Inclusion of Antifungal and Probiotic Lactiplantibacillus plantarum Strains in Edible Alginate Coating as a Promising Strategy to Produce Probiotic Table Grapes and Exploit Biocontrol Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Growth Conditions
2.2. Preparation of the Antifungal and Probiotic Coating-Forming Solutions
2.3. Preparation of Antifungal and Probiotic-Coated Table Grape Berries
2.4. Probiotic Viability
2.5. Fruit Decay Assay
2.6. Sensorial Quality Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Probiotic Viability on Table Grape Berries during Shelf Life
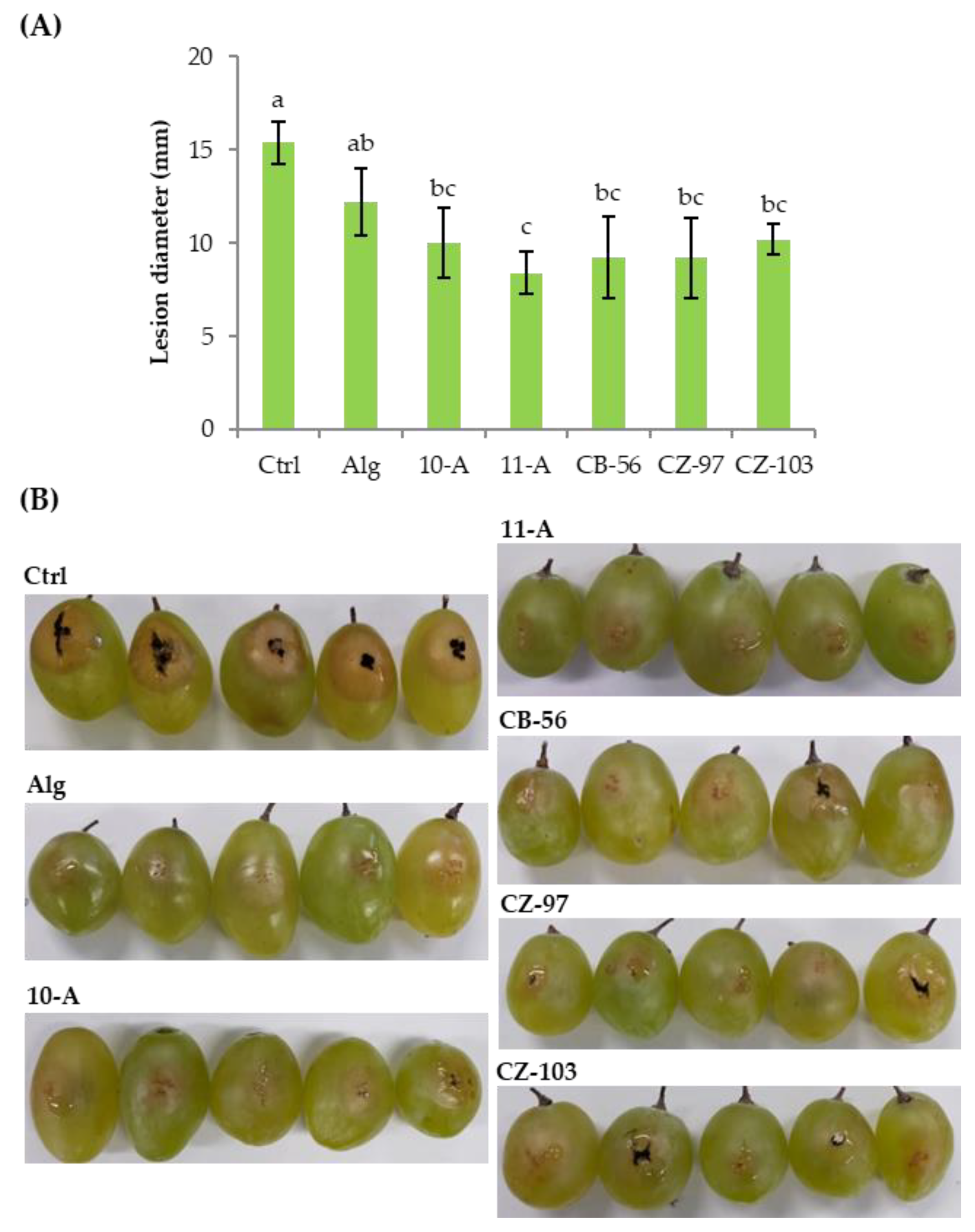
3.2. Fruit Decay Assay
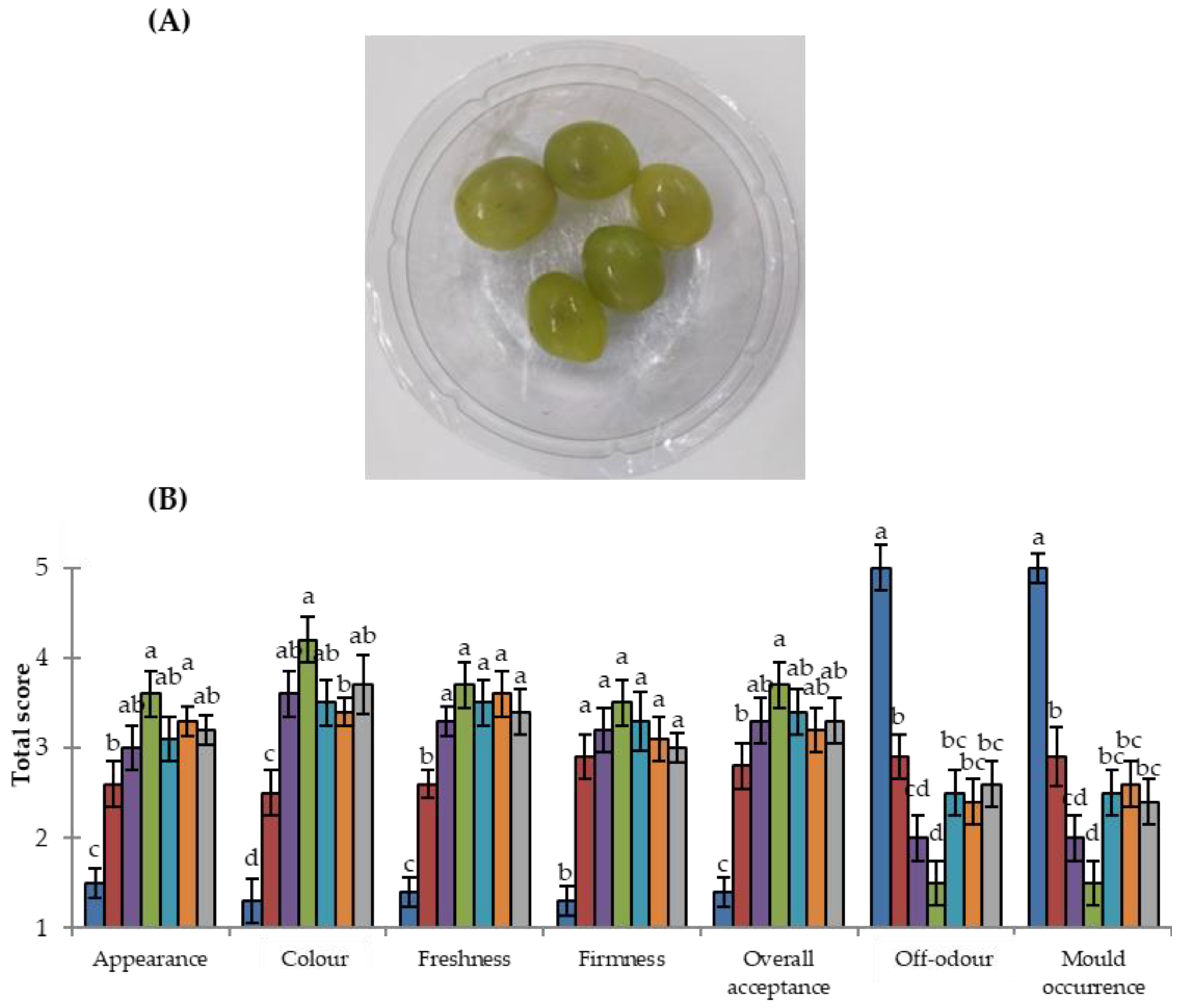
3.3. Sensorial Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.; Liu, X.M.; Zhang, Q.X.; Shen, Z.; Tian, F.W.; Zhang, H.; Sun, Z.H.; Zhang, H.P.; Chen, W. Influence of Consumption of Probiotics on the Plasma Lipid Profile: A Meta-Analysis of Randomised Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 844–850. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal Microbiota in Human Health and Disease: The Impact of Probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef]
- Vijaya Kumar, B.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in Dairy and Non-Dairy Probiotic Products—A Review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef]
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of Vegetable Origin: A New Alternative for the Consumption of Probiotic Bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Gross, K.C.; Wang, C.Y.; Saltveit, M.E. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. In United States Department of Agriculture (USDA)-Agricultural Research Service-Agriculture Handbook No. 66; Gross, K.C., Wang, C.Y., Saltveit, M.E., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2016; p. 780. [Google Scholar]
- Remize, F.; Garcia, C. Fresh-Cut Vegetables and Fruits: Do They Really Meet Sustainability and Nutritional Benefits? Curr. Food Sci. Technol. Rep. 2024, 2, 37–44. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Bambace, M.F.; Quintana, G.; Gomez-Zavaglia, A.; del Rosario Moreira, M. Prebiotic-Alginate Edible Coating on Fresh-Cut Apple as a New Carrier for Probiotic Lactobacilli and Bifidobacteria. LWT 2021, 137, 110483. [Google Scholar] [CrossRef]
- Russo, P.; Peña, N.; de Chiara, M.L.V.; Amodio, M.L.; Colelli, G.; Spano, G. Probiotic Lactic Acid Bacteria for the Production of Multifunctional Fresh-Cut Cantaloupe. Food Res. Int. 2015, 77, 762–772. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Vernile, A.; Amodio, M.L.; Arena, M.P.; Capozzi, V.; Massa, S.; Spano, G. Fresh-Cut Pineapple as a New Carrier of Probiotic Lactic Acid Bacteria. BioMed Res. Int. 2014, 2014, 309183. [Google Scholar] [CrossRef]
