The Effect of Postharvest Storage Temperatures on Fruit Flavor Constituents in ‘Wushancuili’ Plum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Storage Treatment
2.2. High-Performance Liquid Chromatography (HPLC)
2.3. Headspace Solid Phase Microextraction Gas Chromatography Mass Spectrometry (HS-SPME GC–MS)
2.4. Statistical Analysis
3. Results
3.1. Changes in Soluble Sugar Content during Storage at Different Temperatures
3.2. Changes in Organic Acid Content during Storage at Different Temperatures
3.3. Aroma Components Characterized in ‘Wushancuili’ Fruit during Storage at Different Temperatures
3.4. Changes in Major Aroma Components during Storage at Different Temperatures
4. Discussion
4.1. Relationship between Fruit Flavor Quality and Soluble Sugars, Organic Acids, and Aroma Composition
4.2. Effect of Temperature on Soluble Sugar, Organic Acid, and Aroma Composition
4.3. Effect of Temperature on Fruit Flavor Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crisosto, C.H. Establishing a consumer quality index for fresh plums (prunus salicina lindell). Horticulturae 2023, 9, 682. [Google Scholar] [CrossRef]
- Cheng, C.; Lu, J.; Han, W.Q.; Huang, H.; Li, Z.H. Study on processing technology of plum juice beverage with pulp. Food Mach. 2015, 31, 215–218. [Google Scholar]
- Zhou, K.; Wang, J.W.; Pan, L.; Xiang, F.; Zhou, Y.; Xiong, W.; Zeng, M.; Grierson, D.; Kong, W.B.; Hua, L.Y.; et al. A Chromosome-Level Genome Assembly for Chinese Plum ‘Wushancuili’ Reveals the Molecular Basis of its Fruit Color and Susceptibility to Rain-Cracking. Hortic. Plant J. 2023, in press. [Google Scholar] [CrossRef]
- Kou, L.L.; Kong, W.B.; Hu, G.P. Review and Suggestions on Li Production and Sales in Chongqing in 2022. China Fruit News 2022, 39, 23–24. [Google Scholar]
- Cui, Y.C.; Mian, Y.H.; Liu, H.Y.; Han, F.; Guo, W.; Mao, Q.L. Research progress on soluble sugars in fruits. Xinjiang Agric. Sci. Technol. 2019, 2, 44–46. [Google Scholar]
- Ma, B.; Liao, L.; Fang, T.; Peng, Q.; Ogutu, C.; Zhou, H.; Ma, F.; Han, Y. A Ma10 gene encoding P-type ATPase is involved in fruit organic acid accumulation in apple. Plant Biotechnol. J. 2019, 17, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Purvis, A. Moisture loss and juice quality from waxed and individually sealed-packaged citrus fruits. Proc. Fla. State Hort. Soc 1983, 96, 327–329. [Google Scholar]
- Louw, E.D.; Theron, K.I. Volatile dynamics during maturation, ripening and cold storage of three Japanese plum cultivars (Prunus salicina Lindl.). Postharvest Biol. Technol. 2012, 130, 432–440. [Google Scholar] [CrossRef]
