Abstract
The aim of this study was to investigate the effects of different LED lights on the growth of cauliflower microgreens and to determine which combination of LED light best increases tolerance to salt stress and promotes the growth and accumulation of secondary antioxidants in the presence of salt stress in vitro. Plants were grown in a growth chamber under different LED light treatments (red light, blue light, a combination of red and blue light, and cool white light) and in MS media with different NaCl concentrations (0, 50, and 100 mM). The effects of the different light qualities and salt stress conditions on growth, content of photosynthetic pigment, flavonoids, phenol, proline, and antioxidant enzyme activity were measured. The best treatments for microgreen development, according to the data, were those that combined red and blue light. In addition to increasing stress tolerance and enabling plant growth at a lower salt concentration (50 mM NaCl), the combination of blue and red light also enhanced the synthesis and accumulation of secondary metabolites and the antioxidant potential of microgreens grown in vitro, which may have nutritional and pharmaceutical value.
1. Introduction
Cauliflower (Brassica oleracea var. botrytis) microgreens are edible young vegetable greens that are about 2.5–7.5 cm in size. Antioxidants are abundant in microgreens and frequently present in higher concentrations than in mature plants [1]. Thanks to the high amounts of antioxidants they contain, microgreens have benefits for human health: they reduce the risk of heart disease [2,3], diabetes [4], and certain cancers [5]. Vitamins A, C, and E; carotenoids; and phenolic and flavonoid compounds are the main dietary components of vegetables that contribute to antioxidant protection. In plants, phenolic and flavonoid compounds are also crucial for defense against abiotic stressors, herbivores, and pathogens [6].
Various LED lights can be used to regulate plant growth and the production of secondary metabolites [7,8,9]. Both morphogenesis processes and the synthesis and accumulation of secondary metabolites depend on the spectral quality of light [10,11,12,13,14]. Plants use specific photoreceptors to react to both the intensity of the light and its spectral composition. Blue (450 nm) and red (650 nm) light have the greatest effect not only on plant growth and the intensity of photosynthesis due to the absorption peaks of the chlorophyll molecules [15], but also on primary and secondary metabolism [16,17]. Blue and red light activate cryptochromes and phytochromes that stimulate the accumulation of phenols and flavonoids in various plant species during germination [18,19] and in adult plants [20,21,22]. The influence of light of different spectra depends on the plant species, so it needs to be optimized for each plant species and working condition [23]. In Brassicaceae plants, an increased amount of blue light affects the accumulation of phenols, anthocyanins [24], and macro- and micronutrients [25], while in other species (green basil, peas, borage), these processes are more favored by red light [26].
Salt stress in plants causes oxidative stress through the increased production of reactive oxygen species (ROS), which cause structural and functional damage to cell membranes due to lipid peroxidation [27] and consequently halt plant development and growth. Plants possess the capacity to regulate their growth and physical traits by modifying physiological and biochemical mechanisms. This encompasses processes such as the segregation or elimination of detrimental ions and the synthesis of osmolytes and antioxidant components to maintain a balanced redox state [28]. In addition to antioxidant enzymes, nonenzymatic antioxidants such as carotenoids, flavonoids, glutathione, and ascorbic acid are also involved in the elimination of ROS [29,30]. Increased accumulation of these compounds through treatment with LED light can promote plant growth and sometimes enhance plant responses to salt stress [28,29]. On the other hand, the plant species, variety, or an abiotic stress such as salt stress can alter the effects of light treatment and also impair plant growth [31].
The aim of this study was to investigate the effects of different LED lights (blue, red, and cool white) on the growth of cauliflower microgreens in the absence and presence of salt stress in vitro to show which combination of light best helps to increase tolerance to salt stress and allow the growth and accumulation of secondary antioxidants under such conditions. Exposing plants to LED light treatment results in the accumulation of antioxidants, which improves the plant’s ability to cope with salt stress. Conversely, salt stress triggers the creation of reactive oxygen species (ROS), which in turn stimulates the synthesis of antioxidants. The objective was to identify an optimal combination of LED treatment and salt stress level that would facilitate the growth of plants while simultaneously enhancing the production of antioxidants that could be subsequently extracted and used. The combined application of LED light and stress conditions, leading to an increase in the synthesis of secondary metabolites, may be of importance for both plant research and commercially as it increases the nutritional and pharmaceutical value of the plants produced.
2. Materials and Methods
2.1. Material
Mature cauliflower seeds surface-sterilized for 20 min in 20% commercial bleach (8% NaOCl, Sneznik, Pancevo, Serbia) were germinated in 90 mm Petri dishes (15 seeds per dish) with 20 mL of plant growth regulator (PGR)-free Murashige and Skoog (MS) [32] medium containing 2% (w/v) sucrose, and 0.7% (w/v) agar (Institute for Virology, Torlak, Belgrade, Serbia). The pH of the medium was adjusted to 5.8 before autoclaving at 117 °C for 25 min. Immediately after germination, seedlings were transferred into individual jar (5 seedlings per jar) on MS-0, 50 and 100 mM NaCl (Sigma-Aldrich, St. Louis, MO, USA) and grown under different light treatments: LED red (R), blue (B), combination red and blue (1:2 rB, 2:1 Rb, and 1:1 RB) and LED cool white light CW. The PPFD (photosynthetic photon flux density) measured at the top of the plants was 145 μmol/m2s. All plants were grown in a growth chamber at a temperature of 23 ± 2 °C and under a light regime with a long day (16 h day, 8 h night). Their growth and development were monitored to investigate the influence of different light treatments on salt stress tolerance. Growth (length, fresh weight, and dry matter of stems); the content of photosynthetic pigment, flavonoids, phenols, and proline; and antioxidant enzyme activity were measured after 10 days of growth.
