Selective Retention of Cross-Fertilised Fruitlets during Premature Fruit Drop of Hass Avocado
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Sample Processing
2.3. Paternity Analysis
2.4. Mineral Nutrient Analysis
2.5. Fatty Acid Analysis
2.6. Statistical Analysis
3. Results
3.1. Percentages of Retained Fruitlets That Were Cross-Fertilised
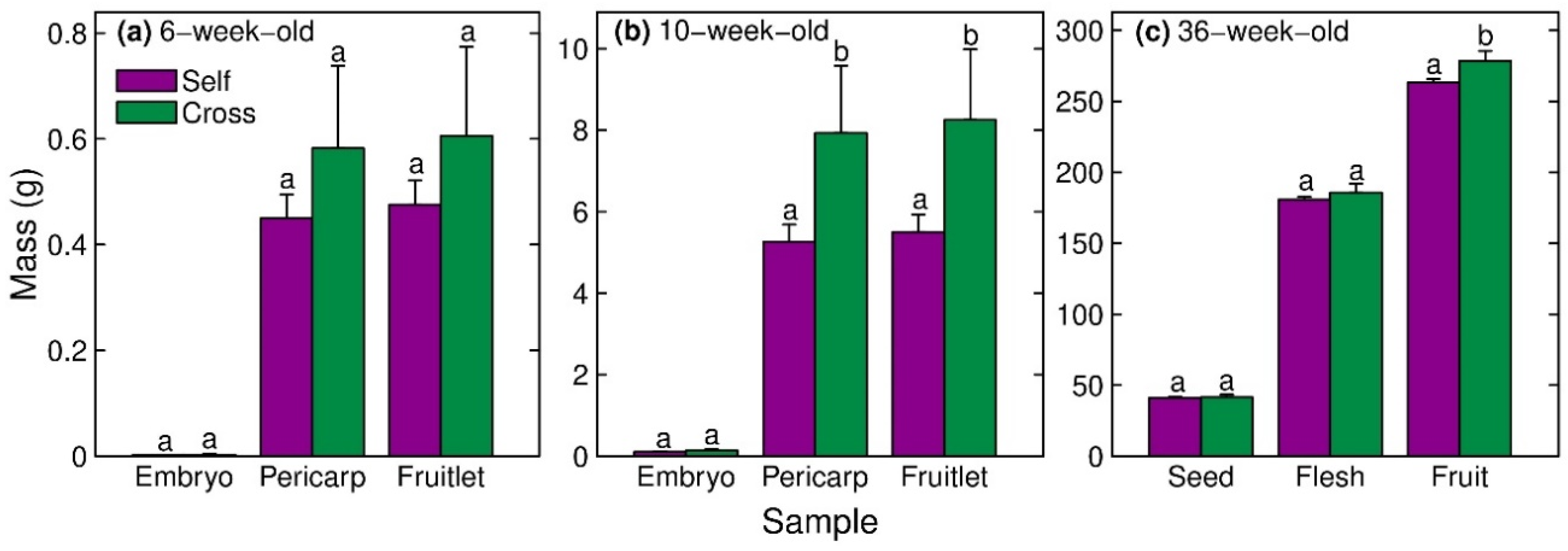
3.2. Cross-Fertilisation Effects on Fruitlet or Fruit Size
3.3. Cross-Fertilisation Effects on Mineral Nutrient Levels and Fatty Acid Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organisation. Fruit and Vegetables—Your Dietary Essentials; FAO: Rome, Italy, 2020. [Google Scholar]
- Production Volume of the Most Produced Food Commodities Worldwide in 2019, by Product. Available online: https://www.statista.com/statistics/1003455/most-produced-crops-and-livestock-products-worldwide/ (accessed on 25 March 2024).
