Exogenous Calcium Alleviates the Photosynthetic Inhibition and Oxidative Damage of the Tea Plant under Cold Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Cold Tolerance Assays
2.3. Quantification of ABA by HPLC
2.4. RNA Isolation
2.5. qRT-PCR Analysis
2.6. Statistical Analysis
3. Results
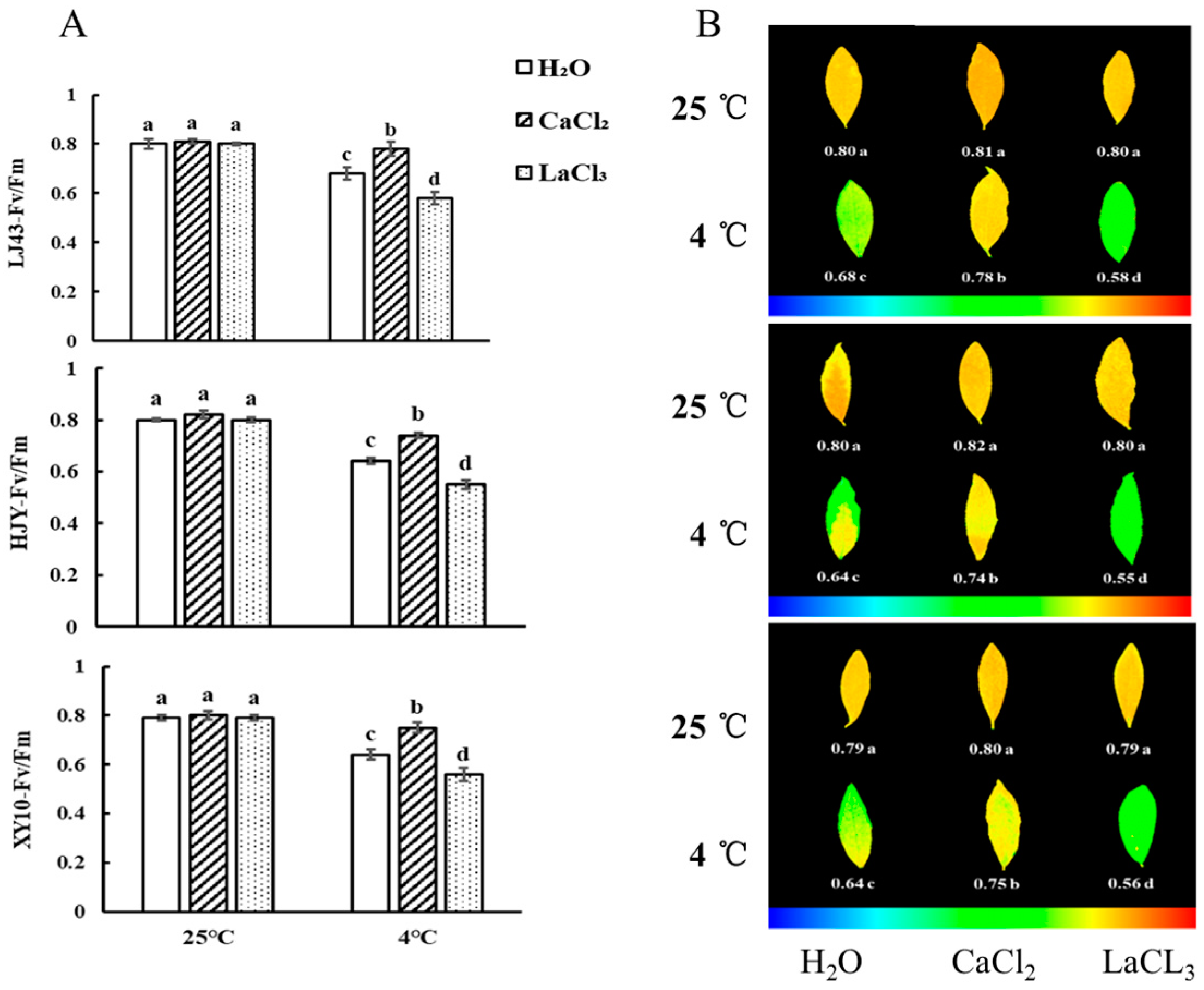
3.1. Effect of CaCl2 and LaCl3 on Chlorophyll Fluorescence of Tea Plants under Cold Stress
3.2. Influence of CaCl2 and LaCl3 on the Relative Conductivity and MDA Levels of Tea Plants under Cold Stress
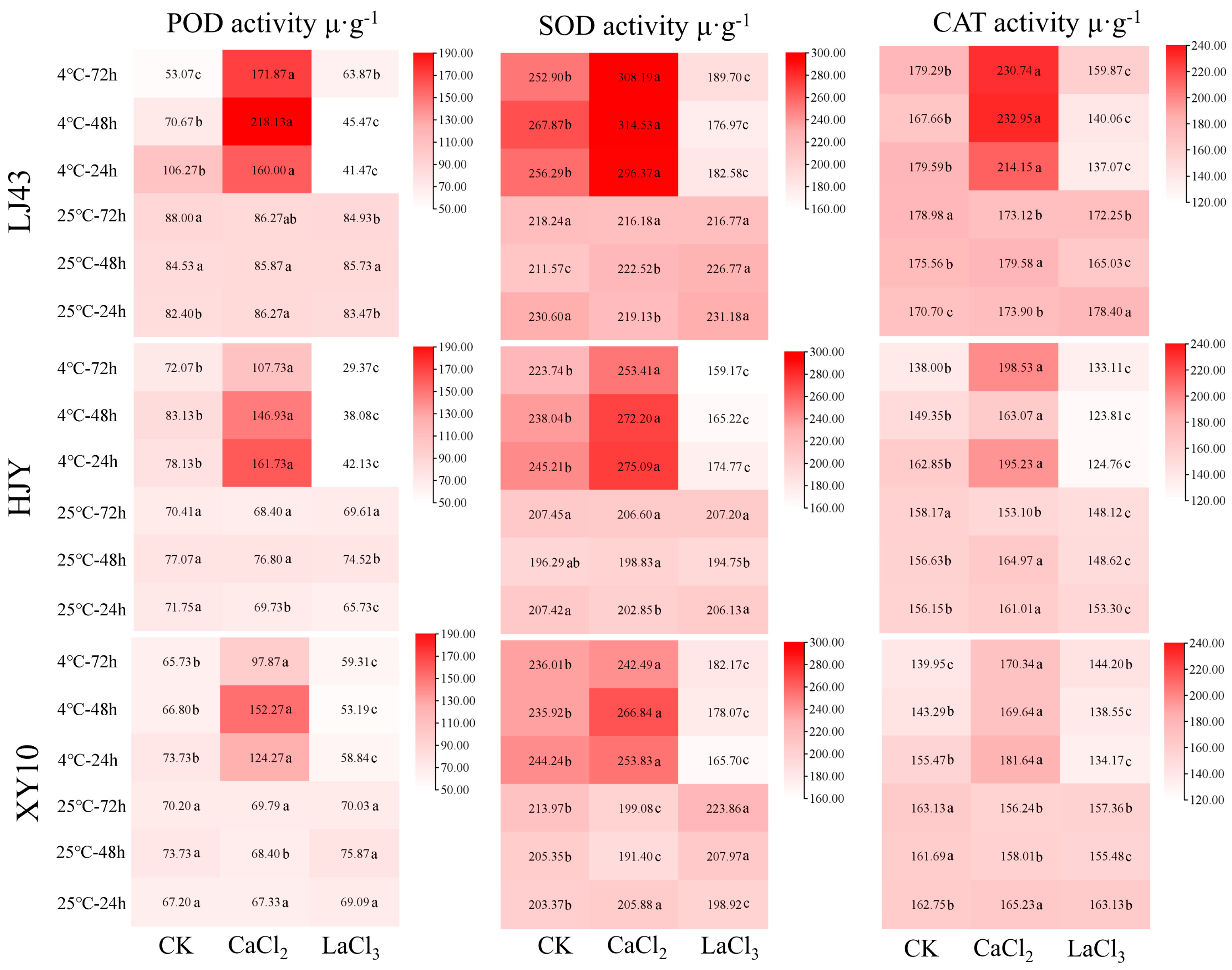
3.3. Effect of CaCl2 and LaCl3 on Antioxidant Enzymes Activities in Tea Plants under Cold Stress
3.4. Effect of CaCl2 and LaCl3 on the ABA Content in Tea Plants under Cold Stress
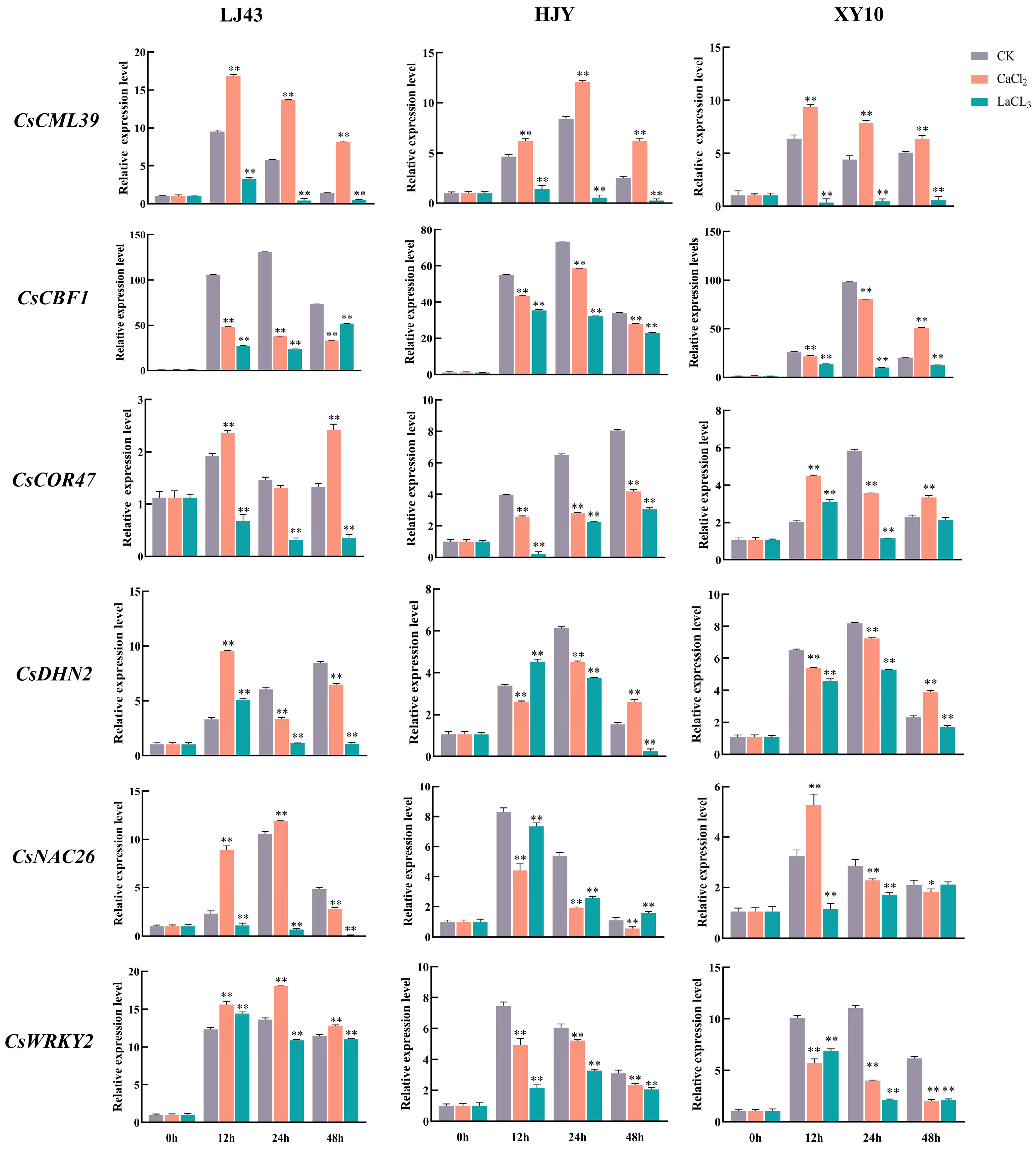
3.5. Effect of CaCl2 and LaCl3 on Cold-Related Gene Expression in Tea Plants under Cold Stress
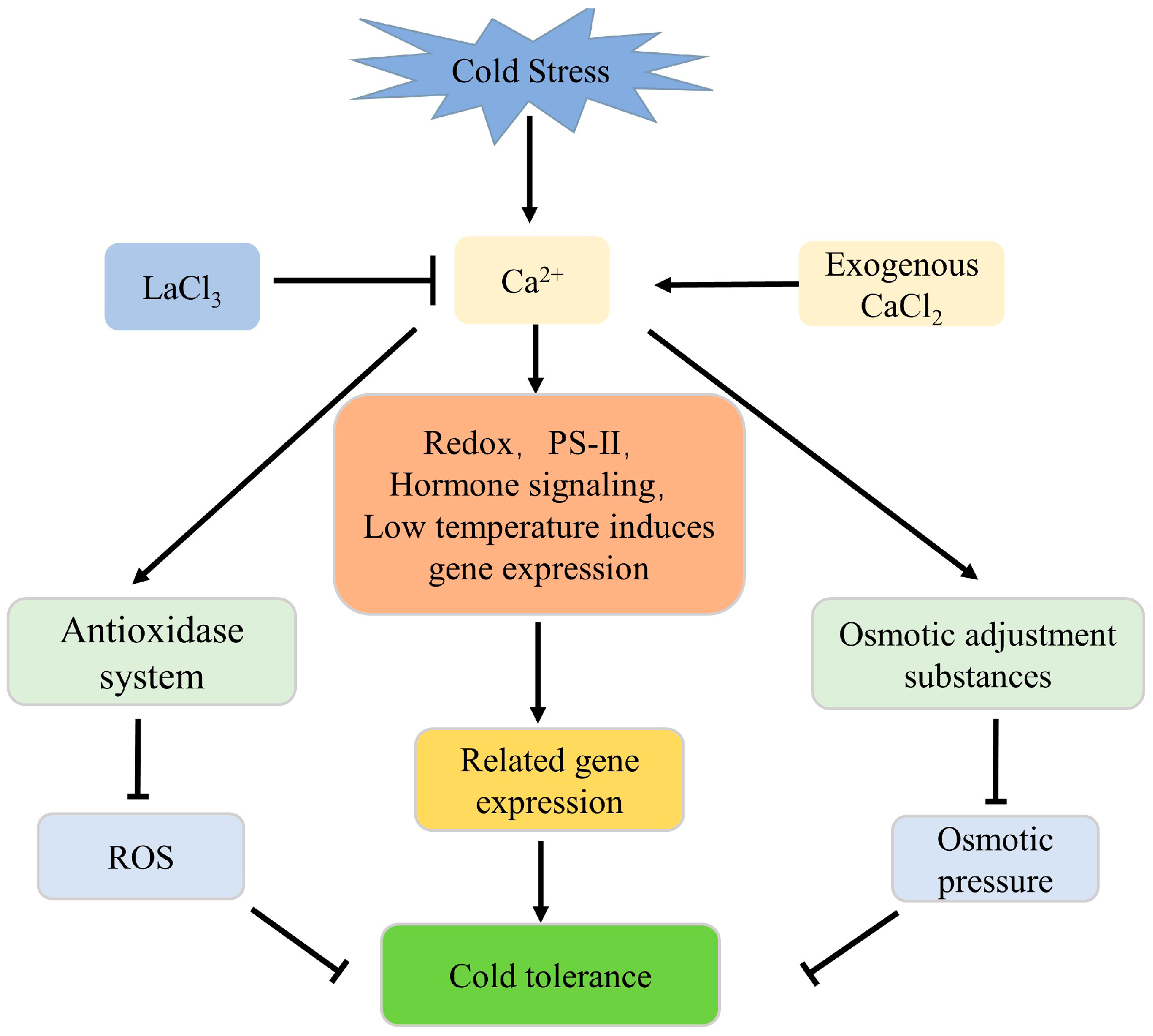
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.; Rout, G.R. Overview of cold stress regulation in plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2011, 63, 43–57. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Chang, Q.; Gu, C.; Chen, F. Cold acclimation induces freezing tolerance via antioxidative enzymes, proline metabolism and gene expression changes in two chrysanthemum species. Mol. Biol. Rep. 2014, 41, 815–822. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Kazemi-Shahandashti, S.S.; Maali-Amiri, R.; Zeinali, H.; Khazaei, M.; Talei, A.; Ramezanpour, S.S. Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J. Plant Physiol. 2014, 171, 1106–1116. [Google Scholar] [CrossRef]
- Dikilitas, M.; Simsek, E.; Roychoudhury, A. Role of proline and glycine betaine in overcoming abiotic stresses. Prot. Chem. Agents Amelior. Plant Abiotic Stress. 2020, 1–23. [Google Scholar] [CrossRef]
- Guan, Y.; Hwarari, D.; Korboe, H.M.; Ahmad, B.; Cao, Y.; Movahedi, A.; Yang, L. Low temperature stress-induced perception and molecular signaling pathways in plants. Environ. Exp. Bot. 2023, 207, 105190. [Google Scholar] [CrossRef]