2.2. Determination of Photosynthetic Pigments
The isolation and determination of the chlorophyll and carotenoid content were carried out according to the method of Brouers and Michel-Wolwertz [33] in 80% (v/v) acetone (Sigma-Aldrich, St. Louis, MO, USA). The total content of chlorophyll (Chl) and carotenoids (TCC) was determined spectrophotometrically (JENWAY 6850, Cole Palmer, IL, USA) by measuring the absorbance at 470 nm for carotenoids and at 645, 652, and 663 nm for chlorophyll according to the formulae of Lichtenthaler [34] and expressed in mg/g fresh weight of the sample.
2.3. Sample Preparation for the Determination of Total Flavonoids and Phenols and Antioxidant Potential
About 0.5 g of the sample was macerated with 5 mL of methanol (Sigma-Aldrich, St. Louis, MO, USA). The extraction lasted 24 h in the dark. Afterwards, the mixture was centrifuged at 6000 rpm for 5 min and the supernatants were stored as prepared extracts at −20 °C until the time of analysis. The extracts were used to determine the total content of flavonoids and phenols and the antioxidant potential. The measurement was performed in triplicate.
2.3.1. Total Flavonoid Content (TFC)
The total flavonoids were determined according to a slightly modified version of the method of Zhishen et al. [35]. Amounts of 40 µL NaNO2 (Sigma-Aldrich, St. Louis, MO, USA) and 70 µL AlCl3 (Sigma-Aldrich, St. Louis, MO, USA) were mixed with 100 µL of methanol extract, and water was added until the total volume reached 1000 µL. After 6 min, NaOH (Sigma-Aldrich, St. Louis, MO, USA) was added to the reaction mixture, and after mixing, the absorbance was measured at 510 nm. The results are expressed as mg rutin equivalents (RE) per gram fresh weight of sample (mgRE/gFW).
2.3.2. Total Phenol Content (TPC)
The total phenols were determined using the Folin–Ciocalteu method [36]. The methanolic extracts were mixed with an aqueous solution of the Folin–Ciocalteu reagent (Sigma-Aldrich, St. Louis, USA); then, Na2CO3 (Sigma-Aldrich, St. Louis, MO, USA) was added. The prepared sample was first incubated in a water bath at 45 °C for 25 min, then in the dark at 25 °C for 2 h, after which the absorbance was measured at 765 nm. The results are expressed as mg gallic acid equivalents (GAE) per gram fresh weight of sample (mgGAE/gFW).
2.3.3. Determination of Antioxidant Potential—DPPH (2,2-Diphenyl-1-picrylhydrazyl) Assay
The total antioxidant potential was determined by the modified DPPH method [37]. The sample extracts (10 µL) were added to 1.1990 mL of 0.01 mM methanol solution of DPPH (Sigma-Aldrich, St. Louis, MO, USA) and incubated in the dark for 30 min, after which the absorbance was measured at 517 nm. The results are expressed as mg Trolox equivalents per gram of fresh sample weight (mgTXE/gFW).
2.4. Proline Content
Proline was extracted from 200 mg of plant tissue with 3% sulfosalicylic acid aqueous solution (Sigma-Aldrich, St. Louis, MO, USA) and its content was determined using the ninhydrin assay of Bates et al. [38]. The absorbance for proline was measured at 520 nm. The concentration of proline was determined using a calibration curve constructed using solutions of known concentrations of proline and expressed as µg per gram fresh weight of sample (µg proline/gFW).
2.5. Determination of Antioxidant Enzyme Activity (POX, CAT, SOD)
To investigate the activity of antioxidant enzymes, 0.5 g of fresh leaves was ground to a powder in liquid nitrogen, and total proteins were extracted according to the method described in Tubić et al. [39]. The supernatant was stored at −70 °C and used for the quantification of total protein and enzymatic assays. Total protein concentrations were determined according to the method of Bradford [40]. The activities of POX, CAT, and SOD were determined spectrophotometrically, and the specific activity of each enzyme was expressed as the rate of the respective product formed or substrate disappeared per minute (U) and per mg of total soluble proteins (U/mg). All measurements were performed in three biological replicates.
Total peroxidase activity (POX) was measured spectrophotometrically according to the modified method of Flatmark [41] by observing the change in absorbance at 430 nm, where the product of peroxidase that catalyzes pyrogallol oxidation and polymerization, purpurogallin, exhibits a maximum absorbance. In a 1.5 mL reaction mixture, 1.38 mL of 0.05 M K-phosphate buffer, pH 6.5, was mixed with 50 µL of 0.6 M pyrogallol (Sigma-Aldrich, St. Louis, MO, USA) and 20 µL of the cauliflower protein extract, with 50 µL of 0.6 M H2O2 added.
The activity of catalases (CAT) was determined by monitoring the kinetics of disappearance of H2O2 at 240 nm by the method of Aebi [42]. The 1.5 mL reaction mixture contained 0.05 M Na-K-phosphate buffer pH 7.0 and 10 μL of sample protein extract. The reaction was initiated by the addition of 30 mM H2O2.
The activity of superoxide dismutase (SOD) was determined according to the modified method of Beyer and Fridovich [43] for monitoring the reduction of NBT substrate at 540 nm. The reaction mixture (1 mL) contained 100 mM potassium phosphate buffer, pH = 7.8 (K-P buffer), 2 mM EDTA(Sigma-Aldrich, St. Louis, MO, USA), 260 mM methionine (Sigma-Aldrich, St. Louis, MO, USA), 1.5 mM nitroblue tetrazolium chloride (NBT) (Sigma-Aldrich, St. Louis, MO, USA), and 0.04 mM riboflavin (Sigma-Aldrich, St. Louis, MO, USA).