- Ickowitz, A.; McMullin, S.; Rosenstock, T.; Dawson, I.; Rowland, D.; Powell, B.; Mausch, K.; Djoudi, H.; Sunderland, T.; Nurhasan, M.; et al. Transforming food systems with trees and forests. Lancet Planet. Health 2022, 6, e632–e639. [Google Scholar] [CrossRef] [PubMed]
- Pallardy, S.G. Reproductive growth. In Physiology of Woody Plants; Pallardy, S.G., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 87–103. [Google Scholar]
- Dag, A.; Eisenstein, D.; Gazit, S.; El-Batsri, R.; Degani, C. Effect of pollinizer distance and selective fruitlet abscission on outcrossing rate and yield in ‘Tommy Atkins’ mango. J. Am. Soc. Hortic. Sci. 1998, 123, 618–622. [Google Scholar] [CrossRef]
- Holanda-Neto, J.P.; Freitas, B.M.; Bueno, D.M.; Araújo, Z.B. Low seed/nut productivity in cashew (Anacardium occidentale): Effects of self-incompatibility and honeybee (Apis mellifera) foraging behavior. J. Hortic. Sci. Biotechnol. 2002, 77, 226–231. [Google Scholar] [CrossRef]
- Trueman, S.J.; Kämper, W.; Nichols, J.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; Bai, S.H.; Wallace, H.M. Pollen limitation and xenia effects in a cultivated mass-flowering tree, Macadamia integrifolia (Proteaceae). Ann. Bot. 2022, 129, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Degani, C.; Stern, R.A.; El-Batsri, R.; Gazit, S. Pollen-parent effect on the selective abscission of ‘Mauritius’ and ‘Floridian’ Lychee Fruitlets. J. Am. Soc. Hortic. Sci. 1995, 120, 523–526. [Google Scholar] [CrossRef]
- Pérez, V.; Herrero, M.; Hormaza, J.I. Self-fertility and preferential cross-fertilization in mango (Mangifera indica). Sci. Hortic. 2016, 213, 373–378. [Google Scholar] [CrossRef]
- Stephenson, A.G. Flower and fruit abortion proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, F.; Guo, S.; Yuan, D.; Niu, G. Self-sterility may be due to prezygotic late-acting self-incompatibility and early-acting inbreeding depression in Chinese chestnut. J. Am. Soc. Hortic. Sci. 2019, 144, 172–181. [Google Scholar] [CrossRef]
- Hao, Y.-Q.; Zhao, X.-F.; She, D.-Y.; Xu, B.; Zhang, D.-Y.; Liao, W.-J. The role of late-acting self-incompatibility and early-acting inbreeding depression in governing female fertility in monkshood, Aconitum kusnezoffii. PLoS ONE 2012, 7, e47034. [Google Scholar] [CrossRef]
- Gibbs, P.E. Late-acting self-incompatibility—The pariah breeding system in flowering plants. New Phytol. 2014, 203, 717–734. [Google Scholar] [CrossRef]
- Seavey, S.R.; Bawa, K.S. Late-acting self-incompatibility in angiosperms. Bot. Rev. 1986, 52, 196–213. [Google Scholar] [CrossRef]
- Sage, T.L.; Sampson, F.B. Evidence for ovarian self-incompatibility as a cause of self-sterility in the relictual woody angiosperm, Pseudowintera axillaris (Winteraceae). Ann. Bot. 2003, 91, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.B.; Meagher, T.R.; Gibbs, P.E. Do s genes or deleterious recessives control late-acting self-incompatibility in Handroanthus heptaphyllus (Bignoniaceae)? A diallel study with four full-sib progeny arrays. Ann. Bot. 2021, 127, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Husband, B.C.; Schemske, D.W. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 1996, 50, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Dag, A.; Degani, C.; Gazit, S. Gene flow in mango orchards and its impact on yield. Acta Hortic. 2009, 820, 347–350. [Google Scholar] [CrossRef]
