without artificial colorants [
15,
19].
The most important problem when processing raw beetroots is color changes. Compared to other plant pigments such as carotenoids, betalains are less resistant to high temperatures, pH, light, oxygen, water activity, metal ions, and enzymatic action [
17]. Long-term treatment at high temperatures promotes betalain degradation [
17,
27].
The aim of this research was to analyze the influence of different drying methods on the chemical composition and color of dried beetroot powder.
3. Results and Discussion
The difference in soluble-solid content among raw beetroots was statistically significant across all cultivars. The highest content was determined in the cultivar ‘Kestrel’ at 14.90%, while the lowest was observed in ‘Jolie’ at 11.30%. The content in ‘Scarlet’ was 14.77% (
Table 1).
The dry matter content in beetroot powder was mainly influenced by the drying method rather than the beetroot cultivar (
Table 2). The highest dry matter content was observed in the spray-dried beetroot powder across all cultivars. Dried material when its dry matter content is higher than 85–90% can be referred to as powder [
36]. However, neither conventional drying nor freeze-drying had a significant effect on the dry matter content in the same cultivar beetroot powder.
The results obtained for the total phenolic content varied according to the drying method used and the cultivar (
Table 3). The highest amount of phenols was obtained in the vacuum freeze-dried ‘Scarlet’ cultivar and spray-dried ‘Jolie’, while the lowest was in conventionally dried ‘Jolie’ and ‘Scarlet’ cultivars beetroot powder. Vacuum freeze-drying resulted in a higher amount of phenols only in the ‘Scarlet’ cultivar beetroot powder, while the others did not show such a tendency. The amount of total phenols determined in dried beetroots in the present study was lower than that of the ethanol extracts of whole beetroot (55.00 ± 1.41 mg GAE g
−1) reported in the study of Rangani and Ranaweera [
37]. According to Kujala et al. [
31], the amount of total phenols in the beetroot depends on the plant part tested and ranges from 4.2 to 15.5 mg GAE g
−1. Vasconsellos et al. [
38] revealed that total phenols in beetroot chips (freeze-dried beetroots) reached 0.75 GAE mg·g
−1, beetroot powder (spray-dried beetroot juice)—0.51 GAE, while in fresh beetroot juice and cooked beetroots, 3.67 and 2.79 mg·GAE g
−1, respectively. However, no significant difference was observed between the total phenol content of beetroot chips and powder. In the present study, the total phenol content was significantly different in conventionally dried, spray-dried and vacuum freeze-dried ‘Scarlet’ cultivar beetroots. The lower amount of total phenols in conventionally dried beetroot may be related to the longer exposure to temperature but not with the loss of water during the drying process, whereas phenolics are hydrosoluble compounds that may have been lost with water [
38].
Betalains, carotenoids and anthocyanins are the main beetroot pigments [
39] where the amount of betalains reaches 606 mg 100 g
−1 [
40]. The amount of carotenoids and anthocyanins in beetroot is very low, and it can be concluded that more than 99.0% of the pigments in the beetroot are betalains, which have the same chemical properties, biological function and color spectrum as anthocyanins [
41,
42]. However, some scientists report that betalains and anthocyanins are never found together in beetroot [
43].
Betalains are nitrogenous water-soluble pigments, and their content in beetroot varies depending on the cultivar, root part (peel or inner part of the beetroot), growing conditions, and processing methods [
44]. Betacyanins and betaxanthins are two groups of betalains with, respectively, red-violet and yellow color. According to the literature, the betacyanin content in the beetroot varies, accounting for about 80–90% [
45] of the total betalains, or in some research, about 50–70% [
43,
46]. Our research shows that regardless of cultivar and drying method, betacyanin content in dried beetroot varies from 88.26% DM in conventionally dried ‘Kestrel’ beetroot to 89.73% DM in vacuum freeze-dried ‘Jolie’ beetroot (
Table 4).