- Kudla, J.; Batistič, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S. Physiological role of signal molecules in improving plant tolerance under abiotic stress. Int. J. Chem. Tech. Res. 2016, 9, 46–60. [Google Scholar]
- Anil, V.S.; Sankara Rao, K. Purification and characterization of a Ca2+-dependent protein kinase from sandalwood (Santalum album L.): Evidence for Ca2+-induced conformational changes. Phytochemistry 2001, 58, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium-and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Poovaiah, B. Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Robe, E.; Jomat, L.; Aldon, D.; Mazars, C.; Galaud, J.P. CML8, an Arabidopsis Calmodulin-like protein.; plays a role in Pseudomonas syringae plant immunity. Plant Cell Physiol. 2016, 58, 307–319. [Google Scholar]
- Vanderbeld, B.; Snedden, W.A. Developmental and stimulus-induced expression patterns of Arabidopsis calmodulin-like genes CML37, CML38 and CML39. Plant Mol. Biol. 2007, 64, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Leba, L.J.; Perochon, A.; Cheval, C.; Ranty, B.; Galaud, J.P.; Aldon, D. CML9, a multifunctional Arabidopsis thaliana calmodulin-like protein involved in stress responses and plant growth? Plant Signal. Behav. 2012, 7, 1121–1124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, G.Y.; Rocha, P.S.C.F.; Wang, M.L.; Xu, M.L.; Cui, Y.C.; Li, L.Y.; Zhu, Y.X.; Xia, X. A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 2011, 234, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Chinpongpanich, A.; Limruengroj, K.; Phean-O-Pas, S.; Limpasen, T.; Buaboocha, T. Expression analysis of calmodulin and calmodulin-like genes from rice, Oryza sativa L. BMC Res. Notes 2012, 5, 625. [Google Scholar] [CrossRef] [PubMed]