2.6. Statistical Analysis
The light treatments were performed in a completely randomized design with three repetitions per treatment with five explants in each jar. All data were statistically analyzed using the program StatSoft Inc. STATISTICA, version 8.0 (Tulsa, OK, USA). The statistical processing of the data included analysis of variance of one-way and two-way ANOVA and separation based on Fisher’s LSD test at a significance level of p ≤ 0.05 and Pearson’s correlation coefficient. The results were presented graphically using the Microsoft Office Excel, version 2405 (Redmond, WA, USA) computer program.
3. Results
In the experiment, the influence of different lights on morphological parameters; the content of pigments, phenols, and flavonoids; and the antioxidative potential of cauliflower microgreens under salt stress conditions were investigated. The effect of complementary light spectra on increasing the tolerance of microgreens to salt stress was also investigated by measuring the content of proline and the activity of antioxidant enzymes. Significant differences were found between the observed light treatments.
After 10 days, plants grown under R light and no stress conditions had the longest stem length, but length was not followed by stem weight. Compared to the plants grown under B light, the stem length of these plants was 20% higher, while the weight was 30% lower (Figure 1A). The plants were thin, pale, and brittle, in contrast to the plants grown under B light, which were more vigorous, although they were slightly shorter than the CW plants. Better growth was observed with the combination of R and B lights, especially with the combinations RB and rB. The highest fresh weight was measured for the plants under RB light (0.142 g), which were 115, 58, and 30% higher than the plants grown under R, B, and CW light, respectively (Figure 1C). Higher weights were also measured in Rb and rB treatments compared to CW.
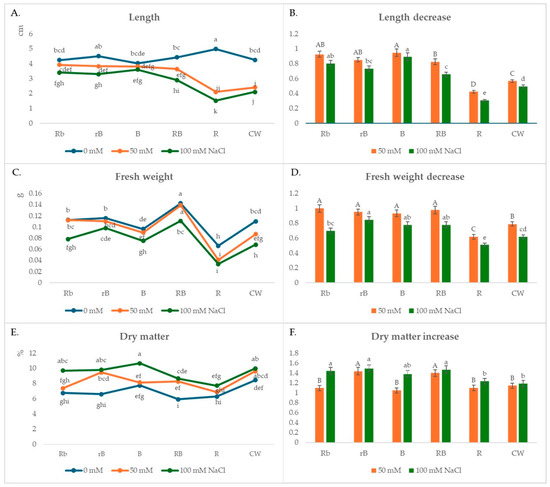
Figure 1.
(A,C,E) The effects of light spectra and salt stress on microgreens’ stem length, fresh weight, and dry matter; (B,D,F) relative changes in stem length, fresh weight, and dry matter obtained with salt treatment (50 mM or 100 mM NaCl) in different light conditions, normalized in relation to the value obtained in the medium without NaCl (=1) under the same light conditions. Values are expressed as means ± SE of three replicates (n = 15). (A,C,E) The different letters represent statistically significant differences (two-way ANOVA); (B,D,F) the different letters represent statistically significant differences between the light treatments within the same concentration of NaCl (50 mM or 100 mM NaCl) (one-way ANOVA).
The presence of NaCl salt in the media had a negative effect on the growth of cauliflower microgreens in all light treatments (Figure 1B,D). The greatest reduction in the length and weight of microgreens at both NaCl concentrations compared to the control plants (growing on medium without NaCl) was observed in the plants growing under R and CW lights. The microgreens were not sufficiently vital; chlorosis appeared in the apical parts of the cotyledons. When the stem growth rate is compared as a function of stress intensity, it is obvious that the plants growing under B light or under combinations of R and B lights are more tolerant to stress (Figure 1B,D).
In the presence of a lower salt concentration of 50 mM NaCl, the highest shoot length value of 3.92 cm was measured under the Rb treatment, while the smallest value was measured under the R treatment (2.1 cm) (Figure 1A). The smallest reduction in stem length compared to the control plants, 6%, was observed in the plants growing under B light, while this reduction was greatest in plants growing under R light (58%) (Figure 1B). The highest fresh weight of the microgreens was also measured under B light (0.138 g), while the lowest was measured under R light (0.041 g) (Figure 1C). The weight loss compared to the control plants was 5% under B light and even 38% under R light. A slight decrease in weight compared to the control plants was also observed in the plants of the Rb, rB, and RB treatments (8, 15, and 18%, respectively) (Figure 1D).
In a solution with twice the concentration of NaCl salt (100 mM), the growth of microgreens under light treatments B, Rb, rB, and RB was reduced but satisfactory. In these light treatments, shoot lengths of 3.6, 3.4, 3.3, and 2.9 cm were measured, which are 10, 20, 27, and 34% less than those of the control plants, respectively (Figure 1B). The weight reduction in these treatments compared to the control plants was 22, 30, 15, and 22% (Figure 1D). The smallest shoot length was measured under R light (1.52 cm), which was 70% less than the control plants. The weight of these plants was reduced by 50–60% compared to the control plants.
