Abstract
Roses (genus Rosa), renowned for their economic significance and aesthetic appeal, face multifaceted challenges in cultivation due to biotic and abiotic stressors. To address these challenges, this study explores the role of osmolytes, particularly polyamines, proline and glycine betaine, as well as antioxidant capacities and condensed tannins, in enhancing stress tolerance in roses. Despite the genetic diversity inherent in roses, the metabolic aspect of stress tolerance has been underexplored in breeding programs. This paper investigates the intraspecific variability among 22 rose cultivars, focusing on osmolyte content (proline and glycine betaine), individual polyamines (putrescine, spermine and spermidine), as well as antioxidant activities, measuring radical scavenging capacity against 2,2′-azinobis(3-ethylbenzothiozoline-6-sulfonic acid (ABTS•+) and NO• radicals. Employing a targeted metabolomic approach, we quantified the levels of individual polyamines in both the petals and leaves of rose cultivars. This was achieved through high-performance liquid chromatography coupled with fluorescent detection following a derivatization pretreatment process. Within the evaluated cultivars, “Unique Aroma”, “Andre Rieu”, “Aroma 3”, “Frayla Marija” and “Trendy Fashion” stood out for their significantly elevated levels of total foliar polyamines. The predominant polyamine detected at both petal and leaf levels was putrescine, with concentrations ranging from 335.81 (“Zora Frayla”) to 2063.81 nmol g−1 DW (“Unique Aroma”) at the leaf level. Following putrescine, foliar spermidine levels varied from 245.08 (“Olivera Frayla”) to 1527.16 nmol g−1 DW (“Andre Rieu”). Regarding antioxidant capacity, the leaf extracts of rose cultivars “Zora Frayla” and “Natalija Frayla” were prominent by showing 68.08 and 59.24 mmol Trolox equivalents (TE) g−1 DW, respectively. The results highlight the intricate biochemical variability across rose cultivars and show that osmolytes, such as glycine betaine, proline and polyamines, and other biochemical markers can be used as reliable criteria for the selection of rose cultivars that are more resilient to biotic stress factors, especially powdery and downy mildew. Bridging fundamental research with practical applications, this study aims to contribute to the development of stress-tolerant rose cultivars adaptable to dynamic environmental conditions.
1. Introduction
Due to their exquisite qualities, rose dominates other ornamental plants and commercial flower crops in terms of economic significance, particularly in the production of cut flowers [1,2]. There is fierce competition among companies in the international market to provide and breed roses with higher quality, which is measured in terms of visual appearance, maintaining quality after harvest, environmental influences and resistance to biotic and abiotic stresses [3].
The genus Rosa, a multispecies mainly tetraploid crop that includes decorative roses, is one of the most prized ornamental plant species in the world because of its visual appeal, aroma and cultural significance. However, a variety of biotic and abiotic environmental stresses pose a considerable challenge to the cultivation of these fragile flowers, having an impact on their development, growth, overall health and lifetime duration [3,4]. Since roses’ considerable genetic diversity dictates their high variability in resistance to numerous diseases, including blackspot, powdery mildew, downy mildew, leafspot and rust, etc., the application of biotechnological tools has become important in recent decades to intervene in rose breeding [4,5]. Early diagnosis by growers is crucial, and in modern times, machine learning is also used to help with this process [6].
Besides biotic stress factors and disease exposure [4], roses are susceptible to a variety of environmental abiotic stressors, such as temperature variations [7], water scarcity [8], the salinity of the soil [9] and insects [10], and these risks can make difficult to cultivate garden roses successfully. In response to these stressors, plants have evolved an array of adaptive strategies, among which the accumulation of osmolytes emerges as a crucial mechanism for maintaining cellular homeostasis and mitigating stress-induced damage, particularly by shielding proteins and other biomolecules from oxidative damage [11,12]. Osmolytes, which play a significant role in a variety of plant species in acclimation to both abiotic and biotic stresses, are low-molecular-weight organic molecules with high osmoprotective, cryoprotective, and antioxidant activities, and among these, mannitol, proline (PRO), glycine betaine (GB) and polyamines (PAs) are the ones that have been most examined [13]. In addition to these functions, proline functions as a multifunctional amino acid that also possesses chaperone-like activity and functions as a signaling molecule to control gene expression and plant development, modify the redox balance, impact pathogen defense and help in plant adaptation to a variety of biotic and abiotic stressors [14,15]. Unlike proline, glycine betaine does not directly contribute to the scavenging of reactive oxygen species (ROS); however, research has shown that it indirectly activates ROS-scavenging enzymes and, as a quaternary ammonium molecule, provides osmotic adjustment and membrane stabilization, as well as protecting the photosynthetic machinery of plants, particularly the oxygen-evolving PSII complex during heat stress [16,17,18]. Furthermore, exogenous application of glycine betaine positively affected the growth and flower yield of Damask rose under salt stress [19].
On the other hand, polyamines as ubiquitous polycations are essential for salinity and thermotolerance because they control ion channels and stabilize negatively charged molecules such as lipid membranes and nucleic acids due to their positive charge at physiological pH [20,21,22]. Most probably, their role in biotic stress and drought mitigation, as well as thermotolerance, is related to their crosstalk with nitric oxide which, as a key signal molecule, is capable of triggering stomata closure and the initiation of a hypersensitive reaction during biotic stress [23,24]. Furthermore, besides being involved in the mitigation of various abiotic and biotic stress, plant growth and development, particularly the enhancement of flowering and delay of senescence, cell division and elongation, PAs are known to contribute to the longevity of the plant [25,26]. Because PAs are essential for floral organ development and flower opening, they are also crucial to the floral industry and are extensively studied not only by plant researchers but also cultivators and breeders [27]. Due to the prominent priming properties of PAs, roses (Rosa hybrida L. ‘Herbert Stevens’) exposed to the exogenous application of different PAs in various concentration ranges exhibited a variety of physiological modifications including the alternation of root length, chlorophyll content, flower longevity and carotenoid, nitrogen and phosphorus content, whereas the concentration of 1 mM had the strongest effects [28]. This research represents the first comprehensive study focused on revealing the dynamics of osmolyte accumulation and distribution patterns and the critical role they play in the stress adaptation of various rose cultivars. Understanding the links to the increased abiotic and biotic stress tolerance of rose cultivars and their regulation mechanisms may be of great importance for rose breeding and selection programs.
