Abstract
Plant hormones regulate adaptive responses to various biotic and abiotic stress factors. Applied exogenously, they trigger the natural plant defense mechanisms, a feature that could be implemented in strategies for supporting crop resilience. The potential of the exogenous cytokinin-like acting compound (kinetin), the auxin analogue 1-naphtyl acetic acid (NAA), abscisic acid (ABA) and the ethyleneprecursor 1-aminocyclopropane-1-carboxylic acid (ACC) to mitigate dehydration was tested on Lactuca sativa (lettuce) grown on 12% polyethylene glycol (PEG). Priming with different blends containing these plant growth regulators (PGRs) applied in bioequivalent concentrations was evaluated through biometric measurements and biochemical analyses. The combined treatment with the four compounds exhibited the best dehydration protective effect. The antioxidative enzyme profiling of the PGR-primed individuals revealed increased superoxide dismutase (SOD), catalase and peroxidase activity in the leaves. Immunodetection of higher levels of the rate-limiting enzyme for proline biosynthesis (delta-pyroline-5-carboxylate synthase) in the primed plants coincided with a significantly higher content of the amino acid measured in the leaves. These plants also accumulated particular dehydrin types, which may have contributed to the observed stress-relieving effect. The four-component mix applied by spraying or through the roots exerted similar stress-mitigating properties on soil-grown lettuce subjected to moderate drought.
1. Introduction
The sustainable food chain is a major concern of modern agriculture put to the test by the continued adverse impact of climate change and the pressing need for innovative approaches to encourage organic plant breeding. The demands of the world’s growing population should be met by maintaining a nutritious and healthy diet with the limited use of pesticides and fertilizers. Several recently introduced policies like the EU “Green Deal” [1] and the EU “Farm to Fork Strategy” [2] aim to significantly reduce the use of chemicals in food production. The new approaches implemented in these initiatives often consider the application of natural biostimulants to foster plant growth and development throughout the crop life cycle [3]. It has been shown that these products could also be used as stress tolerance boosters or abiotic stress recovery promoters, mainly through facilitating nutrient assimilation and translocation or rendering water use efficiency [4]. Biostimulants exert growth-promoting or stress-protective functions in low concentrations through mechanisms that differ from the ones of fertilizers and pesticides. Often, their mode of action is linked to the hormonal impacts of the physiologically active levels of auxins, gibberellins, cytokinins, abscisic acid and jasmonates that are found in the products [5,6,7]. At present, most of the offered formulations labeled as plant growth regulators contain analogues of auxins, gibberellins and cytokinins that are capable of eliciting plant growth, nutrient uptake and stress tolerance responses [8]. The evaluation of their safety and efficiency is necessary to foster the wider application of biostimulants and PGR-based products in “green” agricultural practices.
Drought has a significant impact on plant growth and development, leading to a detrimental impact on the quality and yield of food crops [9,10]. In general, the leafy greens are more vulnerable to drought stress, and vegetables such as lettuce need constant moisture availability. Modern horticulture seeks new methods to improve the drought tolerance of field-grown plants through different agronomic and breeding approaches. These include seed priming, grafting and the use of biostimulants. Some modern genomic techniques like speed breeding, genetic modifications and genome editing have also been recently introduced [11]. These different strategies are largely based on the fortification of the genetically defined capacity of each organism to withstand unfavorable environments. Plants have evolved multiple defense mechanisms to maintain their growth under water-limited conditions comprising enzyme and non-enzyme antioxidants, including metabolites and proteins with protective properties [12]. Together with particular stress-induced anatomical and physiological changes (like stomata closure and increased root growth), these molecular mechanisms facilitate adaptation and survival under limited water supply. When challenged by water scarcity, plant cells regulate their osmotic adjustment through the accumulation of compatible solutes (proline, glycine betaine, fructans, etc.) and protective proteins that can maintain the native structure of the enzymatic complexes [13]. Dehydrins (DHNs) are part of this line of defense, and upon reduced water supply, their levels in the nucleus and cytoplasm usually increase [14]. Their unique molecular properties (thermostability and high hydrophilicity) make them a potent element of molecular defense under stress. They can stabilize large-scale hydrophobic interactions in the plant cell that occur among the various membrane structures or hydrophobic protein clusters. The effects of phytohormone-based biostimulants on the accumulation of DHNs are not yet elucidated but several indicative case studies point to the potential protective effect of these products through the activation of certain dehydrin types [15,16].
Reactive oxygen species (ROS) that accumulate under dehydration provoke oxidative damage to the cellular components (DNA, proteins and membrane lipids). It has been demonstrated that some phytohormone-based growth regulators support the synthesis of essential antioxidant enzymes such as catalase (CAT), peroxidase (POX) and superoxide dismutase, enabling plants to scavenge the harmful free oxygen radicals [17]. Often, exogenously applied PGRs exert their protective effects through the upregulation of genes coding for antioxidant enzymes [18]. Therefore, the analyses of the changes in gene expression of elements from the plant defense system could be helpful for elucidating the antioxidant protective potential of exogenously applied compounds.
The complex physiological reactions and the synthesis of protective compounds that are activated when plants enter a “stress-defense mode” are controlled by a complex regulatory network driven by various hormonal interactions. Based on this, the present study aimed to elucidate the protective potential of abscisic acid and the ethylene precursor 1-aminocyclopropan-carboxylic acid applied in concentrations corresponding to the naturally observed range in drought-stressed plants [19,20]. The positive effect of exogenous auxin on plant performance under dehydration stress was previously observed [21] and we also include in the tests the auxin analogue 1-naphthyl acetic acid. A well-developed root system presents a trait that is crucially important for plant survival under limited water supply. It has been demonstrated that lateral root formation is regulated by the crosstalk of auxins, ethylene and ABA [22,23,24], which presents an additional prospective benefit of their exogenous application. Since the growth and formation of roots are shaped by the signaling pathways of both auxin and cytokinins [25,26], we also considered in the tests the cytokinin-like acting compound kinetin. The broad screen of the pretreatment effects of ABA, ACC, NAA and KIN applied separately or in different combinations resulted in the identification of a PGR blend that mitigates dehydration in Lactuca sativa. The experiments were performed with hydroponically grown plants using 12% polyethylene glycol as a dehydrating agent.
2. Materials and Methods
2.1. Growth Conditions and Plant Material
2.1.1. Hydroponic Experiment with 12% PEG
Lactuca sativa L. cv. Lobjoits, green cos plants (CN seeds Ltd., Cambridgeshire, UK), were grown for 14 days on rockwool cubes (Saint-Gobain Cultilene B.V., Rijen, The Netherlands) soaked in ½ Hoaglands–Arnon nutrient solution under controlled conditions (60% air humidity; 200 μmol m−2 s−1 photon flux density; 21/18 °C day/night temperature; and 14/10 h photoperiod). The treatments were applied on seedlings with a second fully developed leaf at day 15 of the experiment. Each treatment group consisted of 20 individuals that were sprayed with approximately 20 mL/m2 of the following PGR solutions: 2 µM ABA (Carl Roth, Karlsruhe, Germany), 5 µM 1-aminocyclopropane-1-carboxylate (Sigma-Aldrich, Darmstadt, Germany), 4 µM 1-naphthyl acetic acid (Sigma-Aldrich, Darmstadt, Germany) and 0.2 µM kinetin (Sigma-Aldrich, Darmstadt, Germany). In the treatments, varying combinations of the compounds applied in the same concentrations were also included (listed in Table 1). The stress hormone ABA was present in each of them except in Mix 2 which contained only the growth-stimulating hormonal analogues.

Table 1.
Composition of tested PGR mixes.
The controls were sprayed with the same amount of “Mock” solution (distilled H2O and 0.05% Tween-20, used as a surfactant).
Twenty-four hours after PGR application (on day 16 of the experiment), half of the plants from each treatment group were transferred to a nutrient solution with 12% PEG. The remaining individuals were transferred to fresh media without PEG. The nutrient solution (+/− PEG) was refreshed every 5 days to ensure the optimal composition of macro- and microelements.
The fresh (FW) and dry weight (DW) of the plants were measured at day 25 of the experiment (corresponding to 10-day PEG stress). Shoot area was determined by using a Rosette Tracker plug-in in Image J 1.52r according to [27]. Samples derived from the second leaf were collected and preserved for further analyses.
2.1.2. Soil Drought Experiment (“Proof-of-Concept”)
Seeds from Lactuca sativa L. cv. Lobjoits, green cos, were grown on 44 mm Jiffy® Peat Pellets (Jiffy Growing Solutions, Zwijndrecht, The Netherlands) saturated with water at 80% of the substrate’s field capacity (FC). The plants were grown for two weeks under controlled conditions (60% air humidity; 200 μmol m−2 s−1 photon flux density; 21/18 °C day/night temperature; and 14/10 h photoperiod). On day 15, the PGR combination (Mix 7) or a “Mock” solution was administered via foliar spray (20 mL/m2) or through the roots (1 mL per each plant grown separately on a single pellet). After the treatments, the plants were split into control and drought-stressed groups. Moderate drought stress (30% FC) occurred after 5 days [28] and it was maintained for an additional 5-day period. The humidity of the Jiffy pellets in the control groups was kept at 80% FC. Shoot area and fresh and dry weight of individuals subjected to the different treatments were measured at the end of the experiment (day 25).
2.2. Biochemical Analyses
2.2.1. Stress Markers and Non-Enzyme Antioxidants
The leaf samples were grinded in 1% cold trichloroacetic acid (TCA) and centrifuged at 15,000× g for 30 min (at 4 °C). The supernatant was used for biochemical analyses of the stress-related biomarkers malondialdehyde (MDA), hydrogen peroxide and free proline. The same extracts were used for the determination of phenolics and free thiolgroup-containing compounds.