- Wang, X. Analysis of Aromatic Components and Its Characteristics of Different Pear Cultivars. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Youngjae, O.; Christopher, R.B.; Saket, C.; Jinhe, B.; Zhen, F.; Anne, P.; Jeremy, P.; Kevin, M.F.; Vance, M.W.; Seonghee, L. Genomic Characterization of the Fruity Aroma Gene, FaFAD1, Reveals a Gene Dosage Effect on γ-Decalactone Production in Strawberry (Fragaria × ananassa). Front. Plant Sci. 2021, 12, 639345. [Google Scholar]
- Yao, Y.M. Effects of Different Substrates and P, K Levels on Growth Fruit Quality and Flavor Compounds Content of Muskmelon. Master’s Thesis, Shandong Agricultural University, Jinan, China, 2009. [Google Scholar]
- Xie, Y.C.; Chen, Z.G.; Lin, X.S.; Zhang, H.Z. Analysis of Aroma Components of Different Parts of Kumquat and Blending Technology of Natural Kumquat Flavor. Beverage Ind. 2019, 22, 50–55. [Google Scholar]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant Volatiles1. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Chen, X.X.; Wang, N.; Yan, J.; Ma, R.J.; Yu, M.L.; Jiang, W.B. Comparison of near-freezing temperature storage characteristics of different peach varieties based on fruit quality index. Jiangsu J. Agric. Sci. 2021, 37, 998–1009. [Google Scholar]
- Yan, P.L.; Zhang, L.P.; Wang, L.J.; Yuan, Q.F.; Shi, B.B.; Chen, N. Research progress on post harvest storage and preservation technology of passion fruit. Agric. Tech. Serv. 2022, 39, 73–76. [Google Scholar]
- Hao, X.W.; Zhou, Q.; Gao, F.; Zhang, T.T.; Hao, Y.; Jiang, Y.F.; Lu, Y.Z. Effects of Temperature on Storage Quality of Postharvest ‘Guofeng No.7’ Plum Fruit. Packag. Eng. 2023, 44, 10. [Google Scholar]
- Akira, K.; Yuko, T.; Takaya, M.; Mitsuo, O.; Tomoya, A. Differential expression of three sucrose-phosphate synthase isoforms during sucrose accumulation in citrus fruits (Citrus unshiu Marc.). Plant Sci. 1999, 140, 169–178. [Google Scholar]
- Deng, R.; Yuan, Z.Y.; Xia, X.; Liu, Z.Z.; Shi, T.; Gao, H.; Zhao, Z.Y. Apple Influence of bagging on quality of Ruixue. J. Northwest A F Univ. Nat. Sci. Ed. 2018, 46, 117–123. [Google Scholar]
- Kong, W.B.; Xiong, W.; Kou, L.L.; Tang, J.; Xiang, F.; Huang, M.; Qin, J.; Xi, W.P.; Zhang, X.; Li, W. Preliminary exploration of key control factors for postharvest packaging, storage, and transportation of ‘Wushancuili’ plum. South China Fruits 2020, 49, 88–91. [Google Scholar]
- Zhou, J.H. Study on the synthesis of 2-hexene-1-ol. China Surfactant Deterg. Cosmet. 1997, 5, 51–52. [Google Scholar]
- Wang, S.Y.; Hao, X.; Qu, Y.; Chen, Y.Y.; Shen, Y.B. The role of trans-2-hexenal in plant defense responses. Chin. Bull. Bot. 2021, 56, 232–240. [Google Scholar]
- Dong, Q.; Li, S.M.; Gao, S.J.; Li, Y.X.; Duan, H.C.; Zheng, X.H.; Yao, X.F.; Zi, Q. Comparison and comprehensive analysis the quality of Cyphomandra betacea from different provenance. Food Ferment. Ind. 2022, 48, 266–273. [Google Scholar]
- Wu, W.F.; Chen, M.J.; Qi, F.B.; Chen, F.X. Organic Acid Composition Characteristics and Its Correlation with Malate Transporter Genes PsALMT9 and PstDT in Plum Fruit. Acta Bot. Boreali-Occident. Sin. 2020, 40, 1356–1363. [Google Scholar]