- Raz, A.; Goldway, M.; Sapir, G.; Stern, R.A. “Hong Long” lychee (Litchi chinensis Sonn.) is the optimal pollinizer for the main lychee cultivars in Israel. Plants 2022, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Denney, J.O. Xenia includes metaxenia. HortScience 1992, 27, 722–727. [Google Scholar] [CrossRef]
- Yang, Q.; Fu, Y.; Liu, Y.; Zhang, T.; Peng, S.; Deng, J. Novel classification forms for xenia. HortScience 2020, 55, 980–987. [Google Scholar] [CrossRef]
- Wallace, H.M.; Lee, L.S. Pollen source, fruit set and xenia in mandarins. J. Hortic. Sci. Biotechnol. 1999, 74, 82–86. [Google Scholar] [CrossRef]
- Militaru, M.; Butac, M.; Sumedrea, D.; Chiţu, E. Effect of metaxenia on the fruit quality of scab resistant apple varieties. Agric. Agric. Sci. Procedia 2015, 6, 151–156. [Google Scholar] [CrossRef]
- Dung, C.D.; Wallace, H.M.; Bai, S.H.; Ogbourne, S.M.; Trueman, S.J. Cross-pollination affects fruit colour, acidity, firmness and shelf life of self-compatible strawberry. PLoS ONE 2021, 16, e0256964. [Google Scholar] [CrossRef] [PubMed]
- Kämper, W.; Thorp, G.; Wirthensohn, M.; Brooks, P.; Trueman, S.J. Pollen paternity can affect kernel size and nutritional composition of self-incompatible and new self-compatible almond cultivars. Agronomy 2021, 11, 326. [Google Scholar] [CrossRef]
- Trueman, S.J.; Penter, M.G.; Malagodi-Braga, K.S.; Nichols, J.; De Silva, A.L.; Ramos, A.T.M.; Moriya, L.M.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; et al. High outcrossing levels among global macadamia cultivars: Implications for nut quality, orchard designs and pollinator management. Horticulturae 2024, 10, 203. [Google Scholar] [CrossRef]
- Lahav, E.; Zamet, D. Flowers, fruitlets and fruit drop in avocado trees. Rev. Chapingo Ser. Hortic. 1999, 5, 95–100. [Google Scholar]
- Gazit, S.; Degani, C. Reproductive biology. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 101–133. [Google Scholar]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Bai, S.H.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of Hass avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Inadequate pollination is a key factor determining low fruit-to-flower ratios in avocado. Horticulturae 2024, 10, 140. [Google Scholar] [CrossRef]
- Toukem, N.K.; Dubois, T.; Mohamed, S.A.; Lattorff, H.M.G.; Jordaens, K.; Yusuf, A.A. The effect of annual flower strips on pollinator visitation and fruit set of avocado (Persea americana Mill.) in Kenya. Arthropod–Plant Interact. 2023, 17, 19–29. [Google Scholar] [CrossRef]
- D’Asaro, A.; Reig, C.; Martínez-Fuentes, A.; Mesejo, C.; Farina, V.; Agustí, M. Hormonal and carbohydrate control of fruit set in avocado ‘Lamb Hass’. A question of the type of inflorescence? Sci Hortic. 2021, 282, 110046. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Fruit set in avocado: Pollen limitation, pollen load size, and selective fruit abortion. Agronomy 2021, 11, 1603. [Google Scholar] [CrossRef]
- Solares, E.; Morales-Cruz, A.; Balderas, R.F.; Focht, E.; Ashworth, V.E.T.M.; Wyant, S.; Minio, A.; Cantu, D.; Arpaia, M.L.; Gaut, B.S. Insights into the domestication of avocado and potential genetic contributors to heterodichogamy. G3 2023, 13, jkac323. [Google Scholar] [CrossRef]
