Transcriptome and Pigment Analyses Provide Insights into Carotenoids and Flavonoids Biosynthesis in Camellia nitidissima Stamens
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Determination of Pigment Contents
2.2. RNA-Seq Analysis
2.3. Interaction Network and Cluster Heatmap Analysis
2.4. Transcriptome Data Were Validated Using Quantitative Real-Time PCR (qRT-PCR)
2.5. Statistical Analysis
3. Results
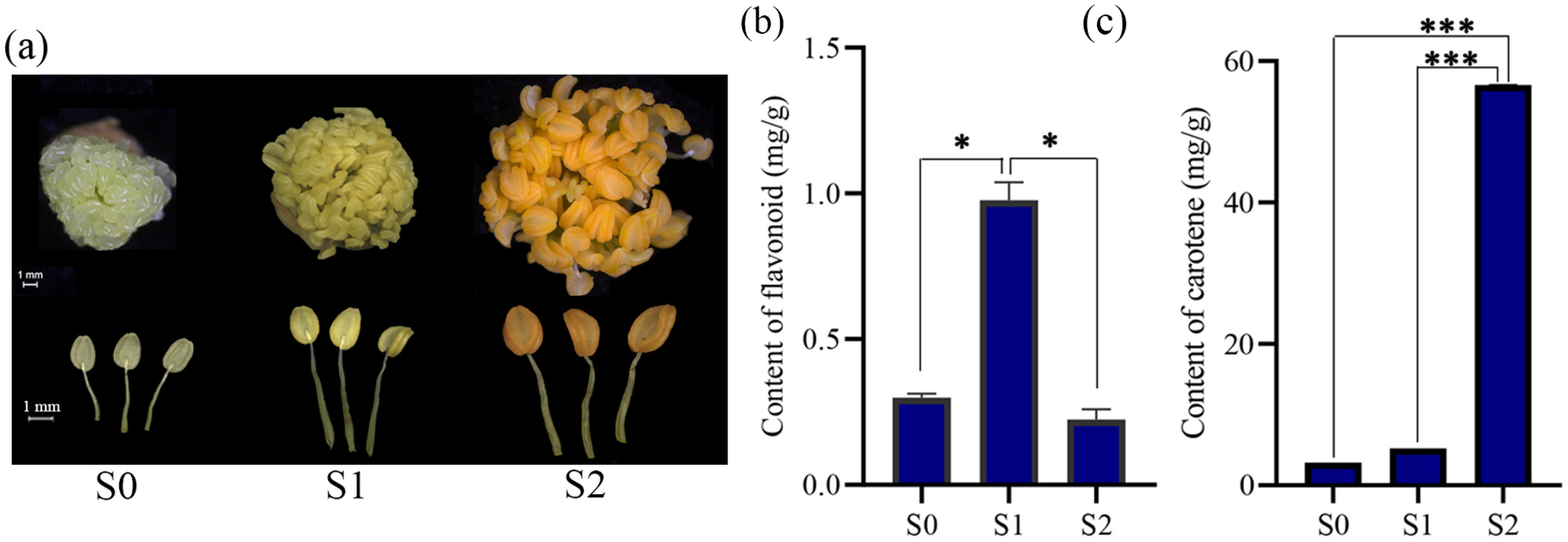
3.1. Stamen Phenotype and Pigment Content Analysis
3.2. Overview of Transcriptome Sequencing and Functional Annotation of Unigenes
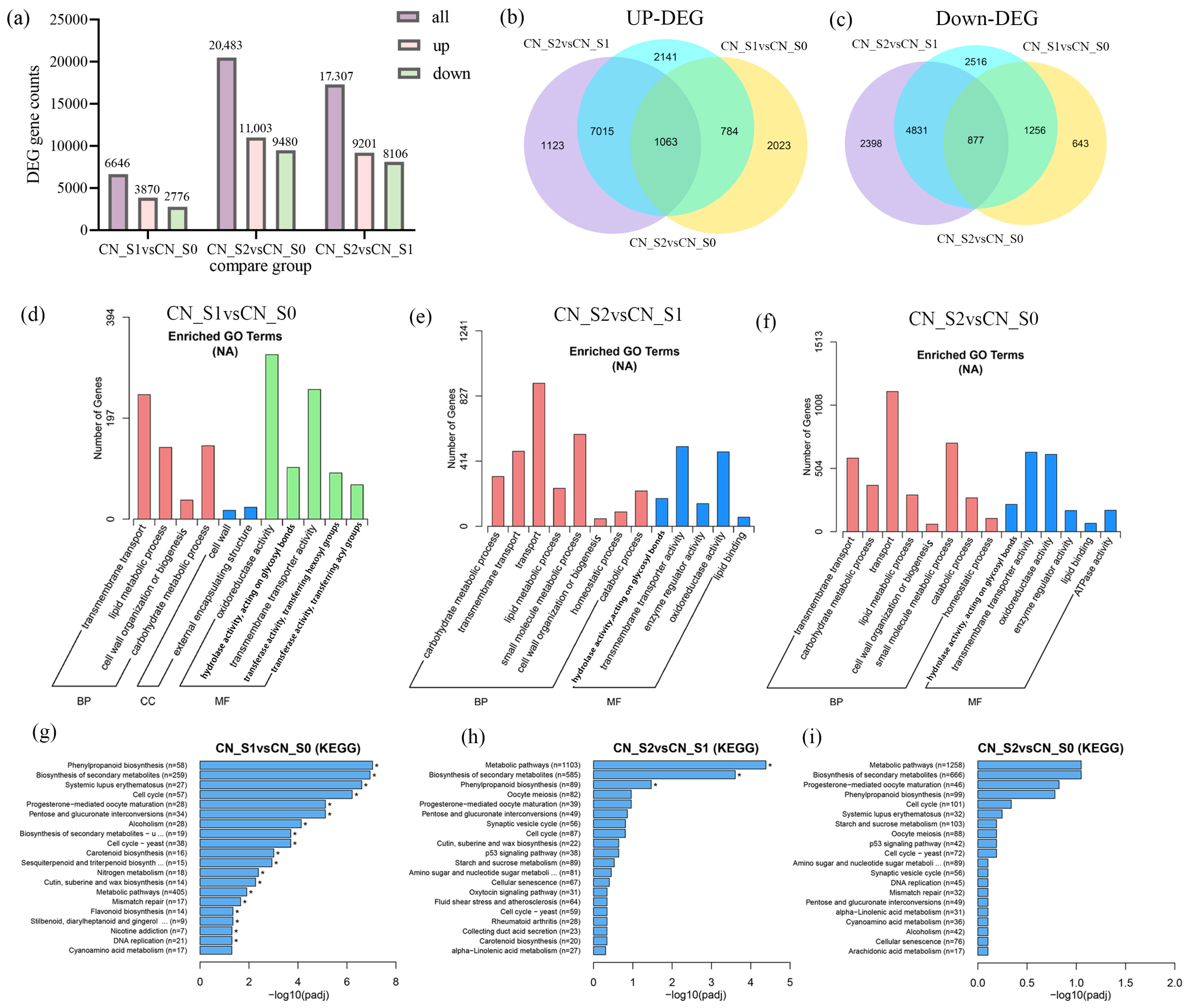
3.3. Identification and Analysis of DEGs
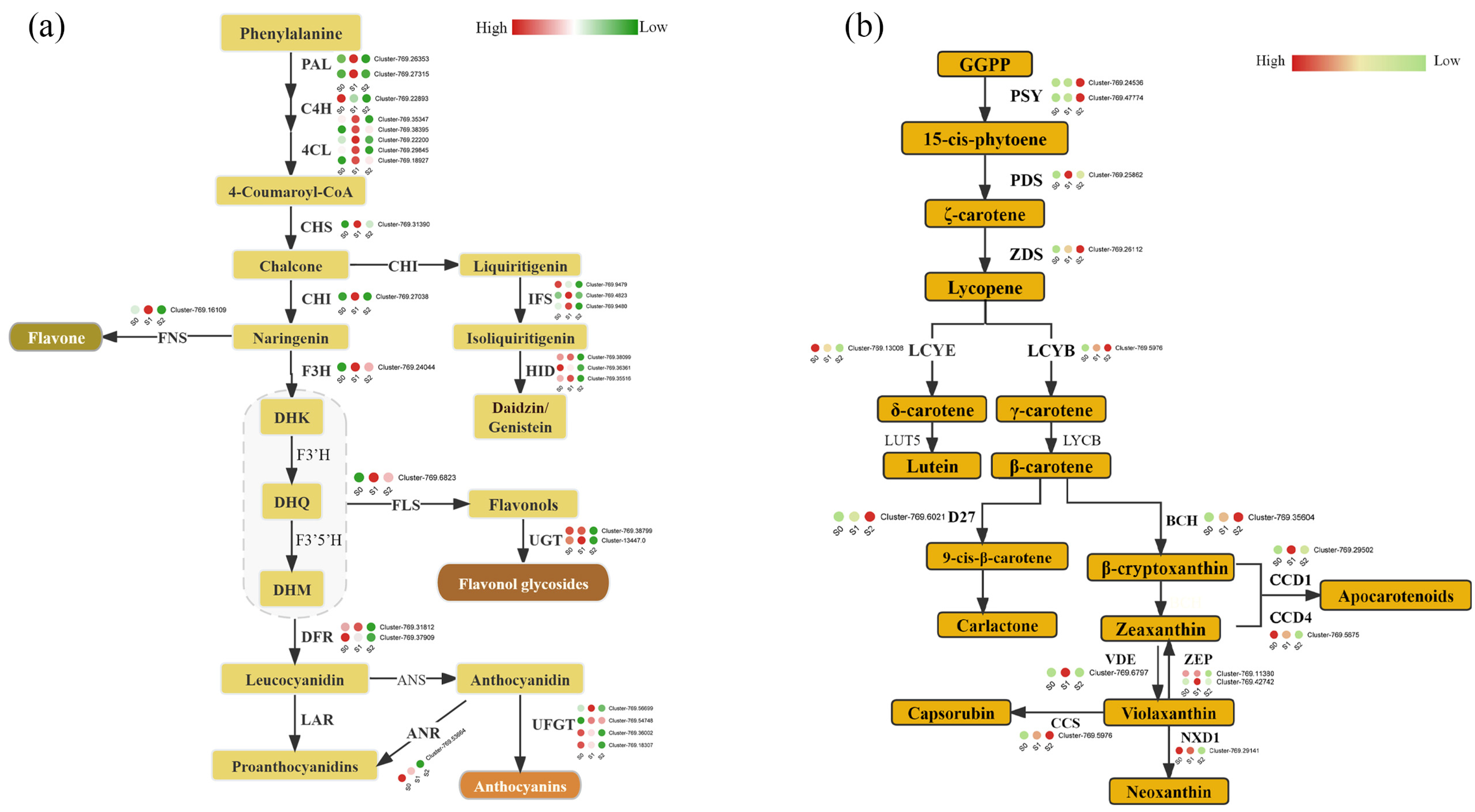
3.4. Flavonoid and Carotenoid Biosynthesis in C. nitidissima Stamens
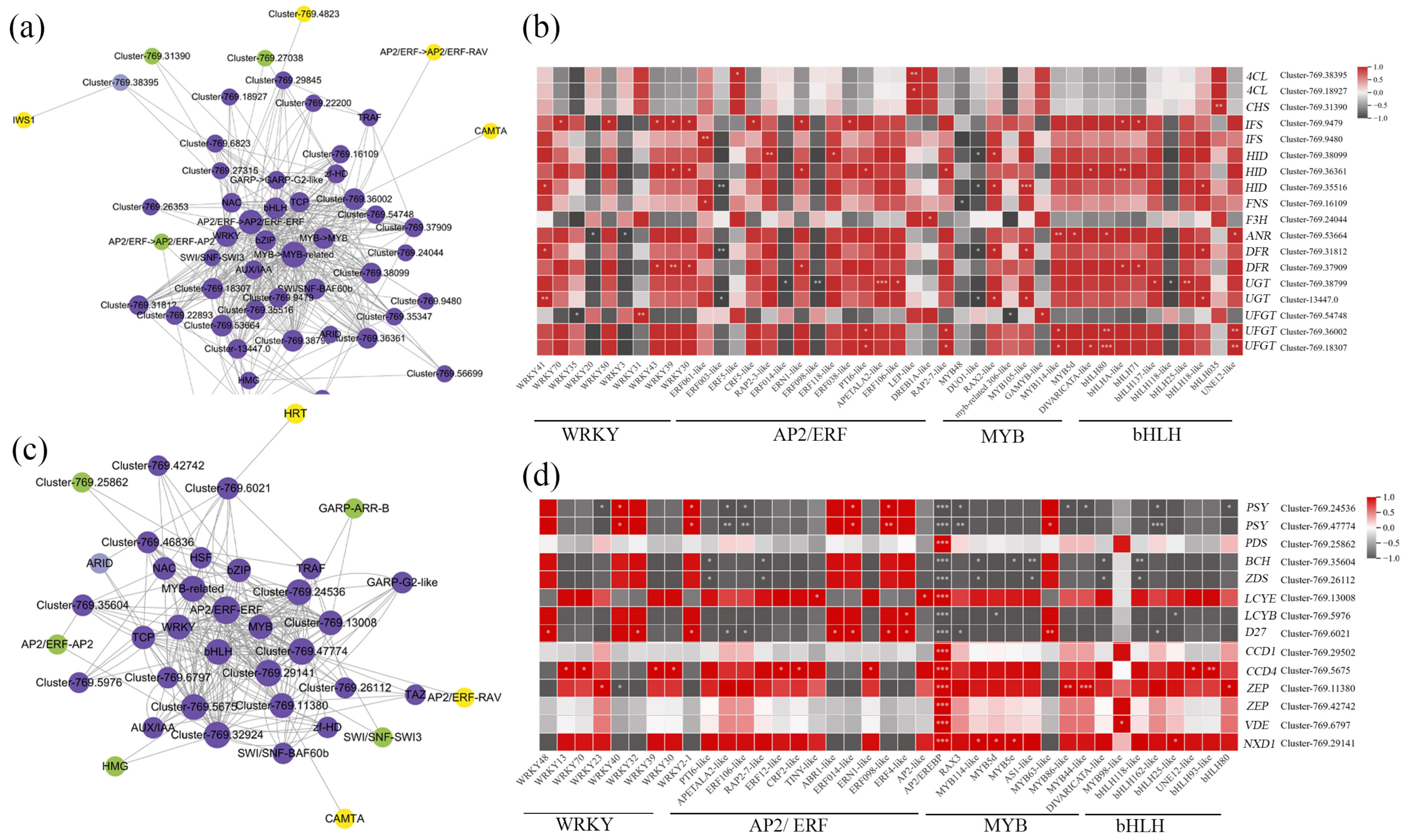
3.5. Identification of Transcription Factors
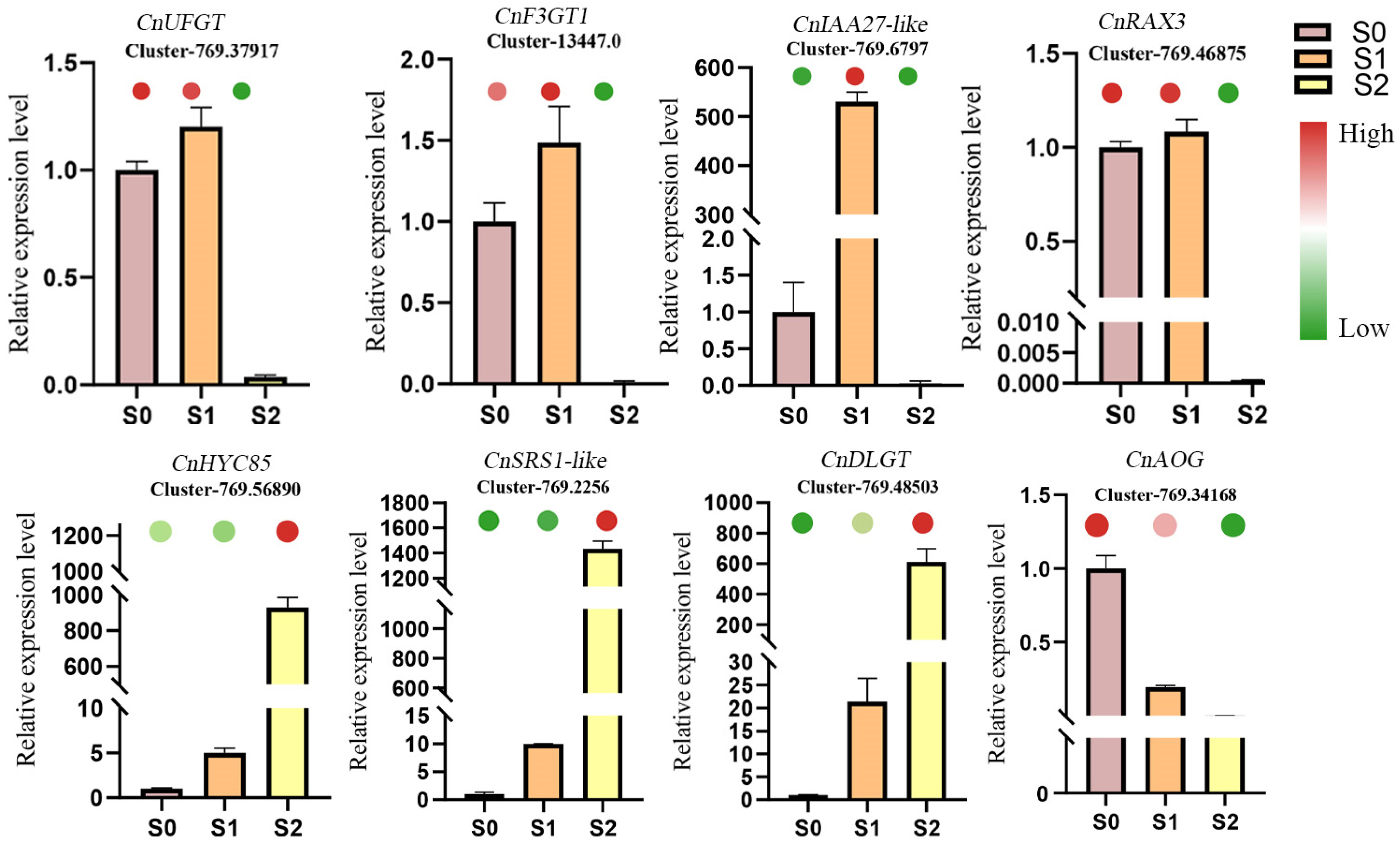
3.6. qRT-PCR Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoshida, K.; Oyama, K.I.; Kondo, T. Insight into chemical mechanisms of sepal color development and variation in hydrangea. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 97, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Li, J.; Wang, Z. Camellias of China; Zhejiang Science and Technology Publishing House: Hangzhou, China, 2014; pp. 395–406. [Google Scholar]
- Wu, H.; Huang, Q.; Li, S.; Qin, X.; Zhong, S.; Chen, J. Component analysis and anti-inflammatory activity of active ingredients from Camellia nitidissima Chi flowers. Food Ferment. Ind. 2024, 1–9. [Google Scholar] [CrossRef]