In the presence of salt with a low concentration of 50 mM NaCl, the highest amount of dry matter was measured in the rB microgreens (9.43%), in which the highest increase in dry matter (43.54%) compared to the control plants was observed (Figure 1E,F). A significant increase in the percentage of dry matter of 39.7% was also observed in the RB plants. For the other plants, the increase ranged from 5% for the B plants to 9% for the R and Rb plants. On a medium with twice the NaCl concentration (100 mM), the greatest increase in dry matter compared to the control plants was again recorded in the rB plants (48.8%), but a significant increase was also observed in the RB, Rb, and B plants (46.7, 44.1, and 31.4%, respectively). The lowest increase in dry matter of 18.2% was found in plants grown under CW light.
In the present study, the different light treatments affected not only the growth of microgreens but also the content of pigments, phenols, and flavonoids. In the absence of salt stress, B, Rb, and rB light increased the total Chl and carotenoid contents compared to CW, while R light decreased them (Figure 2A).
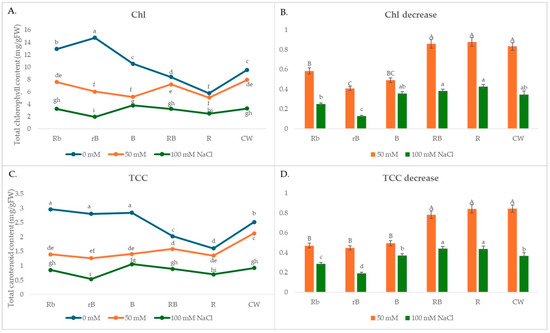
Figure 2.
(A,C) The effects of light spectra and salt stress on the content of chlorophyll and carotenoids; (B,D) relative changes in the content of chlorophyll and carotenoids obtained with salt treatment (50 mM or 100 mM NaCl) in different light conditions normalized in relation to the value obtained in the medium without NaCl (=1) under the same light conditions. Values are expressed as means ± SE of three replicates (n = 15). (A,C) The different letters represent statistically significant differences (two-way ANOVA); (B,D) the different letters represent statistically significant differences between the light treatments within the same concentration of NaCl (50 mM or 100 mM NaCl) (one-way ANOVA).
However, in the presence of salt stress, the smallest decrease in pigment concentration (both chlorophyll and carotenoids) was observed under R and RB light and was similar to the decrease under CW light (Figure 2B,D). The decrease in pigment concentration under B light in the presence of 50 mM NaCl was 45% higher than the decrease under R light, while at higher salt concentrations, the difference was smaller and amounted to 17%.
The highest content of phenols and flavonoids was found in plants grown under CW light, while the lowest content was found in R plants. The content of phenols and flavonoids was higher in plants grown under CW light than in plants grown under R, B, and combinations of R and B light (Figure 3A,C) when no stress was present. The content of phenols was 25% and 45% higher in plants growing under CW light compared to B and R light, while the content of flavonoids was 40% and 51% higher, respectively. The content of both flavonoids and phenols was higher in plants grown under B light compared to those grown under R light by about 25–30%.
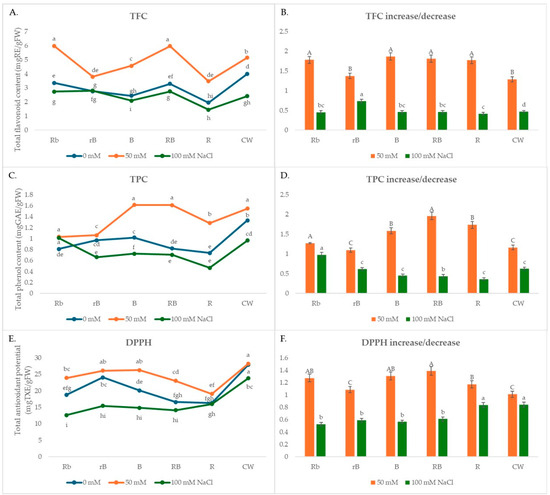
Figure 3.
(A,C,E) The effects of light spectra and salt stress on the content of flavonoids (TFC) and phenols (TPC) and antioxidant potential (DPPH); (B,D,F) relative changes in TFC, TPC, and DPPH obtained with salt treatment (50 mM or 100 mM NaCl) in different light conditions normalized in relation to the value obtained in the medium without NaCl (=1) under the same light conditions. Values are expressed as means ± SE of three replicates (n = 15). (A,C,E) The different letters represent statistically significant differences (two-way ANOVA); (B,D,F) the different letters represent statistically significant differences between the light treatments within the same concentration of NaCl (50 mM or 100 mM NaCl) (one-way ANOVA).
In the presence of 50 mM NaCl, the content of phenols and flavonoids increased in all light treatments (Figure 3B,D). The phenol content increased the most under B, RB, and Rb lights, while B light had a smaller effect on the increase in flavonoid content compared to RB and Rb lights. This suggests that moderate stress conditions can increase the synthesis of secondary metabolites in the plants, which can be used in combination with certain light sources to produce plants with an increased antioxidant status. At higher salt concentrations (100 mM NaCl), the concentration decreased slightly or remained at the level measured in the absence of stress.
In the absence of stress, the total antioxidant potential (DPPH) was 71 and 39% higher in CW light compared to R and B light, respectively (Figure 3E). According to the correlation matrix for phytochemical and antioxidant properties in the absence of salt stress, the total content of carotenoids, phenols, and flavonoids had a positive and significant correlation with the antioxidant DPPH potential (r = 0.54 *, r = 0.61 *, r = 0.93, respectively) (Table 1).

Table 1.
Correlation coefficients between phytochemical compounds and total antioxidant potential of cauliflower microgreen extract.