- Scholefield, P.B. A scanning electron microscope study of flowers of avocado, litchi, macadamia and mango. Sci. Hortic. 1982, 16, 263–272. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Adato, I.; El-Batsri, R. Pollen-parent effect on outcrossing rate, yield, and fruit characteristics of ‘Fuerte’ avocado. HortScience 1990, 25, 471–473. [Google Scholar] [CrossRef]
- Trueman, S.J.; Nichols, J.; Farrar, M.B.; Wallace, H.M.; Hosseini Bai, S. Outcrossing rate and fruit yield of Hass avocado trees decline at increasing distance from a polliniser cultivar. Agronomy 2024, 14, 122. [Google Scholar] [CrossRef]
- Stahl, P.; Mirom, Y.L.; Stern, R.A.; Goldway, M. Comparing ‘Iriet’ and ‘Ettinger’ avocado cultivars as pollinators of ‘Hass’ using SNPs for paternal identification. Sci. Hortic. 2019, 248, 50–57. [Google Scholar] [CrossRef]
- Kämper, W.; Ogbourne, S.M.; Hawkes, D.; Trueman, S.J. SNP markers reveal relationships between fruit paternity, fruit quality and distance from a cross-pollen source in avocado orchards. Sci. Rep. 2021, 11, 20043. [Google Scholar] [CrossRef]
- Degani, C.; El-Batsri, R.; Gazit, S. Outcrossing rate, yield, and selective fruit abscission in ‘Ettinger’ and ‘Ardith’ avocado plots. J. Am. Soc. Hortic. Sci. 1997, 122, 813–817. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Gazit, S. Pollen-parent effect on outcrossing rate in ‘Hass’ and ‘Fuerte’ avocado plots during fruit development. J. Am. Soc. Hortic. Sci. 1989, 114, 106–111. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Gazit, S.; Lavi, U. Genetic selection during the abscission of avocado fruitlets. HortScience 1986, 21, 1187–1192. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Influence of physical distance between cultivars on yield, outcrossing rate and selective fruit drop in avocado (Persea americana, Lauraceae). Ann. Appl. Biol. 2011, 158, 354–361. [Google Scholar] [CrossRef]
- Kämper, W.; Trueman, S.J.; Cooke, J.; Kasinadhuni, N.; Brunton, A.J.; Ogbourne, S.M. Single nucleotide polymorphisms (SNPs) that uniquely identify cultivars of avocado (Persea americana). Appl. Plant Sci. 2021, 9, e11440. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Fazekas, A.J.; Hebert, P.D.N. Semi-automated, membrane-based protocol for DNA isolation from plants. Plant Mol. Biol. Rep. 2008, 26, 186–198. [Google Scholar] [CrossRef]
- McGeehan, S.L.; Naylor, D.V. Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1988, 19, 493–505. [Google Scholar] [CrossRef]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata: Melbourne, Australia, 1992. [Google Scholar]
- Martinie, G.D.; Schilt, A.A. Investigation of the wet oxidation efficiencies of perchloric acid mixtures for various organic substances and the identities of residual matter. Anal. Chem. 1976, 48, 70–74. [Google Scholar] [CrossRef]
- Munter, R.C.; Grande, R.A. Plant tissue and soil extract analysis by ICP-atomic emission spectrometry. In Developments in Atomic Plasma Spectrochemical Analysis; Byrnes, R.M., Ed.; Heyden: London, UK, 1981; pp. 653–672. [Google Scholar]
- Kämper, W.; Trueman, S.J.; Tahmasbian, I.; Bai, S.H. Rapid determination of nutrient concentrations in Hass avocado fruit by Vis/NIR hyperspectral imaging of flesh or skin. Remote Sens. 2020, 12, 3409. [Google Scholar] [CrossRef]