- He, D.; Li, X.; Sai, X.; Wang, L.; Li, S.; Xu, Y. Camellia nitidissima C.W. Chi: A review of botany, chemistry, and pharmacology. Phytochem. Rev. 2017, 17, 327–349. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, Q.X.; Qin, X.M. Camellia nitidissima Chi flower extract alleviates obesity and related complications and modulates gut microbiota composition in rats with high-fat-diet-induced obesity. J. Sci. Food Agric. 2020, 100, 4378–4389. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yu, D.Y.; Feng, B.M.; Wang, Y.Q.; Shi, L.Y. A new acylated flavonoid glycoside from the flowers of Camellia nitidissima and its effect on the induction of apoptosis in human lymphoma U937 cells. J. Asian Nat. Prod. Res. 2012, 14, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, J.-L.; Liang, X.-Q.; Li, L.; Feng, Y.; Liu, H.-Z.; Wei, W.-E.; Ning, S.-F.; Zhang, L.-T. Flowers of Camellia nitidissima cause growth inhibition, cell-cycle dysregulation and apoptosis in a human esophageal squamous cell carcinoma cell line. Mol. Med. Rep. 2016, 14, 1117–1122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, R.; Wang, W.-X.; Chen, H.-J.; He, Z.-C.; Jia, A.-Q. The inhibition of advanced glycation end-products by five fractions and three main flavonoids from Camellia nitidissima Chi flowers. J. Food Drug Anal. 2018, 26, 252–259. [Google Scholar] [CrossRef]
- Liu, W.; Yu, S.; Feng, Y.; Mo, R.; Wang, K.; Fan, M.; Fan, Z.; Yin, H.; Li, J.; Li, X. Comparative Transcriptome and Pigment Analyses Reveal Changes in Gene Expression Associated with Flavonol Metabolism in Yellow Camellia. Forests 2022, 13, 1094. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Noda, N. Recent advances in the research and development of blue flowers. Breed. Sci. 2018, 68, 79–87. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2018, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Wohl, J.; Petersen, M. Functional expression and characterization of cinnamic acid 4-hydroxylase from the hornwort Anthoceros agrestis in Physcomitrella patens. Plant Cell Rep. 2020, 39, 597–607. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Sasaki, N.; Nishihara, M. Transcriptional regulators of flavonoid biosynthesis and their application to flower color modification in Japanese gentians. Plant Biotechnol. 2014, 31, 389–399. [Google Scholar] [CrossRef]
- Jiang, X.; Shi, Y.; Fu, Z.; Li, W.W.; Lai, S.; Wu, Y.; Wang, Y.; Liu, Y.; Gao, L.; Xia, T. Functional characterization of three flavonol synthase genes from Camellia sinensis: Roles in flavonol accumulation. Plant Sci. 2020, 300, 110632. [Google Scholar] [CrossRef]
- Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Capocasa, F.; Sabbadini, S.; Alvarez-Suarez, J.M.; Afrin, S.; Rosati, C.; Pandolfini, T.; et al. Overexpression of the Anthocyanidin Synthase (ANS) Gene in Strawberry Enhances Antioxidant Capacity and Cytotoxic Effects on Human Hepatic Cancer Cells. J. Agric. Food Chem. 2018, 66, 581–592. [Google Scholar] [CrossRef]