- Bambace, M.F.; Alvarez, M.V.; del Rosario Moreira, M. Novel Functional Blueberries: Fructo-Oligosaccharides and Probiotic Lactobacilli Incorporated into Alginate Edible Coatings. Food Res. Int. 2019, 122, 653–660. [Google Scholar] [CrossRef]
- Iglesias, M.B.; Abadias, M.; Anguera, M.; Sabata, J.; Viñas, I. Antagonistic Effect of Probiotic Bacteria against Foodborne Pathogens on Fresh-Cut Pear. LWT-Food Sci. Technol. 2017, 81, 243–249. [Google Scholar] [CrossRef]
- Shigematsu, E.; Dorta, C.; Rodrigues, F.J.; Cedran, M.F.; Giannoni, J.A.; Oshiiwa, M.; Mauro, M.A. Edible Coating with Probiotic as a Quality Factor for Minimally Processed Carrots. J. Food Sci. Technol. 2018, 55, 3712–3720. [Google Scholar] [CrossRef]
- Tapia, M.S.; Rojas-Graü, M.A.; Rodríguez, F.J.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate- and Gellan-Based Edible Films for Probiotic Coatings on Fresh-Cut Fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef]
- Temiz, N.N.; Özdemir, K.S. Microbiological and Physicochemical Quality of Strawberries (Fragaria × Ananassa) Coated with Lactobacillus rhamnosus and Inulin Enriched Gelatin Films. Postharvest Biol. Technol. 2021, 173, 111433. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent Developments in Shelf-Life Extension of Fresh-Cut Fruits and Vegetables by Application of Different Edible Coatings: A Review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. The Use of Packaging Techniques to Maintain Freshness in Fresh-Cut Fruits and Vegetables: A Review. Int. J. Food Sci. Technol. 2009, 44, 875–889. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The Development of a Uniform Alginate-Based Coating for Cantaloupe and Strawberries and the Characterization of Water Barrier Properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef]
- Jin, T.Z.; Chen, W.; Gurtler, J.B.; Fan, X. Effectiveness of Edible Coatings to Inhibit Browning and Inactivate Foodborne Pathogens on Fresh-Cut Apples. J. Food Saf. 2020, 40, e12802. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; de Chiara, M.L.V.; Amodio, M.L.; Brahimi, S.; Colelli, G.; Drider, D.; Spano, G.; Russo, P. Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms 2021, 9, 773. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, e9361614. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, X.; Fu, H.; Wang, X.; Guo, X.; Wang, M. Lactiplantibacillus plantarum: A Comprehensive Review of Its Antifungal and Anti-Mycotoxic Effects. Trends Food Sci. Technol. 2023, 136, 224–238. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, M.; Jiang, Y.; Hu, B.; Guo, X.; Gong, D.; Zhang, Y. Compositional Shifts in Fresh-Cut Apples Microbiome in Response to Application of Lactiplantibacillus plantarum Assessed by Next-Generation Sequencing. LWT 2024, 191, 115627. [Google Scholar] [CrossRef]