MDA was analyzed according to [29] with a reagent containing 0.5% thiobarbituric acid (TBA) in 20% trichloroacetic acid. The absorbance of the substances resulting from the TBA reaction was read at 532 nm and 600 nm. MDA content was calculated using an extinction coefficient of 155 mM cm−1.
Hydrogen peroxide level was measured following a previously described protocol [30]. The absorbance was recorded at 390 nm, and the results were calculated using a standard curve.
The free proline content (L-Pro) was estimated according to [31] with some modifications. In brief, 1 mL of freshly prepared ninhydrin reagent (1.25 g of ninhydrin, 30 mL of CH3COOH, 20 mL of 6M H3PO4) was combined with 0.5 mL supernatant, 0.5 mL 0.1% TCA, and 1 mL CH3COOH. The mix was incubated for 1 h in a 100 °C water bath. The reaction was stopped by putting the samples on ice. The absorbance of the reaction mix was measured at 520 nm and the concentration was calculated using a standard curve.
The content of phenolic compounds was estimated according to a modified version of an established protocol [32]. Aliquots (20 µL) of the supernatant were incubated with 130 µL dH2O and 50 µL Folin–Ciocalteu reagent at room temperature for 3 min. Fifty microliters of 1 M Na2CO3 were added to the reaction mix and after that, it was exposed to light for two hours. The absorbance of the samples was measured at 725 nm. The content of the phenolics was calculated using a standard curve prepared with gallic acid as a referent.
The content of free thiol group-containing compounds (free sulfhydryl groups, –SH groups) was determined according to [33]. The absorbance of the reaction mixture (comprised of 40 µL supernatant and 150 µL Elman’s reagent) was measured at 412 nm after 10 min of incubation at room temperature.
2.2.2. Antioxidant Enzyme Activities
For the assessment of the activities of antioxidant enzymes, approximately 300 mg fresh leaf material was homogenized in cold potassium phosphate buffer (0.1 M) containing 1 mM EDTA, pH 7.0, and 1% PVP. The homogenate was centrifuged at 15,000× g for 30 min (at 4 °C).
Catalase activity (EC 1.11.1.6) was evaluated by monitoring H2O2 degradation for 1 min at 240 nm [34]. The reaction mixture consisted of 100 µL of supernatant, 2.880 mL of reaction potassium phosphate buffer (50 mM, pH 7.0) and 20 µL of freshly prepared 6% hydrogen peroxide.
Guaiacol peroxidase activity (EC 1.11.1.7) was assessed at 470 nm for 1 min by using guaiacol as an electron donor [35]. The reaction mixture contained 100 µL supernatant, 1.020 mL reaction buffer (50 mM potassium phosphate buffer, pH 7.0), 360 µL 1% guaiacol and 20 µL 15% H2O2.
The inhibition of the photochemical reduction of nitroblue tetrazolium was used to measure the activity of superoxide dismutase (EC 1.15.1.1) at 560 nm [36]. One unit of SOD corresponds to an enzyme amount capable of 50% substrate inhibition.
The content of total soluble protein was determined according to [37].
2.3. Immunoblot Analyses of Dehdyrins
Soluble proteins were extracted from 0.3 g leaf samples with ice-cold 100 mM Tris–HCl buffer, pH 7.4, containing 2 mM phenyl-methane-sulfonyl fluoride (PMSF), 12.5% glycerol (v/v), 20 mM β-mercaptho-ethanol and 2% Polyclar® (polyvinyl polypyrrolidone). Equal amounts of total soluble protein (25 µg per lane) were separated by 11% SDS-PAGE in a Mini Protean Dual Slab Cell (BioRad, Hercules, CA, USA) with PageRuler Prestained Protein Ladder 10-170 kDa (Thermo Scientific, Basel, Switzerland) loaded as a molecular weight reference. The separated proteins were transferred to a nitrocellulose membrane using the Trans-Blot system (BioRad, Hercules, CA, USA). The membrane was blocked in TBS buffer (0.1 M Tris, pH 7.9, 0.15 M NaCl) containing 1% bovine serum albumin (BSA) at room temperature for 60 min. The immunodetection protocols using antibodies against K-, S- and Y- segments for identification of dehydrin profiles and anantibody against Delta-1-Pyrroline-5-Carboxylate Synthetase (P5CS, EC:2.7.2.11) are previously described [38,39]. Goat anti-rabbit IgG and peroxidase–anti-peroxidase soluble complex (Sigma-Aldrich, Darmstadt, Germany) were used to visualize the immunoblot signals according to a previously described method [40]. The peroxidase reaction was developed with 4-chloro-alpha-naphtol (Sigma-Aldrich, Darmstadt, Germany).
2.4. RT-qPCR Analysis of SOD, CAT and Peroxidase-Coding Genes
GeneJET Plant RNA Purification Kit (Thermo Scientific, Waltham, MA, USA) was used to extract total RNA. The synthesis of cDNA was performed with 100 ng total RNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific™, Vilnius, Lithuania) according to the manufacturer’s instructions. The gene transcript abundance was evaluated by quantitative real-time RT-PCR (RT-qPCR) using 2× GreenMasterMix No ROXTM (GENAXXON Bioscience, Ulm, Germany) with ‘PikoReal’ Real-Time PCR System (Thermo Scientific, Basel, Switzerland). The PCR program settings were as follows: 95 °C for 15 min and 45 cycles of 95 °C for 15 s followed by 60 °C for 30 s, and melting curve analysis with a temperature range of 60–95 °C in 0.2 °C increments for 60 s.
The relative expression of the target genes was calculated by using the ΔΔCq method [41] using actin (Gene Bank ID: XM_023878805) and 18S ribosomal RNA (Gene Bank ID: AH001680) as references. Lactuca sativa genes coding for the different antioxidant enzymes that were analyzed and the used respective primers are listed in Table 2.

Table 2.
Oligonucleotides for RT-qPCR analyses of transcripts coding for antioxidant enzymes in Lactuca sativa.
2.5. Statistical Analyses
A completely randomized experimental design was implemented. The presented results are based on three independent experiments and the graphs reflect a representative dataset. The error bars depict the calculated standard error (SE). The statistical significance of the results was evaluated by one-way ANOVA with Duncan’s multiple range test and Students’s t-test at p < 0.05.
3. Results and Discussion
Exogenous application of plant growth-regulating substances is a common practice for improving plant productivity and resilience, including facilitating drought survival (reviewed in [42]). The PGR products that are offered on the market include mainly growth-stimulating compounds like gibberelic acid or cytokinin and auxin analogues (Ascend® SL, Planofix®, CytoGro®, etc.). ABA and ethylene-like acting compounds, such as Ethrel ®, are mainly used for pre-and postharvest management. This is based on their ability to stimulate the maturation and ripening of non-climacteric (mainly ABA) and climacteric fruits (ethylene and ABA). ABA also provokes abscission and seed dormancy, which is particularly important for post-harvest management in grain crops.
A recent study reports that when treated with ethylene in darkness and subsequently exposed to light, plants exhibited enhanced root growth, denser lateral roots and increased fresh weight of aerial tissues, a phenomenon observed both in the model species Arabidopsis thaliana, as well as in various crops like tomatoes, cucumbers and wheat [43]. This newly identified property of the gaseous hormone opens up a new potential for its use in agricultural practice as a growth-boosting compound that fortifies plant vigor and resilience.
The results obtained in the present study suggest that combining stress hormones (ABA, ethylene or its precursor ACC) with growth-stimulating substances (auxins and cytokinins) in bioequivalent amounts and applying them before dehydration could subsequently improve plant performance under water limitation. The strategy is based on the understanding that drought response engages multiple physiological, biochemical and morphological changes driven by a complex network of hormonal inputs.
3.1. The Combined Pretreatment with Bioequivalent Amounts of ABA, ACC, NAA and KIN Ameliorates the Negative Effect of Dehydration Stress Provoked by 12% PEG in Hydroponically Grown Lactuca sativa
3.1.1. Growth Parameters
The effect of exogenous ABA, ACC, NAA and kinetin applied in the intrinsic concentration range of the respective phytohormones was screened.