- Hu, P.Z.; Ju, Y.W.; Gai, H.Q.; Gai, F.Y.; Kai, J.Q.; Li, F.W.; Shao, L.Z. The role of sucrose-metabolizing enzymes in pear fruit that differ in sucrose accumulation. Acta Physiol. Plant. 2014, 36, 71–77. [Google Scholar]
- Zheng, L.J.; Nie, J.Y.; Yan, Z.; Xu, G.F.; Wang, K.; Gao, Y.; Ye, M.L. Studies on the Characteristics of the Composition and Content of Soluble Sugars in Apple Fruit. Acta Hortic. Sin. 2015, 42, 950–960. [Google Scholar]
- Chai, Q.Q.; Wu, B.H.; Liu, W.S.; Wang, L.J.; Yang, C.X.; Wang, Y.J.; Fang, J.B.; Liu, Y.C.; Li, S.H. Volatiles of plums evaluated by HS-SPME with GC–MS at the germplasm level. Food Chem. 2012, 130, 432–440. [Google Scholar] [CrossRef]
- Diao, Y.; Zeng, S.D.; Gao, L.J.; Zeng, X.F.; Cheng, Y.; Shang, S. The effect of modified atmosphere packaging with silicon windows on the postharvest storage quality of green crisp plums. South China Agric. 2019, 13, 76–80. [Google Scholar]
- Zhang, B.; Tieman, D.M.; Jiao, C.; Xu, Y.M.; Chen, K.S.; Fei, Z.J.; Giovannoni, J.J.; Klee, H.J. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl. Acad. Sci. USA 2016, 113, 12580–12585. [Google Scholar] [CrossRef] [PubMed]
- Lu, L. Study on Postharvest Storage Characteristics of New Red-Fleshed Apple Cultivar Meihong. Master’s Thesis, Shandong Agricultural University, Jinan, China, 2020. [Google Scholar]
- Zhang, X.Y.; Wu, G.C.; Wang, L.; Zhao, Z.L. Analysis of Volatile Aroma Component of “Oishiwase” Plum by SPME and GC-MS. Food Ind. 2018, 39, 178–181. [Google Scholar]
- Wang, Z.H.; Jia, C.S.; Wang, W.H.; Tong, W.; Jiang, Y.B. Effects of Low Temperature Storage on Energy Metabolism, Related Physiology and Quality in ‘Jinhong’ Apple Fruit. Acta Hortic. Sin. 2020, 47, 2277–2289. [Google Scholar]
- Zhu, Y.C. Analysis of Mechanisms for Postharvest Chilling Injury and the Effect of Ethylene on Alleviating Chilling Injury in Peach. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2020. [Google Scholar]
- Qu, X.Q. Regulation of trans-2-Hexenal on Chloroplast Degradation and Fruit Softening in Tomato. Master’s Thesis, Qilu University of Technology, Jinan, China, 2023. [Google Scholar]
- Jin, J.; Zhao, M.; Jing, T.; Wang, J.; Lu, M.; Pan, Y.; Du, W.; Zhao, C.; Bao, Z.; Zhao, W.; et al. (Z)-3-hexenol integrates drought and cold stress signaling by activating abscisic acid glucosylation in tea plants. Plant Physiol. 2023, 193, 1491–1507. [Google Scholar] [CrossRef]
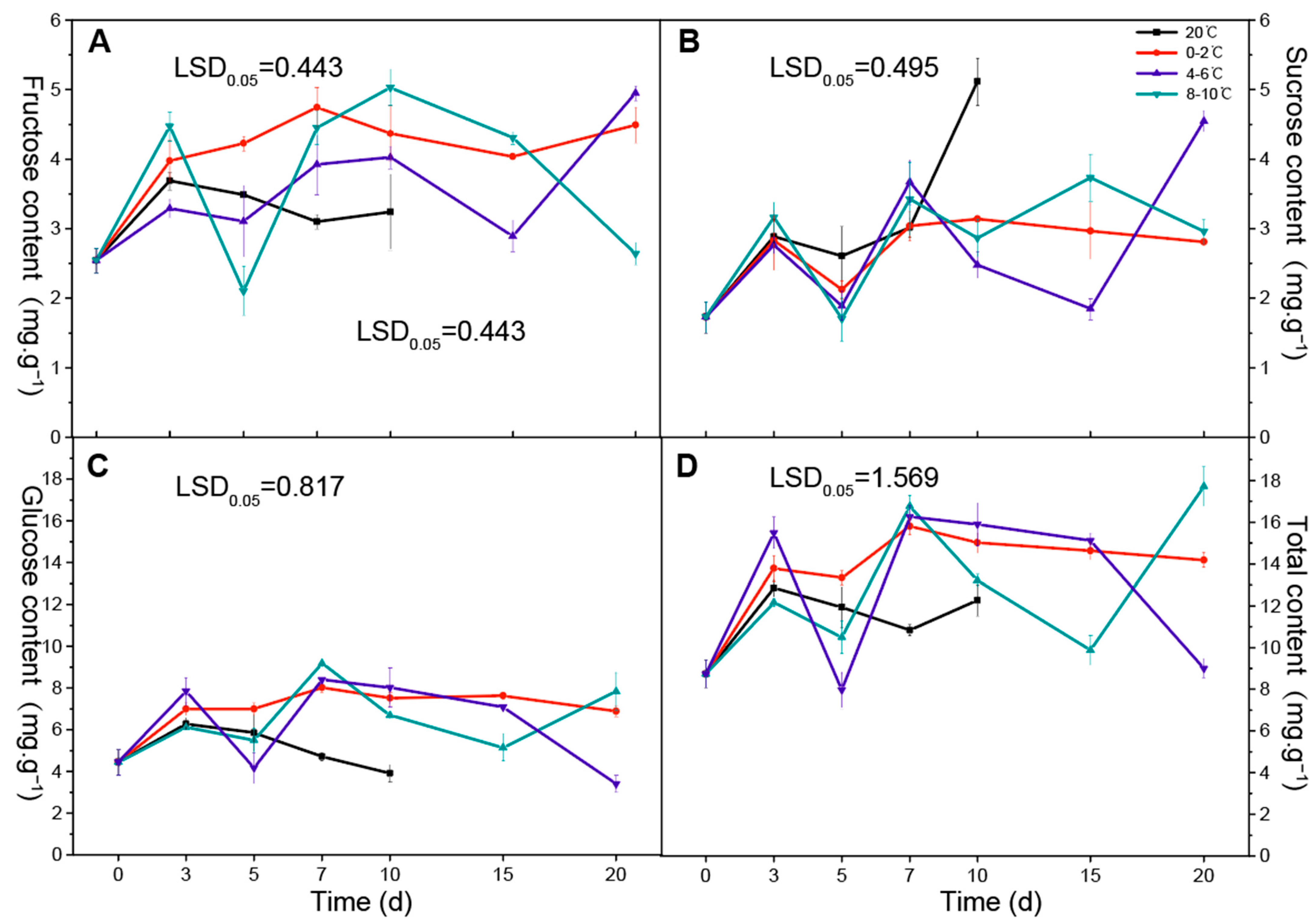
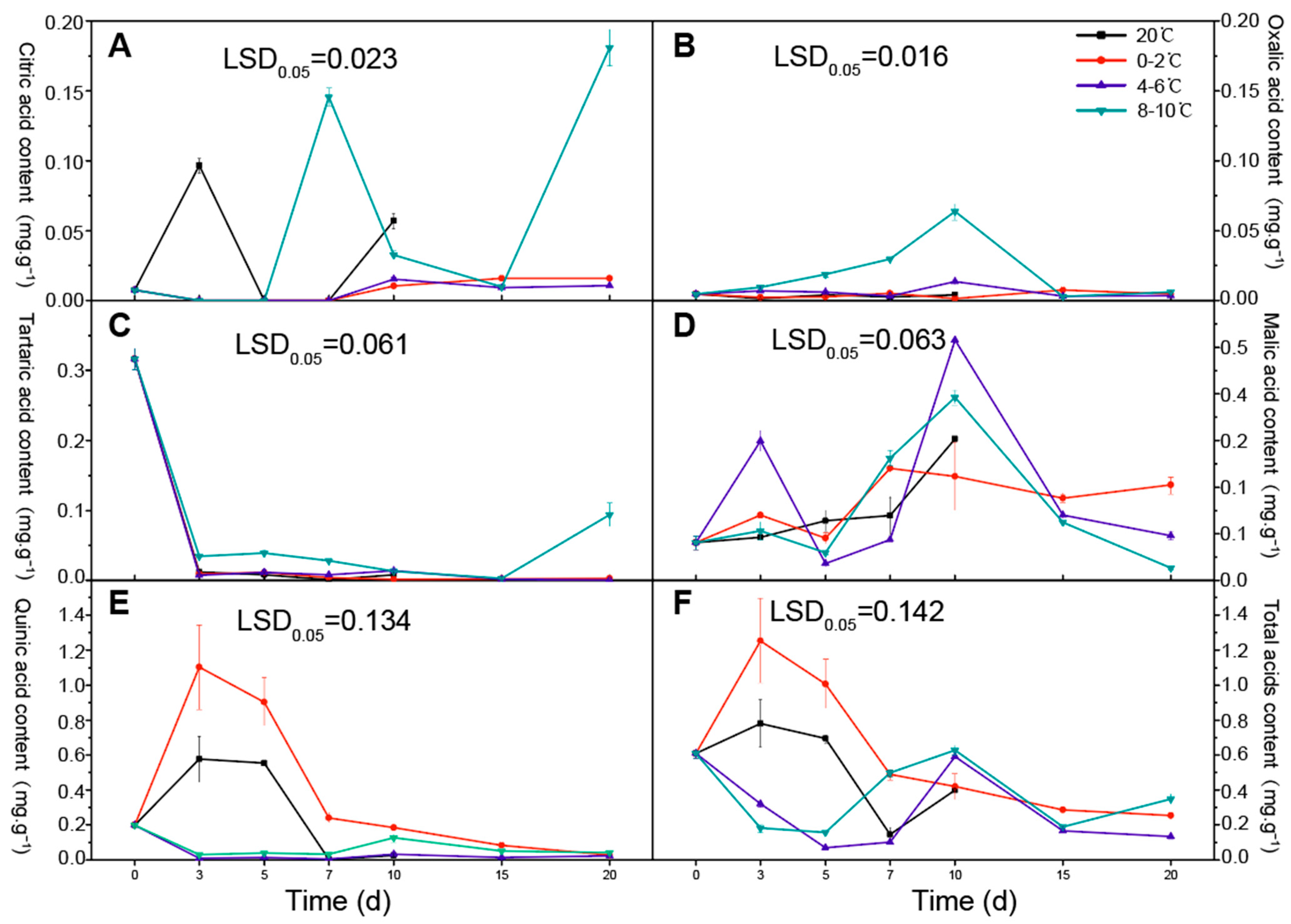
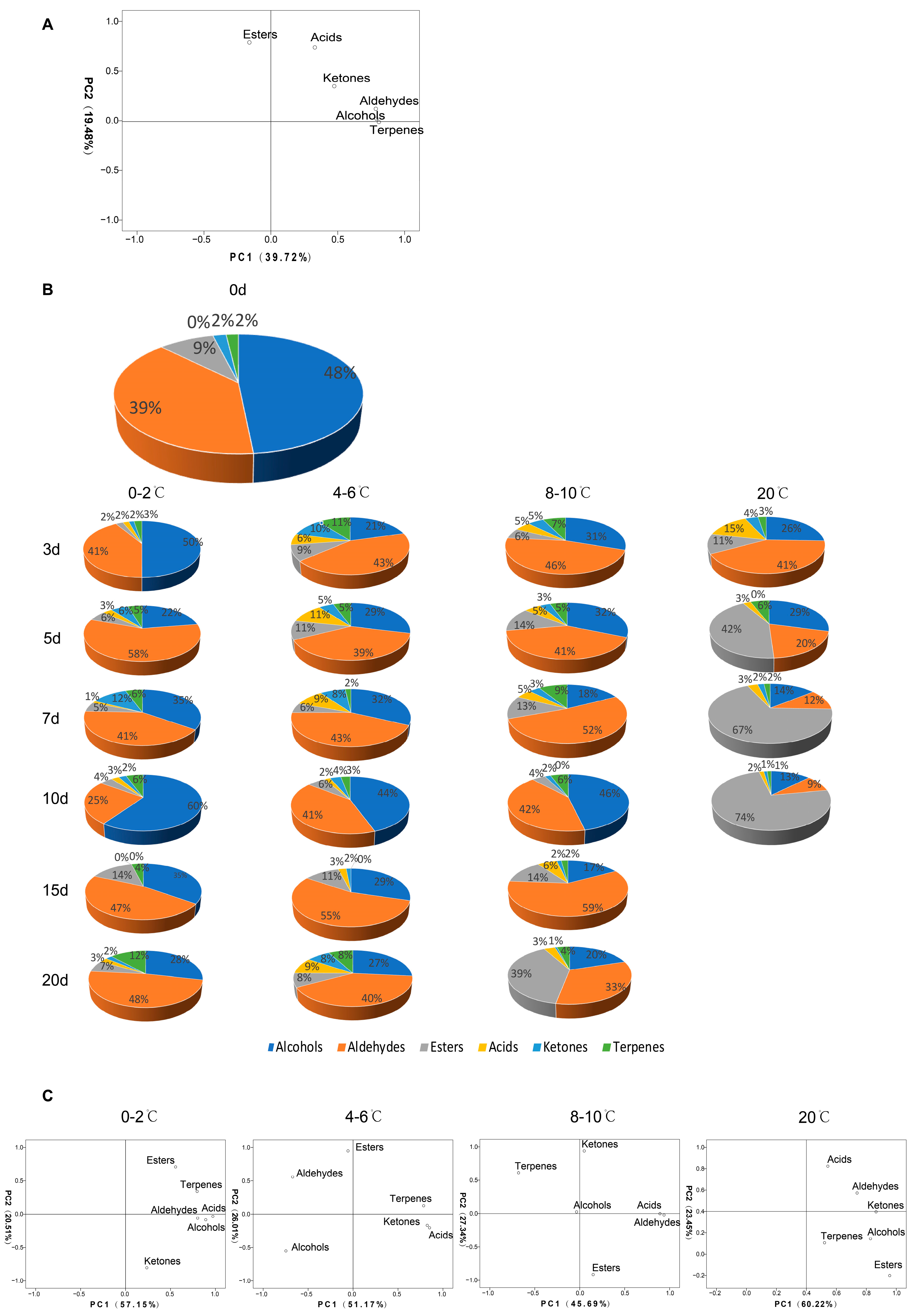
| Category | Compound | Aroma Character | Temperature Treatment (°C) | 0 d | 3 d | 5 d | 7 d | 10 d | 15 d | 20 d | Significances | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | D | T × D | |||||||||||