- Li, K.; Zhang, W.; Kwok, L.-Y.; Menghe, B. Screening of Lactobacillus plantarum with Broad-Spectrum Antifungal Activity and Its Application in Preservation of Golden-Red Apples. Czech J. Food Sci. 2020, 38, 5. [Google Scholar] [CrossRef]
- Islam, S.; Biswas, S.; Jabin, T.; Moniruzzaman, M.; Biswas, J.; Uddin, M.S.; Akhtar-E-Ekram, M.; Elgorban, A.M.; Ghodake, G.; Syed, A. Probiotic Potential of Lactobacillus plantarum DMR14 for Preserving and Extending Shelf Life of Fruits and Fruit Juice. Heliyon 2023, 9, 6. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, S.; Fang, X.; Wang, Z.; Jiang, Y.; Guo, X.; Zhu, J.; Zhang, Y. The Effect of Lactiplantibacillus plantarum BX62 Alone or in Combination with Chitosan on the Qualitative Characteristics of Fresh-Cut Apples during Cold Storage. Microorganisms 2021, 9, 2404. [Google Scholar] [CrossRef]
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an Exopolysaccharide-Based Edible Coating and Lactic Acid Bacteria with Antifungal Activity to Preserve the Postharvest Quality of Cherry Tomato. LWT 2021, 151, 112225. [Google Scholar] [CrossRef]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Lactic Acid Bacteria Incorporated into Edible Coatings to Control Fungal Growth and Maintain Postharvest Quality of Grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z. Influence of Bioactive Edible Coatings Loaded with Lactobacillus plantarum on Physicochemical Properties of Fresh Strawberries. Postharvest Biol. Technol. 2019, 156, 110944. [Google Scholar] [CrossRef]
- Speranza, B.; Campaniello, D.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Viability of Lactobacillus plantarum on Fresh-Cut Chitosan and Alginate-Coated Apple and Melon Pieces. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Chang, S.; Guo, Q.; Du, G.; Tang, J.; Liu, B.; Shao, K.; Zhao, X. Probiotic-Loaded Edible Films Made from Proteins, Polysaccharides, and Prebiotics as a Quality Factor for Minimally Processed Fruits and Vegetables: A Review. Int. J. Biol. Macromol. 2023, 253, 127226. [Google Scholar] [CrossRef]
- Guo, Q.; Tang, J.; Li, S.; Qiang, L.; Chang, S.; Du, G.; Yue, T.; Yuan, Y. Lactobacillus plantarum 21805 Encapsulated by Whey Protein Isolate and Dextran Conjugate for Enhanced Viability. Int. J. Biol. Macromol. 2022, 216, 124–131. [Google Scholar] [CrossRef]
- Romano, N.; Tavera-Quiroz, M.J.; Bertola, N.; Mobili, P.; Pinotti, A.; Gómez-Zavaglia, A. Edible Methylcellulose-Based Films Containing Fructo-Oligosaccharides as Vehicles for Lactic Acid Bacteria. Food Res. Int. 2014, 64, 560–566. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-Based Biocontrol Solutions for Fruits and Vegetables: Recent Insight, Patents, and Innovative Trends. Recent Pat. Food Nutr. Agric. 2021, 12, 3–18. [Google Scholar] [CrossRef]
