Vermicompost Rate Effects on Soil Fertility and Morpho-Physio-Biochemical Traits of Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Site Location
2.3. Experimental Soil Collection and Characterization
2.4. Vermicompost
2.5. Experimental Design
2.6. Data Collection
2.6.1. Growth Measurements
2.6.2. Biochemical Analysis
2.6.3. Post-Harvest Soil Physiochemical Analysis
2.6.4. Post-Harvest Soil Biological Analysis
2.6.5. Enzymatic Activity
2.7. Statistical Analysis
3. Results
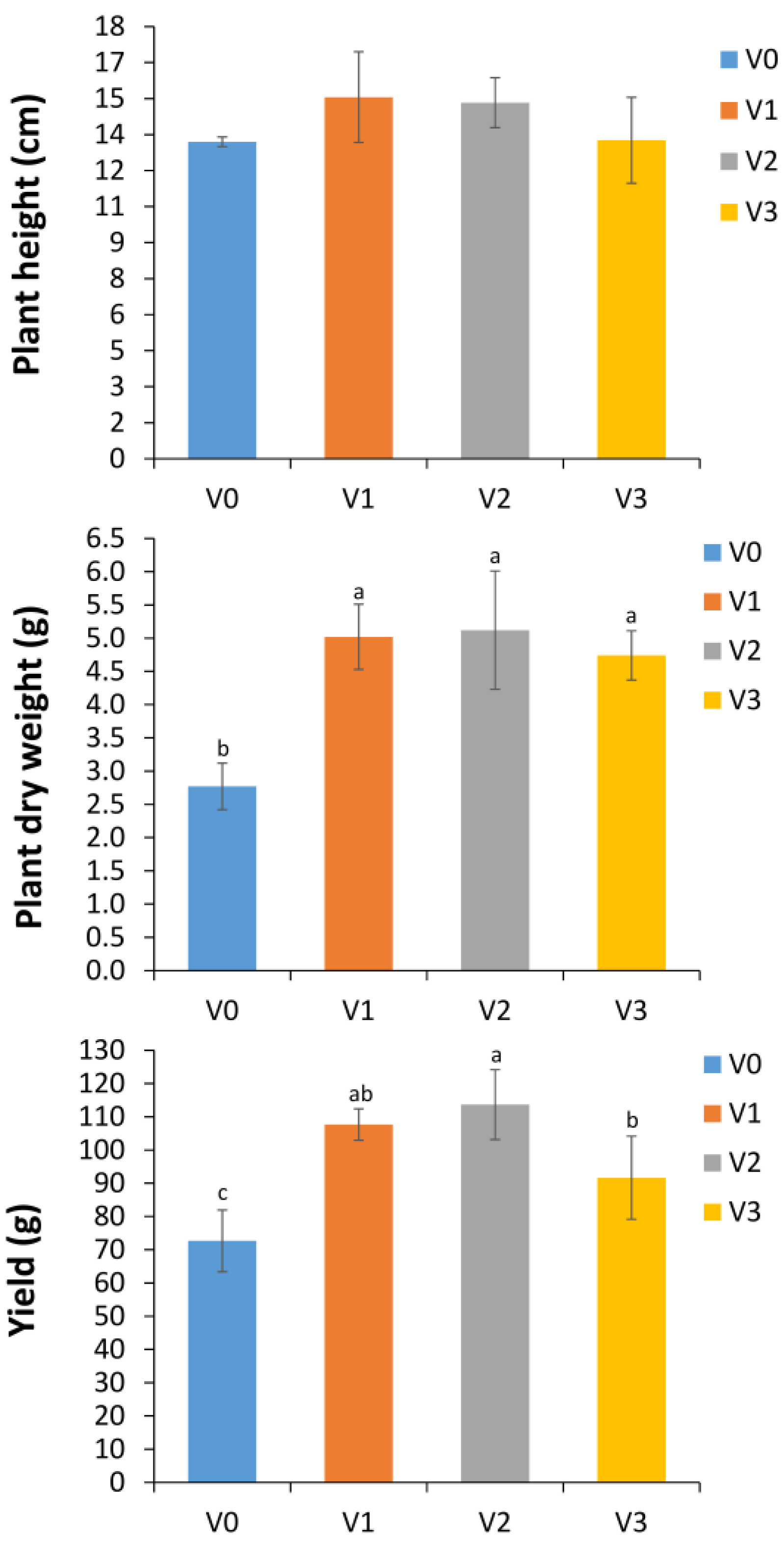
3.1. Effect of Vermicompost on Lettuce Growth and Yield
3.2. Impact of Vermicompost Application on Soil Properties and Nutrient Dynamics
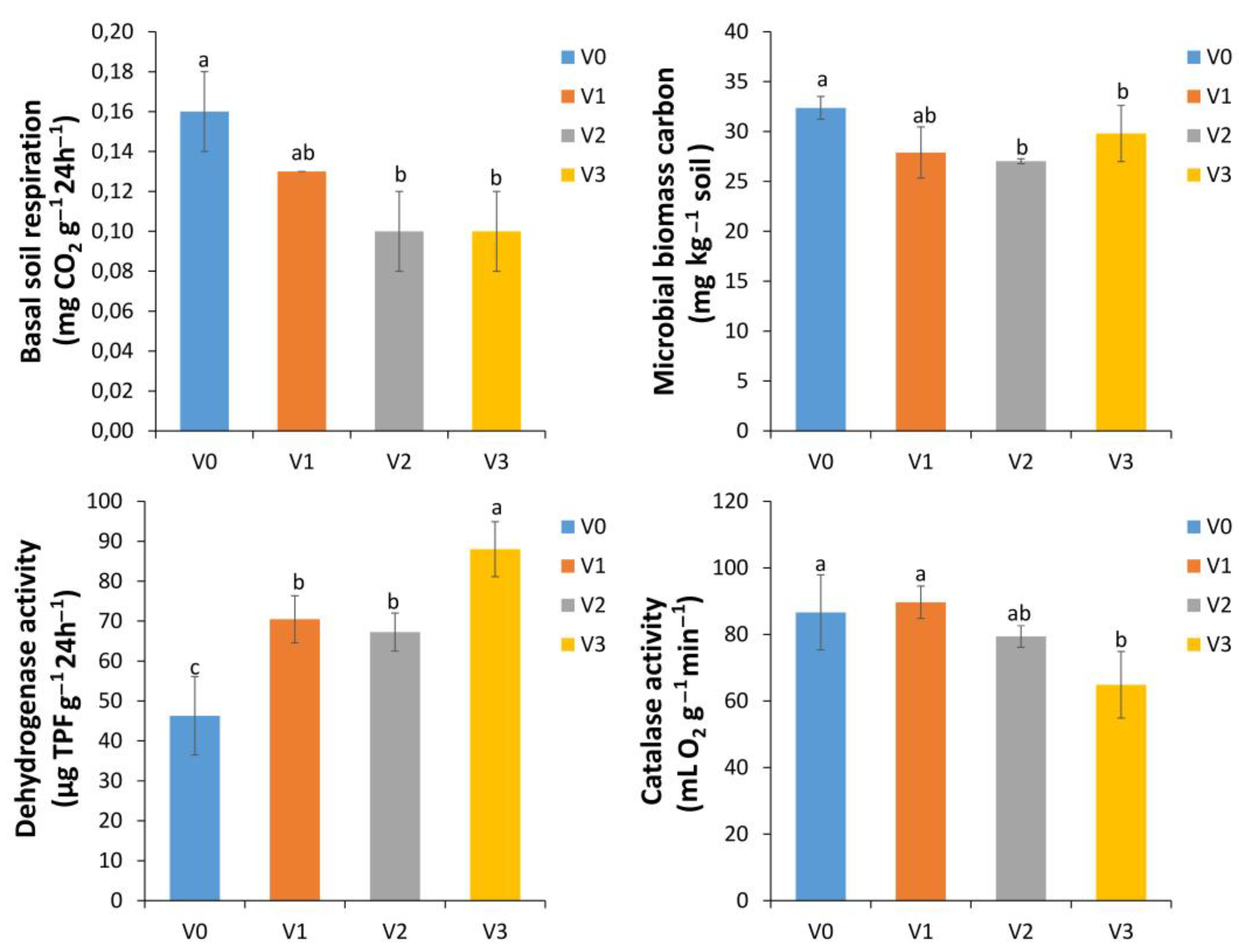
3.3. Impact of Vermicompost Application on Soil Biological Parameters
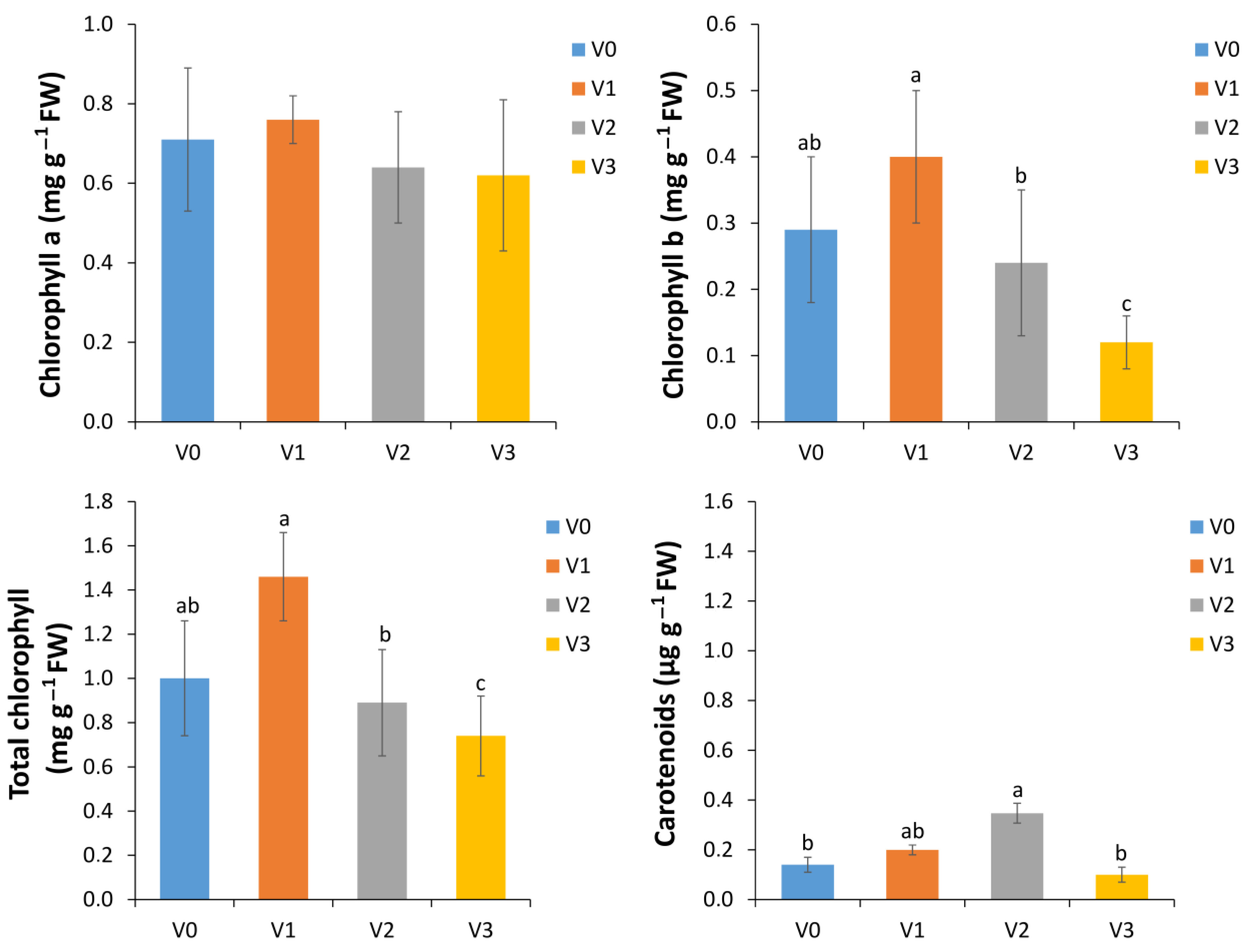