- Richards, T.E.; Kämper, W.; Trueman, S.J.; Wallace, H.M.; Ogbourne, S.M.; Brooks, P.R.; Nichols, J.; Bai, S.H. Relationships between nut size, kernel quality, nutritional composition and levels of outcrossing in three macadamia cultivars. Plants 2020, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Tagliavini, M.; Zavalloni, C.; Rombolà, A.D.; Quartieri, M.; Malaguti, D.; Mazzanti, F.; Millard, P.; Marangoni, B. Mineral nutrient partitioning to fruits of deciduous trees. Acta Hortic. 2000, 512, 131–140. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef]
- Dung, C.D.; Wallace, H.M.; Bai, S.H.; Ogbourne, S.M.; Trueman, S.J. Biomass and mineral nutrient partitioning among self-pollinated and cross-pollinated fruit on the same strawberry plant. PLoS ONE 2022, 17, e0269485. [Google Scholar] [CrossRef]
- Vrecenar-Gadus, M.; Ellstrand, N.C. The effect of planting design on out-crossing rate and yield in the ‘Hass’ avocado. Sci. Hortic. 1985, 27, 215–221. [Google Scholar] [CrossRef]
- Garner, L.C.; Ashworth, V.E.T.M.; Clegg, M.T.; Lovatt, C.J. The impact of outcrossing on yields of ‘Hass’ avocado. J. Am. Soc. Hortic. Sci. 2008, 133, 648–652. [Google Scholar] [CrossRef]
- Kobayashi, M.; Lin, J.-Z.; Davis, J.; Francis, L.; Clegg, M.T. Quantitative analysis of avocado outcrossing and yield in California using RAPD markers. Sci. Hortic. 2000, 86, 135–149. [Google Scholar] [CrossRef]
- Bittencourt, N.S. Evidence for post-zygotic self-incompatibility in Handroanthus impetiginosus (Bignoniaceae). Plant Reprod. 2017, 30, 69–79. [Google Scholar]
- Whiley, A.W. Crop management. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 231–253. [Google Scholar]
- Lovatt, C.J. Hass Avocado Nutrition Research in California; University of California: Riverside, CA, USA, 2013. [Google Scholar]
- Lovatt, C.; Zheng, Y.; Khuong, T.; Campisi-Pinto, S.; Crowley, D.; Rolshausen, P. Yield characteristics of ‘Hass’ avocado trees under California growing conditions. In Proceedings of the VIII World Avocado Congress: Management and Techniques of Cultivation; ProHass: Lima, Peru, 2015; pp. 336–341. [Google Scholar]
- Avocados Australia. Avocados Australia Best Practice Resource—Packaging for Export; Avocados Australia: Rocklea, Australia, 2018. [Google Scholar]
- Fulgoni, V.L.; Dreher, M.; Davenport, A.J. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: Results from the national health and nutrition examination survey (NHANES) 2001–2008. Nutr. J. 2013, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L.; Cheng, F.W.; Ford, N.A. A comprehensive review of Hass avocado clinical trials, observational studies, and biological mechanisms. Nutrients 2021, 13, 4376. [Google Scholar] [CrossRef] [PubMed]
- Stolp, L.J.; Kodali, D.R. Naturally occurring high-oleic oils: Avocado, macadamia, and olive oils. In High Oleic Oils. Development, Properties, and Uses; Flider, F.J., Ed.; Elsevier: London, UK, 2021; pp. 7–52. [Google Scholar]
- Pacheco, L.S.; Li, Y.; Rimm, E.B.; Manson, J.E.; Sun, Q.; Rexrode, K.; Hu, F.B.; Guasch-Ferré, M. Avocado consumption and risk of cardiovascular disease in US adults. J. Am. Heart Assoc. 2022, 11, e024014. [Google Scholar] [CrossRef]