- Yuenyong, W.; Chinpongpanich, A.; Comai, L.; Chadchawan, S.; Buaboocha, T. Downstream components of the calmodulin signaling pathway in the rice salt stress response revealed by transcriptome profiling and target identification. BMC Plant Biol. 2018, 18, 335. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Liu, H.; Xing, Y.; Hussain, S.; Ouyang, B.; Zhang, Y.; Li, H.; Ye, Z. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016, 6, 31772. [Google Scholar] [CrossRef]
- Li, C.; Meng, D.; Zhang, J.; Cheng, L. Genome-wide identification and expression analysis of calmodulin and calmodulin-like genes in apple (Malus × domestica). Plant Physiol. Biochem. 2019, 139, 600–612. [Google Scholar] [CrossRef]
- Lu, L.; Rong, W.; Zhou, R.H.; Huo, N.X.; Zhang, Z.Y. TaCML36, a wheat calmodulin-like protein, positively participates in an immune response to. Crop J. 2019, 7, 608–618. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Kiselev, K.V.; Ogneva, Z.V.; Dubrovina, A.S. The grapevine Calmodulin-like protein gene CML21 is regulated by alternative splicing and involved in abiotic stress pesponse. Int. J. Mol. Sci. 2020, 21, 7939. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Li, J.; Yang, W.; Ci, J.; Ren, X.; Wang, W.; Wang, Y.; Jiang, L.; Yang, W. Identification and expression analysis revealed drought stress-responsive Calmodulin and Calmodulin-like genes in maize. J. Plant Interact. 2022, 17, 450–461. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liang, S.; Chen, X.; Liu, J.; Zhang, Y.; Wang, A. Identification of CDPK gene family in Solanum habrochaites and its function analysis under stress. Int. J. Mol. Sci. 2022, 23, 4227. [Google Scholar] [CrossRef]
- Ma, S.Y.; Wu, W.H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol. Biol. 2007, 65, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Mo, Z.; Yuan, G.; Xiang, H.; Visser, R.G.F.; Bai, Y.; Liu, H.; Wang, Q.; van der Linden, C.G. The CBL-CIPK network is involved in the physiological crosstalk between plant growth and stress adaptation. Plant Cell Environ. 2022, 46, 3012–3022. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, Y.; Pang, J.; Yong, J.W.H.; Chen, Y.; Bai, C.; Gille, C.; Shi, Q.; Wu, D.; Han, X. Supplementary calcium restores peanut (Arachis hypogaea) growth and photosynthetic capacity under low nocturnal temperature. J. Plant Physiol. 2020, 258, 153373. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yang, S.; Wang, Y.; Lu, L.; Sun, M.; He, C.; Wang, J.; Li, Y.; Yu, X.; Li, Q. Physiological and molecular mechanisms of ABA and CaCl2 regulating chilling tolerance of Cucumber seedlings. Plants 2021, 10, 2746. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, H.; Ma, H.; Lyu, D. Differential proteomic analysis reveals the effect of calcium on Malus baccata Borkh. leaves under temperature stress. Int. J. Mol. Sci. 2017, 18, 1755. [Google Scholar] [CrossRef]
- Liu, Y.F.; Qi, M.F.; Li, T.L. Photosynthesis, photoinhibition, and antioxidant system in tomato leaves stressed by low night temperature and their subsequent recovery. Plant Sci. 2012, 34, 263–273. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Ni, Y.; Meng, Z.; Lu, T.; Li, T. Exogenous calcium alleviates low night temperature stress on the photosynthetic apparatus of tomato leaves. PLoS ONE 2014, 9, e97322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Zhang, G.X.; Qi, M.F.; Li, T.L. Effects of calcium on photosynthesis, antioxidant system and chloroplast ultrastructure in tomato leaves under low night temperature stress. J. Plant Growth Regul. 2015, 34, 263–273. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Z.; Zheng, Y.; Jin, P. Effects of CaCl2 treatment alleviates chilling injury of Loquat fruit (Eribotrya japonica) by modulating ROS homeostasis. Foods 2021, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, Y.; Zhang, L.; Zhao, M.; Wang, C. Adapting tea production to climate change under rapid economic development in China from 1987 to 2017. Agronomy 2022, 12, 3192. [Google Scholar] [CrossRef]