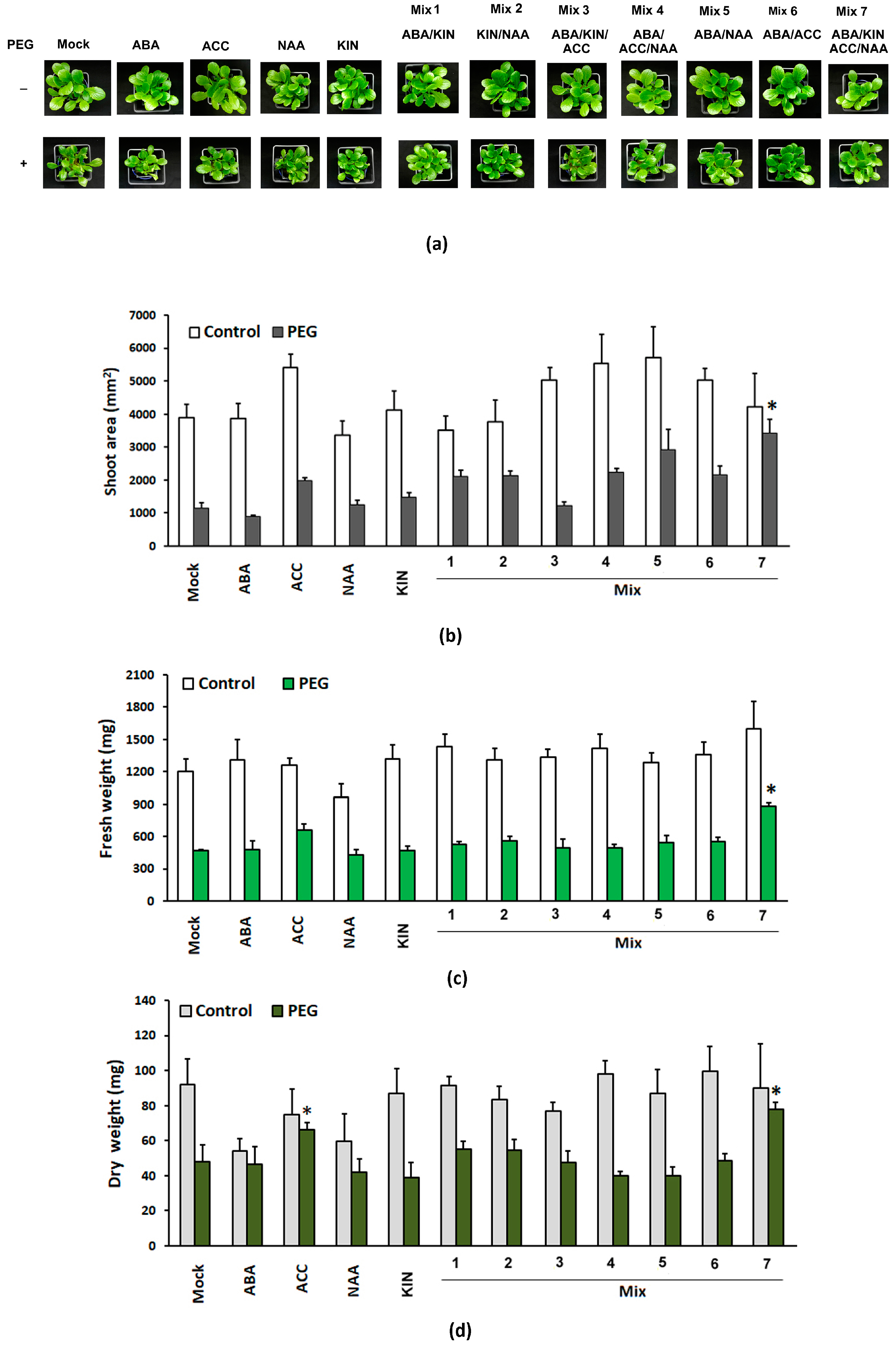
The phenotypes of the different control and PEG-stressed groups are presented in Figure 1a. We did not observe any significant differences in shoot area or fresh and dry weights of the control groups subjected to PGR treatments. This means that if applied exogenously, these compounds do not interfere with the intrinsic mechanisms controlling growth and development under normal conditions. However, in the PEG-stressed variants, the PGR combination comprising the full spectrum of the tested compounds (Mix 7) significantly affected the three growth parameters, with well-sustained shoot growth indicated by the relatively bigger shoot area (Figure 1b). The pretreated group had up to a 20% higher fresh weight and around a 30% higher dry weight than the PEG-stressed non-primed plants (Figure 1c,d). We also observed a weaker positive effect of the ACC application on the growth parameters of the pretreated PEG-stressed plants which exhibited a statistically significant higher dry weight compared to the “Mock” PEG-grown variant (Figure 1d). It is well documented that ethylene (respectively, its precursor ACC) promotes the expansion of root surface area and biomass by manipulating local auxin and cytokinin biosynthesis and transport [44,45]. The higher dry weight of ACC-treated individuals subjected to dehydration could be linked to the root-specific effect of the ethylene precursor (i.e., increased formation of secondary roots and root hairs). This result is in agreement with the recently reported positive effect of ethylene priming that leads to increased growth and stress tolerance [43]. Interestingly, this effect is sustained only in the mix that also contains the auxin and cytokinin analogues (Mix 7), which is related to the proposed mechanism by which ethylene controls root growth and architecture [26]. The observed fluctuations in the growth indexes in the other treatment groups were not found to be statistically significant.
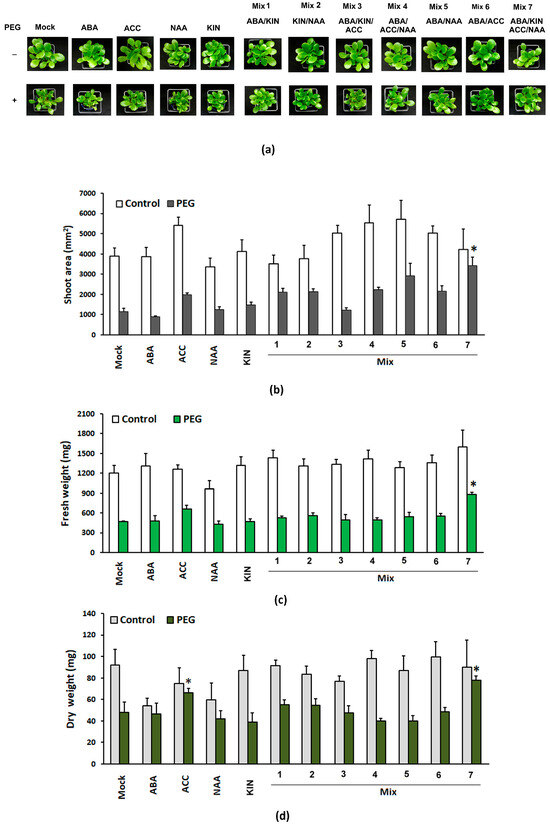
Figure 1.
Representative phenotype (a), shoot area (b), and fresh (c) and dry weight (d) of the aboveground part of Lactuca sativa plants subjected to different PGR pretreatments and subsequent 10-day dehydration stress provoked by 12% PEG. The graphs show the absolute values of the parameters and the error bars indicate the standard error (SE, n = 20). The asterisk marks statistically significant differences with the PEG-affected “Mock”-treated group (one-way ANOVA with Student’s t-test).
3.1.2. Malondialdehyde (MDA), Free Sulfhydryl Groups (SH-Groups) and Total Phenolic Compounds
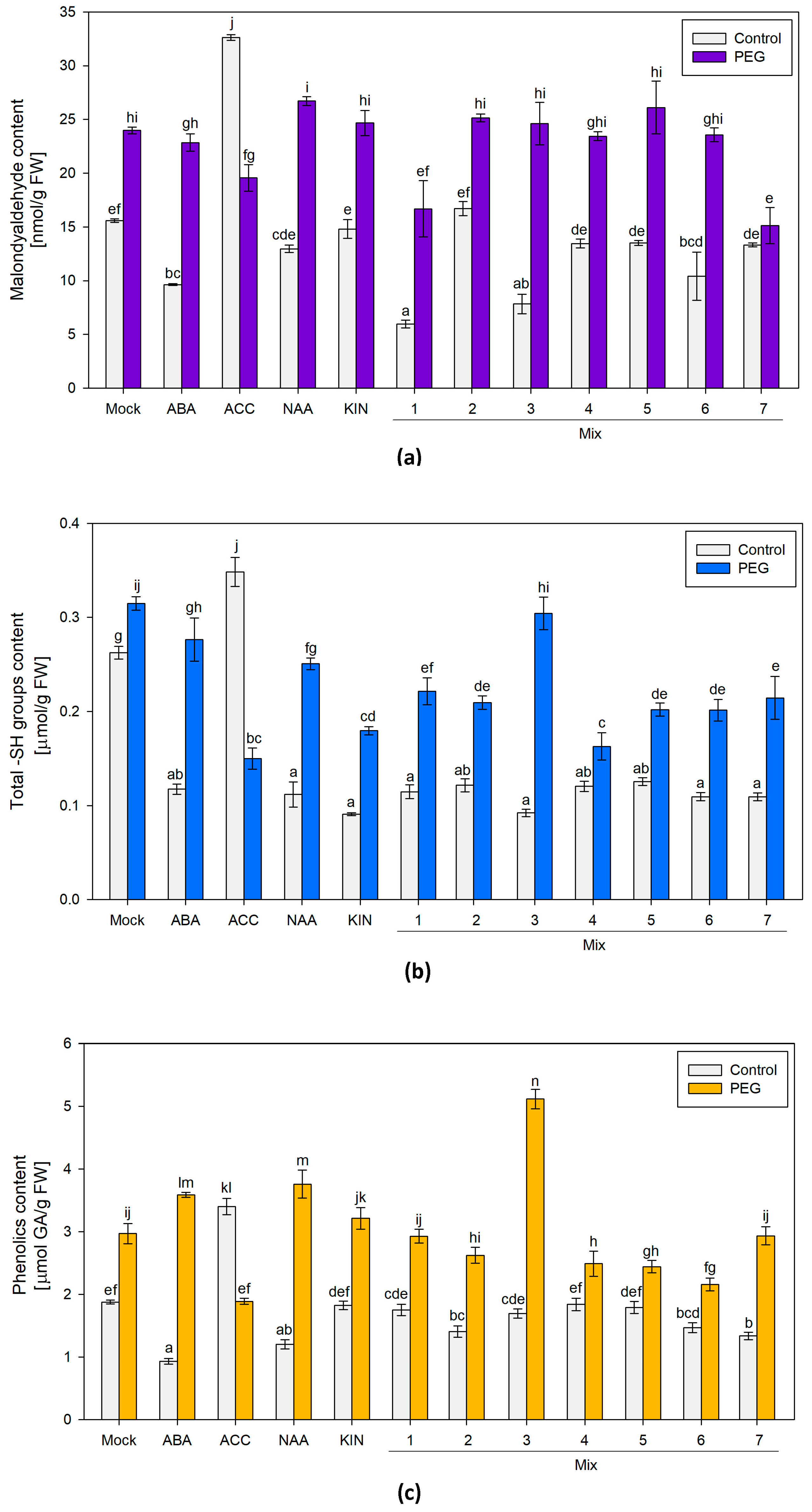
Malondialdehyde (MDA) levels and the total content of sulfhydryl groups and polyphenolic compounds were assessed. The broad screen of the different PGR-treated groups exposed to PEG showed an elevated MDA content comparable to the one measured in the “Mock”-treated plants. The only pretreatment that sustained lower MDA levels in plants exposed to PEG was the group that received Mix 7 (the combination of the four PGRs) (Figure 2a). An interesting observation was made in the ACC-only-treated plants. The ACC-treated individuals grown under optimal conditions had the highest level of the stress marker, while in the similarly treated plants, subjected to osmotic stress, the measured MDA level was comparable to that of the respective “Mock” group (Figure 2a). MDA is a conventional stress marker that is linked to the preserved functionality of the cellular membranes, as it is a product of lipid peroxidation induced by reactive oxygen species (ROS). The lower MDA level in the plants primed with the four-component mix suggests improved capacity to withstand the applied PEG stress.