| Alcohols | 1-Octanol | Pungent aroma | 0–2 | 0.24 ± 0.35 a | 0.77 ± 0.38 a | 0.55 ± 0.16 a | 0.15 ± 0.70 ab | 0.13 ± 0.11 b | 0.15 ± 0.61 ab | - | ns | ns | * |
| 4–6 | 0.32 ± 0.31 ab | 0.14 ± 0.88 ab | 0.49 ± 0.14 ab | 0.42 ± 0.25 ab | 0.15 ± 0.22 b | - | |||||||
| 8–10 | 0.36 ± 0.15 ab | 0.67 ± 0.12 a | 0.14 ± 0.14 b | 0.74 ± 0.44 ab | 0.15 ± 0.32 ab | 0.65 ± 0.76 ab | |||||||
| 20 | 0.38 ± 0.82 ab | 0.12 ± 0.26 b | 0.19 ± 0.16 b | 0.15 ± 0.28 ab | - | - | |||||||
| α-terpineol | Lilac aroma | 0–2 | - | - | - | - | - | - | - | - | - | - | |
| 4–6 | - | - | - | - | - | - | |||||||
| 8–10 | - | - | - | - | 0.18 ± 0.22 a | - | |||||||
| 20 | - | 0.24 ± 0.26 a | 0.15 ± 0.99 a | 0.75 ± 0.92 a | - | - | |||||||
| (E)-2-Hexen-1-ol | Faint scent | 0–2 | 5.81 ± 0.19 a | 14.27 ± 6.87 a | 2.13 ± 0.58 c | 4.42 ± 0.54 b | 24.48 ± 19.42 a | 2.99 ± 0.43 bc | 5.27 ± 0.85 a | ns | ns | * | |
| 4–6 | 2.23 ± 0.19 c | 2.37 ± 0.16 c | 2.81 ± 0.29 c | 5.15 ± 2.75 abc | 3.22 ± 0.36 abc | 3.14 ± 0.39 bc | |||||||
| 8–10 | 4.29 ± 0.52 b | 4.55 ± 0.42 a | 2.13 ± 0.27 c | 6.52 ± 3.70 abc | 4.86 ± 0.68 ab | 2.89 ± 0.46 bc | |||||||
| 20 | 2.39 ± 0.75 c | 3.87 ± 0.32 b | 5.39 ± 0.91 a | 6.50 ± 0.79 abc | - | - | |||||||
| (E)-3-Hexen-1-ol | Faint scent | 0–2 | 0.48 ± 0.17 c | 1.26 ± 0.48 ab | - | 0.22 ± 0.24 cd | 0.57 ± 0.24 bc | - | 1.17 ± 0.13 a | ns | ns | * | |
| 4–6 | 0.13 ± 0.14 d | 0.76 ± 0.14 b | 0.42 ± 0.43 bcd | 2.59 ± 1.53 a | 0.32 ± 0.73 abcd | 0.28 ± 0.12 cd | |||||||
| 8–10 | 0.17 ± 0.24 cd | 0.86 ± 0.16 b | 0.68 ± 0.13 bc | 0.17 ± 0.28 cd | 0.37 ± 0.24 cd | 0.47 ± 0.25 bcd | |||||||
| 20 | 0.39 ± 0.91 abcd | 0.40 ± 0.81 abcd | 0.42 ± 0.44 bcd | - | - | - | |||||||
| Aldehydes | Furfural | Bitter almond aroma | 0–2 | - | 0.17 ± 0.80 bc | 0.26 ± 0.25 c | 0.28 ± 0.84 c | 0.18 ± 0.18 c | 0.46 ± 0.49 c | 1.16 ± 0.17 c | * | ns | * |
| 4–6 | 0.56 ± 0.80 c | - | 0.14 ± 0.16 c | - | - | 2.69 ± 0.32 a | |||||||
| 8–10 | 0.33 ± 0.34 c | 1.92 ± 0.44 b | 1.54 ± 0.20 bc | 0.87 ± 0.38 c | 0.54 ± 0.12 c | 0.25 ± 023 c | |||||||
| 20 | 0.90 ± 0.28 c | 0.47 ± 0.82 c | 0.57 ± 0.13 c | 0.68 ± 0.32 c | - | - | |||||||
| Heptanal | Strong unpleasant fat odor | 0–2 | 0.15 ± 0.12 d | 0.62 ± 0.21 ab | 0.19 ± 0.27 c | 0.19 ± 0.27 d | 0.37 ± 0.35 abcd | 0.11 ± 0.13 d | 0.58 ± 0.95 abcd | ns | ns | * | |
| 4–6 | 0.70 ± 0.11 ab | 0.68 ± 0.14 ab | 0.49 ± 0.11 bc | 0.18 ± 0.11 d | 0.65 ± 0.97 abcd | - | |||||||
| 8–10 | 0.40 ± 0.13 c | 0.12 ± 0.20 d | 0.12 ± 0.12 d | 0.20 ± 0.11 d | 0.64 ± 0.24 ab | 0.97 ± 0.16 a | |||||||
| 20 | 0.92 ± 0.20 a | 0.74 ± 0.88 abcd | 0.96 ± 0.16 ab | 0.18 ± 0.54 d | - | - | |||||||
| Benzaldehyde | Almond aroma | 0–2 | 0.48 ± 0.35 ab | 2.66 ± 0.95 a | 0.73 ± 0.56 ab | 0.70 ± 0.17 a | 3.72 ± 3.22 a | 0.52 ± 0.29 ab | 0.54 ± 0.76 ab | ns | ns | * | |