Betacyanin content was significantly different in all beetroot cultivars, but the highest was observed in a spray-dried ‘Scarlet’, while the lowest was in conventionally dried ‘Kestrel’ beetroot powder (
Table 4). In terms of betaxanthins, the highest amount was in vacuum freeze-dried ‘Scarlet’ and the lowest in conventionally dried ‘Jolie’ cultivar beetroot powder. The color of beetroot varies depending on the proportions of betacyanins and betaxanthins. The ratio of betacyanins to betaxanthins varied from 6.93 in conventionally dried ‘Kestrel’ to 8.73 in vacuum freeze-dried ‘Jolie’ beetroot powder.
The higher betacyanin content gives the beetroot its typical red-purple pigmentation [
47]. This is also shown by the results of a present study, where the spray-dried beetroot powder of ‘Scarlet’ cultivar with the highest betacyanin content had the highest color a* coordinate value (
Table 4 and Table 6).
The stability of the pigments depends on intrinsic as well as extrinsic factors. Contradictory findings regarding the thermal stability of beetroot betacyanins and betaxanthins have been reported by different scientists. According to some researchers, betacyanins are more stable under temperature treatment [
9,
48], whereas according to others—betaxanthins [
49].
The tendencies for both betacyanins and betaxanthins are similar, with conventional drying resulting in the lowest amounts of betalains (
Table 4). This may be related to endogenous enzymes such as
β-glucosidase, polyphenoloxidases, and peroxidases, which can be inhibited at low and relatively high temperatures, although they may be active during conventional drying and lead to much greater betalain degradation [
50,
51]. Secondary, prolonged heating results in the degradation of these pigments and color changes [
52]. Otherwise, thermal treatment not only determines the degradation of betacyanins, but it has also been observed that prolonged thermal treatment time increases the amount of betaxanthins. These changes may occur due to the condensation of free amino acids with betalamic acid generated by betacyanin hydrolysis [
53,
54]. Nevertheless, the conventionally dried beetroot powder showed the lowest statistically significant amount of betacyanins and betaxanthins, with these compounds demonstrating the highest stability in the ‘Scarlet’ cultivar beetroot powder.
The color coordinate values of raw beetroot juice differed from those of grated beetroot (
Table 5). Beetroot juice lightness (L*) of the different cultivars did not differ statistically, but ‘Jolie’ juice a* coordinate values were the highest (juice had more intense red color), while ‘Kestrel’ had the lowest. The color coordinate b* values of the juice samples were statistically significantly different in ‘Jolie’ and ‘Scarlet’ cultivar beetroot juice. The ‘Scarlet’ beetroot cultivar juice was the most yellowish, while the ‘Jolie’ juice was the most blueish. In terms of grated beetroot color, the lightness of all beetroots cultivars differs significantly. The darkest and the most blue was ‘Scarlet’, while the lightest and most intense red was ‘Jolie’ beetroot.
All drying methods used resulted in color changes compared to beetroot juice and grated beetroots (
Table 5 and
Table 6).
According to Ochoa-Martinez et al. [
55], thermal treatment causes some degradation in red-orange betanins, increasing the values of the color parameter a*. Depending on the cultivar and drying method, the a* value in dried beetroot powder increased by 6.42 units in conventionally dried ‘Jolie’ and 30.4 units in spray-dried ‘Scarlet’ cultivar beetroot powder (
Table 5 and
Table 6). All color parameter values, regardless of the cultivar and drying method, compared with the grated beetroots and beetroot juice, increased.
Spray-dried ‘Kestrel’ cultivar beetroot powder was statistically significantly the lightest, while conventionally dried ‘Kestrel’ and ‘Scarlet’ were the darkest (
Table 6). The highest a* value was observed in ‘Jolie’ and the lowest in conventionally dried ‘Kestrel’ cultivar beetroot. The most intense blue color was observed in the vacuum freeze-dried ‘Jolie’ beetroot powder, while the most intense yellow color was observed in the spray-dried ‘Jolie’ beetroot powder. Betalamic acid and cyclo-DOPA 5-O-glucoside, bright yellow and colorless compounds, respectively, are formed during the degradation of betacyanins [
52]. According to Liu et al. [
56], the L* coordinate value of freeze-dried beetroots was 43.52, a*—27.76 and b*—4.02.