In the presence of 50 mM NaCl, only the total carotenoid content showed a positive and significant correlation with the antioxidant DPPH potential (r = 0.58 *), while in the presence of a higher NaCl concentration (100 mM), no correlation was observed between any component and DPPH (Table 1). According to the correlation matrix for phytochemical and antioxidant properties in the presence of salt stress, high positive correlations were observed for TFC-TPC (0.85 *) and TCC-TPC (0.48 *).
Plants exposed to B light and combinations of B/R light were shown to have higher levels of proline in the absence of stress (Figure 4A), and in these plants, a significant increase was noted under stressful conditions too. At both NaCl concentrations, rB plants showed the largest increase in proline content under stress, around 60% more than CW (Figure 4B). The smallest increases were observed in plants grown under R light.
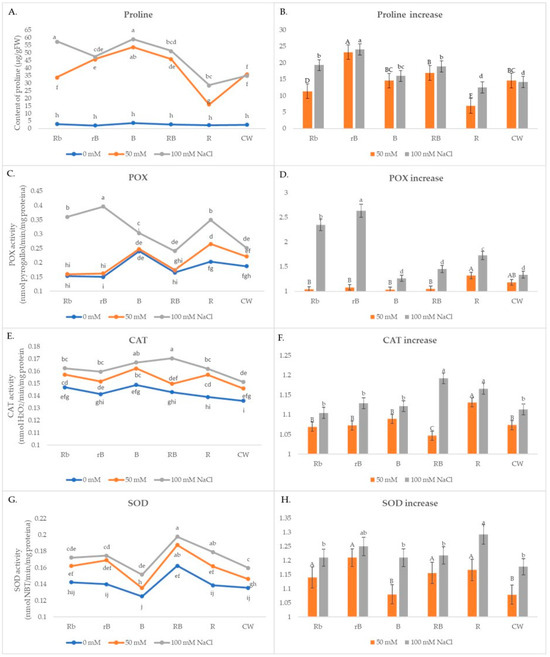
Figure 4.
(A,C,E,G) The effects of light spectra and salt stress on the content of proline and POX, CAT, and SOD activity; (B,D,F,H) relative changes in proline content and peroxidase (POX), catalases (CAT), and superoxide dismutase (SOD) activity obtained with salt treatment (50 mM or 100 mM NaCl) in different light conditions normalized in relation to the value obtained in the medium without NaCl (=1) under the same light conditions. Values are expressed as means ± SE of three replicates (n = 15). (A,C,E,G) The different letters represent statistically significant differences (two-way ANOVA); (B,D,F,H) the different letters represent statistically significant differences between the light treatments within the same concentration of NaCl (50 mM or 100 mM NaCl) (one-way ANOVA).
In the presence of 50 mM NaCl, there was a slight increase in POX activity in all light treatments, while a significant increase of 30% occurred only in R plants (Figure 4C,D). Under stress conditions of 100 mM NaCl, there was a significant increase in treatments rB and Rb. CAT activity increased significantly in all light treatments compared to CW, and the most significant increase was recorded in B and Rb lights (Figure 4E,F). It was a similar case with SOD activity (Figure 4G,H). At lower salt concentrations, the highest increase of 21% compared to the control was recorded in the rB plants, 13% higher compared to CW, while at higher concentrations, the highest increase of 29% compared to the control was recorded in the R treatment. Increases in activity greater than 20% compared to the control were also recorded in the rB, Rb, RB, and B plants.
4. Discussion
Light-emitting diode (LED) lamps have been developed as an alternative light source for plants. LED light stimulates the regulation of metabolic pathways in plants, which directly affect plant growth, physiology, and secondary metabolism. This regulation is mediated by photoreceptors, such as phytochromes (in response to red and far-red wavelengths), cryptochromes, and phototropins (in response to blue wavelengths) [44,45]. In this study, the plants grown under R light were thin, pale, and brittle, in contrast to the plants grown under B light, which were more vigorous, although they were slightly shorter than the CW plants. According to Wei et al. [46], red light induces endogenous gibberellins, which are involved in cell elongation and root inhibition. Similar results were observed in different crops such as R. glutinosa, potato, and strawberry [7,47,48]. In our study, better growth was observed with the combination of R and B lights, especially with the combinations RB and rB. Higher weights were also measured in Rb and rB treatments compared to CW. In contrast, Edesi et al. [49] demonstrated that a wide spectrum (white LED) produced the highest fresh weight of potato shoots and roots.
An experiment involving two genotypes of basil showed that when subjected to salt stress, each genotype exhibited distinct responses to identical wavelengths of LED lights [50]. The presence of NaCl salt in the media had a negative effect on the growth of cauliflower microgreens in all light treatments. The outcomes are consistent with the findings of a study on the velvet plant [51]. Additionally, Kaouther et al. [52] discovered that when salinity increased, Capsicum frutescens L.’s fresh weight decreased.
In the presence of salt with a low concentration of 50 mM NaCl, the highest amount of dry matter was measured in rB cauliflower microgreens, in which the highest increase in dry matter was observed compared to the control plants. In his studies, Munns [53] explains that under conditions of increased salt concentration, the decrease in fresh weight and the change in dry matter of the plants are caused by a suppression of plant growth, which is caused either by a decrease in water availability or by NaCl toxicity. The proportion of dry matter was lower because seedlings grown in environments with lower salt concentrations absorbed more water from the solution than in environments with higher concentrations [54]. Additionally, He et al. [55] found a significant interaction between salinity and LED red/blue ratio on shoot FW and DW, indicating that light quality had an impact on salinity’s effect on shoot FW and DW.