- Monge, A.; Stern, D.; Cortés-Valencia, A.; Catzín-Kuhlmann, A.; Lajous, M.; Denova-Gutiérrez, E. Avocado consumption is associated with a reduction in hypertension incidence in Mexican women. Br. J. Nutr. 2023, 129, 1976–1983. [Google Scholar] [CrossRef]
- Read, S.F.J.; Howlett, B.G.; Jesson, L.K.; Pattemore, D.E. Insect visitors to avocado flowers in the Bay of Plenty, New Zealand. N. Z. Plant Prot. 2017, 70, 38–44. [Google Scholar] [CrossRef]
- Peña, J.F.; Carabalí, A. Effect of honey bee (Apis mellifera L.) density on pollination and fruit set of avocado (Persea americana Mill.) cv. Hass. J. Apic. Sci. 2018, 62, 5–14. [Google Scholar] [CrossRef]
- Sagwe, R.N.; Peters, M.K.; Dubois, T.; Steffan-Dewenter, I.; Lattorff, H.M.G. Insect pollination and pollinator supplementation enhances fruit weight, quality, and marketability of avocado (Persea americana). Arthropod–Plant Interact. 2023, 17, 753–763. [Google Scholar] [CrossRef]
- Dymond, K.; Celis-Diez, J.L.; Potts, S.G.; Howlett, B.G.; Wilcox, B.K.; Garratt, M.P.D. The role of insect pollinators in avocado production: A global review. J. Appl. Entomol. 2021, 145, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Faber, B.; Frankie, G.; Pawelek, J.; Chase, M.; Witt, S. Native pollinators of avocado as affected by constructed pollinator habitat gardens in southern California. Acta Hortic. 2020, 1299, 329–332. [Google Scholar] [CrossRef]
- Celis-Diez, J.L.; García, C.B.; Armesto, J.J.; Abades, S.; Garratt, M.P.D.; Fontúrbel, F.E. Wild floral visitors are more important than honeybees as pollinators of avocado crops. Agronomy 2023, 13, 1722. [Google Scholar] [CrossRef]
- Muñoz, A.E.; Plantegenest, M.; Amouroux, P.; Zavieso, T. Native flower strips increase visitation by non-bee insects to avocado flowers and promote yield. Basic Appl. Ecol. 2021, 56, 369–378. [Google Scholar] [CrossRef]

| Cultivar | Common Avocado Allele | Private Cultivar Allele | DNA Sequence with SNP | Hass | Maluma Hass | Shepard |
|---|---|---|---|---|---|---|
| Hass | T | A | TGCAGAAAGCAGTTTCAGATGCCAGAGTCGGAGAACCATTTTTGCAGTCTTATATGAGAGGAGGTAGAAAAACACTAGGGAGGAGAAATTGTTTTGCCA[T/A]ACCCAACAGTAGAATATGTTACTATTGAAAAAGAAATCTTCA | AA | TT | TT |
| Hass | G | T | TGCAGAGCAGAAAAGCACATGACTTGGAACTTCAACAAGAGCCTGCATCTTGAGTCTGTC[G/T]GGAGGAACTGATCGCCTGATGGCAAATTAGGAATCTTGTTGGTGATGAGAGAGGTTTTGCCCTCCCAAATTGCATCAGTC | TT | GG | GG |
| Maluma Hass | T | C | TGCAGTAGAAGAAAGAAGGATGTAATGATCTTGGCTCCAAAGAGAAACTCTCC[T/C]TTTCTTTTCCCTCTTTTTCTCCACTTGAGAAGGAAAACCATAGTCACATCAATGAAAAATATACCTCCTTTTTTATTATTCCTGTCT | TT | CC | TT |
| Maluma Hass | A | G | TGCAGCTATTATTTATATCACATGATTTTTTCCATTCTATCAGGCGTTGGAGAAAACCCATCACCTGAAAGCAAGAAT[A/G]CATTACATTAGTCTACATCCAGTTTAGCCTGAGTGGGCCCCGCTATTGAGTGATCCAACTCA | AA | GG | AA |
| Maluma Hass | A | G | TGCAGCCTGGAGCTGTTGCTGTTATAGTTGTGTTTTGAGAGTGCGGCGAGGGAAGGACACAGCAGACAAGAGTACAGACTAGACGAAACTCAAAACCCTCGGG[A/G]CAAATGGCTGTGTGTTTTCCCCATTGCATTGCATTG | AA | GG | AA |
| Shepard | G | C | TGCAGGCCAAGCCGAAACTGAGCTCAAGGGAAAGCGTGGAGGAGGTGAAAAGGAAAAGCGTGCTAAAAAAAAGCAGGTCT[G/C]CAACTGTATATTCTTTATGTTCTTCATAGAGTATCTTTTCCATGGATCCTTGACTCCTCG | GG | GG | CC |
| Shepard | A | G | TGCAGTTGTGCTATCTATGTGGTCCCTGCT[A/G]GCTAACTGTGTTTTATCATGTGTAGACTCTTTGGATGGTTGAGATGAGTGTGATTCTTCTACACAATTGAATGGTCAGAATTCATGAATGGTACTGGACCGGCCTAAGAT | AA | AA | GG |