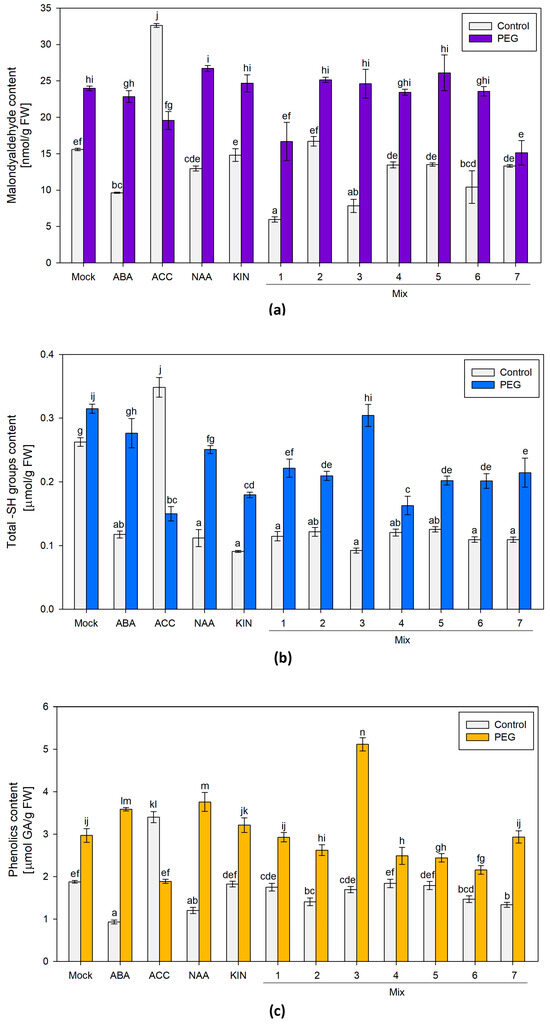
Figure 2.
(a) Malondialdehyde (MDA); (b) free sulfhydryl groups (SH-groups); (c) total phenolic compounds in differently pretreated Lactuca sativa plants grown on nutrient media −/+12% PEG for 10 days. The graphs show the absolute values of the parameters and the error bars indicate the standard error (SE, n = 6). The lowercase letters designate statistically different results (one-way ANOVA with Duncan’s multiple range test at p < 0.05).
Overall, a comparable –SH content was measured in all of the plants subjected to various PGR treatments and grown under optimal conditions (Figure 2b). In most of them, the measured level was lower than the one of the “Mock” control. The differently PGR-treated stressed plants exhibited an increased total content of sulfhydryl groups similar to or slightly below the respective control values. The only exception to these trends was the ACC-treated groups. The plants that received only a dose of the ethylene precursor and were grown under optimal conditions had the highest –SH level among all of the tested treatments. In contrast, a reverse trend of diminishing sulfhydryl group content was observed in the ACC-pretreated individuals upon PEG exposure (Figure 2b). The total –SH group level is a molecular indicator of the intact physiological capacity of the plant to respond to various developmental and environmental cues. Low-molecular sulfhydryl groups (–SH groups), mainly associated with potent non-enzymatic antioxidants like glutathione and cysteine, are highly reactive to oxidative stress that occurs along with dehydration [46]. The sulfhydryl groups in proteins, on the other hand, are actively involved in enzyme-based catalysis, metal coordination, protein folding and protein–protein interactions [46].
The accumulation of phenolics is a common reaction of plants challenged by various environmental stressors. Due to their chemical characteristics, these compounds can operate as non-enzymatic antioxidants. Therefore, this parameter was also included in the broad stress-response screen of the PGR pretreatments and their effect upon dehydration (Figure 2c). The highest amount of polyphenolics was detected in the PEG-treated group primed with Mix 3 (a combination of ABA, KIN and ACC). The other stressed plants also accumulated higher polyphenol levels comparable to or slightly below the “Mock”-treated dehydrated individuals. Both ACC-treated groups again showed an odd response, i.e., higher polyphenolics were detected in the controls and lower ones in the PEG-affected individuals treated with the ethylene precursor (Figure 2c). Although the changes in polyphenolics upon dehydration did not show any significant deviations as a result of Mix 7 priming, the combination of ABA, NAA, KIN and ACC exerted a protective effect, resulting in better growth indexes compared to the other tested PGR combinations.
The data obtained in the broad screen of differently primed experimental groups motivated the subsequent detailed analyses of the Mix 7 combination as it exhibited the best stress-mitigating effect.
3.2. Combined Pretreatment with ABA, ACC, NAA and KIN Activates Enzymatic Antioxidant Defense
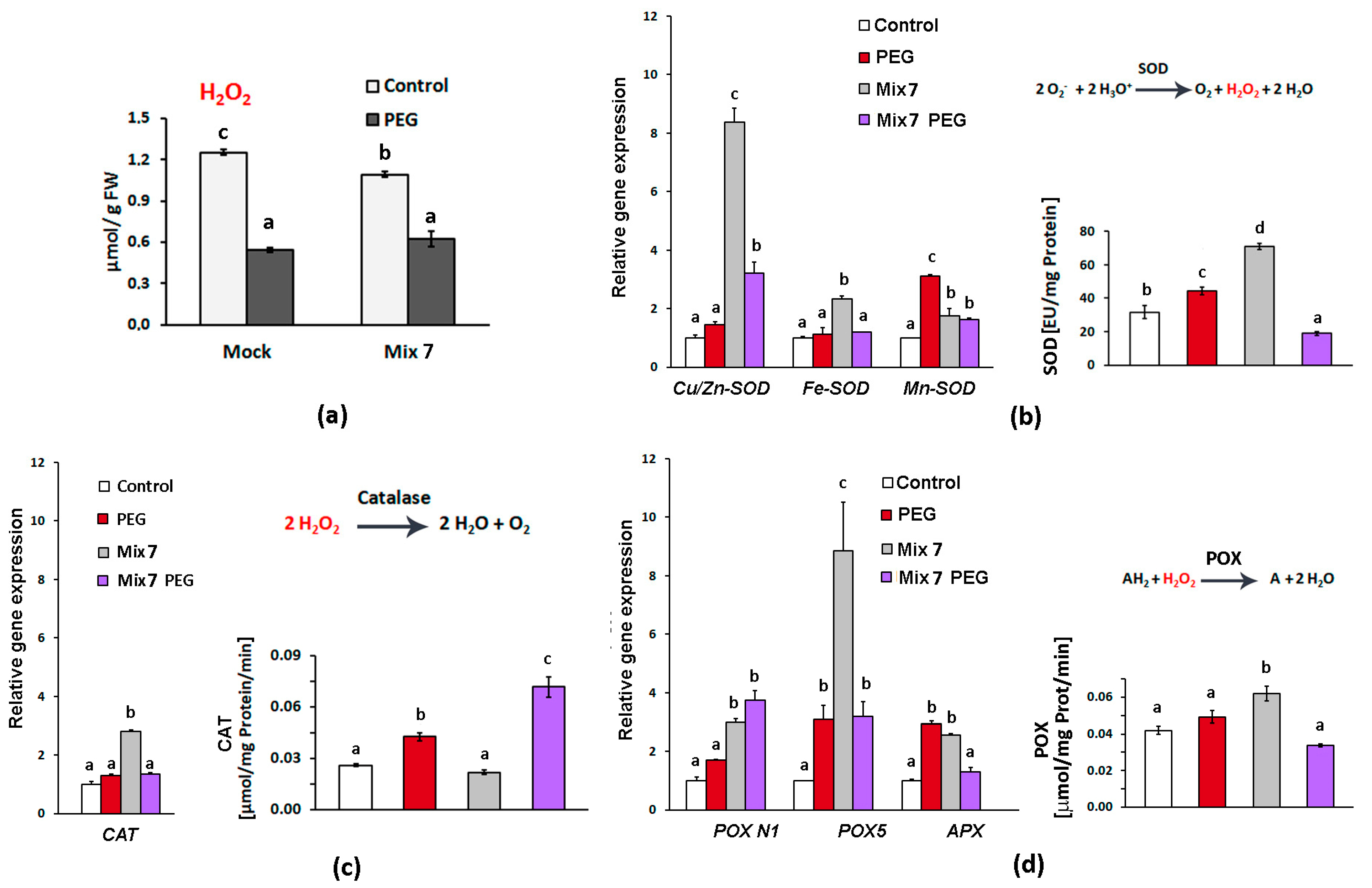
Drought is accompanied by cellular oxidative stress which triggers multiple molecular components of the antioxidative defense system. The excessive amounts of hydrogen peroxide and the superoxide radical O2˙− that usually accumulate upon dehydration can cause oxidative damage to the cellular membrane and macromolecules. Hydrogen peroxide is a relatively stable ROS that can also act as a stress signal transducer [47]. The levels of H2O2 measured in the second true leaves of the PEG-grown plants, both “Mock”- and Mix 7-treated ones, were lower than the controls, reflecting the effectively controlled local content (Figure 3a). This could be explained by the efficient antioxidant enzyme activities that handled the excessive hydrogen peroxide produced therein. Among them are the core ROS scavenging enzymes superoxide dismutase, catalase and peroxidase. SOD neutralizes the extremely harmful superoxide radical O2˙− by converting it into molecular oxygen and H2O2 (Figure 3b).