In addition to PAs, polyphenolic compounds—also referred to as proanthocyanidins or condensed tannins (CTs)—also have osmoprotective, antioxidant, membrane-stabilizing, antifeedant and allelopathic properties that help rose hybrid cultivars tolerate and resist biotic and abiotic stress conditions [29].
Large genetic diversity in the Rosa genus results from complex genetic interactions between species as a result of intraspecific hybridization and species variability, particularly for cultivars [30]. Strict selection is carried out based on a range of criteria, including color and shape, the lack of stem bending, production (many stems per square meter), thornlessness and long vase life. To investigate the interplay between large genetic diversity in roses and their varying resistance to various diseases and tolerance to abiotic stress factors, numerous biotechnological technologies have been used, including gene expression research, QTL mapping and microsatellite markers, next-generation sequencing facilitated in the creation of transcriptomes and large numbers of single-nucleotide polymorphism (SNP) markers [5,31,32] while the metabolic approach has been mostly neglected in the process of rose cultivar selection.
Therefore, this paper aims to elucidate the role of osmolytes, particularly polyamines, proline and glycine betaine, in conferring stress tolerance in various rose cultivars and to reveal how these secondary metabolites can serve as a reliable criterion for the selection of more resilient cultivars to biotic and abiotic stresses. To achieve that, intraspecific variability among 22 rose cultivars was inspected regarding the following parameters at both the leaf and flower levels:
- Proline and glycine betaine content.
- The amount of individual polyamines such as putrescine, spermidine and spermine.
- Antioxidant activities against ABTS and NO radicals as well as total condensed tannin amounts.
By bridging the gap between fundamental research and practical applications, this study endeavors to contribute toward the development of stress-tolerant rose varieties tailored to withstand the challenges of a rapidly changing environment.
2. Materials and Methods
2.1. Plant Material and Sample Preparation
The garden roses (Rosa hybrida L.) used in this study were aged for two years and cultivated under open field conditions at the PhenoGeno Roses private breeding company in Temerin, Northern Serbia (45.2401900° N 19.5301300° E/45.105166° N 19.886833° E). The rose nursery is situated roughly 20 km away from Novi Sad, Serbia, and is subjected to a typical continental climate featuring warm summers and cold winters. In autumn 2019, the experimental field, spanning 30 m in length and 20 m in width, was established via in situ bud grafting. Each cultivar comprised 150 grafted plants, spaced 10 cm apart within rows spaced one meter apart. During the initial flowering period in June 2021, thirty flowers from each cultivar were harvested and promptly frozen in liquid nitrogen and stored at −80 °C for subsequent analysis. All additional characteristics including detailed phenotyping and disease resistance data for each rose cultivar were enlisted in a separate table (Supplementary Material Table S1). Cultivars included in this study (Table 1) were selected based on earlier research conducted on collections grouped by common characteristics, due to criteria such as high aesthetic appeal and high fragrance intensity (scoring above 3) as well high cultivar resilience to common diseases like powdery and downy mildew grown in the open field under given agroecological conditions.

Table 1.
Differences in the flower colors among the rose cultivars examined in this study.
The sampling procedure for leaves and petals from roses in our study adhered to a rigorous protocol to ensure the collection of high-quality material for subsequent analysis. Harvesting was conducted during the early morning on a sunny day to ensure that leaves and petals were well hydrated and at their metabolic peak. Only mature rose plants exhibiting excellent fitness were selected, with three representative individuals from each cultivar in the same growth stage included. Criteria for leaf selection included fully developed, characteristic, undamaged leaves from the upper third of the stem, devoid of any pathological symptoms and exposed to full sunlight. Approximately 100 g of fresh-weight leaves or petals per individual of each cultivar was collected, labeled and documented with cultivar name, date and location and promptly stored in a transport fridge to maintain freshness during transportation to the laboratory. Upon arrival, leaves were immediately frozen using liquid nitrogen and stored at −80 °C until further processing. Subsequent steps involved freeze drying (by using lyophilizer for 24 h at −70 °C (model Alpha 1-2 LDplus, Martin Christ, Osterode am Harz, Germany)), powdering and storage in a cold and dark environment to preserve sample integrity prior to analysis (Table 1).
2.2. Phytochemical Characterization
2.2.1. HPLC Quantification of Polyamines (PAs)
Approximately 20 mg of lyophilized plant material (petal and leaf) (dry weight (DW)) was extracted using a solution containing 4% perchloric acid (v/v) in a volume ten times that of the sample. After cooling the resulting homogenate on ice for 1 h, it was centrifuged at 15,000× g for 30 min. Supernatant samples, along with standard solutions containing putrescine (PUT), spermidine (SPD) and spermine (SPM), underwent dansylation treatment according to the procedure outlined by Scaramagli et al. [33] and Biondi et al. [34]. A total of 200 µL of supernatant was mixed with 200 µL of dansyl-chloride solution (5 mg ml−1 previously prepared in acetone) and 200 µL of 1.5 M solution of sodium carbonate (Na2CO3). Tubes were briefly vortexed and incubated in a water bath at 60 °C for 1 h. The resulting dansylated derivatives were then extracted using toluene (600 µL), dried and reconstituted in acetonitrile. The dilution factor for both petal and leaf samples was 20 times, and prior to that, the injection samples were filtered through disposable membrane syringe filters (dimensions 0.2 µm × 25 mm, Chromafil, Macherey Nagel, Duren, Germany). Polyamine-dansyl derivates were separated and quantified using high-performance liquid chromatography (HPLC) with fluorescent detection (FD) (Nexera XR, Shimadzu, Kyoto, Japan), employing a reverse-phase C18 column (Spherisorb ODS, 2.5-μm particle diameter, 4.6 × 250 mm, Waters, Wexford, Ireland) and a programmed acetonitrile–water gradient [33,34]. Acetonitrile–water (v/v) gradient consisted of five steps as follows: 60 to 70% of acetonitrile in 5.5 min, 70 to 80% in 1.5 min, 80 to 100% in 2 min, 100% in 2 min, 100 to 70% in 2 min and 70 to 60% in 2 min at a flow rate of 1.5 mL min−1 for a total run time of 15 min. Eluted dansylated PA derivates were detected at excitation wavelength of 365 nm and emission of 510 nm with fluorescent detector (RF-20A-xs, Shimadzu, Kyoto, Japan). The integration of peaks was manually performed utilizing the Lab solution program, and the quantification of individual PAs was determined based on the peaks of analytical standards, expressed as nanomoles per gram of dry weight (nmol g−1 DW).