3.4. Impact of Vermicompost Application on Biochemical Parameters of Lettuce
3.5. Effect of Vermicompost on Soil Enzyme Activities
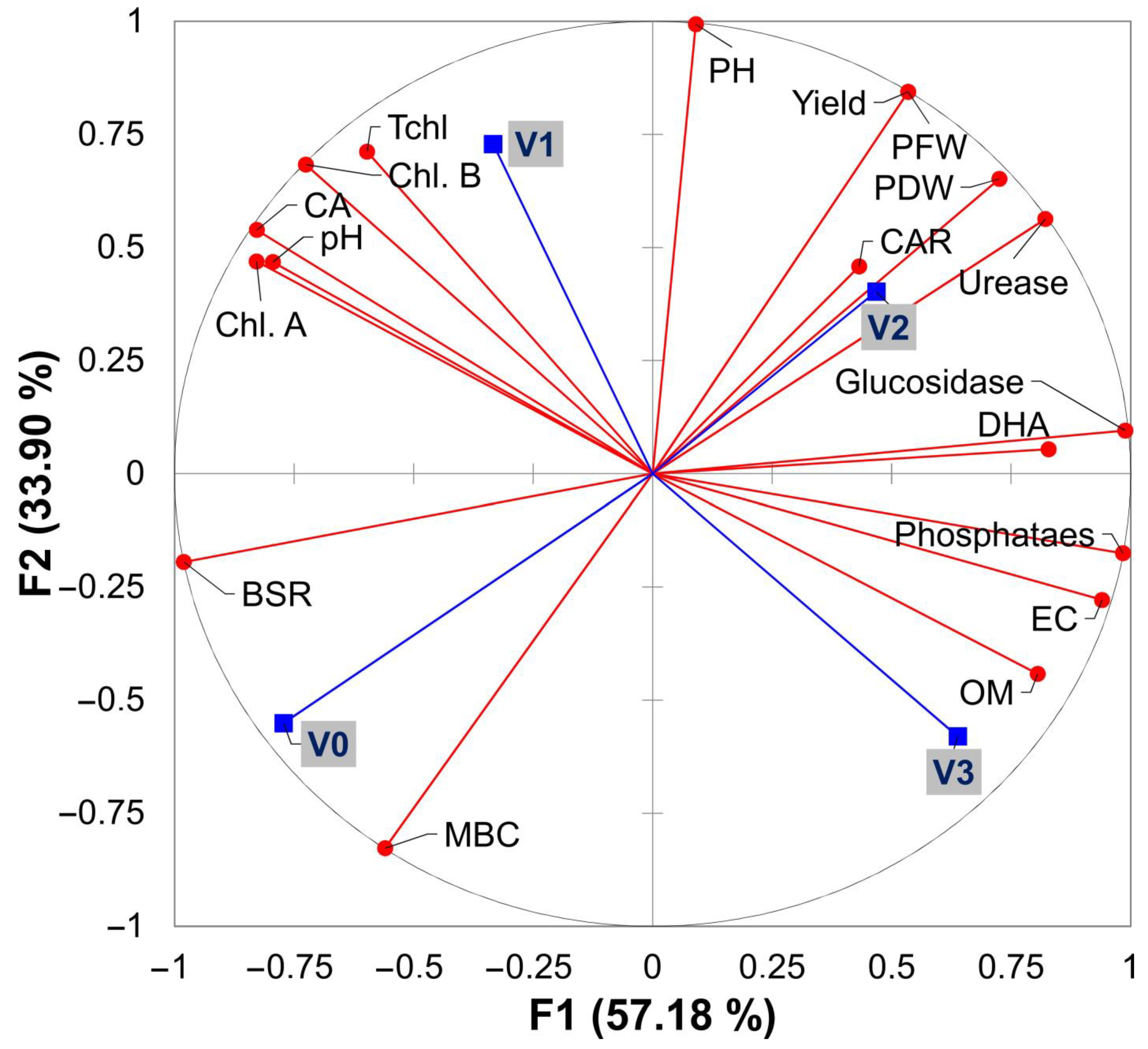
3.6. Principal Component Analysis and Heat Map Pearson Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M.; Rosado, P. Fertilizers. Published Online at OurWorldInData. 2022. Available online: https://ourworldindata.org/land-use (accessed on 15 November 2023).