| Shepard | C | T | TGCAGCAAAGCATCACGGTGCCTTCATTTGCCCGTGTCTATATTTGGATGCCAAATTTTTATAGCAGTTAGAAGCACTGATAACAGCAACCAAA[C/T]AAATAATCTGGTGCATACAGATAAAATACAACCCAGGATATCTAC | CC | CC | TT |
| Time after Peak Anthesis | Sample Location | |
|---|---|---|
| Ground | Canopy | |
| 6 weeks | 1.6 ± 0.8 a | 4.6 ± 1.2 b * |
| 10 weeks | 1.0 ± 0.6 a | 6.5 ± 1.4 b |
| 36 weeks (mature) | – | 10.7 ± 1.6 * |
| Fruit Parameter | Pollen Parent | |
|---|---|---|
| Hass (Self) | Shepard (Cross) | |
| Length (cm) | 9.93 ± 0.04 a | 10.00 ± 0.11 a |
| Diameter (cm) | 7.32 ± 0.02 a | 7.49 ± 0.07 b |
| Nutrient | Time after Peak Anthesis | |||
|---|---|---|---|---|
| 6 Weeks | 10 Weeks | |||
| Pollen Parent | ||||
| Hass (Self) | Shepard (Cross) | Hass (Self) | Shepard (Cross) | |
| P | 95.7 ± 2.0 a | 95.1 ± 6.0 a | 70.7 ± 1.5 a | 67.0 ± 3.9 a |
| K | 364 ± 7 a | 367 ± 17 a | 317 ± 4 a | 307 ± 13 a |
| Al | 0.615 ± 0.048 a | 0.478 ± 0.068 a | 0.317 ± 0.019 a | 0.276 ± 0.025 a |
| B | 1.68 ± 0.20 a | 1.84 ± 0.52 a | 2.28 ± 0.21 a | 2.10 ± 0.13 a |
| Ca | 85.1 ± 3.0 a | 68.4 ± 7.5 b | 46.0 ± 2.4 a | 40.3 ± 3.4 a |
| Fe | 1.33 ± 0.06 a | 1.32 ± 0.16 a | 1.32 ± 0.08 a | 1.27 ± 0.07 a |
| Mg | 39.4 ± 1.0 a | 37.3 ± 3.2 a | 39.0 ± 6.0 a | 31.2 ± 1.6 a |
| Mn | 5.74 ± 0.30 a | 4.51 ± 0.74 b | 3.12 ± 0.16 a | 3.01 ± 0.27 a |
| Na | 1.63 ± 0.17 a | 1.59 ± 0.34 a | 1.78 ± 0.56 a | 1.03 ± 0.19 a |
| S | 50.5 ± 1.0 a | 49.2 ± 3.3 a | 41.1 ± 5.2 a | 35.0 ± 2.1 a |
| Zn | 1.51 ± 0.05 a | 1.50 ± 0.16 a | 0.94 ± 0.03 a | 0.91 ± 0.06 a |
| Nutrient | Time after Peak Anthesis | |||
|---|---|---|---|---|
| 6 Weeks | 10 Weeks | |||
| Pollen Parent | ||||
| Hass (Self) | Shepard (Cross) | Hass (Self) | Shepard (Cross) | |
| P | 0.51 ± 0.04 a | 0.53 ± 0.12 a | 3.03 ± 0.21 a | 4.58 ± 0.85 b |
| K | 1.98 ± 0.19 a | 2.12 ± 0.52 a | 14.30 ± 1.16 a | 22.51 ± 4.43 b |
| Al | 0.003 ± <0.001 a | 0.002 ± 0.001 a | 0.013 ± 0.001 a | 0.020 ± 0.004 b |
| B | 0.009 ± 0.002 a | 0.008 ± 0.002 a | 0.119 ± 0.026 a | 0.168 ± 0.037 a |
| Ca | 0.46 ± 0.04 a | 0.36 ± 0.07 a | 1.87 ± 0.13 a | 2.81 ± 0.58 b |
| Fe | 0.007 ± 0.001 a | 0.006 ± 0.001 a | 0.060 ± 0.006 a | 0.102 ± 0.021 b |
| Mg | 0.22 ± 0.02 a | 0.27 ± 0.07 a | 1.61 ± 0.19 a | 2.22 ± 0.41 a |
| Mn | 0.029 ± 0.002 a | 0.022 ± 0.004 a | 0.135 ± 0.012 a | 0.207 ± 0.040 b |
| Na | 0.008 ± 0.001 a | 0.007 ± 0.001 a | 0.069 ± 0.020 a | 0.083 ± 0.023 a |
| S | 0.27 ± 0.02 a | 0.27 ± 0.06 a | 1.77 ± 0.22 a | 2.47 ± 0.48 a |
| Zn | 0.008 ± 0.001 a | 0.007 ± 0.002 a | 0.041 ± 0.003 a | 0.063 ± 0.012 b |
| Nutrient | Fruit Part | |||
|---|---|---|---|---|
| Seed | Flesh | |||
| Pollen Parent | ||||
| Hass (Self) | Shepard (Cross) | Hass (Self) | Shepard (Cross) | |
| N | 522 ± 13 a | 519 ± 24 a | 472 ± 12 a | 486 ± 21 a |
| P | 67.3 ± 1.5 a | 64.4 ± 2.8 a | 58.0 ± 1.3 a | 60.0 ± 2.7 a |
| K | 570 ± 11 a | 540 ± 18 a | 572 ± 10 a | 572 ± 23 a |
| Al | 0.069 ± 0.006 a | 0.072 ± 0.013 a | 0.053 ± 0.004 a | 0.049 ± 0.005 a |
| B | 2.34 ± 0.14 a | 2.65 ± 0.40 a | 3.60 ± 0.13 a | 3.39 ± 0.19 a |
| Ca | 8.12 ± 0.49 a | 7.77 ± 0.76 a | 6.50 ± 0.19 a | 6.37 ± 0.35 a |
| Fe | 1.04 ± 0.03 a | 1.11 ± 0.06 a | 0.62 ± 0.02 a | 0.60 ± 0.04 a |
| Mg | 38.1 ± 1.2 a | 37.3 ± 1.8 a | 28.2 ± 0.4 a | 28.4 ± 0.6 a |
| Mn | 0.61 ± 0.02 a | 0.53 ± 0.04 a | 0.48 ± 0.02 a | 0.50 ± 0.04 a |
| Na | 1.07 ± 0.10 a | 0.98 ± 0.25 a | 7.88 ± 0.41 a | 6.40 ± 0.48 a |