- Wang, Y.; Samarina, L.; Mallano, A.I.; Tong, W.; Xia, E. Recent progress and perspectives on physiological and molecular mechanisms underlying cold tolerance of tea plants. Front. Plant Sci. 2023, 14, 1145609. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Lee, U.; Vierling, E. Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol. 2003, 132, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.F.; Li, Y.; Chen, Y.; Zhou, Y.; Yu, T.; Zhou, Y.; Yang, Y. Spectral light quality regulates the morphogenesis, architecture, and flowering in pepper (Capsicum annuum L.). J. Photochem. Photobiol. B 2023, 241, 112673. [Google Scholar] [CrossRef]
- Hao, X.Y.; Horvath, D.P.; Chao, W.S.; Yang, Y.J.; Wang, X.C.; Xiao, B. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze). Int. J. Mol. Sci. 2014, 15, 22155–22172. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Dhingra, M. Physiological responses and tolerance mechanisms of low temperature stress in plants. Int. J. Adv. Res. 2015, 3, 637–646. [Google Scholar]
- Ding, Y.; Shi, Y.; Yang, S. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 2004, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Browse, J.; Xin, Z. Temperature sensing and cold acclimation. Curr. Opin. Plant Biol. 2001, 4, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Yu, Q.; Ma, Y.; Gu, W.; Yang, D. Physiological changes associated with enhanced cold resistance during maize (Zea mays) germination and seedling growth in response to exogenous calcium. Crop Pasture Sci. 2020, 71, 529–538. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Zhong, B.; Liu, X.; Chan, Z. Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J. Integr. Plant Biol. 2014, 56, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N. Abscisic acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, J.; Yuan, Y.; Chen, L.; Ma, J.; Li, X.; Li, J. The mechanism of abscisic acid regulation of wild Fragaria species in response to cold stress. BMC Genom. 2022, 23, 670. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Niu, L.; Jameson, P.E.; Zhou, W. Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant Physiol. 