- Sarim, M.; Jan, T.; Khattak, S.A.; Mihoub, A.; Jamal, A.; Saeed, M.F.; Soltani-Gerdefaramarzi, S.; Tariq, S.R.; Fernández, M.P.; Mancinelli, R. Assessment of the Ecological and Health Risks of Potentially Toxic Metals in Agricultural Soils from the Drosh-Shishi Valley, Pakistan. Land 2022, 11, 1663. [Google Scholar] [CrossRef]
- De Castro, F.; Vergaro, V.; Benedetti, M.; Baldassarre, F.; Del Coco, L.; Dell’Anna, M.M.; Mastrorilli, P.; Fanizzi, F.P.; Ciccarella, G. Visible light-activated water-soluble platicur nanocolloids: Photocytotoxicity and metabolomics studies in cancer cells. ACS Appl. Bio Mater. 2020, 3, 6836–6851. [Google Scholar] [CrossRef]
- Jamal, A.; Saeed, M.F.; Mihoub, A.; Hopkins, B.G.; Ahmad, I.; Naeem, A. Integrated use of phosphorus fertilizer and farmyard manure improves wheat productivity by improving soil quality and P availability in calcareous soil under subhumid conditions. Front. Plant Sci. 2023, 14, 1034421. [Google Scholar] [CrossRef]
- Oyege, I.; Balaji Bhaskar, M.S. Effects of Vermicompost on Soil and Plant Health and Promoting Sustainable Agriculture. Soil Syst. 2023, 7, 101. [Google Scholar] [CrossRef]
- Toor, M.D.; Kizilkaya, R.; Ullah, I.; Koleva, L.; Basit, A.; Mohamed, H.I. Potential Role of Vermicompost in Abiotic Stress Tolerance of Crop Plants: A Review. J. Soil Sci. Plant Nutr. 2023, 23, 4765–4787. [Google Scholar] [CrossRef]
- Lirikum; Kakati, L.; Thyug, L.; Mozhui, L. Vermicomposting: An eco-friendly approach for waste management and nutrient enhancement. Trop. Ecol. 2022, 63, 325–337. [Google Scholar] [CrossRef]
- Ali, U.; Sajid, N.; Khalid, A.; Riaz, L.; Rabbani, M.M.; Syed, J.H.; Malik, R.N. A review on vermicomposting of organic wastes. Environ. Prog. Sustain. Energy 2015, 34, 1050–1062. [Google Scholar] [CrossRef]
- Katiyar, R.B.; Sundaramurthy, S.; Sharma, A.K.; Arisutha, S.; Pratap-Singh, A.; Mishra, S.; Ayub, R.; Jeon, B.H.; Khan, M.A. Vermicompost: An Eco-Friendly and Cost-Effective Alternative for Sustainable Agriculture. Sustainability 2023, 15, 14701. [Google Scholar] [CrossRef]
- Yatoo, A.M.; Bhat, S.A.; Ali, M.N.; Baba, Z.A.; Zaheen, Z. Production of nutrient-enriched vermicompost from aquatic macrophytes supplemented with kitchen waste: Assessment of nutrient changes, phytotoxicity, and earthworm biodynamics. Agronomy 2022, 12, 1303. [Google Scholar] [CrossRef]
- Hoque, T.S.; Hasan, A.K.; Hasan, M.A.; Nahar, N.; Dey, D.K.; Mia, S.; Solaiman, Z.M.; Kader, M.A. Nutrient release from vermicompost under anaerobic conditions in two contrasting soils of Bangladesh and its effect on wetland rice crop. Agriculture 2022, 12, 376. [Google Scholar] [CrossRef]
- Aslam, Z.; Bashir, S.; Hassan, W.; Bellitürk, K.; Ahmad, N.; Niazi, N.K.; Khan, A.; Khan, M.I.; Chen, Z.; Maitah, M. Unveiling the efficiency of vermicompost derived from different biowastes on wheat (Triticum aestivum L.) plant growth and soil health. Agronomy 2019, 9, 791. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. The use of PGPB to promote plant hydroponic growth. Plants 2022, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.Y.; Ajeng, A.A.; Cheah, W.Y.; Ng, E.-P.; Abdullah, R.; Ling, T.C. Towards circular economy: Potential of microalgae–bacterial-based biofertilizer on plants. J. Environ. Manag. 2024, 349, 119445. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Shah, S.; Jamal, A.; Saeed, M.F.; Mihoub, A.; Zia, A.; Ahmed, I.; Seleiman, M.F.; Mancinelli, R.; Radicetti, E. Combined Effect of Biochar and Plant Growth-Promoting Rhizbacteria on Physiological Responses of Canola (Brassica napus L.) Subjected to Drought Stress. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Salman, M.; Inamullah; Jamal, A.; Mihoub, A.; Saeed, M.F.; Radicetti, E.; Ahmad, I.; Naeem, A.; Ullah, J.; Pampana, S. Composting sugarcane filter mud with different sources differently benefits sweet maize. Agronomy 2023, 13, 748. [Google Scholar] [CrossRef]