2.2.2. Carbon and Nitrogen Elemental Analysis
Tiny tin capsules, each containing about 25 mg of powdered plant sample (petal and leaf), underwent combustion in the combustion box of an elemental analyzer (model Elementar Vario EL III, Hanau, Germany) for approximately two minutes at 900 °C. During this process, carbon (C) and nitrogen (N) from the samples were converted into CO2 and mixture of nitric oxides, which were consequently reduced to N2. The resulting gases were separated using analytical columns, and their quantification relative to the standard was achieved by adjusting the thermal conductivity detector (TCD). Acetanilide, with a known C content of 71.09% and N content of 10.36%, was used as the standard, and the percentages of carbon and nitrogen in the samples were automatically calculated following the method of Karthikeyan and Kumaravel [35].
2.2.3. Quantification of Free Proline (PRO)
In summary, 20 mg of freeze-dried petal and leaf powder was blended with 1 mL of sulfosalicylic acid (3% w/v). After centrifugation at 4000 rpm for 10 min, 0.7 mL of the supernatant was combined with 0.7 mL of acid ninhydrin solution (2.5% ninhydrin in glacial acetic acid, distilled water and 85% orthophosphoric acid in a 6:3:1 ratio) and 0.7 mL of glacial acetic acid, then heated at +95 °C for 1 h. Subsequently, the reaction was halted by transferring the mixture to an ice bath. The resultant compound from the proline and ninhydrin reaction was extracted with 2 mL of toluene via vigorous vortexing. Proline concentration was determined spectrophotometrically using the MultiScan GO (ThermoScientific, Karlsruhe, Germany), as previously outlined by Bates et al. [36]. The concentration of free PRO was determined using a standard calibration curve and expressed as micromoles per gram dry weight (μmol g−1 DW).
2.2.4. Quantification of Glycine Betaine (GB) as a Predominant Quaternary Ammonium Compound
To extract glycine betaine, 20 mg of freeze-dried plant material was mixed with 500 µL of 1 M H2SO4 and vigorously shaken on a vortex. Ultrasonication was applied for 10 min to enhance extraction. After centrifugation at 13,200 rpm at 4 °C for 30 min, the supernatant was combined with cold KI/I2 solution and left overnight at 4 °C. Following a second centrifugation under the same conditions, QAC-periodide crystals in the sediment were dissolved in 9 mL of 1,2-dichloroethane using an ultrasonic bath. The absorbance of the mixture was measured at a wavelength of 365 nm using a MultiScan GO spectrophotometer (Multiscan GO, ThermoScientific, Germany). GB concentration was determined from a standard calibration curve, and the GB content was expressed as µmol g−1 DW [37].
2.2.5. Quantification of Condensed Tannins (CTs)
By using the butanol-HCl-Fe (III) method, the amount of CTs in methanolic extracts was determined [38]. After adding 25 µL of 0.04 M ammonium ferric sulfate (NH4 Fe(SO4)2) solution into the tubes containing 75 µL of methanolic extracts and freshly prepared acidic butanol reagent (1-butanol:hydrochloric acid (95:5 v/v)), the tubes were incubated at 80 °C for one hour. Using a spectrophotometer (Multiscan GO, ThermoScientific, Germany), after the reaction mixture was cooled in an ice bath, the absorbances were measured at wavelengths of 555 nm. The results were presented using leucocyanidin equivalents (LE) per unit dry weight (DW).
2.3. Evaluation of Antioxidant Capacity
2.3.1. Extract Preparation
Antioxidant capacity was assessed using 80% methanol (MeOH) extracts derived from 23 different garden rose genotypes. The extraction process followed the methodology outlined by Rašeta et al. [39], wherein 20 mg of freeze-dried plant material, including petals and leaves separately, was macerated with 2 mL of 80% MeOH using a magnetic stirrer (MS-3000 High-speed magnetic stirrer, Biosan, Riga, Latvia) for 20 min. Subsequently, the mixture underwent centrifugation at 4 °C for 20 min (Microcentrifuge 5424 R, Eppendorf, Germany). The resulting extracts were then analyzed, and the filtrate was stored at +4 °C, yielding a final concentration of 10 mg/mL.
2.3.2. Assays of Antioxidant Defense Systems
The ABTS assay was utilized to evaluate total antioxidant capacity, which involves monitoring the conversion of the cationic radical 2,2′-azinobis(3-ethylbenzothiozoline-6-sulfonic acid), ABTS•+, from a blue-green color to its neutral and colorless form at 734 nm (MultiScan GO, ThermoScientific, Germany). This method followed the protocol outlined by Miller and Rice-Evans [40], and the outcomes are expressed as mg Trolox equivalents per gram of dry weight (mg TE g−1 DW).
The inhibition of the nitric oxide radical (NO•) was assessed through the Griess diazotization process, following the methodology developed by Green et al. [41]. The degree of inhibition was determined by measuring the absorbance of the resulting chromophore at 546 nm (MultiScan GO, ThermoScientific, Germany). This approach facilitated the quantification of the NO• inhibitory effects, with results presented as mmol TE g−1 DW.
2.4. Chemicals and Reagents
The following chemicals were procured from company Sigma-Aldrich (Steinheim, Germany) and used for analysis: analytical standards such as putrescine (CAS-No: 110-60-1), spermidine (CAS-No: 124-20-9) and spermine (CAS-No: 71-44-3); solvents such as toluene (CAS-No: 108-88-3, anhydrous 99.8%), acetonitrile (CAS-No: 75-05-8, HPLC grade, purity ≥ 99.9%), 1,2-dichloroethane (CAS-No: 107-06-2, purity ACS reagent, ≥99.0%), n-butanol (CAS-NO: 71-36-3, ACS reagent, ≥99.4%) and methanol (CAS-No: 67-56-1, ACS reagent, ≥99.8%); reagents such as ninhydrin (CAS-No: 485-47-2, ACS reagent), proline (CAS-No: 147-85-3, purity ≥ 99.0%), glycine betaine (CAS-No: 107-43-7, BioUltra, purity ≥ 99.0%), sulphuric acid (CAS-No: 7664-93-9, ACS reagent 95–98%), ammonium ferric sulfate (CAS-No: 7783-83-7, ACS reagent, 99%), 2,2′-azinobis(3-ethylbenzothiozoline-6-sulfonic acid) (CAS-No: 30931-67-0, purity ≥ 98%) and (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox®, CAS-No: 53188-07-1, purity 97%). Perchloric acid (CAS-NO: 7601-90-3, ASC reagent, 70%), 5-sulfosalicylic acid (CAS-NO: 5965-83-3, ACS reagent, ≥99%), glacial acetic acid (CAS-NO: 64-19-7, ACS reagent, ≥99.7%) and orthophosphoric acid (CAS-NO: 7664-38-2, ACS reagent, ≥85%) were supplied by Merck Millipore (Darmstadt, Germany) and were either analytical or HPLC grade.