2021, 186, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shi, H.; Hu, Z.; Liu, A.; Amombo, E.; Chen, L.; Fu, J. ABA is involved in regulation of cold stress response in bermudagrass. Front. Plant Sci. 2017, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Castro-Cegrí, A.; Sierra, S.; Hidalgo-Santiago, L.; Esteban-Muñoz, A.; Jamilena, M.; Garrido, D.; Palma, F. Postharvest treatment with abscisic acid alleviates chilling injury in Zucchini fruit by regulating phenolic metabolism and non-enzymatic antioxidant system. Antioxidants 2023, 12, 211. [Google Scholar] [CrossRef]
- Pospíšilová, J.; Synková, H.; Haisel, D.; Baťková, P. Effect of abscisic acid on photosynthetic parameters during ex vitro transfer of micropropagated tobacco plantlets. Biol. Plant. 2009, 53, 11–20. [Google Scholar] [CrossRef]
- Verslues, P.E.; Zhu, J.K. Before and beyond ABA: Upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem. Soc. Trans. 2005, 33, 375–379. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, P.; Bai, Z.; Herde, M.; Ma, Y.; Li, N.; Liu, S.; Huang, C.F.; Yang, Z.B. Calmodulin-like protein CML24 interacts with CAMTA2 and WRKY46 to regulate ALMT1-dependent Al resistance in Arabidopsis thaliana. New Phytol. 2005, 233, 2471–2487. [Google Scholar] [CrossRef]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The central role of transcription factors in bridging biotic and abiotic stress responses for plants’ resilience. New Crops. 2024, 1, 100005. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.; Teng, R.; Wang, Y.; Wang, W.; Zhuang, J. Genomic and transcriptomic analyses of HD-Zip family transcription factors and their responses to abiotic stress in tea plant (Camellia sinensis). Genomics 2018, 111, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Tang, H.; Wang, B.; Yue, C.; Wang, L.; Zeng, J.; Yang, Y.; Wang, X. Integrative transcriptional and metabolic analyses provide insights into cold spell response mechanisms in young shoots of the tea plant. Tree Physiol. 2018, 38, 1655–1671. [Google Scholar] [CrossRef]