- Chen, A.; Han, Z.; Xie, X.; Song, C.; Zhang, X.; Zhao, Y. Co-composting sugar-containing waste with chicken manure–A new approach to carbon sequestration. J. Environ. Manag. 2024, 356, 120609. [Google Scholar] [CrossRef] [PubMed]
- Filipović, V.; Ugrenović, V.; Popović, V.; Dimitrijević, S.; Popović, S.; Aćimović, M.; Dragumilo, A.; Pezo, L. Productivity and flower quality of different pot marigold (Calendula officinalis L.) varieties on the compost produced from medicinal plant waste. Ind. Crops Prod. 2023, 192, 116093. [Google Scholar] [CrossRef]
- Mihoub, A.; Koull, N.; Helimi, S.; Elhafed Kherraze, M.; Mokhtari, S.; Pulido Fernández, M. Developing scoring functions for soil quality to assess land suitability for irrigated wheat in Southern Algeria. Soil Use Manag. 2022, 38, 262–276. [Google Scholar] [CrossRef]
- Khan, I.; Amanullah; Jamal, A.; Mihoub, A.; Farooq, O.; Farhan Saeed, M.; Roberto, M.; Radicetti, E.; Zia, A.; Azam, M. Partial substitution of chemical fertilizers with organic supplements increased wheat productivity and profitability under limited and assured irrigation regimes. Agriculture 2022, 12, 1754. [Google Scholar] [CrossRef]
- Toor, M.D.; Anwar, A.; Koleva, L.; Eldesoky, G.E. Effects of vermicompost on soil microbiological properties in lettuce rhizosphere: An environmentally friendly approach for sustainable green future. Environ. Res. 2024, 243, 117737. [Google Scholar] [CrossRef]
- Hemati, A.; Alikhani, H.A.; Ajdanian, L.; Babaei, M.; Asgari Lajayer, B.; van Hullebusch, E.D. Effect of different enriched vermicomposts, humic acid extract and indole-3-acetic acid amendments on the growth of Brassica napus. Plants 2022, 11, 227. [Google Scholar] [CrossRef]
- Enebe, M.C.; Erasmus, M. Mediators of biomass transformation–a focus on the enzyme composition of the vermicomposting process. Environ. Chall. 2023, 12, 100732. [Google Scholar] [CrossRef]
- Hanc, A.; Dume, B.; Hrebeckova, T. Differences of enzymatic activity during composting and vermicomposting of sewage sludge mixed with straw pellets. Front. Microbiol. 2022, 12, 801107. [Google Scholar] [CrossRef]
- Becagli, M.; Arduini, I.; Cardelli, R. Using biochar and vermiwash to improve biological activities of soil. Agriculture 2022, 12, 178. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Liu, X.; Chang, T.; Wang, Q.; Shaghaleh, H.; Hamoud, Y.A. Effects of biochar and vermicompost on microorganisms and enzymatic activities in greenhouse soil. Front. Environ. Sci. 2023, 10, 1060277. [Google Scholar] [CrossRef]
- Karasahin, M. Effects of vermicompost and inorganic fertilizer applications in different forms and doses on grain corn. J. Plant Nutr. 2023, 46, 3002–3017. [Google Scholar] [CrossRef]
- Sainova, G.; Kaliyeva, N.; Yuldashbek, D.K.; Akbasova, A. The Influence of Vermicompost and Various Concentrations of Lead on the Enzymatic Activity of Sierozem Soils of Kazakhstan. Scientifica 2023, 2023, 8490234. [Google Scholar] [CrossRef]
- McLean, E. Soil pH and lime requirement. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; 1983; Volume 9, pp. 199–224. Available online: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2134/agronmonogr9.2.2ed.c12. (accessed on 20 December 2023).