- Serra, R.; Lourenço, A.; Alípio, P.; Venâncio, A. Influence of the Region of Origin on the Mycobiota of Grapes with Emphasis on Aspergillus and Penicillium Species. Mycol. Res. 2006, 110, 971–978. [Google Scholar] [CrossRef]
- Lasram, S.; Oueslati, S.; Mliki, A.; Ghorbel, A.; Silar, P.; Chebil, S. Ochratoxin A and Ochratoxigenic Black Aspergillus Species in Tunisian Grapes Cultivated in Different Geographic Areas. Food Control 2012, 25, 75–80. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Russo, P.; De Simone, N.; Capozzi, V.; Spano, G.; Fiocco, D. Immunomodulatory Activity on Human Macrophages by Cell-Free Supernatants to Explore the Probiotic and Postbiotic Potential of Lactiplantibacillus plantarum Strains of Plant Origin. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef]
- De Simone, N.; López, L.; Ciudad, C.S.; Scauro, A.; Russo, P.; Rodríguez, J.; Spano, G.; Martínez, B. Antifungal Activity of Lactiplantibacillus plantarum Isolated from Fruit and Vegetables and Detection of Novel Antifungal VOCs from Fungal-LAB Co-Cultures. Food Biosci. 2024, 58, 103824. [Google Scholar] [CrossRef]
- Plessas, S. Advancements in the Use of Fermented Fruit Juices by Lactic Acid Bacteria as Functional Foods: Prospects and Challenges of Lactiplantibacillus (Lpb.) plantarum subsp. plantarum Application. Fermentation 2022, 8, 6. [Google Scholar] [CrossRef]
- Shi, C.; Chen, Y.; Li, C.; Al-Asmari, F.; Cui, H.; Lin, L. Potential Application of Lactiplantibacillus plantarum in Food Bio-Preservation—A Comprehensive Review with a Focus on the Antibacterial and Anti-Virulence Effects on Foodborne Pathogens. Food Rev. Int. 2024, 1–27. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, M.; Xue, R.; Liu, T.; Yang, Z.; Yang, Z. Purification and Characterization of a Novel Low-Molecular-Weight Antimicrobial Peptide Produced by Lactiplantibacillus plantarum NMGL2. Int. J. Biol. Macromol. 2023, 248, 125932. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Weng, P.; Wu, Z.; Liu, Y. Purification and Antimicrobial Mechanism of a Novel Bacteriocin Produced by Lactiplantibacillus plantarum FB-2. LWT 2023, 185, 115123. [Google Scholar] [CrossRef]
- Lappa, I.K.; Mparampouti, S.; Lanza, B.; Panagou, E.Z. Control of Aspergillus Carbonarius in Grape Berries by Lactobacillus plantarum: A Phenotypic and Gene Transcription Study. Int. J. Food Microbiol. 2018, 275, 56–65. [Google Scholar] [CrossRef]
- De Simone, N.; Rocchetti, M.T.; la Gatta, B.; Spano, G.; Drider, D.; Capozzi, V.; Russo, P.; Fiocco, D. Antimicrobial Properties, Functional Characterisation and Application of Fructobacillus fructosus and Lactiplantibacillus plantarum Isolated from Artisanal Honey. Probiotics Antimicrob. Proteins 2022, 15, 1406–1423. [Google Scholar] [CrossRef]