- Paul, A.; Kumar, S. Dehydrin2 is a stress-inducible, whereas Dehydrin1 is constitutively expressed but up-regulated gene under varied cues in tea [Camellia sinensis (L.) O. Kuntze]. Mol. Biol. Rep. 2013, 40, 3859–3863. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Xie, X.; Shu, X.; Deng, W.; Jiang, C. Cloning and expression analysis of dehydrin gene (csDHN) in tea plant (Camellia sinensis). J. Agric. Biotechnol. 2016, 24, 332–341. [Google Scholar]
- Wang, Y.; Shu, Z.; Wang, W.; Jiang, X.; Li, D.; Pan, J.; Li, X. CsWRKY2, a novel WRKY gene from Camellia sinensis, is involved in cold and drought stress responses. Biol. Plant. 2016, 60, 443–451. [Google Scholar] [CrossRef]
- Samarina, L.S.; Malyukova, L.S.; Efremov, A.M.; Simonyan, T.A.; Matskiv, A.O.; Koninskaya, N.G.; Rakhmangulov, R.S.; Gvasaliya, M.V.; Malyarovskaya, V.I.; Ryndin, A.V. Physiological, biochemical and genetic responses of Caucasian tea (Camellia sinensis (L.) Kuntze) genotypes under cold and frost stress. PeerJ 2020, 8, e9787. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, Z.W.; Wu, Z.J.; Li, H.; Zhuang, J. Transcriptome-wide identification and expression analysis of the NAC gene family in tea plant [Camellia sinensis (L.) O. Kuntze]. PLoS ONE 2016, 11, e0166727. [Google Scholar] [CrossRef]
- Wayne, A.S.; Hillel, F. Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 2001, 151, 35–66. [Google Scholar]
- Tang, M.; Xu, C.; Cao, H.; Shi, Y.; Chen, J.; Chai, Y.; Li, Z. Tomato calmodulin-like protein SlCML37 is a calcium (Ca2+) sensor that interacts with proteasome maturation factor SlUMP1 and plays a role in tomato fruit chilling stress tolerance. J. Plant Physiol. 2021, 258, 153373. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, L.; Hao, Y.; Xiao, S.; Wu, Z.; Chen, W.; Li, X.; Zhu, X. Genome-wide identification and expression analyses of the calmodulin and calmodulin-like proteins reveal their involvement in stress response and fruit ripening in papaya. Postharvest Biol. Technol. 2018, 143, 13–27. [Google Scholar] [CrossRef]