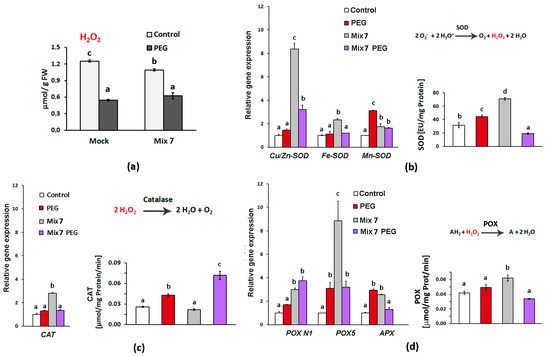
Figure 3.
Mix 7’s priming effect on antioxidant enzymes in the second true leaf of Lactuca sativa plants grown on nutrient media −/+ 12% PEG for 10 days. (a) Hydrogen peroxide level in the second true leaves of the differently treated plants; (b) Cu/Zn-SOD, Fe-SOD and Mn-SOD transcript accumulation and total SOD activity; (c) CAT transcript accumulation and total CAT activity; (d) POX N1, POX5 and APX transcript accumulation and total guaiacol POX activity. The error bars indicate the standard error (SE, n = 3). The lowercase letters designate statistically different results (one-way ANOVA with Duncan’s multiple range test at p < 0.05).
The registered slightly higher total SOD enzyme activity in the leaves of the “Mock”-treated PEG-grown L. sativa plants correlated with the relatively higher accumulation of Mn-SOD transcripts coding for the SOD isoenzyme that operates mainly in the mitochondrion and the peroxisomes [48]. The transcript profiling of SOD-coding genes showed that Mix 7 selectively upregulated the expression of Cu/Zn-SOD and this aligned with the higher total SOD enzymatic activity measured in the control Mix 7-treated samples (Figure 3b). The expression of the Cu/Zn-SOD gene in the Mix 7-primed plants remained elevated upon PEG exposure, in contrast to the plants that were not subjected to PGR pretreatment. However, the measured total SOD activity in the Mix 7/PEG individuals was lower than that in the “Mock”/PEG-treated plants. It should be noted that the increased transcript abundance could compensate for damaged or inactivated enzymes; therefore, the measured total enzyme activity could remain constant or could exhibit changes that do not always align with the transcript profiles. It should be outlined that Cu/Zn-SOD isoenzymes are the most abundant isoform in the leaves, usually found in the chloroplast, the cytosol and the extracellular space [48]. The observed targeted activation of this particular SOD type due to Mix 7 priming could be an important element contributing to the overall protective effect.
CAT neutralizes H2O2 in peroxisomes releasing H2O and O2 [49]. The pretreatment with Mix 7 had a slight but significant effect on CAT gene expression in the leaves of the control group. Nevertheless, a significant increase in enzyme activity was registered in the Mix 7-primed group subjected to PEG-provoked dehydration (Figure 3c).
Peroxidase enzymes operate in the vacuoles, the cell walls and the cytosol [49]. They use aromatic compounds (marked with “A” in Figure 3d) such as guaiacol as electron donors to convert H2O2 into two water molecules. Total peroxidase activity measured in the PEG-treated plants that did not receive the PGR combination was slightly higher compared to the control but on the level of gene expression, we registered moderate (approximately 3-fold) upregulation of POX 5 and APXtranscripts (Figure 3d). Mix 7 priming exerted stimulation of POX gene expression, with particularly significant upregulation of POX 5 transcripts (up to ten-fold in the primed plants grown under normal conditions). The effect was confirmed at the level of total enzyme activity measured in the Mix 7-primed control group, whereas POX activity in the PEG-stressed individuals that received the PGR blend was lower than that in the respective ”Mock”-treated group (Figure 3d).
The consistently observed increased SOD and peroxidase activity in the control group of the Mix 7-pretreated plants could be a result of the ABA component. The potential of exogenous ABA as a priming agent that could lower oxidative damage under stress by activating antioxidant enzymes has been previously demonstrated [50,51]. The prior-to-dehydration elevated levels of these enzymes might be advantageous during water limitation, as it would lessen the ROS burden on the plant cells.
3.3. Combined Pretreatment with ABA, ACC, NAA and KIN Positively Affects the Accumulation of Free Proline upon Dehydration
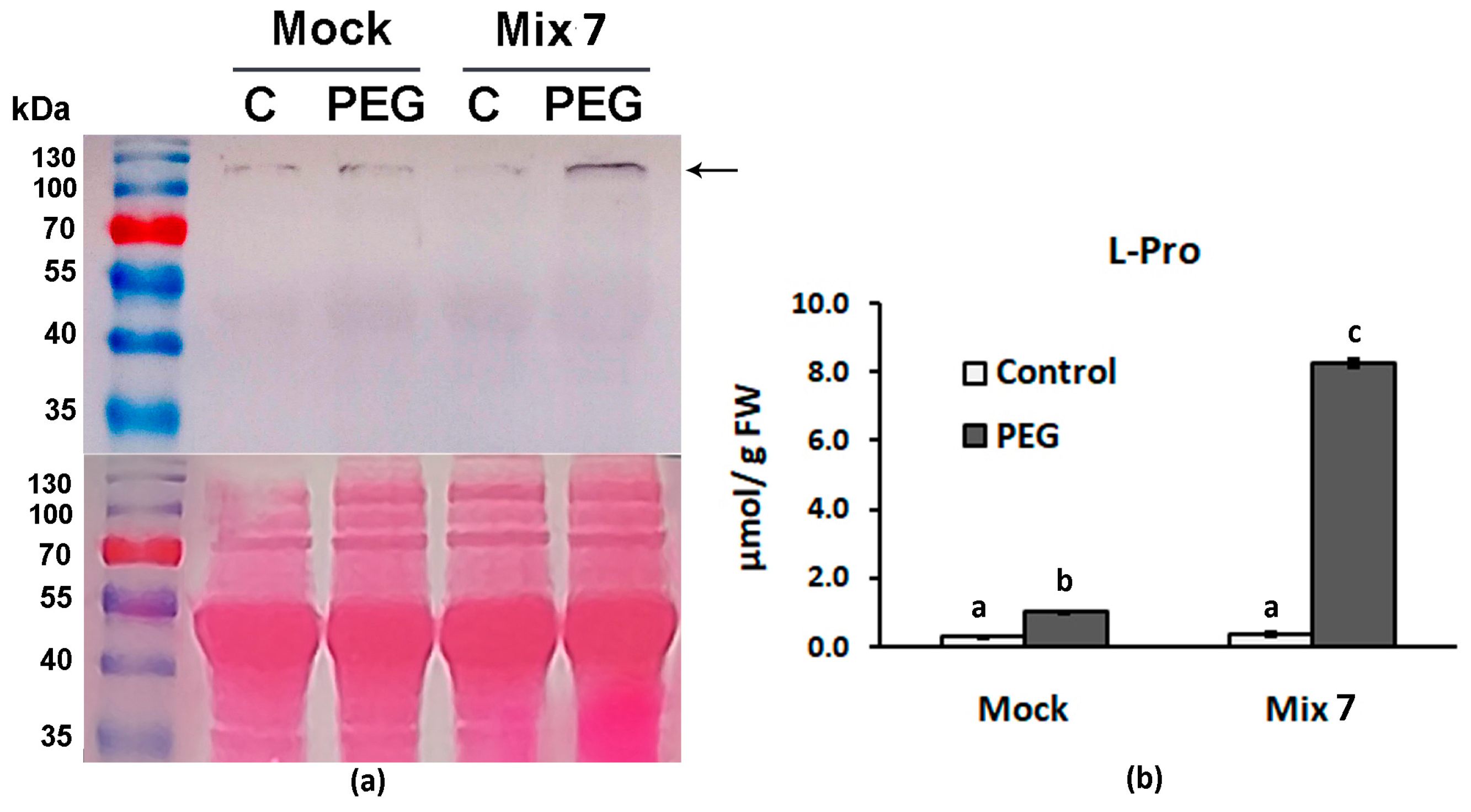
It is well known that during water limitation, plants alter sink/source allocation [52], slow down respiration and photosynthesis, and start to accumulate high levels of dehydrins, chaperons and osmotically active substances like sugars, polyols and free amino acids like proline [53]. Delta-1-pyrroline-5-carboxylate synthase catalyzes the conversion of L-glutamate to pyrroline-5-carboxylate which is the limiting step for the biosynthesis of two essential proteinogenic amino acids L-proline and L-arginine [54]. Free L-Pro in particular is considered a stress defense molecule capable of adjusting cellular osmotic potential. The immunoblot analyses of the samples derived from Mix 7-pretreated individuals that have experienced dehydration showed increased levels of P5CS compared to the respective “Mock”-treated PEG experimental group (Figure 4a). The positive effect of Mix 7 on proline biosynthesis was further confirmed by the measured elevated free L-Pro content in the same treatment group (Figure 4b).
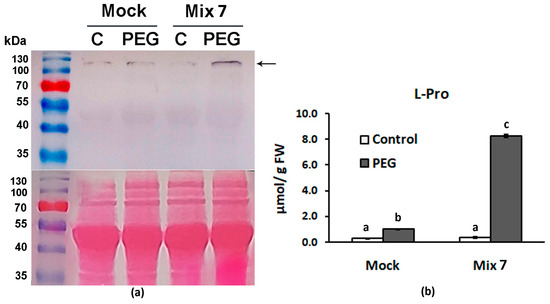
Figure 4.