| 4–6 | 0.34 ± 0.43 ab | 0.20 ± 0.26 b | 0.77 ± 0.25 a | 0.54 ± 0.29 ab | 1.86 ± 0.15 a | 0.44 ± 0.24 ab | |||||||
| 8–10 | 0.58 ± 0.73 ab | 1.35 ± 0.27 a | 1.81 ± 0.29 a | 0.89 ± 0.57 ab | 0.36 ± 0.44 ab | 1.37 ± 0.46 a | |||||||
| 20 | 0.53 ± 0.12 ab | 0.17 ± 0.28 b | 0.55 ± 0.45 ab | 1.53 ± 0.39 a | - | - | |||||||
| Octanal | Strong fruity aroma | 0–2 | 0.16 ± 0.12 c | 0.22 ± 0.85 abc | 0.95 ± 0.21 a | 0.58 ± 0.73 abc | 0.38 ± 0.35 abc | 0.34 ± 0.22 bc | 0.15 ± 0.28 bc | ns | ns | * | |
| 4–6 | 0.57 ± 0.12 b | 0.43 ± 0.67 abc | 0.57 ± 0.82 abc | 0.16 ± 0.71 abc | 0.95 ± 0.22 a | 0.17 ± 0.29 bc | |||||||
| 8–10 | 0.14 ± 0.86 abc | 0.56 ± 0.19 ab | 0.75 ± 0.87 abc | - | - | - | |||||||
| 20 | - | - | - | - | - | - | |||||||
| Nonanal | Rose and citrus aromas; strong oily aroma | 0–2 | 0.39 ± 0.75 a | 1.13 ± 0.43 a | 0.24 ± 0.90 a | 0.31 ± 0.34 a | 1.72 ± 1.58 a | 0.16 ± 0.76 a | 0.13 ± 0.13 a | ns | ns | ns | |
| 4–6 | 0.17 ± 0.13 a | 0.36 ± 0.25 a | 0.73 ± 0.54 a | 0.59 ± 0.36 a | 0.15 ± 0.28 a | - | |||||||
| 8–10 | 0.14 ± 0.17 a | 0.38 ± 0.39 a | 0.34 ± 0.44 a | 0.49 ± 0.22 a | 0.32 ± 0.66 a | 0.38 ± 0.27 a | |||||||
| 20 | 0.14 ± 0.30 a | 0.15 ± 0.29 a | 0.23 ± 0.49 a | 0.51 ± 0.47 a | - | - | |||||||
| (E)-2-Nonenal | Cucumber fragrance | 0–2 | 0.34 ± 0.42 ab | 0.83 ± 0.37 a | 0.57 ± 0.16 a | 0.24 ± 0.57 ab | 0.18 ± 0.94 ab | 0.13 ± 0.16 b | - | ns | ns | * | |
| 4–6 | 0.23 ± 0.39 ab | 0.48 ± 0.41 ab | 0.37 ± 0.68 ab | 0.43 ± 0.23 ab | - | - | |||||||
| 8–10 | 0.39 ± 0.59 ab | 0.29 ± 0.19 ab | 0.43 ± 0.57 ab | 0.51 ± 0.31 ab | 0.53 ± 0.66 ab | - | |||||||
| 20 | 0.18 ± 0.53 ab | - | - | - | - | - | |||||||
| Decanal | Strong aldehyde aroma | 0–2 | 0.51 ± 0.56 a | 0.13 ± 0.53 a | 0.83 ± 0.12 a | 0.82 ± 0.28 a | 0.22 ± 0.19 a | 0.27 ± 0.35 a | 0.19 ± 0.35 a | ns | ns | ns | |
| 4–6 | 0.12 ± 0.17 a | 0.13 ± 0.14 a | 0.14 ± 0.22 a | 0.19 ± 0.14 a | 0.88 ± 0.19 a | - | |||||||
| 8–10 | 0.14 ± 0.50 a | 0.19 ± 0.23 a | 0.17 ± 0.12 a | 0.88 ± 0.63 a | 0.26 ± 0.33 a | 0.16 ± 0.12 a | |||||||
| 20 | 0.15 ± 0.96 a | 0.16 ± 0.29 a | 0.10 ± 0.94 a | - | - | - | |||||||
| 1-Cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl | Fruit and faint scent | 0–2 | 0.18 ± 0.98 ab | 0.30 ± 0.14 b | 0.20 ± 0.59 a | 0.13 ± 0.25 a | 0.47 ± 0.38 ab | 0.16 ± 0.47 b | 0.76 ± 0.16 b | * | ns | * | |
| 4–6 | 0.12 ± 0.13 b | 0.13 ± 0.13 b | 0.15 ± 0.32 a | 0.34 ± 0.22 ab | 0.19 ± 0.29 b | - | |||||||
| 8–10 | 0.44 ± 0.35 b | 1.17 ± 0.18 a | 0.24 ± 0.32 a | 0.25 ± 0.17 b | 0.61 ± 0.25 b | 0.19 ± 0.23 b | |||||||
| 20 | 0.22 ± 0.29 b | 0.38 ± 0.91 ab | 0.43 ± 0.84 ab | 0.32 ± 0.58 b | - | - | |||||||
| 2-Hexenal | Fruit aroma and green leaf fragrance | 0–2 | 2.71 ± 0.27 c | 6.78 ± 2.49 b | 2.74 ± 0.81 c | 2.24 ± 0.41 c | 2.74 ± 18.48 abcde | 1.86 ± 0.19 c | 6.78 ± 1.14 b | * | ns | ** | |