- Morelli, L.; Rodriguez-Concepcion, M. Open avenues for carotenoid biofortification of plant tissues. Plant Commun. 2023, 4, 100466. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, Y.; Wang, J.; Qu, J.; Chen, Y.; Chen, X.; Huang, H.; Dai, S. Flower color classification and correlation between color space values with pigments in potted multiflora chrysanthemum. Sci. Hortic. 2021, 283, 110082. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Zhu, Y.; Ni, S.; Chen, J.; Feng, X.; Zhang, Y.; Li, S.; Zhu, H.; Wen, Y. De novo Assembly of the Camellia nitidissima Transcriptome Reveals Key Genes of Flower Pigment Biosynthesis. Front. Plant Sci. 2017, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Q.; Chen, Y.; Zhu, Y.; Zhou, X.; Li, B. Full-length transcriptome sequencing provides insights into flavonoid biosynthesis in Camellia nitidissima Petals. Gene 2023, 850, 146924. [Google Scholar] [CrossRef]
- Yu, S.; Li, J.; Peng, T.; Ni, S.; Feng, Y.; Wang, Q.; Wang, M.; Chu, X.; Fan, Z.; Li, X.; et al. Identification of Chalcone Isomerase Family Genes and Roles of CnCHI4 in Flavonoid Metabolism in Camellia nitidissima. Biomolecules 2023, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L. Study on Flower Color Formation Metabolism Mechanism and Key Genes Function of Camellia nitidissima. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2020. [Google Scholar]
- McQuinn, R.P.; Gapper, N.E.; Gray, A.G.; Zhong, S.; Tohge, T.; Fei, Z.; Fernie, A.R.; Giovannoni, J.J. Manipulation of ZDS in tomato exposes carotenoid- and ABA-specific effects on fruit development and ripening. Plant Biotechnol. J. 2020, 18, 2210–2224. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Zheng, X.; Zhu, K.; Xu, Q.; Deng, X. Molecular characterization, critical amino acid identification, and promoter analysis of a lycopene β-cyclase gene from citrus. Tree Genet. Genomes 2016, 12, 106. [Google Scholar] [CrossRef]