The highest total color change was detected in all cultivars of spray-dried beetroot (
Table 7). Conventional drying resulted in the least color change in beetroot powder of all cultivars. In terms of browning and yellowness indexes, there were not observed any tendencies considering the cultivar or drying method. The highest browning index was observed in the spray-dried and the lowest in conventionally dried ‘Jolie’ cultivar beetroot, while the yellowness index in spray-dried and freeze-dried ‘Jolie’ beetroot, respectively. The increase in the browning index is a consequence of the formation of pigments as a result of enzymatic and non-enzymatic reactions [
27].
The increase in yellowness index values can be attributed to the pigment degradation and the occurrence of the Maillard reaction [
27].
Higher values of chroma parameter indicate an increase in color purity [
49].
The highest chroma value had spray-dried ‘Scarlet’ cultivar beetroot powder, while the lowest—conventionally dried ‘Kestrel’ beetroot powder (
Table 7).
The results of other researchers indicate that higher a* and chroma values were observed in samples with the lower betacyanin content, but our observations do not show this tendency. According to [
57], dark pigmented compounds mask color; therefore, less-pigmented samples have higher color parameter values.
The highest rehydration ratio was observed in conventionally dried beetroots, while the lowest was in spray-dried beetroots, regardless of beetroot cultivar (
Table 8). Meanwhile, according to [