| 4–6 | 1.95 ± 0.15 c | 1.88 ± 0.36 c | 1.52 ± 0.15 c | 3.14 ± 1.75 bc | 1.99 ± 0.26 c | 1.30 ± 0.14 d | |||||||
| 8–10 | 4.61 ± 0.29 b | 1.56 ± 0.66 cde | 2.25 ± 0.40 c | 4.62 ± 1.94 bc | 15.27 ± 2.42 a | 2.93 ± 0.23 c | |||||||
| 20 | 1.84 ± 0.23 c | 0.87 ± 0.15 e | 2.00 ± 0.40 c | 2.17 ± 0.54 b | - | - | |||||||
| (E,E)-2,4-hexadienal | Green plant scent | 0–2 | 0.34 ± 0.67 ab | 0.62 ± 0.25 a | 0.18 ± 0.23 b | 0.14 ± 0.44 b | 0.69 ± 0.87 ab | 0.19 ± 0.77 ab | 0.62 ± 0.86 ab | * | ns | * | |
| 4–6 | 0.84 ± 0.12 a | 0.11 ± 0.12 b | 0.14 ± 0.16 b | 0.14 ± 0.67 ab | 0.17 ± 0.33 b | 0.60 ± 0.15 ab | |||||||
| 8–10 | 0.18 ± 0.2 b | 0.34 ± 0.11 b | 0.99 ± 0.29 a | 0.17 ± 0.12 b | 0.17 ± 0.21 b | 0.59 ± 0.79 ab | |||||||
| 20 | 0.27 ± 0.66 ab | 0.22 ± 0.60 ab | - | - | - | - | |||||||
| Esters | cis-3-hexenyl acetate | Intense banana aroma | 0–2 | 0.32 ± 0.56 ab | 0.14 ± 0.42 b | 0.33 ± 0.44 ab | 0.46 ± 0.86 ab | 0.41 ± 0.39 b | 0.23 ± 0.50 a | 0.56 ± 0.29 ab | ns | ns | * |
| 4–6 | 0.19 ± 0.37 ab | 0.12 ± 0.30 b | 0.20 ± 0.32 b | 0.34 ± 0.18 b | 0.12 ± 0.38 b | 0.13 ± 0.13 b | |||||||
| 8–10 | 0.31 ± 0.12 b | 0.86 ± 0.11 a | 0.81 ± 0.28 a | 0.39 ± 0.28 ab | 0.12 ± 0.15 b | 0.49 ± 0.33 ab | |||||||
| 20 | 0.47 ± 0.67 ab | 0.23 ± 0.82 ab | 0.84 ± 0.11 a | 1.42 ± 0.34 a | - | - | |||||||
| Acetic acid, hexyl ester | Fruit and faint scent | 0–2 | 0.56 ± 0.80 c | 0.33 ± 0.93 c | 0.18 ± 0.43 c | 0.19 ± 0.50 c | 0.96 ± 0.70 c | 0.94 ± 0.35 c | 0.93 ± 0.22 c | * | * | * | |
| 4–6 | 0.14 ± 0.27 c | 0.43 ± 0.44 c | 0.28 ± 0.16 c | 0.16 ± 0.17 c | 0.51 ± 0.74 c | - | |||||||
| 8–10 | 0.34 ± 0.43 c | 0.86 ± 0.11 c | 0.34 ± 0.32 c | 0.23 ± 0.12 c | 0.13 ± 0.16 c | 0.48 ± 0.32 c | |||||||
| 20 | 0.43 ± 0.12 c | 0.63 ± 0.95 c | 8.64 ± 0.82 b | 35.22 ± 6.58 a | - | - | |||||||
| Butanoic acid, 3-hexenyl ester | The faint scent of fruits | 0–2 | 0.12 ± 0.29 b | 0.31 ± 0.13 b | 0.17 ± 0.13 b | 0.11 ± 0.34 b | 0.34 ± 0.33 b | 0.13 ± 0.19 b | - | * | ns | * | |
| 4–6 | 0.83 ± 0.13 a | 0.68 ± 0.53 ab | 0.18 ± 0.19 b | 0.32 ± 0.19 b | 0.15 ± 0.18 b | 0.43 ± 0.53 ab | |||||||
| 8–10 | 0.15 ± 0.99 ab | 0.25 ± 0.53 ab | 0.21 ± 0.42 b | 0.29 ± 0.14 b | 0.88 ± 0.11 a | 0.34 ± 0.27 b | |||||||
| 20 | 0.24 ± 0.67 ab | 0.36 ± 0.94 ab | 0.43 ± 0.68 ab | 0.43 ± 0.19 ab | - | - | |||||||
| Butanoic acid, hexyl ester | The sweetness of fruits | 0–2 | 0.16 ± 0.23 d | - | - | - | - | - | - | * | * | * | |
| 4–6 | 0.13 ± 0.53 d | 0.65 ± 0.55 d | 0.76 ± 0.14 bd | - | 0.66 ± 0.94 d | - | |||||||
| 8–10 | 0.27 ± 0.34 d | 5.54 ± 0.58 b | 18.75 ± 6.33 a | 4.58 ± 8.80 abcd | 2.99 ± 0.46 c | 6.67 ± 0.82 b | |||||||
| 20 | - | - | - | - | - | - | |||||||
| Acids | Acetic acid | Strong pungent aroma | 0–2 | - | 0.52 ± 0.19 a | - | - | 0.62 ± 0.59 a | - | - | ns | ns | ns |
| 4–6 | 0.57 ± 0.58 a | - | 0.22 ± 0.16 a | - | 0.13 ± 0.27 a | 0.53 ± 0.19 a | |||||||