2.5. Statistical Analysis
Quantitative data were presented with mean and standard deviation, while differences among rose cultivars and organs were examined using two-way ANOVA and Pearson’s correlations, visually depicted on a correlation matrix. Additionally, principal component analysis (PCA) and dendrogram hierarchical clustering were employed to categorize cultivars based on tracked biochemical parameters. ANOVA results were assessed using the Fisher (F) test and their statistical significance levels (p). Statistical analyses were conducted within an R environment. Basic descriptive statistics and ANOVA were computed using the “rstatix” R package (Version: 0.7.2), while clustering analyses utilized the “denextend” R package (Version: 1.17.1). Visual representations were generated using the “ggplot2” R package (Version: 3.5.0) [42,43,44].
3. Results
3.1. Osmolyte Variability among Inspected Rose Cultivars
High levels of intraspecific heterogeneity were detected regarding proline (PRO) and glycine betaine (GB) content within the 22 distinct rose cultivars that were subjected to this investigation, both at the leaf and petal levels (Figure 1). Based on two-way ANOVA results, higher variations were noticed between organs than among cultivars and their interactions (Table S2). The mean concentration of free PRO and GB found in petals was generally higher than that found at the leaf level, with a more pronounced difference observed for PRO (Figure 1a). The PRO level in various rose cultivars varied from 0.064 (“Natalija Frayla”) to 4.465 (“Fun Fashion”) mmol g−1 DW at the petal level and from 0.008 (“Katarina Frayla”) to 0.366 (“Zora Frayla”) mmol g−1 DW at the leaf level. Rose cultivars “Zora Frayla” and “Chick Fashion” were the most prevalent at the leaf level in terms of PRO content, whereas the “experimental cultivar” and “Fun Fashion” cultivar showed the greatest amounts of PRO (4.306 and 4.465 mmol g−1 DW, respectively) at the petal levels. Glycine betaine (GB) levels varied from 15.98 (“Perfume Fashion”) to 28.46 µmol g−1 DW (“Katarina Frayla”) at the petal level and from 15.62 (“Katarina Frayla”) to 22.86 µmol g−1 DW (“Unique Aroma”) at the leaf level among different rose cultivars (Figure 1b).
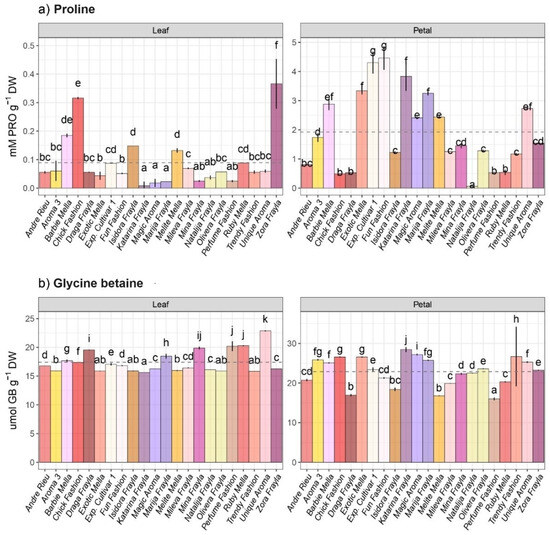
Figure 1.
Intraspecific variability in main osmolytes at leaf and petal level regarding (a) free proline and (b) glycine betaine across 22 rose cultivars. Distinct lowercase letters denote significant differences among rose cultivars based on Tukey’s honestly significant difference (HSD) post hoc test (p ≤ 0.05). The data are presented as mean ± standard deviation (SD).
3.2. Polyamine Profiling within Inspected Rose Cultivars
Among the 22 different rose cultivars, the mean concentrations of all PAs were higher at the leaf level compared to the petal level (Figure 2), whereas organ specificity was statistically proven. Predominantly, PUT emerged as the most prevalent PA at both leaf and petal levels among the majority of examined rose cultivars, with subsequent amounts of SPD and SPM. Both leaves and petals were significantly differing for all detected PAs while higher variations were noticed among organs (leaf and petal). The mean value of PUT was considerably higher in the leaves than in the petals of most of the inspected rose cultivars (Figure 2a). Specifically, PUT levels in various rose cultivars varied from 335.81 (“Zora Frayla”) to 2063.81 nmol g−1 DW (“Unique Aroma”) in leaves, while the concentration of PUT in petals ranged from 59.31 (“Isidora Frayla”) to 2140.39 nmol g−1 DW (“Trendy Fashion”). Remarkably, in terms of PUT content, rose cultivars “Unique Aroma” and “Andre Rieu” exhibited heightened prevalence at the leaf level, while “Exotic Mella” and “Trendy Fashion” had the highest PUT content in petals, respectively. Conversely, the cultivars “Zora Frayla” and “Katarina Frayla” exhibited the lowest foliar PUT levels, whereas “Draga Frayla” and “Isidora Frayla” demonstrated the lowest PUT content in petals.
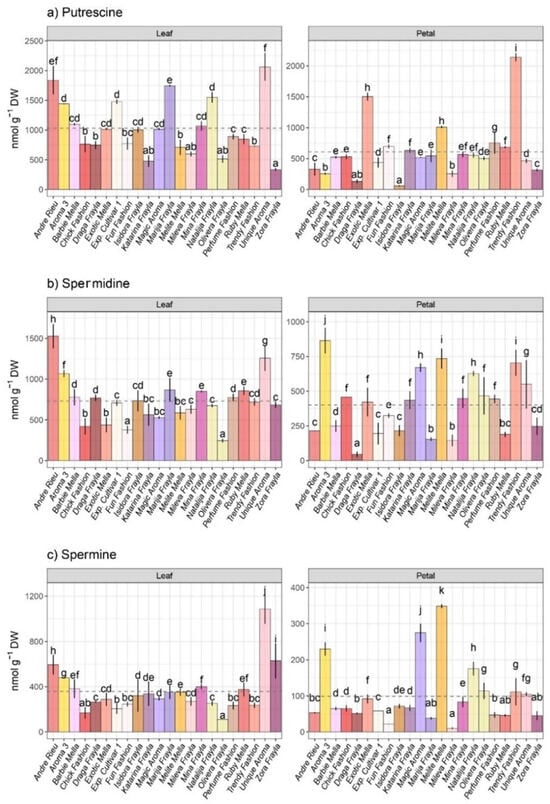
Figure 2.