- Hadjipieri, M.; Georgiadou, E.C.; Marin, A.; Diaz-Mula, H.M.; Goulas, V.; Fotopoulos, V.; Tomás-Barberán, F.A.; Manganaris, G.A. Metabolic and transcriptional elucidation of the carotenoid biosynthesis pathway in peel and flesh tissue of loquat fruit during on-tree development. BMC Plant Biol. 2017, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Abuauf, H.; Haider, I.; Jia, K.-P.; Ablazov, A.; Mi, J.; Blilou, I.; Al-Babili, S. The Arabidopsis DWARF27 gene encodes an all-trans-/9-cis-β-carotene isomerase and is induced by auxin, abscisic acid and phosphate deficiency. Plant Sci. 2018, 277, 33–42. [Google Scholar] [CrossRef]
- Hinz, M.; Wilson, I.W.; Yang, J.; Buerstenbinder, K.; Llewellyn, D.; Dennis, E.S.; Sauter, M.; Dolferus, R. ArabidopsisRAP2.2: An Ethylene Response Transcription Factor That Is Important for Hypoxia Survival. Plant Physiol. 2010, 153, 757–772. [Google Scholar] [CrossRef]
- Palozza, P. Can β-carotene regulate cell growth by a redox mechanism? An answer from cultured cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1740, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Bashandy, H.; Ainasoja, M.; Kontturi, J.; Pietiainen, M.; Laitinen, R.A.E.; Albert, V.A.; Valkonen, J.P.T.; Elomaa, P.; Teeri, T.H. Functional diversification of duplicated chalcone synthase genes in anthocyanin biosynthesis of Gerbera hybrida. New Phytol. 