56], the lowest rehydration ratio indicated the most severe damage to the cell structure of the beetroot, and the rehydration ratio of freeze-dried beetroot was more than 4.
The extent to which rehydration occurs is influenced by the degree of cellular and structural damage sustained by the product during treatment. If the treatment causes irreversible rupture and displacement, leading to a loss of cellular integrity and resulting in a dense, collapsed structure, the sample may not be fully rehydrated [
57,
58]. According to Ng and Sulaiman [
35], the higher the rehydration ratio of beetroot powder, the greater its ability to rehydrate in water. Samples with the highest rehydration ratio had the lowest bulk density. Spray-dried beetroot powder dissolved in water unlike conventionally dried beetroots; however, their particle and surface properties influenced a higher bulk density.
Our research shows that the bulk density of beetroot powder ranged from 0.450 to 0.630, with the highest being found in spray-dried samples. Bulk density is regulated by the particle shape and size and the surface properties of powders. According to William L. Kerr and Audrey Varner [
59], powders with larger particles, a wide range of sizes and irregular shape are more difficult to fit in a given volume. Conversely, smaller and more uniform particles are easier to compact in bulk.
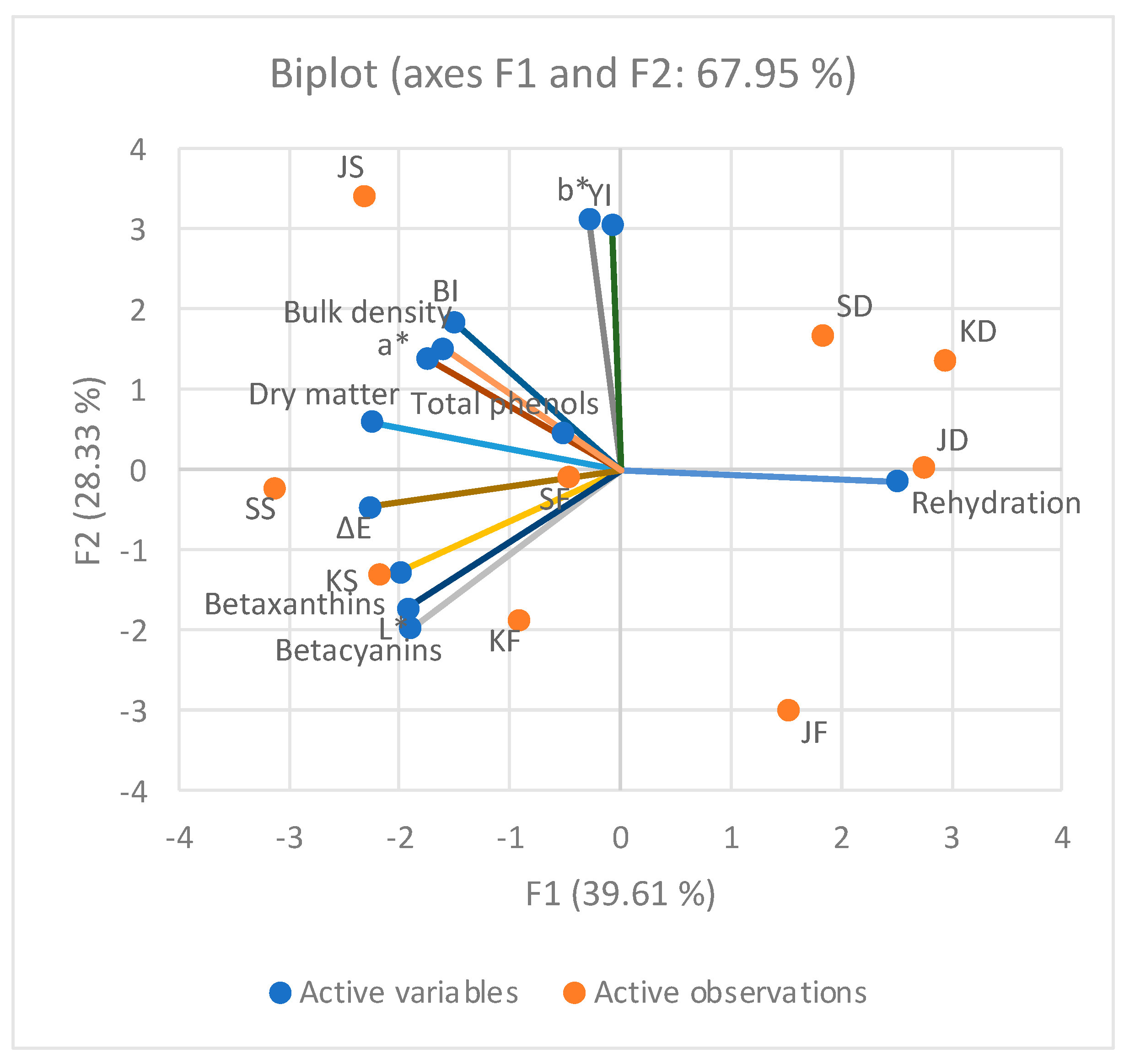
PCA analysis was performed based on the chemical composition, color parameters and physical properties of beetroot powder (
Figure 1).