In the present study, the different light treatments affected not only the growth of microgreens but also the content of pigments, phenols, and flavonoids, which have potential nutritional and pharmaceutical value. LED light treatments have the ability to induce changes in both the primary and secondary metabolism of plants, which are associated with their ability to tolerate abiotic stress. This ultimately results in enhanced plant growth and improved resilience to various pressures. The enhancement of antioxidant status with LED light treatment plays a vital role in promoting stress tolerance in plants [55]. In the absence of salt stress, B, Rb, and rB light increased the total Chl and carotenoid contents in cauliflower microgreens compared to CW, while R light decreased them. These results are in contrast to those obtained in oilseed rape [56], where red light increases and blue light decreases pigment content. In R. glutinosa, red LED light treatment led to a considerable increase in chlorophyll synthesis, which was subsequently followed by blue LED light treatment [7]. On the other hand, in an oriental melon investigation, chlorophyll content was highest under blue light [57]. Several reports have shown that the different responses of plants to the same light treatments are determined by genetic diversity both between plant species and between different varieties within a species [47,49,58].
Several studies have indicated that LED light treatments enhanced the activity of important enzymes involved in the shikimate and phenylpropanoid pathways or in the flavonoid synthesis pathway [59]. In our cauliflower microgreens, the content of both flavonoids and phenols was higher in plants grown under B light compared to those grown under R light. The higher accumulation of polyphenolic compounds of plants exposed to LEDs, in particular under B, is probably due to the fact that blue light activates phototropins and cryptochromes, which affect primary and secondary metabolism [60]. In a study on Rehmannia glutinosa, blue LED treatment significantly increased the total phenol content by 17.7% more than red LED treatment, while the total flavonoid contents were enhanced by the red LED treatment [7]. In the presence of NaCl, the content of phenols and flavonoids increased in all light treatments. A high content of phenols enhances the antioxidant properties of plants and contributes to protection against oxidative damage [61].
The correlation matrix indicates that in the absence of salt stress, there was a positive and substantial association between the overall concentration of carotenoids, phenols, and flavonoids and the antioxidant DPPH potential. The strongest association was seen between flavonoids and DPPH potential, indicating that flavonoids play the most significant role in the antioxidant potential of cauliflower microgreen extract, particularly in the absence of stress. Similar results were obtained in previous studies with other crops [62,63]. The antioxidant activity of Lantana camara leaf extract was found to positively correlate with its total phenol content but not with its flavonoid level [6]. In the presence of 50 mM NaCl, only the total carotenoid content showed a positive and significant correlation with the antioxidant DPPH potential. Carotenoids contribute the most to the antioxidant potential under moderate-stress conditions, and their main function is to protect the photosynthetic apparatus from photooxidative damage [64].
The combination of hyperosmotic and hyperionic stressors, followed by salt, has a detrimental impact on crucial cellular processes, ultimately resulting in a decrease in plant growth. Plants utilize antioxidant enzymes and nonenzymatic antioxidants, like proline, in response to osmotic stress following salt stress [65,66]. Proline aids in membrane stabilization and osmoregulation, as well as in the elimination of reactive oxygen species (ROS) [67], aiding in recovery and growth [68]. Utilizing LED light can enhance the proline content, which possesses the capability to diminish lipid peroxidation by immediately eliminating reactive oxygen species (ROS) and stabilizing proteins and membranes. This procedure aids in the elimination of hydrogen peroxide (H2O2) that is associated with salt stress, therefore preserving cellular homeostasis. Cao et al. [69] and He et al. [70] reported similar findings. Cauliflower microgreens exposed to B light and combinations of B/R light were shown to have higher levels of proline in the absence of stress, and in these plants, a significant increase was noted under stressful conditions too. Cao et al. [69] suggested in their study that tomato seedlings, when subjected to salt stress and cultivated under lower red-to-far-red (R:FR) light conditions, showed less cellular damage and lipid peroxidation. The lower accumulation of reactive oxygen species, including H2O2 and superoxide anion, as well as reduced amounts of malondialdehyde (MDA) and electrolytic leakage, was the cause of this phenomenon.
In general, the increase in ROS production triggers the activities of antioxidant enzymes to prevent cell damage due to oxidative stress. The results of a study with bean plants under three salinity levels showed that the activities of CAT, APX, SOD, and POD increased [70]. Under stress conditions, the activity of antioxidant enzymes saw a significant increase in B and Rb treatments. In concordance with our results, enhanced antioxidant enzyme activities were induced by blue LED treatments in both leaf and root extracts of R. glutinosa upon [7]. The increased levels of catalase (CAT) and superoxide dismutase (SOD) under red (R) and red–blue (RB) light conditions may be a result of oxygen-producing photosynthetic activity stimulated by the red light [71]. Overall, one of the reasons why plants exposed to LEDs, especially under RB and R lights, have improved development and stress tolerance is due to their enhanced antioxidant capacity [72].
5. Conclusions
In the present study, the different light treatments had an impact on the content of pigments, phenols, and flavonoids, in addition to the cauliflower microgreens’ growth. The damaging effects of salt stress can be mitigated by choosing the right-light spectrum. Combinations of blue and red light increased stress tolerance and enabled plant growth under conditions of lower salt concentration (50 mM NaCl). In combination with lower salt stress and red and blue lights, microgreens cultured in vitro increased the synthesis and accumulation of secondary metabolites and antioxidant potential. These findings may have applications in nutrition and pharmacy. In conclusion, the obtained data indicate that blue and/or red LEDs have the potential to stimulate the growth of cauliflower microgreens in vitro, as well as to improve their morphological and phytochemical parameters under salt stress. The acquired results have the potential to be used in future studies, and they may be employed in the production of cauliflower microgreens under hydroponic circumstances or in a substrate in greenhouses. This is significant because microgreens grown in this way have the potential to be directly used in human nutrition.