Intraspecific variability at leaf and petal level in major polyamines (PAs) within roses: (a) putrescine (PUT), (b) spermidine (SPD) and (c) spermine (SPM), across 22 rose cultivars. Distinct lowercase letters denote significant differences among rose cultivars based on Tukey’s honestly significant difference (HSD) post hoc test (p ≤ 0.05). The data are presented as mean ± standard deviation (SD).
The PA SPD, derived from PUT, manifested substantial cultivar-dependent variability within rose cultivars (F 44.72; p 5.06 × 10−38) as well as organs (F 876.50; p 1.57 × 10−47). Foliar SPD levels exhibited fluctuations ranging from 245.08 nmol g−1 DW found in the cultivar “Olivera Frayla” to 1527.16 nmol g−1 DW that was recorded in the rose cultivar “Andre Rieu”, while petal-level SPD concentrations ranged from 45.49 in “Draga Frayla” to 864.20 nmol g−1 DW found in “Aroma 3” (Figure 2b). Rose cultivars displaying relatively elevated foliar SPD levels included “Andre Rieu”, “Unique Aroma”, “Aroma 3”, “Mina Frayla” and ”Marija Frajla”, whereas cultivars such as “Olivera Frayla”, “Fun Fashion” and “Chick Fashion” exhibited lower foliar SPD amounts. Conversely, at the petal level, cultivars “Aroma 3”, “Trendy Fashion”, “Melite Mella” and “Magic Aroma” demonstrated the highest SPD abundance, while “Draga Frayla“, “Marija Frayla” and “Mileva Frayla” were characterized by lower levels of SPD (Figure 2b).
Similar to the other PAs examined, the mean concentrations of the tetraamine SPM were found to be higher at the leaf level compared to the petals in rose cultivars (Figure 2c). Compared with other PAs, SPM varied most significantly between organs (F 2403.25, p 1.12 × 10−65) as well as cultivars (F 81.09; p 1.73 × 10−48). Foliar levels of SPM ranged from 110.93 (“Olivera Frayla”) to 1085.49 nmol g−1 DW (“Unique Aroma”) across rose cultivars, while petal SPM levels ranged between 10.04 (“Mileva Frayla”) and 348.36 nmol g−1 DW (“Melite Mella”) (Figure 2c). Among the inspected rose cultivars, those exhibiting the highest foliar SPM content were “Unique Aroma”, “Zora Frayla”, “Andre Rieu” and “Aroma 3”. On the other hand, at the petal level, “Melite Mella”, “Magic Aroma” and “Aroma 3” demonstrated the most prominent SPM levels.
Inspected rose cultivars demonstrated considerable heterogeneity in total PA abundance. Among the cultivars assessed, “Unique Aroma”, “Andre Rieu”, “Aroma 3”, “Frayla Marija” and “Trendy Fashion” exhibited notably high levels of total foliar PAs. On the contrary, “Trendy Fashion”, “Melite Mella”, “Exotic Mella” and “Magic Aroma” were identified as the most abundant cultivars in terms of total PAs at the petal level.
3.3. Variability in Antioxidant Capacities and Condensed Tannins among Examined Rose Cultivars
In assessing the total antioxidant capacity against the commercially available chromophore ABTS, methanolic extracts derived from the leaves of various rose cultivars exhibited higher mean activity compared to those obtained from extracts made of various rose petals (Figure 3a). Notably, leaf extracts from rose cultivars “Zora Frayla” and “Natalija Frayla” displayed noteworthy total antioxidant activity as estimated by the ABTS test, with values of 68.08 and 59.24 mmol Trolox equivalents (TE) per gram of DW, respectively, while leaf extracts from “Aroma 3” and “Unique Aroma” had the lowest antioxidant activities according to this assay (7.89 and 8.86 mM TE g−1 DW). Petal extracts derived from rose cultivars “Fun Fashion” and “Perfume Fashion” exhibited the highest antioxidant activities estimated by the ABTS test, 28.45 and 28.81 mmol TE g−1 DW, respectively (Figure 3a).
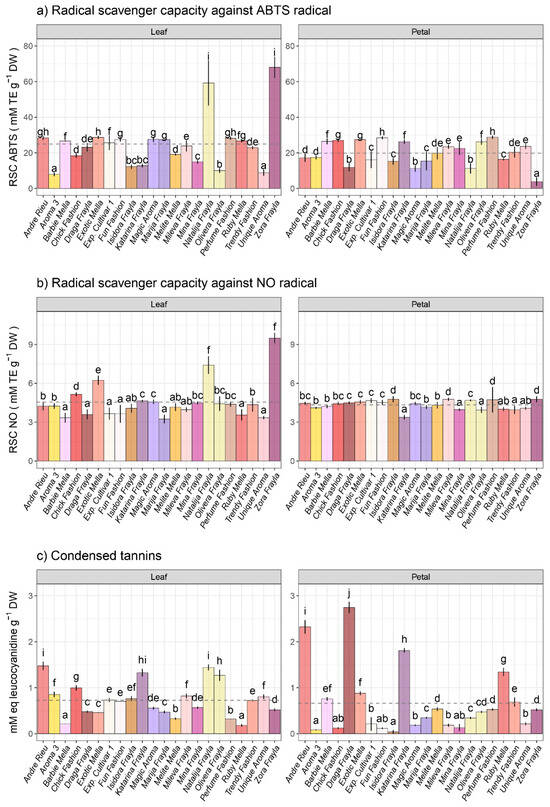
Figure 3.
Intraspecific variability in antioxidant activities at leaf and petal level regarding (a) radical scavenging capacity against ABTS•+, (b) radical scavenging capacity against NO radical and (c) total condensed tannins (CTs) across 22 rose cultivars. Distinct lowercase letters denote significant differences among rose cultivars based on Tukey’s honestly significant difference (HSD) post hoc test (p ≤ 0.05). The data are represented as mean ± standard deviation (SD).
In evaluating the efficacy of methanolic extracts from rose leaves against the NO radical, the most effective extracts were derived from cultivars “Zora Frayla”, “Natalija Frayla” and “Exotic Mella”, exhibiting NO radical scavenging capacities equivalent to 9.49, 7.41 and 6.23 mmol of Trolox equivalents per gram of dry weight (eq. TE g−1 DW), respectively. Conversely, among the methanolic extracts from rose petals, no cultivar demonstrated significant prominence in NO radical scavenging capacity, with values ranging from 3.37 to 4.77 mmol of TE g−1 DW (Figure 3b).