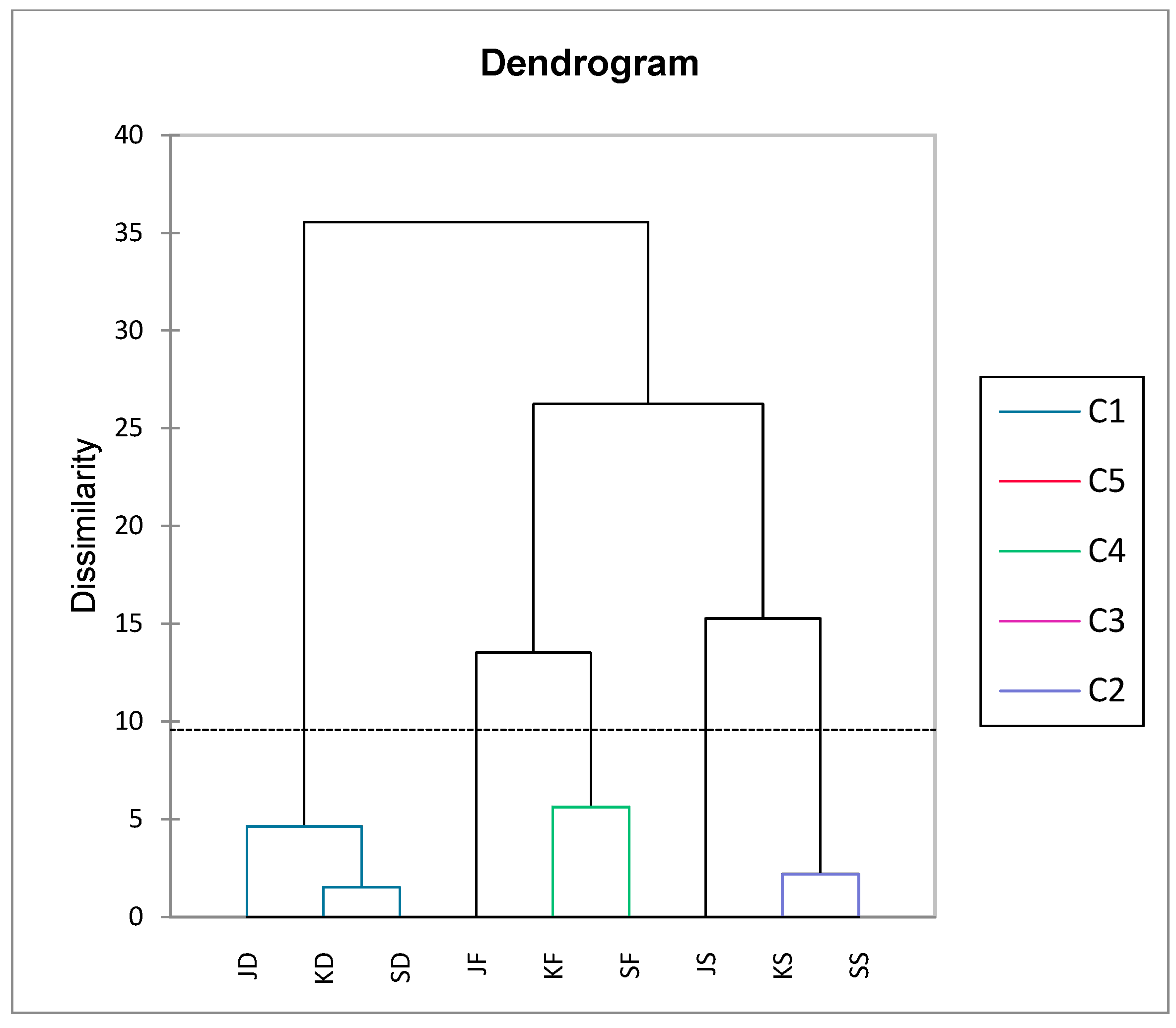
PC1 retained 39.61% of the data variation and differentiated the beetroot powder samples according to the betacyanin, betaxanthin, and dry matter contents, L*, a*, ΔE, and rehydration ratio. Similarly, PC2 explains 28.33% of the variability and separates beetroot powder based on b*, BI, and YI. All cultivars of conventionally dried beetroot powder samples were separated and related to the rehydration ratio. Based on the HCA, the beetroot powder samples were grouped into five clusters (C1, C2, C3, C4, and C5) (
Figure 2). The first cluster (C1) was formed by all conventionally dried beetroot cultivars powders with the lowest phenols content and the highest rehydration ratio; the second cluster (C2) by spray-dried ‘Kestrel’ and ‘Scarlet’ beetroot powders with the lowest rehydration ratio; the third cluster (C3) by ‘Jolie’ cultivar spray-dried beetroot powder, also with the lowest rehydration ratio as well as the highest content of phenols; the fourth cluster (C4) by freeze-dried ‘Kestrel’ and ‘Scarlet’ cultivar beetroot powders with the highest content of betaxanthins; and the fifth cluster (C5) by freeze-dried ‘Jolie’ cultivar beetroot powder with the highest content of betacyanins (
Figure 2).