Author Contributions
Conceptualization, S.P.; methodology, S.P., J.M. and Z.G.; validation, Z.G., J.D. and O.Đ.M.; formal analysis, S.P., Z.G., J.M. and J.D.; investigation, S.P., Z.G., J.M. and J.D.; resources, S.R.N. and V.M.S.; writing—original draft preparation, S.P.; writing—review and editing, Z.G., J.M., J.D., S.R.N. and O.Đ.M.; visualization, Z.G. and V.M.S.; supervision, S.P.; project administration, S.P. and J.D.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science, Technological Development and Innovations of the Republic of Serbia (No. 451-03-66/2024-03/200015; 451-03-66/2024-03/200054; 451-03-66/2024-03/200007).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, Inflammation, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2013, 15, 110714. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, X.; Xiao, Z.; Yu, L.; Pham, Q.; Sun, J.; Chen, P.; Yokoyama, W.; Yu, L.L.; Luo, Y.S.; et al. Red Cabbage Microgreens Lower Circulating Low-Density Lipoprotein (LDL), Liver Cholesterol, and Inflammatory Cytokines in Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2016, 64, 9161–9171. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, S.; Tripathi, J.; Gautam, S. In vitro regulation of enzymatic release of glucose and its uptake by Fenugreek microgreen and Mint leaf extract. Int. J. Food Sci. Technol. 2017, 53, 320–326. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Kayani, W.K.; Dilshad, E.; Rani, R.; Khan, R.S. Production of biomass and medicinal metabolites through adventitious roots in Ajuga bracteosa under different spectral lights. J. Photochem. Photobiol. B Biol. 2019, 193, 109–117. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Jones, M.A. Using light to improve commercial value. Hortic. Res. 2018, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L.A. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef]
- Mark, G.L.; Dean, A.K.; Carl, E.S. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An Overview of LEDs’ Effects on the Production of Bioactive Compounds and Crop Quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue:Red LED light proportion affects vegetative parameters, pigment content, and oxidative status of einkorn (Triticum monococcum L. ssp. monococcum) wheatgrass. J. Agric. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef]
- Acharya, J.; Rechner, O.; Neugart, S.; Schreiner, M.; Poehling, H.M. Effects of light-emitting diode treatments on Brevicoryne brassicae performance mediated by secondary metabolites in Brussels sprouts. J. Plant Dis. Prot. 2016, 123, 321–330. [Google Scholar] [CrossRef]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef]
- Kim, E.Y.; Park, S.A.; Park, B.J.; Lee, Y.; Oh, M.M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Gu, M.; Chen, X.; Chen, X.; Yang, J.; Zhao, F.; Ye, N. Exploration of the Effects of Different Blue LED Light Intensities on Flavonoid and Lipid Metabolism in Tea Plants via Transcriptomics and Metabolomics. Int. J. Mol. Sci. 2020, 21, 4606. [Google Scholar] [CrossRef]
- Liang, D.; Yousef, A.F.; Wei, X.; Ali, M.M.; Yu, W.; Yang, L.; Oelmüller, R.; Chen, F. Increasing the performance of Passion fruit (Passiflora edulis) seedlings by LED light regimes. Sci. Rep. 2021, 11, 20967. [Google Scholar] [CrossRef]
- Ying, Q.; Jones-Baumgardt, C.; Zheng, Y.; Bozzo, G. The Proportion of Blue Light from Light-emitting Diodes Alters Microgreen Phytochemical Profiles in a Species-specific Manner. HortScience 2021, 56, 13–20. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of Different Ratios of Blue and Red LED Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Bantis, F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants 2021, 10, 2182. [Google Scholar] [CrossRef]
- Boguszewska, D.; Zagdanska, B. ROS as Signaling Molecules and Enzymes of Plant Response to Unfavorable Environmental Conditions. In Oxidative Stress—Molecular Mechanisms and Biological Effects; Lushchak, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 341–362. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Francisco Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Greer, SC, USA, 2018. [Google Scholar]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Shamsabad, M.R.M.; Esmaeilizadeh, M.; Roosta, H.R.; Dehghani, M.R.; Dąbrowski, P.; Kalaji, H.M. The effect of supplementary light on the photosynthetic apparatus of strawberry plants under salinity and alkalinity stress. Sci. Rep. 2022, 12, 13257. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium of rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Brouers, M.; Michel-Wolwertz, M.R. Estimation of protochlorophyll(ide) contents in plant extracts; reevaluation of the molar absorption coefficient of protochlorophyll(ide). Photosynth. Res. 1983, 4, 265–270. [Google Scholar] [CrossRef]
- Lichtenhaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Tubić, L.; Savić, J.; Mitić, N.; Milojević, J.; Janošević, D.; Budimir, S.; Zdravković-Korać, S. Cytokinins differentially affect regeneration, plant growth and antioxidative enzymes activity in chive (Allium schoenoprasum L.). Plant Cell Tissue Organ Cult. 2016, 124, 1–14. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of proteins utilising the principal of protein–dye binding. Anal. Biochem. 1976, 72, 24–54. [Google Scholar] [CrossRef]