The quantities of condensed tannins (CTs) extracted from leaves using methanol ranged from 0.181 to 1.476 millimoles of leucocyanidin equivalents per gram of dry weight (mmol LE g−1 DW), whereas the levels identified in petals ranged from 0.039 to 2.746 mmol LE g−1 DW (Figure 3c).
Among the 22 examined rose cultivars, varieties such as “Andre Rieu”, “Katarina Frayla”, ”Natalija Frayla” and “Olivera Frayla” demonstrated the highest abundance of CTs in their leaves, while “Barbie Mella” and “Ruby Mella” exhibited the lowest levels of foliar CTs. On the contrary, at the petal level, cultivars “Andre Rieu”, “Draga Frayla” and “Katarina Frayla” showed the highest concentrations of CTs (Figure 3c).
The rose cultivar “Andre Rieu” exhibited the highest carbon content, whereas the cultivar “Chick Fashion” showed the lowest carbon foliar content among the inspected cultivars. No statistically significant differences were observed between leaf and petal carbon levels (Figure 4a). Nitrogen levels displayed significant variations between leaf and petal tissues. Notably, “Draga Frayla” and “Mina Frayla” stood out in terms of nitrogen content in leaves, while “Aroma 3” and “Katarina Frayla” exhibited the highest nitrogen levels among rose cultivars at the petal level (Figure 4b).
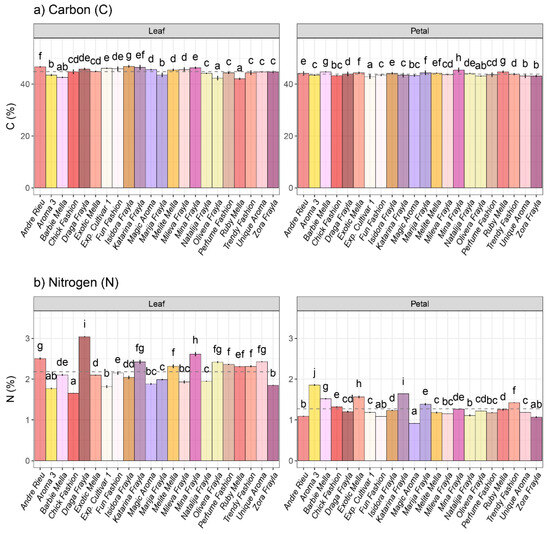
Figure 4.
Intraspecific variability in (a) carbon and (b) nitrogen amounts at leaf and petal level among 22 rose cultivars. Distinct lowercase letters denote significant differences among rose cultivars based on Tukey’s honestly significant difference (HSD) post hoc test (p ≤ 0.05). The data are represented as mean ± standard deviation (SD).
3.4. PCA Analysis, Hierarchical Clustering and Correlation Matrix
The biochemical profiles encompassing osmolytes, PAs, antioxidant capacities and carbon and nitrogen content across diverse rose cultivars were subjected to principal component analysis (PCA) and Pearson correlation analysis, considering a significance threshold of p < 0.05. In leaf samples, PCA revealed that the first two principal components (PCs) collectively accounted for 51.16% of the total variation among cultivars, with PC1 explaining 31.27% and PC2 explaining 19.89% of the variation (Figure 5a). Meanwhile, the PCA analysis for petals elucidated 47.31% of the variability. Notably, in the PCA graph for leaves, parameters such as GB and CT predominantly aligned with PC1, whereas PA parameters (PUT, SPM, SPD) were distributed evenly across both PC1 and PC2 within quadrant I. Additionally, PRO and radical scavenging capacities against nitric oxide (NO) and ABTS were evenly dispersed between PC1 and PC2 within quadrant II. Henceforth, the distinctive positioning of rose cultivars such as “Andre Rieu” and “Unique Aroma” within quadrant I can be attributed to their foliar PA levels (PUT, SPM, SPD), as evidenced by their proximity to the loadings of these parameters. Conversely, the unique placement of rose cultivars “Natalija Frayla” and “Zora Frayla”, distinct from the other grouped cultivars, is primarily influenced by their strong foliar radical scavenger capacities, estimated by parameters such as RSC ABTS and RSC NO (Figure 5a). Through the examination of PCA and the distribution of rose cultivars based on the biochemical parameters of their petals, it becomes evident that variables such as PUT, GB and radical scavenger capacity against nitric oxide (RSC NO) exhibit a stronger association with PC1. This close relationship outlined the positioning of cultivars such as “Trendy Fashion”, “Perfume Fashion”, “Isidora Frayla” and “Unique Aroma”. Furthermore, cultivars such as “Natalija Frayla”, “Mina Frayla” and “Zora Frayla” stood out and clustered around the loadings of SPM and SPD (Figure 5b).
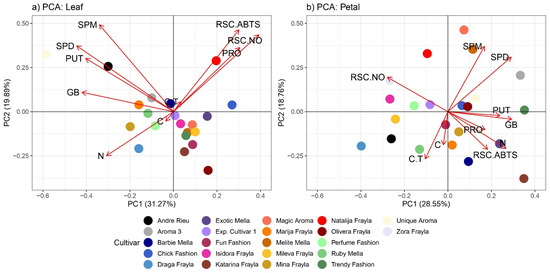
Figure 5.
Principal component analysis (PCA) of all assessed parameters across different rose cultivars at (a) leaf and (b) petal levels. Abbreviations correspond to following parameters: SPD: spermidine; SPM: spermine; PUT: putrescine; RSC ABTS: radical scavenger capacity against 2,2′-azinobis(3-ethylbenzothiozoline)-6-sulfonic acid ABTS•+; PRO: free proline; GB: glycine betaine; C. tann: condensed tannins; C: carbon; N: nitrogen content.
A Pearson correlation matrix (Figure 6) illustrates a strong positive correlation between nitrogen levels and PUT, as well as higher PAs such as SPM and SPD. Furthermore, the concentration of free PRO exhibited a positive correlation with GB but displayed negative correlations with individual PAs, namely PUT, SPM and SPD. Additionally, PRO content demonstrated a negative correlation with nitrogen levels. The total antioxidant capacity, as estimated by radical scavenger capacity against nitric oxide (RSC NO), exhibited negative correlations with all individual PAs, while RSC ABTS displayed positive correlations with all PAs. Carbon content demonstrated positive correlations with all individual aliphatic PAs as well as with CT. Meanwhile, GB exhibited negative correlations with individual polyamines as well as RSC NO (Figure 6).