- Alegre, I.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Microbiological and Physicochemical Quality of Fresh-Cut Apple Enriched with the Probiotic Strain Lactobacillus rhamnosus GG. Food Microbiol. 2011, 28, 59–66. [Google Scholar] [CrossRef]
- Konuk Takma, D.; Korel, F. Impact of Preharvest and Postharvest Alginate Treatments Enriched with Vanillin on Postharvest Decay, Biochemical Properties, Quality and Sensory Attributes of Table Grapes. Food Chem. 2017, 221, 187–195. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Sánchez-González, L.; Muneret, L.; Jeandel, C.; Hamdi, M.; Desobry, S. Alginate Coatings Containing Grapefruit Essential Oil or Grapefruit Seed Extract for Grapes Preservation. Int. J. Food Sci. Technol. 2014, 49, 952–959. [Google Scholar] [CrossRef]
- Petkova, M.; Gotcheva, V.; Dimova, M.; Bartkiene, E.; Rocha, J.M.; Angelov, A. Screening of Lactiplantibacillus plantarum Strains from Sourdoughs for Biosuppression of Pseudomonas syringae Pv. syringae and Botrytis cinerea in Table Grapes. Microorganisms 2022, 10, 2094. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Simone, N.; Scauro, A.; Fatchurrahman, D.; Russo, P.; Capozzi, V.; Spano, G.; Fragasso, M. Inclusion of Antifungal and Probiotic Lactiplantibacillus plantarum Strains in Edible Alginate Coating as a Promising Strategy to Produce Probiotic Table Grapes and Exploit Biocontrol Activity. Horticulturae 2024, 10, 419. https://doi.org/10.3390/horticulturae10040419
De Simone N, Scauro A, Fatchurrahman D, Russo P, Capozzi V, Spano G, Fragasso M. Inclusion of Antifungal and Probiotic Lactiplantibacillus plantarum Strains in Edible Alginate Coating as a Promising Strategy to Produce Probiotic Table Grapes and Exploit Biocontrol Activity. Horticulturae. 2024; 10(4):419. https://doi.org/10.3390/horticulturae10040419
Chicago/Turabian StyleDe Simone, Nicola, Angela Scauro, Danial Fatchurrahman, Pasquale Russo, Vittorio Capozzi, Giuseppe Spano, and Mariagiovanna Fragasso. 2024. "Inclusion of Antifungal and Probiotic Lactiplantibacillus plantarum Strains in Edible Alginate Coating as a Promising Strategy to Produce Probiotic Table Grapes and Exploit Biocontrol Activity" Horticulturae 10, no. 4: 419. https://doi.org/10.3390/horticulturae10040419
APA StyleDe Simone, N., Scauro, A., Fatchurrahman, D., Russo, P., Capozzi, V., Spano, G., & Fragasso, M. (2024). Inclusion of Antifungal and Probiotic Lactiplantibacillus plantarum Strains in Edible Alginate Coating as a Promising Strategy to Produce Probiotic Table Grapes and Exploit Biocontrol Activity. Horticulturae, 10(4), 419. https://doi.org/10.3390/horticulturae10040419