2014, 201, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.C.; Martin, C.; Toledo-Ortiz, G.; Quail, P.H.; Huq, E.; Heim, M.A.; Jakoby, M.; Werber, M.; Weisshaar, B. Update on the Basic Helix-Loop-Helix Transcription Factor Gene Family in Arabidopsis thaliana. Plant Cell 2003, 15, 2497–2502. [Google Scholar] [CrossRef]
- Hichri, I.; Deluc, L.; Barrieu, F.; Bogs, J.; Mahjoub, A. A single amino acid change within the R2 domain of the VvMYB5b transcription factor modulates affinity for protein partners and target promoters selectivity. BMC Plant Biol. 2011, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Spelt, C.; Quattrocchio, F.; Mol, J.N.M.; Koes, R. Anthocyanin1 of petunia encodes a basic helixloop-helix protein that directly activates transcription of structural anthocyanin genes. Plant Cell 2000, 12, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, T.; Pan, Q.; Anupol, N.; Chen, H.; Shi, J.; Liu, F.; Deqiang, D.; Wang, C.; Zhao, J.; et al. RrMYB5- and RrMYB10-regulated flavonoid biosynthesis plays a pivotal role in feedback loop responding to wounding and oxidation in Rosa rugosa. Plant Biotechnol. J. 2019, 17, 2078–2095. [Google Scholar] [CrossRef]
- Verweij, W.; Spelt, C.E.; Bliek, M.; de Vries, M.; Wit, N.; Faraco, M.; Koes, R.; Quattrocchio, F.M. Functionally Similar WRKY Proteins Regulate Vacuolar Acidification in Petunia and Hair Development in Arabidopsis. Plant Cell 2016, 28, 786–803. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.; Brockman, A.; Aguirre, L.; Campbell, A.; Bean, A.; Cantero, A.; Gonzalez, A. Advances in the MYB-bHLH-WD Repeat (MBW) Pigment Regulatory Model: Addition of a WRKY Factor and Co-option of an Anthocyanin MYB for Betalain Regulation. Plant Cell Physiol. 2017, 58, 1431–1441. [Google Scholar] [CrossRef]