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; John Wiley & Sons: Madison, WI, USA, 1983; Volume 9, pp. 539–579. [Google Scholar]
- Soltanpour, P.; Schwab, A. A new soil test for simultaneous extraction of macro-and micro-nutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; Volume 5, pp. 1085–1121. [Google Scholar]
- Marshall, M.R. Ash analysis. In Food Analysis, 4th ed; Nielsen, S.S., Ed.; Springer: New York, NY, USA, 2010; Chapter 7; pp. 105–116. [Google Scholar]
- Rowell, D.L. Soil Science: Methods & Applications; Routledge: London, UK, 2014. [Google Scholar]
- Witham, F.H.; Blaydes, D.F.; Devlin, R.M. Experiments in Plant Physiology, Van Nostrand Reinhold Co.: New York, NY, USA, 1971.
- Jones, J.B., Jr.; Wolf, B.; Mills, H.A. Plant Analysis Handbook. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing, Inc.: Athens, GA, USA, 1991. [Google Scholar]
- Lindsay, W.L.; Norvell, W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Anderson, J.P.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Anderson, J.P. Soil respiration. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Wiley: Hoboken, NJ, USA, 1982; pp. 831–871. [Google Scholar]
- Pepper, I.L.; Gerba, C.P.; Brendecke, J.W. Environmental Microbiology: A Laboratory Manual; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Beck, E.H. Catalase activity in soil. Plant Soil 1971, 35, 179–192. [Google Scholar]
- Eivazi, F.; Tabatabai, M. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Yao, X.-h.; Min, H.; Lü, Z.-h.; Yuan, H.-p. Influence of acetamiprid on soil enzymatic activities and respiration. Eur. J. Soil Biol. 2006, 42, 120–126. [Google Scholar] [CrossRef]
- Schneider, K.; Turrión, M.B.; Gallardo, J.F. Modified method for measuring acid phosphatase activities in forest soils with high organic matter content. Commun. Soil Sci. Plant Anal. 2000, 31, 3077–3088. [Google Scholar] [CrossRef]
- Rehman, S.u.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: Enhancing Plant Growth and Combating Abiotic and Biotic Stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble salts in compost and their effects on soil and plants: A review. Compost Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Alam, M.A.; Alauddin, M.; Rahman, M.; Alauddin, M.; Rahman, M.S.; Mohsin, G.; Rahman, M.K. Vermicompost induced growth and yield performance of capsicum (Capsicum annuum L.) at sustainable rooftop farming system. J. Phytol. 2023, 15, 94–100. [Google Scholar] [CrossRef]
- Raza, S.T.; Zhu, B.; Yao, Z.; Wu, J.; Chen, Z.; Ali, Z.; Tang, J.L. Impacts of vermicompost application on crop yield, ammonia volatilization and greenhouse gases emission on upland in Southwest China. Sci. Total Environ. 2023, 860, 160479. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, C.; Domínguez, J. The use of vermicompost in sustainable agriculture: Impact on plant growth and soil fertility. Soil Nutr. 2011, 10, 187. [Google Scholar]
- Kansotia, B.; Sharma, Y.; Meena, R. Effect of vermicompost and inorganic fertilizers on soil properties and yield of Indian mustard (Brassica juncea L.). J. Oilseed Brassi. 2016, 1, 198–201. [Google Scholar]
- Ansari, A.A.; Ori, L.; Ramnarain, Y.I. An effective organic waste recycling through vermicompost technology for soil health restoration. In Soil Health Restoration and Management; Springer: Singapore, 2020; pp. 83–112. [Google Scholar]
- Adiloğlu, S.; Eryılmaz Açıkgöz, F.; Solmaz, Y.; Çaktü, E.; Adiloğlu, A. Effect of vermicompost on the growth and yield of lettuce plant (Lactuca sativa L. var. crispa). Int. J. Plant Soil Sci. 2018, 21, 1–5. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, R.; Yu, W.; Liang, J.; Liao, X. Influences of a vermicompost application on the phosphorus transformation and microbial activity in a paddy soil. Soil Water Res. 2020, 15, 199–210. [Google Scholar] [CrossRef]
- Nuraini, Y.; Prasetya, B.; Handayanto, E. Effectiveness of compost and vermicompost from market organic waste to improve soil chemical properties. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 012068. [Google Scholar]
- Atiyeh, R.; Edwards, C.; Subler, S.; Metzger, J. Pig manure vermicompost as a component of a horticultural bedding plant medium: Effects on physicochemical properties and plant growth. Bioresour. Technol. 2001, 78, 11–20. [Google Scholar] [CrossRef]