| S | 36.1 ± 1.2 a | 37.7 ± 2.5 a | 29.3 ± 1.1 a | 29.3 ± 2.0 a |
| Zn | 0.631 ± 0.016 a | 0.568 ± 0.029 b | 0.759 ± 0.013 a | 0.763 ± 0.027 a |
| Nutrient | Sample | |||||
|---|---|---|---|---|---|---|
| Seed | Flesh | Fruit | ||||
| Pollen Parent | ||||||
| Hass (Self) | Shepard (Cross) | Hass (Self) | Shepard (Cross) | Hass (Self) | Shepard (Cross) | |
| N | 224 ± 9 a | 225 ± 18 a | 878 ± 31 a | 949 ± 68 a | 1102 ± 37 a | 1175 ± 82 a |
| P | 28.5 ± 1.0 a | 27.8 ± 2.1 a | 107.7 ± 3.6 a | 117.9 ± 9.6 a | 136.2 ± 4.1 a | 145.7 ± 11.2 a |
| K | 241 ± 8 a | 235 ± 17 a | 1061 ± 32 a | 1117 ± 80 a | 1302 ± 37 a | 1351 ± 90 a |
| Al | 0.029 ± 0.002 a | 0.033 ± 0.010 a | 0.095 ± 0.006 a | 0.095 ± 0.011 a | 0.124 ± 0.008 a | 0.128 ± 0.016 a |
| B | 0.97 ± 0.07 a | 1.08 ± 0.15 a | 6.67 ± 0.29 a | 6.54 ± 0.52 a | 7.64 ± 0.34 a | 7.62 ± 0.65 a |
| Ca | 3.3 ± 0.2 a | 3.2 ± 0.4 a | 11.7 ± 0.3 a | 11.9 ± 0.5 a | 15.0 ± 0.4 a | 15.1 ± 0.7 a |
| Fe | 0.44 ± 0.02 a | 0.48 ± 0.04 a | 1.13 ± 0.05 a | 1.15 ± 0.08 a | 1.57 ± 0.05 a | 1.63 ± 0.11 a |
| Mg | 16.0 ± 0.6 a | 15.9 ± 1.1 a | 52.0 ± 1.4 a | 55.0 ± 3.2 a | 67.9 ± 1.6 a | 70.9 ± 3.8 a |
| Mn | 0.25 ± 0.01 a | 0.22 ± 0.02 a | 0.86 ± 0.03 a | 0.94 ± 0.07 a | 1.11 ± 0.03 a | 1.16 ± 0.08 a |
| Na | 0.44 ± 0.04 a | 0.46 ± 0.13 a | 16.9 ± 1.5 a | 13.5 ± 1.6 a | 17.3 ± 1.6 a | 14.0 ± 1.7 a |
| S | 15.3 ± 0.7 a | 16.4 ± 1.5 a | 53.5 ± 2.2 a | 56.1 ± 4.6 a | 68.8 ± 2.6 a | 72.5 ± 5.9 a |
| Zn | 0.27 ± 0.01 a | 0.25 ± 0.02 a | 1.40 ± 0.04 a | 1.48 ± 0.10 a | 1.67 ± 0.05 a | 1.73 ± 0.11 a |
| Fatty Acid (%) | Pollen Parent | |
|---|---|---|
| Hass (Self) | Shepard (Cross) | |
| Palmitic acid (C16:0) | 26.1 ± 0.3 a | 27.0 ± 0.4 a |
| Palmitoleic acid (C16:1 cis) | 5.34 ± 0.14 a | 5.65 ± 0.25 a |
| Stearic acid (C18:0) | 0.186 ± 0.003 a | 0.189 ± 0.007 a |
| Oleic acid (C18:1 cis) | 48.6 ± 0.3 a | 47.6 ± 0.5 a |
| Elaidic acid (C18:1 trans) | 10.6 ± 0.2 a | 10.7 ± 0.4 a |
| Linoleic acid (C18:2) | 9.21 ± 0.16 a | 8.82 ± 0.28 a |
| Total UFAs | 73.8 ± 0.3 a | 72.8 ± 0.4 a |
| Total SFAs | 26.2 ± 0.3 a | 27.2 ± 0.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hapuarachchi, N.S.; Kämper, W.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Wallace, H.M.; Trueman, S.J. Selective Retention of Cross-Fertilised Fruitlets during Premature Fruit Drop of Hass Avocado. Horticulturae 2024, 10, 591. https://doi.org/10.3390/horticulturae10060591
Hapuarachchi NS, Kämper W, Hosseini Bai S, Ogbourne SM, Nichols J, Wallace HM, Trueman SJ. Selective Retention of Cross-Fertilised Fruitlets during Premature Fruit Drop of Hass Avocado. Horticulturae. 2024; 10(6):591. https://doi.org/10.3390/horticulturae10060591
Chicago/Turabian StyleHapuarachchi, Nimanie S., Wiebke Kämper, Shahla Hosseini Bai, Steven M. Ogbourne, Joel Nichols, Helen M. Wallace, and Stephen J. Trueman. 2024. "Selective Retention of Cross-Fertilised Fruitlets during Premature Fruit Drop of Hass Avocado" Horticulturae 10, no. 6: 591. https://doi.org/10.3390/horticulturae10060591
APA StyleHapuarachchi, N. S., Kämper, W., Hosseini Bai, S., Ogbourne, S. M., Nichols, J., Wallace, H. M., & Trueman, S. J. (2024). Selective Retention of Cross-Fertilised Fruitlets during Premature Fruit Drop of Hass Avocado. Horticulturae, 10(6), 591. https://doi.org/10.3390/horticulturae10060591