Mix 7 priming effect on L-Proline biosynthesis in Lactuca sativa grown on nutrient media −/+ 12% PEG for 10 days. (a) Immunodetection of P5CS signal (marked with arrow) in leaves of control and PEG-stressed plants that have received “Mock” or “Mix 7” pretreatments. Ponceao-S staining of membrane is shown below the immunoblot. (b) Leaf L-Pro content measured in samples derived from the same individuals analyzed in the immunoblot. Error bars indicate standard error (SE, n = 6). Lowercase letters designate statistically different results (one-way ANOVA with Duncan’s multiple range test at p < 0.05).
The increased proline biosynthesis in the PEG-stressed plants resulting from the combined PGR pretreatment may significantly contribute to a better physiological status. The multifaceted function of proline in plant growth and development has been acknowledged by multiple studies that gave evidence supporting its active involvement in sustaining cellular redox balance and osmoregulation [55]. Previously published research suggests that ABA and ethylene signals regulate P5CS gene expression [56,57]. The obtained results align well with these findings as the observed stimulated activity of the enzyme in the primed plants corresponds to the presence of the ethylene precursor and ABA in the mix.
3.4. Combined Pretreatment with ABA, ACC, NAA and KIN Stimulates the Accumulation of Certain Dehydrin Types
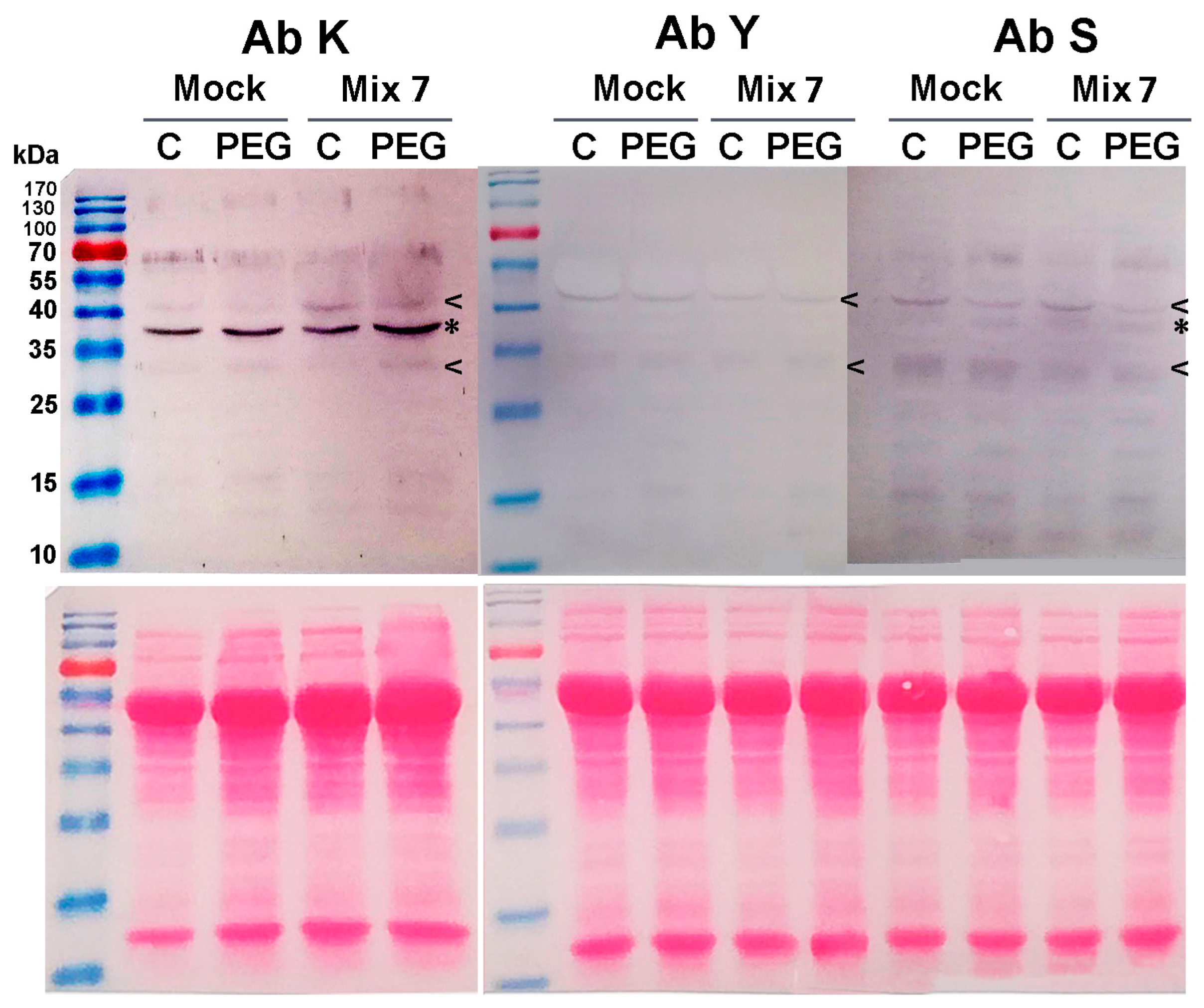
The induction of specific dehydrin signals was immunodetected in leaf samples derived from the Mix 7-treated plants (Figure 5). The bands aligned with approximate positions of 34 kDa and 40 kDa and were visualized in the membranes probed with the three primary antibodies (marked with “<” symbol in Figure 5). This classifies them as YSK-type dehydrins. On the K- and the S-probed membranes, a visualized immunosignal of about 36 kDa exhibited higher intensity in the PGR-pretreated samples (marked with “*” in Figure 5). The accumulation of specific dehydrin types as a result of the priming could also partially contribute to the better physiological status of the plants grown in the presence of 12% PEG.
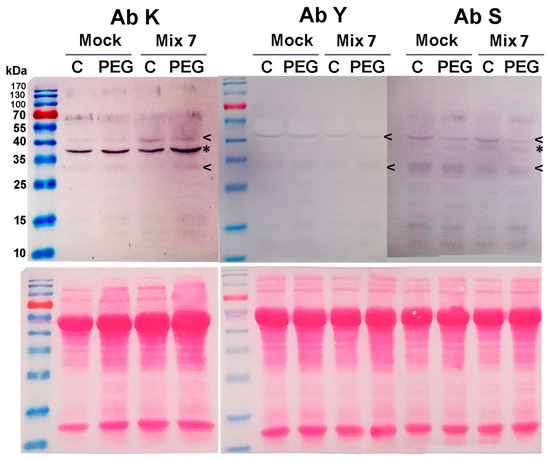
Figure 5.
Mix 7 priming effect on dehydrin profiles in Lactuca sativa grown on nutrient media −/+ 12% PEG for 10 days. Immunodetection with K-, Y- and S primary antibodies is presented. Mix 7-induced “KYS” signals are marked with “<”, and “KS” with “*”. Ponceao-S staining of the same membranes is shown below the immunoblots to visualize equal protein loading.
Dehydrins belong to a broad class of stress-inducible proteins that are an essential part of the drought stress response. Due to their unique molecular characteristics, they are capable of stabilizing biomolecules and membranes, acting as chaperones, but they have also been recently found to regulate stress-responsive genes (reviewed in [58]). DHNs are characterized by the presence of one or more copies of a highly conserved amphipathic α-helix-forming domain of a 15-residue consensus sequence called the K-segment (EKKGIMDKIKEKLPG). It is usually situated near the C-end of the DHN molecule. Many dehydrins also contain a serine-rich S-segment, which is prone to phosphorylation. Another dehydrin subfamily is characterized by the presence of the so-called Y-segments that are usually found close to the N terminus of the amino acid chain. The performed immunoblot analyses using antibodies against the three conservative DHN domains (K, Y and S) allow us to identify the molecular type of the detected dehydrin signals. However, it should be noted that the absence of a signal upon immunoblotting with a given antibody must be interpreted conditionally. The lack of immunosignal could be due to transient spatial inaccessibility of the epitope resulting from dimerization, post-translational modifications, etc.