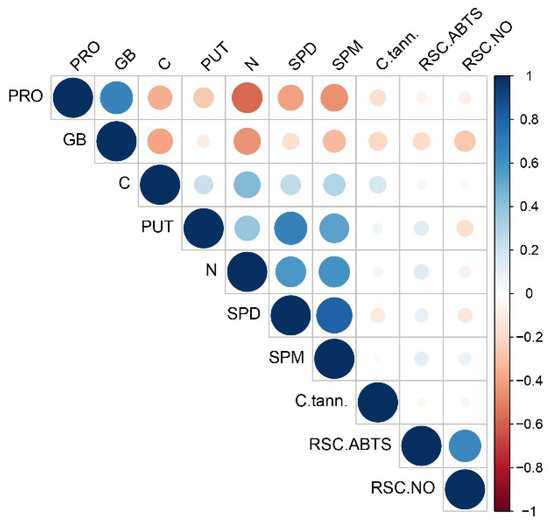
Figure 6.
The Pearson correlation coefficient matrix of the analyzed parameters in 22 rose cultivars. Blue squares indicate a strong and significant correlation among the examined parameters, whereas red squares indicate a lower level of interaction based on the corresponding Pearson coefficient. The abbreviations correspond to the following parameters: SPD: spermidine; SPM: spermine; PUT: putrescine; RSC ABTS: radical scavenger capacity against 2,2′-azinobis(3-ethylbenzothiozoline)-6-sulfonic acid ABTS•+; PRO: free proline; GB: glycine betaine; C. tann: condensed tannins; C: carbon; N: nitrogen content.
Through hierarchical clustering, all investigated rose cultivars were categorized into four distinct clusters. Notably, “Zora Frayla” and “Natalija Frayla” were grouped together in one cluster, while “Andre Rieu” and “Unique Aroma” formed another cluster based on their foliar biochemical properties (Figure 7a).
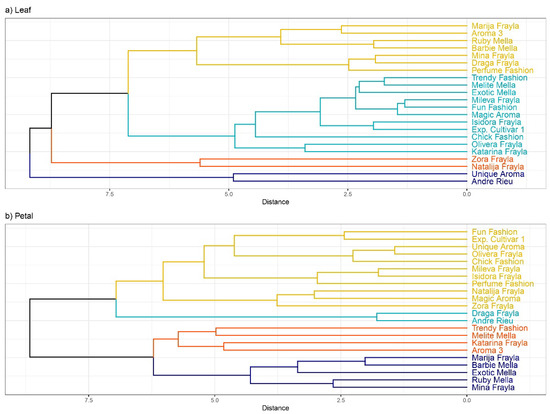
Figure 7.
Hierarchical clustering of 22 analyzed rose cultivars according to inspected parameters at (a) leaf and (b) petal level.
In another cluster analysis focused on petal biochemical properties, “Draga Frayla” and “Andre Rieu” emerged distinctly in separate clusters, whereas cultivars “Marija Frayla” and “Mina Frayla” clustered together with “Barbie Mella”, “Exotic Mella” and “Ruby Mella” (Figure 7b).
4. Discussion
This study represents a pioneering endeavor aimed at selecting rose cultivars that exhibit enhanced levels of osmolytes such as glycine betaine and proline, as well as individual polyamines quantified through a targeted metabolic approach, that may affect the resilience of selected cultivars to abiotic and biotic stressors. To the best of the authors’ knowledge, such comprehensive selection has not been previously conducted with such a large number of rose cultivars and inspected biochemical parameters. Certain edible rose cultivars from the same collection have undergone screening based on their morphological and phytochemical properties, as well as clustering via the essential oil analysis of 30 dominant volatile organic compounds and the assessment of main phenolic compounds and their contribution to antioxidant capacities in the context of nutrition and human health benefits [45]. Our study builds upon prior research by integrating additional metabolic markers to facilitate a more comprehensive selection of cultivars. Furthermore, we expanded our investigation to include non-edible cultivars with a particular emphasis on evaluating links between osmolyte amounts and the adaptability and resilience of rose cultivars to both biotic and abiotic stresses. The cultivar “Frayla Marija”, which was highlighted as the most notable in previous research [45] due to its strong antioxidant capacities as estimated by FRAP and DPPH assays, demonstrated moderate antioxidant activities estimated by RSC ABTS and RSC NO assays in the present study when compared to other examined rose cultivars.
Extensive literature emphasizes the strong correlation between elevated endogenous PRO levels and its multifaceted role in increasing stress tolerance against various abiotic stressors, including salt stress [46], drought [47,48] and temperature extremes [7]. These functions are attributed to proline’s antioxidant, osmoprotective and cryoprotective properties, along with its function as a chaperone and modulator of gene expression [49]. Additionally, the advantageous impacts of exogenous PRO were associated with its priming capabilities, as evidenced by spray application methods, which have been documented to mitigate oxidative stress in roses [50]. This is achieved through the enhancement of endogenous PRO levels and the upregulation of superoxide dismutase (ZnMnSOD) [50] which subsequently resulted in extending the longevity of vase life in Rosa hybrida L. (cultivar “Grand Gala”) by 3.3 days compared to non-treated controls [50]. Furthermore, this effect is probably associated with proline’s role in delaying senescence in both leaves and petals, through the modulation of ROS scavenger pathways [51]. On the other hand, PRO plays a role in biotic interactions, as evidenced by studies indicating that during hypersensitive reactions, elevated levels of ROS, particularly hydrogen peroxide (H2O2), promote the expression of the biosynthetic enzyme, namely, pyrroline-5-carboxylate synthetase (P5CS) [52]. Additionally, elevated ROS levels have been shown to negatively impact the activity of proline dehydrogenase (ProDH), the catalytic enzyme involved in proline metabolism [52]. In this study, rose cultivar “Zora Frayla”, which had the most abundant foliar levels of proline, was proven to be resilient to powdery mildew as shown in Supplementary Material (Table S1) which is consistent with the previously observed findings of the protective role of proline in cucumbers [53] and oaks infected with powdery mildew [54]. The negative correlation observed between proline and individual polyamines such as putrescine, spermine and spermidine SPD can be attributed to the shared intermediary metabolite, ornithine, within their biosynthetic pathways [55]. The cultivars “Frayla Zora” and “Chick Fashion”, which stood out for their high foliar proline levels in this study, could be potential subjects for future research focused on estimating whether these cultivars, with their elevated proline levels, demonstrate enhanced tolerance to frost, heat or drought or other abiotic stressors. Additionally, future research may compare their vase life longevity with other cultivars having lower proline levels to confirm the potential increased tolerance to abiotic stress factors. These cultivars have previously been confirmed to exhibit greater resistance to typical rose pathogens like powdery mildew or leafspots, which may be attributed to their elevated proline levels.