- Bi, M.; Liang, R.; Wang, J.; Qu, Y.; Liu, X.; Cao, Y.; He, G.; Yang, Y.; Yang, P.; Xu, L.; et al. Multifaceted roles of LhWRKY44 in promoting anthocyanin accumulation in Asiatic hybrid lilies (Lilium spp.). Hortic. Res. 2023, 10, uhad167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Zhao, K.; Li, J.; Wang, M.; Yin, H.; Fan, Z.; Li, X.; Liu, W. Transcriptome and Pigment Analyses Provide Insights into Carotenoids and Flavonoids Biosynthesis in Camellia nitidissima Stamens. Horticulturae 2024, 10, 420. https://doi.org/10.3390/horticulturae10040420
Feng Y, Zhao K, Li J, Wang M, Yin H, Fan Z, Li X, Liu W. Transcriptome and Pigment Analyses Provide Insights into Carotenoids and Flavonoids Biosynthesis in Camellia nitidissima Stamens. Horticulturae. 2024; 10(4):420. https://doi.org/10.3390/horticulturae10040420
Chicago/Turabian StyleFeng, Yi, Kunkun Zhao, Jiyuan Li, Minyan Wang, Hengfu Yin, Zhengqi Fan, Xinlei Li, and Weixin Liu. 2024. "Transcriptome and Pigment Analyses Provide Insights into Carotenoids and Flavonoids Biosynthesis in Camellia nitidissima Stamens" Horticulturae 10, no. 4: 420. https://doi.org/10.3390/horticulturae10040420
APA StyleFeng, Y., Zhao, K., Li, J., Wang, M., Yin, H., Fan, Z., Li, X., & Liu, W. (2024). Transcriptome and Pigment Analyses Provide Insights into Carotenoids and Flavonoids Biosynthesis in Camellia nitidissima Stamens. Horticulturae, 10(4), 420. https://doi.org/10.3390/horticulturae10040420