| 8–10 | 0.14 ± 0.20 a | 0.17 ± 0.45 a | - | - | 0.28 ± 0.62 a | - | |||||||
| 20 | 0.77 ± 0.13 a | - | 0.19 ± 0.22 a | 0.33 ± 0.45 a | - | - | |||||||
| Benzoic acid | Slight smell of benzoin | 0–2 | - | 0.13 ± 0.39 b | 0.19 ± 0.26 b | - | 0.45 ± 0.40 b | - | 0.57 ± 0.92 ab | * | * | * | |
| 4–6 | - | 0.46 ± 0.60 ab | 0.52 ± 0.39 b | - | - | - | |||||||
| 8–10 | 0.50 ± 0.27 b | 0.57 ± 0.11 b | - | - | 1.23 ± 0.25 a | - | |||||||
| 20 | 0.53 ± 0.61 ab | 0.40 ± 0.36 b | 0.57 ± 0.37 ab | 0.25 ± 0.34 b | - | - | |||||||
| Nonanoic acid | Light fat fragrance | 0–2 | - | - | 0.18 ± 0.14 b | 0.17 ± 0.35 ab | - | - | - | ns | ns | * | |
| 4–6 | 0.24 ± 0.21 ab | 0.81 ± 0.49 a | 0.35 ± 0.59 ab | 0.27 ± 0.16 ab | 0.19 ± 0.25 ab | 0.66 ± 0.13 a | |||||||
| 8–10 | 0.18 ± 0.26 ab | 0.30 ± 0.44 ab | 0.38 ± 0.81 ab | - | 0.22 ± 0.39 ab | 0.17 ± 0.65 ab | |||||||
| 20 | 0.50 ± 0.19 a | - | 0.36 ± 0.23 ab | 0.15 ± 0.25 ab | - | - | |||||||
| Hexanoic acid | Smells like sheep | 0–2 | - | - | - | - | - | - | - | - | - | - | |
| 4–6 | - | - | - | - | - | - | |||||||
| 8–10 | - | - | 0.46 ± 0.58 a | - | 0.27 ± 0.33 a | 0.55 ± 0.31 a | |||||||
| 20 | - | - | 0.27 ± 0.12 a | 0.18 ± 0.15 a | - | - | |||||||
| Ketones | 2-Butanone, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl) | Woody, floral, and fruity aromas | 0–2 | - | 0.14 ± 0.73 a | 0.45 ± 0.83 a | 0.49 ± 0.30 a | 0.21 ± 0.20 a | - | - | - | - | - |
| 4–6 | 0.40 ± 0.39 a | 0.37 ± 0.56 a | 0.57 ± 0.43 a | - | - | - | |||||||
| 8–10 | - | - | - | - | - | - | |||||||
| 20 | - | - | - | - | - | - | |||||||
| 5,9-Undecadien-2-one, 6,10-dimethyl | Fruit, wax, wood, and faint aromas | 0–2 | - | - | - | - | - | - | - | - | - | - | |
| 4–6 | 0.79 ± 0.72 a | 0.15 ± 0.12 a | - | - | - | ||||||||
| 8–10 | 0.17 ± 0.32 a | 0.24 ± 0.32 a | 0.18 ± 0.27 a | - | - | - | |||||||
| 20 | 0.16 ± 0.16 a | - | 0.18 ± 0.11 a | 0.27 ± 0.34 a | - | - | |||||||
| 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl) | Wood aroma | 0–2 | 0.28 ± 0.29 a | 0.42 ± 0.14 a | 0.24 ± 0.34 a | 1.15 ± 14.31 a | 0.83 ± 0.80 a | - | 0.49 ± 0.14 a | ns | ns | ns | |
| 4–6 | 0.17 ± 0.46 a | 0.18 ± 0.39 a | 0.24 ± 0.11 a | 0.49 ± 0.30 a | 0.20 ± 0.26 a | 0.99 ± 0.17 a | |||||||
| 8–10 | 0.65 ± 0.28 a | 0.41 ± 0.40 a | 0.42 ± 0.85 a | 0.39 ± 0.24 a | 0.45 ± 0.14 a | 0.14 ± 0.70 a | |||||||
| 20 | 0.38 ± 0.73 a | - | 0.69 ± 0.69 a | 0.36 ± 0.82 a | - | - | |||||||
| Alkanes | 7-Oxabicyclo [4.1.0] heptane | No fragrance | 0–2 | 0.81 ± 0.58 a | 0.34 ± 0.82 a | 0.22 ± 0.80 a | 0.27 ± 0.36 a | 1.65 ± 1.45 a | 0.33 ± 0.32 a | 0.55 ± 0.78 a | ns | ns | ns |
| 4–6 | 0.42 ± 0.15 a | 0.23 ± 0.16 a | 0.27 ± 0.11 a | 0.33 ± 0.20 a | 0.15 ± 0.22 a | 0.84 ± 0.19 a | |||||||
| 8–10 | 0.80 ± 0.24 a | 0.86 ± 0.11 a | 0.49 ± 0.87 a | 0.68 ± 0.29 a | 0.19 ± 0.40 a | 0.73 ± 0.52 a | |||||||