3.5. Testing of the Stress-Mitigating Potential of Mix 7 on Soil-Grown L. sativa Plants Subjected to Moderate Drought
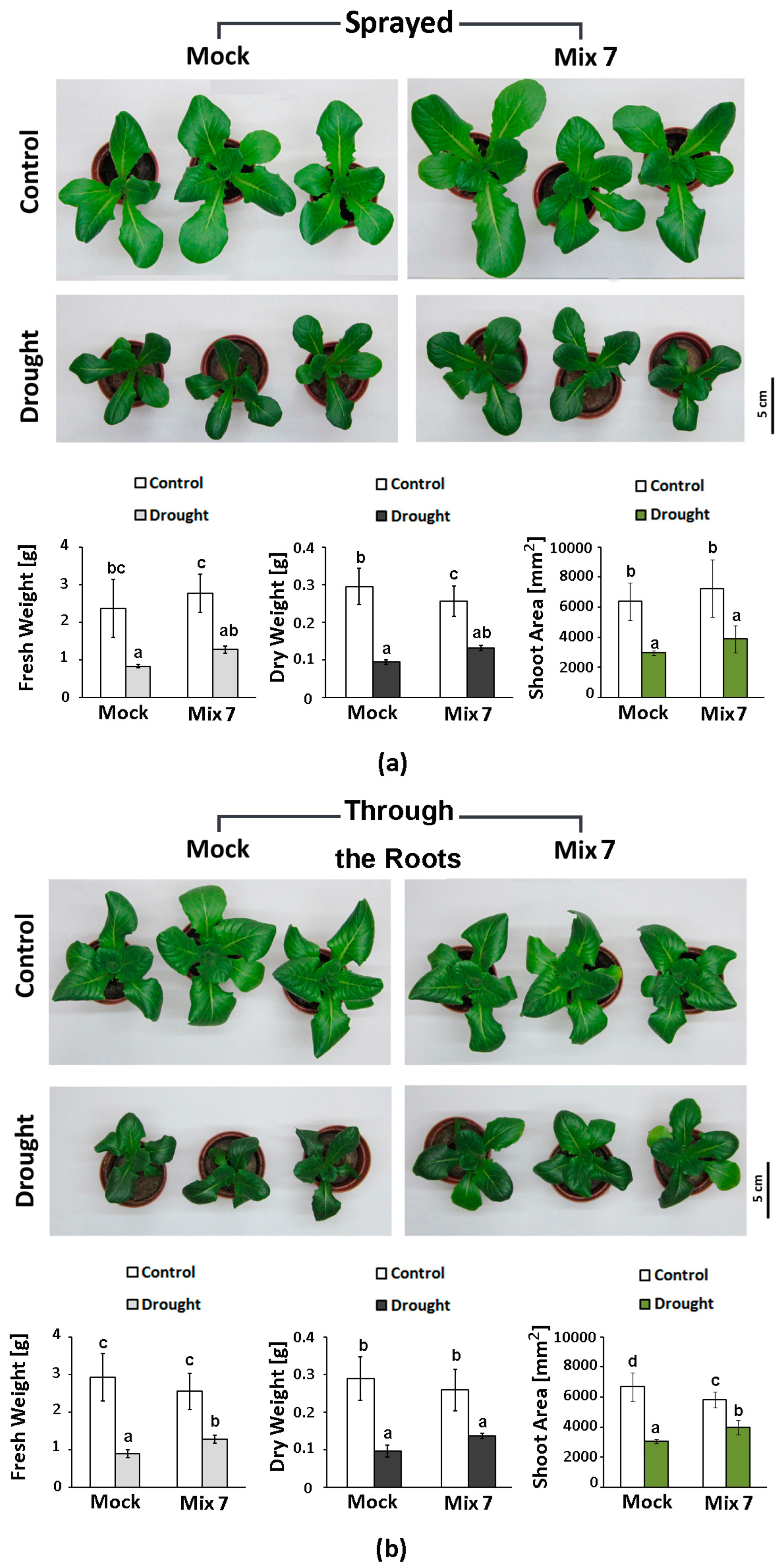
The effect of the PGR mix was evaluated on soil-grown lettuce plants subjected to moderate drought. The solution was administered either by spraying, using the same amount of preparation as in the PEG experiment (Figure 6a), or it was applied through the roots in a 1 mL dose per plant (Figure 6b). Overall, we observed a similar beneficial effect of the combined PGR pretreatment which was not influenced by the application method. The primed plants had a 15–20% higher fresh and dry weight compared to the “Mock”-treated drought-stressed individuals and a relatively larger leaf area. The variations in the growth indexes of the differently treated control plants were not statistically significant.
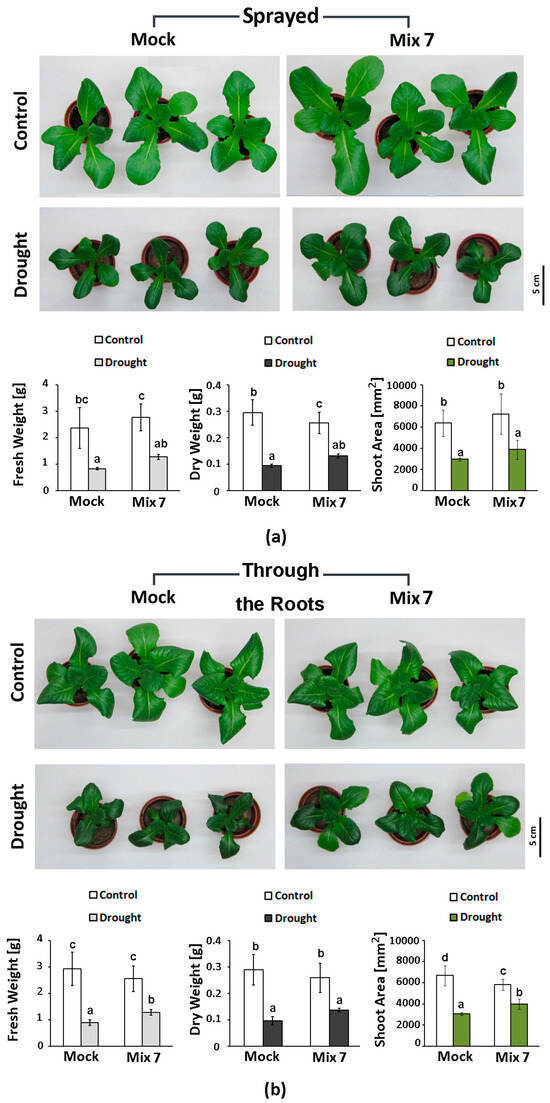
Figure 6.
Representative phenotype, fresh weight, dry weight and shoot area of Lactuca sativa plants pretreated with Mix 7 by spraying (a) or through the roots (b) and subjected to moderate soil drought. The error bars indicate the standard deviation (SD, n = 10). The lowercase letters designate statistically different results (one-way ANOVA with Duncan’s multiple range test at p < 0.05).
Testing the “transferability” of the positive effect of a hormonal preparation observed in a particular experimental setting is mandatory to confirm its consistent action. This is largely due to the extremely versatile and dynamic nature of the PGR’s inputs which strongly depend on the developmental stage, the plant species and the particular environmental factors in which the trials are conducted. The validated drought-mitigating properties of the mix on soil-grown plants suggest its consistent action in alleviating the negative impact of dehydration in this vegetable crop. Additional extended testing of the ABA-ACC-NAA-KIN combination in field conditions is necessary to confirm its efficiency as a drought-protective agent in other leafy greens.
3.6. Comprehensive Analysis of the Observed Drought-Mitigating Property of the Applied PGR Combination
To elaborate on the protective effect of Mix 7, one should consider several well-established properties of its comprising components as elements of the hormonal blueprint that governs the drought response. For example, ethylene and ABA control the synthesis of many stress-inducible proteins, including dehydrins and antioxidant enzymes [59,60,61,62]. ABA is also considered the master regulator of stomata closure and opening [63] and hence, it is a core factor preventing water loss via transpiration, justifying the imperative presence of this hormone in the blend. As the best ethylene proxy, we used ACC, which in theory should provide an equimolar concentration of ethylene gas [64].
The other two components in the preparation, the auxin and the cytokinin analogue, target the appropriate hormonal background [26,52] for establishing a strong root system on which plant survival under limiting water and nutrient availability depends. In our approach, we also take into account the fact that the intrinsic hormonal interactions can be synergistic or antagonistic depending on the physiological and developmental context [65,66]. Auxins and cytokinins are known to form such a complex partnership [52]. They synergistically control the shoot stem cell niche while acting as antagonists in maintaining the root meristem [26]. This qualifies them as important regulatory components establishing the shoot-to-root ratio under given environmental requirements.
A previous study has demonstrated that exogenously applied auxin is capable of upregulating multiple transcription factor genes responding to drought [21]. In the same study, conducted on white clover grown on PEG, the authors showed that exogenous auxin induced the endogenous levels of ABA and jasmonate. The capacity of exogenous PGRs to activate hormonal crosstalk networks is a central feature that integrates adequate and physiologically relevant responses to the imposed stressor. Consequently, we assume that Mix 7 alleviates dehydration through a multiplied exogenous effect on various signaling pathways that activate different protective mechanisms.
4. Conclusions
The present study demonstrates that ABA and ACC applied in concentrations mimicking hormetic stress exposure, in combination with auxin and cytokinin analogues, have drought protective effect in lettuce. The results confirm the advantages of combined PGR treatment for drought stress management, as this approach can activate multiple protective mechanisms. The blend containing ABA, ACC, NAA and KIN activated major antioxidative enzymes, as well as promoted the accumulation of free proline and particular dehydrin types. The drought-mitigating effect of the four-component PGR combination was validated on soil-grown plants subjected to moderate stress. “Bioequivalence” strategies in priming with exogenous stress hormones could be a practical and safe solution for the improvement of crop resilience. This concept needs to be tested in other experimental models to confirm its efficiency.
Author Contributions
Conceptualization, I.I.V., D.T. and I.S.; methodology, I.I.V., D.T., I.S. and M.U.; formal analysis, I.I.V., D.T. and I.S.; investigation, I.I.V., I.S. and D.T.; resources, L.S.-S., G.S., and D.T.; data curation, I.S. and D.T.; writing—original draft preparation, I.I.V.; writing—review and editing, I.S., D.T., L.S.-S., M.U. and G.S.; visualization, I.I.V. and I.S.; funding acquisition, I.I.V. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by a joint research project “Physiological and molecular mechanisms of phytohormones-mediated drought stress tolerance—HormOnDrought” under the agreement for Scientific Cooperation between the Bulgarian Academy of Sciences (BAS) and the Lithuanian Academy of Sciences.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors are very grateful to Urs Feller who provided the specific primary antibodies against P5CS and against the three conservative amino acid sequences (K-, Y- and S-segments) of dehydrin proteins used in the present study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Commission. The European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 31 March 2024).
- European Commission. Farm to Fork Strategy Action Plan. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 31 March 2024).