- Flatmark, T. Studies on the Peroxidase effect of Cytochrome c. Acta Chem. Scand. 1964, 18, 2269–2279. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Christie, J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef]
- Galvao, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, S.W.; Ma, J.J.; Liu, H.M.; Han, F.X.; Li, Y.; Niu, S.H. Gibberellin signaling is required for far-red light-induced shoot elongation in Pinus tabuliformis seedlings. Plant Physiol. 2020, 182, 658–668. [Google Scholar] [CrossRef]
- Rocha, P.S.G.; Oliveira, R.P.; Scivittaro, W.B. New light sources for in-vitro potato micropropagation. Biosci. J. 2015, 31, 1312–1318. [Google Scholar] [CrossRef]
- Sivakumar, G.; Heo, J.W.; Kozai, T.; Paek, K.Y. Effect of continuous or intermittent radiation on sweet potato plantlets in vitro. J. Hortic. Sci. Biotechnol. 2006, 81, 546–548. [Google Scholar] [CrossRef]
- Edesi, J.; Pirttilä, A.M.; Häggman, H. Modified light spectral conditions prior to cryopreservation alter growth characteristics and cryopreservation success of potato (Solanum tuberosum L.) shoot tips in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 128, 409–421. [Google Scholar] [CrossRef]
- Rafeie, M.; Shabani, L.; Sabzalian, M.; Gharibi, S. Pretreatment with LEDs regulates antioxidant capacity and polyphenolic profile in two genotypes of basil under salinity stress. Protoplasma 2022, 259, 1567–1583. [Google Scholar] [CrossRef]
- Sayyed, A.; Gul, H.L.; Ullah, Z.; Hamayun, M. 2014. Effect of salt stress on growth of Tagetes erecta L. Pakhtunkhwa J. Life Sci. 2014, 2, 96–106. [Google Scholar]
- Kaouther, Z.; Nina, H.; Rezwan, A.; Cherif, H. Evaluation of Salt Tolerance (NaCl) in Tunisian Chili Pepper (Capsicum frutescens L.) on Growth, Mineral Analysis and Solutes Synthesis. J. Stress Physiol. Biochem. 2013, 9, 209–228. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Akram, M.; Ashraf, M.Y.; Ahnad, R.; Waraich, E.A.; Iqbal, J.; Mohsan, M. Screening for salt tolerance in maize (Zea mays L.) hybrids at an early seedling stage. Pak. J. Bot. 2010, 42, 141–154. [Google Scholar]
- He, J.; Koh, D.J.Q.; Qin, L. LED spectral quality and NaCl salinity interact to affect growth, photosynthesis and phytochemical production of Mesembryanthemum crystallinum. Funct. Plant Biol. 2021, 12, 686910. [Google Scholar] [CrossRef]
- Saleem, M.H.; Rehman, M.; Fahad, S.; Tung, S.A.; Iqbal, N.; Hassan, A.; Ayub, A.; Wahid, M.A. 2020 Leaf gas exchange, oxidative stress, and physiological attributes of rapeseed (Brassica napus L.) grown under different light-emitting diodes. Photosynthetica 2020, 58, 836–845. [Google Scholar] [CrossRef]
- Cui, X.H.; Guo, X.O.; Sun, T.Y.; Qi, H.Y. Effects of LED supplementary lighting on seedling growth and fruit quality of oriental melon. Plant Physiol. J. 2017, 53, 657–667. [Google Scholar] [CrossRef]
- Paradiso, R.; Arena, C.; Rouphael, Y.; d’Aquino, L.; Makris, K.; Vitaglione, P.; De Pascale, S. Growth, photosynthetic activity and tuber quality of two potato cultivars in controlled environment as affected by light source. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 153, 725–735. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and opportunities of light-emitting diode (LED) as key to modulate antioxidant compounds in plants. Antioxidants 2021, 10, 42. [Google Scholar] [CrossRef]
- Abney, K.R.; Kopsell, D.A.; Sams, C.E.; Zivanovic, S.; Kopsell, D.E. UV-B radiation impacts shoot tissue pigment composition in Allium fistulosum L. cultigens. Sci. World J. 2013, 513867. [Google Scholar] [CrossRef]
- Reddy, N.S.; Navanesan, S.; Sinniah, S.K.; Wahab, N.A.; Sim, K.S. Phenolic content, antioxidant effect and cytotoxic activityof Leea indica leaves. BMC Complement. Altern. Med. 2012, 12, 128–134. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Wiśniewski, R. Determination of triterpenoids, carotenoids, chlorophylls, and antioxidant capacity in Allium ursinum L. at different times of harvesting and anatomical parts. Eur. Food Res. Technol. 2018, 244, 1269–1280. [Google Scholar] [CrossRef]
- Gordanić, S.G.; Radovanović, D.; Vuković, S. Phytochemical characterization and antioxidant potential of Allium ursinum L. cultivated on different soil types—A preliminary study. Emir. J. Food Agric. 2022, 34, 904–914. [Google Scholar] [CrossRef]
- Liang, L.D.; Zhu, T.; Ni, Z.; Lin, L.; Tang, Y.; Wang, Z.; Wang, X.; Wang, J.; Lv, X.; Xia, H. Ascorbic acid metabolism during sweet cherry (Prunus avium) fruit development. PLoS ONE 2017, 12, e0172818. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.B.; Ghneim-Herrera, T.; Romdhane, W.B.; Dabbous, A.; Saad, R.B.; Brini, F.; Abdelly, C.; Hamed, K.B. Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima. Funct. Plant Biol. 2020, 47, 912–924. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef]
- Wu, H. Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). BioMed Res. Int. 2016, 7383918. [Google Scholar] [CrossRef]
- Mirzahosseini, Z.; Shabani, L.; Sabzalian, M.R. LED lights increase an antioxidant capacity of Arabidopsis thaliana under wound induced stresses. Funct. Plant Biol. 2020, 47, 853–864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).