- Arancon, N.; Edwards, C.; Bierman, P.; Welch, C.; Metzger, J. Influences of vermicomposts on field strawberries: 1. Effects on growth and yields. Bioresour. Technol. 2004, 93, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ramos, S.; Escamilla-Silva, E.; Dendooven, L. Vermicomposting of biosolids with cow manure and oat straw. Biol. Fertil. Soils 2005, 41, 190–198. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.; Kumar, S.; Gupta, R.; Patil, R. Vermicompost substitution influences growth, physiological disorders, fruit yield and quality of strawberry (Fragaria x ananassa Duch.). Bioresour. Technol. 2008, 99, 8507–8511. [Google Scholar] [CrossRef] [PubMed]
- Ceritoğlu, M.; Şahin, S.; Erman, M. Effects of vermicompost on plant growth and soil structure. Selcuk J. Agric. Food Sci. 2018, 32, 607–615. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y.; Lim, P.N.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant growth-promoting bacteria (PGPB) with biofilm-forming ability: A multifaceted agent for sustainable agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- Babar, S.; Jilani, G.; Mihoub, A.; Jamal, A.; Ahmad, I.; Chaudhary, A.N.; Saeed, M.F.; Alam, T. Bacterial redox cycling of manganese in calcareous soil enhances the nutrients bioavailability to wheat. J. Soil Sci. Plant Nutr. 2022, 22, 1215–1223. [Google Scholar] [CrossRef]
- Kumari, E.; Kumari, S.; Das, S.S.; Mahapatra, M.; Sahoo, J.P. Plant Growth-Promoting Bacteria (PGPB) for Sustainable Agriculture: Current Prospective and Future Challenges. AgroEnvironmental Sustain. 2023, 1, 274–285. [Google Scholar] [CrossRef]
- Qasim, M.; Ju, J.; Zhao, H.; Bhatti, S.M.; Saleem, G.; Memon, S.P.; Ali, S.; Younas, M.U.; Rajput, N.; Jamali, Z.H. Morphological and Physiological Response of Tomato to Sole and Combined Application of Vermicompost and Chemical Fertilizers. Agronomy 2023, 13, 1508. [Google Scholar] [CrossRef]
- Yadav, A.; Garg, V. Recycling of organic wastes by employing Eisenia fetida. Bioresour. Technol. 2011, 102, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Przemieniecki, S.W.; Zapałowska, A.; Skwiercz, A.; Damszel, M.; Telesiński, A.; Sierota, Z.; Gorczyca, A. An evaluation of selected chemical, biochemical, and biological parameters of soil enriched with vermicompost. Environ. Sci. Pollut. Res. 2021, 28, 8117–8127. [Google Scholar] [CrossRef] [PubMed]
- Tammam, A.A.; Rabei Abdel Moez Shehata, M.; Pessarakli, M.; El-Aggan, W.H. Vermicompost and its role in alleviation of salt stress in plants–I. Impact of vermicompost on growth and nutrient uptake of salt-stressed plants. J. Plant Nutr. 2023, 46, 1446–1457. [Google Scholar] [CrossRef]
- Zeng, J.; Hu, H.; He, X.; Song, W.; Wang, F.; Zhang, Y.; Qin, S. N2O emissions, microbial community composition and genes expressions in soil amended with vermicomposts derived from different feedstocks. Eur. J. Soil Biol. 2023, 115, 103473. [Google Scholar] [CrossRef]
- Jha, S.; Verma, A.; Bhattacharyya, P. Assessing the Effectiveness of Vermicomposted Products and Predicting Potential Hazards From Metal Contaminated Steel Waste Through Multi-model Analysis. Water Air Soil Pollut. 2023, 234, 679. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Z.; Ye, Q.; Peng, Z.; Zhu, S.; Chen, H.; Liu, D.; Li, Y.; Deng, L.; Shu, X. Positive Effects of Organic Amendments on Soil Microbes and Their Functionality in Agro-Ecosystems. Plants 2023, 12, 3790. [Google Scholar] [CrossRef]
- Cui, J.; Yang, B.; Zhang, M.; Song, D.; Xu, X.; Ai, C.; Liang, G.; Zhou, W. Investigating the effects of organic amendments on soil microbial composition and its linkage to soil organic carbon: A global meta-analysis. Sci. Total Environ. 2023, 894, 164899. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.-H.; Zhao, X.-L. Effects of Organic Amendments on Microbial Biomass Carbon and Nitrogen Uptake by Corn Seedlings Grown in Two Purple Soils. Huan Jing Ke Xue 2019, 40, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, M.; Sirousmehr, A.; Ansari, M.H.; Ghanbari, A. Organic soil amendments using vermicomposts under inoculation of N2-fixing bacteria for sustainable rice production. PeerJ 2021, 9, e10833. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.D.; Singh, N.; Sharma, A.; Joshi, R.; Srivastava, P. Hydrogen peroxide regulates antioxidant responses and redox related proteins in drought stressed wheat seedlings. Physiol. Mol. Biol. Plants 2021, 27, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.; Edwards, C.A. Biology and ecology of earthworm species used for vermicomposting. In Vermiculture Technology: Earthworms, Organic Waste and Environmental Management; CRC Press: Boca Raton, FL, USA, 2011; pp. 27–40. [Google Scholar]