- De Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and their role in improving plant growth under abiotic stresses. Biostimulants Plant Science. Intech. Open 2020, 88829, 1–14. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Conejo, M.I.; Prieto-Fernández, Á.; Kidd, P.S. Exogenous treatments with phytohormones can improve growth and nickel yield of hyperaccumulating plants. Sci. Total Environ. 2014, 494–495, 1–8. [Google Scholar] [CrossRef]
- Jiang, K.; Asami, T. Chemical regulators of plant hormones and their applications in basic research and agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 1265–1300. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 28, 259. [Google Scholar] [CrossRef]
- Khalid, M.F.; Huda, S.; Yong, M.; Li, L.; Li, L.; Chen, Z.-H.; Ahmed, T. Alleviation of drought and salt stress in vegetables: Crop responses and mitigation strategies. Plant Growth Regul. 2023, 99, 177–194. [Google Scholar] [CrossRef]
- Kaldate, R.; Singh, S.K.; Guleria, G.; Soni, A.; Naikwad, D.; Kumar, N.; Meshram, S.; Rana, M. Current approaches in horticultural crops to mitigate the effect of drought stress. In Stress Tolerance in Horticultural Crops; Rai, A.C., Rai, A., Rai, K.K., Rai, V.P., Kumar, A., Eds.; Woodhead Publishing: Sawston, UK; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 213–240. [Google Scholar]
- Movahedi, A.; Dzinyela, R.; Aghaei-Dargiri, S.; Alhassan, A.R.; Yang, L.; Xu, C. Advanced study of drought-responsive protein pathways in plants. Agronomy 2023, 13, 849. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buintink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: A Commonalty in the response of plants to dehydration and low temperature. Physiol. Plant 1997, 100, 291–296. [Google Scholar] [CrossRef]
- González-Morales, S.; Solís-Gaona, S.; Valdés-Caballero, M.V.; Juárez-Maldonado, A.; Loredo-Treviño, A.; Benavides-Mendoza, A. Transcriptomics of biostimulation of plants under abiotic stress. Front. Genet. 2021, 12, 583888. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.I.; Simova-Stoilova, L.; Kostadinova, A.; Yuperlieva-Mateeva, B.; Karakicheva, T.; Vassileva, V. Heat-stress-mitigating effects of a protein-hydrolysate-based biostimulant are linked to changes in Protease, DHN, and HSP gene expression in maize. Agronomy 2022, 12, 1127. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Chang, X.; Li, Q.; Abbasi, A.M. Influence of plant growth regulators on key-coding genes expression associated with phytochemicals biosynthesis and antioxidant activity in soybean (Glycine max (L.) Merr) sprouts. Int. J. Food. Sci. Technol. 2019, 54, 771–779. [Google Scholar] [CrossRef]
- Guerrero, F.; Mullet, J.E. Increased abscisic acid biosynthesis during plant dehydration requires transcription. Plant Physiol. 1986, 80, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.A.; Santos, I.S.; Torres, M.E.L.; Cardon, C.H.; Caldeira, C.F.; Lima, R.R.; Chalfun-Junior, A. Drought and re-watering modify ethylene production and sensitivity, and are associated with coffee anthesis. Environ. Exp. Bot. 2021, 181, 104289. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.Y.; Azhar, N.; Hussain, M. Indole acetic acid (IAA) induced changes in growth, relative water contents and gas exchange attributes of barley (Hordeum vulgare L.) grown under water stress conditions. Plant Growth Regul. 2006, 50, 85–90. [Google Scholar] [CrossRef]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Muday, G.K.; Dubrovsky, J.G. Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008, 55, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Ramireddy, E.; Hosseini, S.A.; Eggert, K.; Gillandt, S.; Gnad, H.; von Wirén, N.; Schmülling, T. Root engineering in barley: Increasing cytokinin degradation produces a larger root system, mineral enrichment in the shoot and improved drought tolerance. Plant Physiol. 2018, 177, 1078–1095. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Jervis, G.; Topping, J.F.; Chen, C.; Liu, J.; Lindsey, K. A predictive model for ethylene-mediated auxin and cytokinin patterning in the Arabidopsis root. Plant Commun. 2024, 100886. [Google Scholar] [CrossRef] [PubMed]
- De Vylder, J.; Vandenbussche, F.; Hu, Y.; Philips, W.; Van Der Straeten, D. Rosette tracker: An open source image analysis tool for automatic quantification of genotype effects. Plant Physiol. 2012, 160, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef]
- Kramer, G.; Norman, H.; Krizek, D.; Mirecki, R. Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 1991, 30, 2101–2108. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Swain, T.; Goldstein, L. Methods in Polyphenol Chemistry; Pridham, J.B., Ed.; Pergamon Press: Oxford, UK, 1964; pp. 131–146. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–75. [Google Scholar] [CrossRef]
- Aebi, M. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Dias, I.; Costa, M. Effect of low salt concentration on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase. Improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.; Akiscan, Y.; Simova-Stoilova, L.; Kostadinova, A.; Nenkova, R.; Anders, I.; Feller, U.; Demirevska, K. Antioxidant response to drought in red and white clover. Acta Physiol. Plant. 2012, 34, 1689–1699. [Google Scholar] [CrossRef]
- Vaseva, I.; Anders, I.; Feller, U. Identification and expression of different dehydrin subclasses involved in the drought response of Trifolium repens. J. Plant Physiol. 2014, 171, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, W.; Feller, U. Effects of light and external solutes on the catabolism of nuclear-encoded stromal proteins in intact chloroplasts isolated from pea leaves. Plant Physiol. 1992, 100, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCq method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 2022, 3, 100228. [Google Scholar] [CrossRef] [PubMed]
- Brenya, E.; Dutta, E.; Herron, B.; Walden, L.H.; Roberts, D.M.; Binder, B.M. Ethylene-mediated metabolic priming increases photosynthesis and metabolism to enhance plant growth and stress tolerance. PNAS Nexus 2023, 2, pgad216. [Google Scholar] [CrossRef]
- Růzicka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Zdarska, M.; Cuyacot, A.R.; Tarr, P.T.; Yamoune, A.; Szmitkowska, A.; Hrdinova’, V.; Gelova’, Z.; Meyerowitz, E.M.; Hejatko, J. ETR1 integrates response to ethylene and cytokinins into a single multistep phosphorelay pathway to control root growth. Mol. Plant 2019, 12, 1338–1352. [Google Scholar] [CrossRef]
- Most, P.; Papenbrock, J. Possible roles of plant sulfurtransferases in detoxification of cyanide, reactive oxygen species, selected heavy metals and arsenate. Molecules 2015, 20, 1410–1423. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Editorial: Recent insights into the double role of hydrogen peroxide in plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Kurepa, J.; Smalle, J.A. Auxin/cytokinin antagonistic control of the shoot/root growth ratio and its relevance for adaptation to drought and nutrient deficiency stresses. Int. J. Mol. Sci. 2022, 23, 1933. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365e2384. [Google Scholar] [CrossRef]
- Hu, C.A.; Delauney, A.J.; Verma, D.P. A bifunctional enzyme (delta 1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. USA 1992, 89, 9354–9358. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; László Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Shrestha, A.; Cudjoe, D.K.; Kamruzzaman, M.; Siddique, S.; Fiorani, F.; Léon, J.; Naz, A.A. Abscisic acid-responsive element binding transcription factors contribute to proline synthesis and stress adaptation in Arabidopsis. J. Plant Physiol. 2021, 261, 153414. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Simova-Stoilova, L.; Kirova, E.; Mishev, K.; Depaepe, T.; Van Der Straeten, D.; Vassileva, V. Ethylene signaling in salt-stressed Arabidopsis thaliana ein2-1 and ctr1-1 mutants—A dissection of molecular mechanisms involved in acclimation. Plant Physiol. Biochem. 2021, 167, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan, C.; Turkan, I.; Sekmen, A.H.; Seckin, B. Abscisic acid-regulated responses of aba2-1 under osmotic stress: The abscisic acid-inducible antioxidant defence system and reactive oxygen species production. Plant Biol. 2012, 14, 337–346. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, G.; Wang, L.; Tang, Y. Effects of spraying abscisic acid on growth and antioxidant enzyme of lettuce seedlings under salt stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 032015. [Google Scholar] [CrossRef]
- Tiwari, P.; Chakrabarty, D. Dehydrin in the past four decades: From chaperones to transcription co-regulators in regulating abiotic stress response. Curr. Res. Biotech. 2021, 3, 249–259. [Google Scholar] [CrossRef]
- Dalal, M.; Tayal, D.; Chinnusamy, V.; Bansal, K.C. Abiotic stress and ABA-inducible Group 4 LEA from Brassica napus plays a key role in salt and drought tolerance. J Biotech. 2009, 139, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA enhances the antioxidant defense system of maize by regulating the AsA-GSH cycle under drought stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Sehar, Z.; Gautam, H.; Masood, A.; Khan, N.A. Ethylene- and proline-dependent regulation of antioxidant enzymes to mitigate heat stress and boost photosynthetic efficacy in wheat plants. J. Plant Growth Regul. 2023, 42, 2683–2697. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, J.; Chen, K.; Amombo, E.; Chen, L.; Fu, J. Effects of ethylene on photosystem II and antioxidant enzyme activity in Bermuda grass under low temperature. Photosynth. Res. 2016, 128, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Leckie, C.P.; McAinsh, M.R.; Allen, G.J.; Sanders, D.; Hetherington, A.M. Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 15837–15842. [Google Scholar] [CrossRef]
- Zarembinski, T.I.; Theologis, A. Ethylene biosynthesis and action: A case of conservation. In Signals and Signal Transduction Pathways in Plants; Palme, K., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 343–361. [Google Scholar]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef]
- Lee, Z.H.; Hirakawa, T.; Yamaguchi, N.; Ito, T. The roles of plant hormones and their interactions with regulatory genes in determining meristem activity. Int. J. Mol. Sci. 2019, 20, 4065. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).