- Delk, N.A.; Johnson, K.A.; Chowdhury, N.I.; Braam, J. CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol. 2005, 139, 240–253. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Primer Sequences (5′–3′) | Primer Sequences (5′–3′) |
|---|---|---|
| CsCML39 | F: TGGACTCCGATGGAAGCCTAAC | R: GCCTCGCTCATATCGGGTAAAA |
| CsCBF1 | F: AACTGAAACTGCGACTGAGACGA | R: AGGCGGTGGAGGAGGTAGC |
| CsCOR47 | F: CACAAATGTAGAAACCATCCCC | R: CTCCTTCCTTCAAATCTACAACAGT |
| CsDHN2 | F: ACT TATGGCACCGGCACTA | R: CCTTCCTCCTCCCTCCTTGAC |
| CsWRKY2 | F: GAGACAGAAATGAGCAGGGAAAA | R: TGTATCGGTGTCAGTTGGGTAGA |
| CsNAC26 | F: ACAAACTACGCCACAATGC | R: AGGGAGGGTTCTTTTCAGG |
| CsPTB | F: ACCAAGCACACTCCACACTATCG | R: TGCCCCCTTATCATCATCCACAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, L.; Kang, R.; Liu, C.; Xing, L.; Wu, S.; Wang, Z.; Wu, C.; Zhou, Q.; Zhao, R. Exogenous Calcium Alleviates the Photosynthetic Inhibition and Oxidative Damage of the Tea Plant under Cold Stress. Horticulturae 2024, 10, 666. https://doi.org/10.3390/horticulturae10070666
Chen S, Wang L, Kang R, Liu C, Xing L, Wu S, Wang Z, Wu C, Zhou Q, Zhao R. Exogenous Calcium Alleviates the Photosynthetic Inhibition and Oxidative Damage of the Tea Plant under Cold Stress. Horticulturae. 2024; 10(7):666. https://doi.org/10.3390/horticulturae10070666
Chicago/Turabian StyleChen, Siwen, Long Wang, Rui Kang, Chunhui Liu, Liyuan Xing, Shaobo Wu, Zhihui Wang, Chunlai Wu, Qiongqiong Zhou, and Renliang Zhao. 2024. "Exogenous Calcium Alleviates the Photosynthetic Inhibition and Oxidative Damage of the Tea Plant under Cold Stress" Horticulturae 10, no. 7: 666. https://doi.org/10.3390/horticulturae10070666
APA StyleChen, S., Wang, L., Kang, R., Liu, C., Xing, L., Wu, S., Wang, Z., Wu, C., Zhou, Q., & Zhao, R. (2024). Exogenous Calcium Alleviates the Photosynthetic Inhibition and Oxidative Damage of the Tea Plant under Cold Stress. Horticulturae, 10(7), 666. https://doi.org/10.3390/horticulturae10070666