- Aslam, Z.; Ahmad, A.; Bellitürk, K.; Kanwal, H.; Asif, M.; Ullah, E. Integrated use of simple compost, vermicompost, vermi-tea and chemical fertilizers NP on the morpho-physiological, yield and yield related traits of tomato (Solanum lycopersicum L.). J. Innov. Sci. 2023, 9, 1–12. [Google Scholar] [CrossRef]
- Khourchi, S.; Elhaissoufi, W.; Loum, M.; Ibnyasser, A.; Haddine, M.; Ghani, R.; Barakat, A.; Zeroual, Y.; Rchiad, Z.; Delaplace, P. Phosphate solubilizing bacteria can significantly contribute to enhance P availability from polyphosphates and their use efficiency in wheat. Microbiol. Res. 2022, 262, 127094. [Google Scholar] [CrossRef]
- Turp, G.A.; Ozdemir, S.; Yetilmezsoy, K.; Oz, N.; Elkamel, A. Role of Vermicomposting Microorganisms in the Conversion of Biomass Ash to Bio-Based Fertilizers. Sustainability 2023, 15, 8984. [Google Scholar] [CrossRef]
- da Silva, L.F.; da Silva, E.F.; Morais, F.M.S.; Portela, J.C.; de Oliveira, F.H.T.; de Freitas, D.F.; de Almeida Ferreira, E.; Gurgel, M.T.; Pinheiro, A.M.; Lima, R.B. Potential of vermicomposting with mixtures of animal manure and vegetable leaves in the development of Eisenia foetida, microbial biomass, and enzymatic activity under semi-arid conditions. J. Environ. Manag. 2023, 330, 117169. [Google Scholar] [CrossRef]



| Vermicompost (%) | pH | EC (μS cm−1) | OM (%) |
|---|---|---|---|
| V0 | 7.13 ± 0.02 ab | 1030.00 ± 51.5 c | 2.07 ± 0.18 c |
| V1 | 7.21 ± 0.02 a | 1216.00 ± 60.8 c | 2.24 ± 0.04 bc |
| V2 | 7.02 ± 0.12 bc | 1669.33 ± 84.97 b | 2.53 ± 0.07 b |
| V3 | 7.01 ± 0.02 c | 2213.33 ± 110.67 a | 3.83 ± 0.29 a |
| N (%) | P (mg kg−1) | K (cmol(+) kg−1) | Ca (cmol(+) kg−1) | Mg (cmol(+) kg−1) | Na (cmol(+) kg−1) | Fe (mg kg−1) | Mn (mg kg−1) | Cu (mg kg−1) | Zn (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| 0.45 ± 0.01 c | 36.49 ± 3.63 d | 0.75 ± 0.03 c | 25.68 ± 0.36 a | 15.58 ± 0.34 d | 1.62 ± 0.07 c | 49.40 ± 2.83 a | 68.8 ± 0.28 a | 13.9 ± 2.34 a | 9.46 ± 1.27 d |
| 0.4 ± 0.01 d | 70.38 ± 4.20 c | 0.98 ± 0.04 c | 24.64 ± 0.43 a | 17.67 ± 0.37 c | 2.02 ± 0.10 b | 42.89 ± 2.79 b | 53.2 ± 0.17 b | 12.12 ± 3.12 b | 15.32 ± 1.02 c |
| 0.52 ± 0.03 b | 104.54 ± 10.46 b | 1.44 ± 0.02 b | 24.39 ± 1.36 a | 20.82 ± 1.29 b | 2.06 ± 0.05 b | 42.59 ± 1.59 b | 51.5 ± 0.84 b | 12.27 ± 1.96 b | 22.49 ± 1.00 b |
| 0.61 ± 0.02 a | 164± 4.9 a | 2.41 ± 0.32 a | 20.26 ± 0.50 b | 29.39 ± 072 a | 3.22 ± 0.04 a | 31.60 ± 1.84 c | 45.2 ± 0.22 c | 11.39 ± 1.95 b | 36.66 ± 1.96 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toor, M.D.; Ay, A.; Ullah, I.; Demirkaya, S.; Kızılkaya, R.; Mihoub, A.; Zia, A.; Jamal, A.; Ghfar, A.A.; Di Serio, A.; et al. Vermicompost Rate Effects on Soil Fertility and Morpho-Physio-Biochemical Traits of Lettuce. Horticulturae 2024, 10, 418. https://doi.org/10.3390/horticulturae10040418
Toor MD, Ay A, Ullah I, Demirkaya S, Kızılkaya R, Mihoub A, Zia A, Jamal A, Ghfar AA, Di Serio A, et al. Vermicompost Rate Effects on Soil Fertility and Morpho-Physio-Biochemical Traits of Lettuce. Horticulturae. 2024; 10(4):418. https://doi.org/10.3390/horticulturae10040418
Chicago/Turabian StyleToor, Muhammad Danish, Abdurrahman Ay, Izhar Ullah, Salih Demirkaya, Rıdvan Kızılkaya, Adil Mihoub, Adil Zia, Aftab Jamal, Ayman A. Ghfar, Annamaria Di Serio, and et al. 2024. "Vermicompost Rate Effects on Soil Fertility and Morpho-Physio-Biochemical Traits of Lettuce" Horticulturae 10, no. 4: 418. https://doi.org/10.3390/horticulturae10040418
APA StyleToor, M. D., Ay, A., Ullah, I., Demirkaya, S., Kızılkaya, R., Mihoub, A., Zia, A., Jamal, A., Ghfar, A. A., Di Serio, A., & Ronga, D. (2024). Vermicompost Rate Effects on Soil Fertility and Morpho-Physio-Biochemical Traits of Lettuce. Horticulturae, 10(4), 418. https://doi.org/10.3390/horticulturae10040418