| 20 | - | 0.99 ± 0.12 a | 0.76 ± 0.21 a | 0.13 ± 0.22 a | - | - | |||||||
| Aromatic | o-Xylene | Smells like toluene | 0–2 | 0.58 ± 0.19 a | 0.13 ± 0.59 a | 0.58 ± 0.86 a | 0.48 ± 0.13 a | 0.47 ± 0.39 a | 0.52 ± 0.65 a | - | ns | ns | ns |
| 4–6 | 0.12 ± 0.12 a | 0.35 ± 0.40 a | - | - | - | - | |||||||
| 8–10 | 0.73 ± 0.26 a | - | 0.14 ± 0.29 a | 0.65 ± 0.48 a | 0.13 ± 0.16 a | 0.44 ± 0.12 a | |||||||
| 20 | 0.43 ± 0.20 a | 0.32 ± 0.84 a | 0.41 ± 0.11 a | 0.64 ± 0.23 a | - | - | |||||||
| Terpenes | Limonene | Smells like lemon | 0–2 | 0.26 ± 0.31 a | 0.59 ± 0.24 a | 0.41 ± 0.16 a | 0.26 ± 0.40 a | 2.17 ± 1.83 a | 0.36 ± 0.43 a | 1.27 ± 0.34 a | ns | ns | ns |
| 4–6 | 0.46 ± 0.43 a | 0.21 ± 0.24 a | - | 0.29 ± 0.15 a | - | 0.43 ± 0.65 a | |||||||
| 8–10 | 0.33 ± 0.27 a | 0.59 ± 0.50 a | 0.27 ± 0.24 a | 1.22 ± 0.69 a | - | 0.16 ± 0.62 a | |||||||
| 20 | 0.12 ± 0.25 a | 0.35 ± 0.43 a | 0.58 ± 0.74 a | 0.31 ± 0.24 a | - | - | |||||||
| linalool | Lily of the valley aroma | 0–2 | - | 0.26 ± 0.72 b | 0.14 ± 0.12 b | 0.52 ± 0.76 b | 0.16 ± 0.95 b | - | 1.49 ± 0.17 a | * | ns | * | |
| 4–6 | 0.93 ± 0.86 ab | 0.43 ± 0.44 b | 0.21 ± 0.53 b | 0.14 ± 0.73 b | - | 0.59 ± 0.13 b | |||||||
| 8–10 | 0.82 ± 0.59 ab | 0.46 ± 0.65 b | 1.27 ± 0.66 ab | - | 0.68 ± 0.14 b | 0.68 ± 0.94 ab | |||||||
| 20 | 0.21 ± 0.51 b | 0.62 ± 0.36 b | 0.36 ± 0.22 b | 0.16 ± 0.34 b | - | - | |||||||
| Heterocyclic compound | 2-Furancarboxaldehyde, 5-(hydroxymethyl) | Chamomile fragrance | 0–2 | - | - | - | - | - | - | 1.26 ± 0.67 c | - | - | - |
| 4–6 | - | - | 0.12 ± 0.18 d | - | - | - | |||||||
| 8–10 | - | 6.43 ± 0.32 a | - | - | 1.14 ± 0.18 c | - | |||||||
| 20 | 5.92 ± 1.17 b | 1.48 ± 0.94 c | 0.72 ± 0.46 c | 0.28 ± 0.25 c | - | - | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Wang, Z.; Xiong, W.; Kong, W.; Huang, M.; Xi, W.; Zhou, K. The Effect of Postharvest Storage Temperatures on Fruit Flavor Constituents in ‘Wushancuili’ Plum. Horticulturae 2024, 10, 414. https://doi.org/10.3390/horticulturae10040414
Feng Q, Wang Z, Xiong W, Kong W, Huang M, Xi W, Zhou K. The Effect of Postharvest Storage Temperatures on Fruit Flavor Constituents in ‘Wushancuili’ Plum. Horticulturae. 2024; 10(4):414. https://doi.org/10.3390/horticulturae10040414
Chicago/Turabian StyleFeng, Qinyu, Zhichao Wang, Wei Xiong, Wenbin Kong, Ming Huang, Wanpeng Xi, and Kun Zhou. 2024. "The Effect of Postharvest Storage Temperatures on Fruit Flavor Constituents in ‘Wushancuili’ Plum" Horticulturae 10, no. 4: 414. https://doi.org/10.3390/horticulturae10040414
APA StyleFeng, Q., Wang, Z., Xiong, W., Kong, W., Huang, M., Xi, W., & Zhou, K. (2024). The Effect of Postharvest Storage Temperatures on Fruit Flavor Constituents in ‘Wushancuili’ Plum. Horticulturae, 10(4), 414. https://doi.org/10.3390/horticulturae10040414