Regarding biotic stress factors, as demonstrated in Supplementary Material Table S1, it is evident that only one of the 22 rose cultivars exhibits resistance to downy mildew, namely “Unique Aroma”. Intriguingly, this cultivar was also shown to possess the highest levels of SPM and SPD among the rose cultivars examined in this study (Figure 3c). However, the precise mechanism underlying the role of SPM and SPD in mitigating specifically downy mildew infection in roses remains unclear and undisclosed; therefore, this rose cultivar represents a suitable candidate for further research. Nevertheless, the involvement of PAs, particularly SPM, in alleviating biotic stress has been previously outlined in the hypersensitive response of plants to pathogens such as powdery mildew [54,56], tobacco mosaic virus (TMV) [57] and Fusarium pathogen strains [58], through mechanisms such as the binding and strengthening of cell walls. Furthermore, hydrogen peroxide produced via PA catabolism may play a role in triggering hypersensitive reactions, as well as modulating defense gene expression through the activation of serine/threonine kinases from the MAPK group which can lead to cell death [59]. Additionally, it may contribute to strengthening and thickening the cell wall, thereby aiding in the prevention of fungal or insect attacks [60]. In addition to their involvement in generating ROS, PAs play a significant role in plant immunity and the amplification of pattern-triggered immunity (PTI) responses [24].
Another noteworthy aspect of PAs pertains to their involvement in expediting the flowering process and regulating bud dormancy [61,62]. The exogenous application of SPD on “Fuji” apples stimulated floral induction and the production of endogenous PAs [27]. Intriguingly, the application of exogenous PAs in marigold resulted in an increase in both flower carotenoid content and essential oil yield [63]. This targeted metabolomics-driven selection of rose cultivars based on PA levels may pave the way for further investigations pertaining to the selection of early and late flowering cultivars and the elucidation of flowering time patterns, along with their correlation with PA content. Interestingly, cultivars with the highest levels of polyamines, like “Unique Aroma” and “Andre Rieu”, also exhibited the greatest number of petals among all cultivars, which reaffirms the significant role of polyamines in flower development. Among other secondary metabolites, condensed tannins (CTs), as polymeric end-products of the flavonoid pathway, play a crucial role in plants’ defense mechanisms against biotic stress [54,64] but also abiotic stress [65]. In this research, rose cultivars with drastically increased CT levels, “Andre Rieu” and “Natalija Frayla”, were previously empirically characterized as highly resistant cultivars toward various biotic stress factors (as shown in Supplementary Table S1). On the other hand, the data regarding CTs in edible rose petals (rose cultivars “Marija Frayla”, “Olivera Frayla” and “Katarina Frayla”) also hold significance, as tannins found in food possess antioxidant and antimicrobial properties, thereby potentially reducing the risk of heart disease and cancer [66]. Among the surveyed rose cultivars, the edible cultivar “Katarina Frayla” exhibited exceptional abundance in condensed tannins.
5. Conclusions
This investigation revealed significant intraspecific heterogeneity in proline and glycine betaine content among 22 distinct rose cultivars, both at leaf and petal levels. Notably, proline and glycine betaine concentrations were generally higher in petals than leaves. Among polyamines, putrescine emerged as the most prevalent at both leaf and petal levels, followed by spermidine and spermine. Total PA abundance varied considerably among cultivars, with “Unique Aroma”, “Andre Rieu” and “Aroma 3” exhibiting notably high foliar PA levels, while “Trendy Fashion” and “Melite Mella” demonstrated prominence in PA abundance at the petal level. Methanolic leaf extracts from cultivars such as “Zora Frayla” and “Natalija Frayla” displayed significant total antioxidant activity, while cultivars “Andre Rieu” and “Natalija Frayla” stood out regarding foliar tannin levels. Rose cultivars abundant in individual PAs, osmolytes or tannins present potential candidates for subsequent experiments aimed to assess their resilience to specific abiotic or biotic stressors and further transcriptomic and metabolomic analysis. Pearson correlation analysis revealed associations between biochemical parameters, with nitrogen levels positively correlated with putrescine, spermine and spermidine, while proline content showed negative correlations with nitrogen levels and positive correlations with glycine betaine. Overall, these findings underscore the complex biochemical diversity within rose cultivars and demonstrate that inspected biochemical markers, osmolytes and polyamines may serve as reliable criteria for the selection of more resilient rose cultivars.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10040401/s1, Table S1: Phenotypical and disease resistance data about inspected rose cultivars; Table S2: Two-way ANOVA results of variable effects on inspected parameters.
Author Contributions
Conceptualization, M.K., B.B.T. and M.R.; methodology, M.K. and M.R.; software, S.K.; formal analysis, M.K., M.R. and V.V.; data curation, S.K., M.R. and M.K.; writing—original draft preparation, M.K.; writing—review and editing, M.R., S.K., V.V., S.O., B.B.T. and O.I.; visualization, S.K. and O.I.; supervision, S.O.; funding acquisition, B.B.T. and S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, Grant No. 451-03-66/2024-03/200197.
Data Availability Statement
The original contributions presented in the study are included in the article and supplementary material, further inquiries can be directed to the corresponding authors.
Acknowledgments
Special thanks to Biljana Božanić Tanjga, the Director of PhenoGenoRoses Company in Serbia, for generously providing exceptionally beautiful rose plant materials.
Conflicts of Interest
In the spirit of transparency and to address any potential conflicts of interest, authors Biljana Božanić Tanjga and Olivera Ilić acknowledge their affiliation with the breeding company PhenoGenoRoses. It is important to note that the employees of this breeding company had no involvement in the design of the trial, acquisition of results, data processing and interpretation and formulation of conclusions. Authors Biljana Božanić Tanjga and Olivera Ilić have no financial or non-financial interests to disclose that could potentially influence the interpretation or bias of the findings presented in this manuscript. We assert that there are no conflicts of interest among the authors that could affect the integrity, objectivity or validity of the findings presented in this manuscript.
References
- Ji, S. The true value of flowers and their effect on the Dutch economy. An interdisciplinary relationship between Art and Economics. Res. Econ. 2020, 74, 228–232. [Google Scholar] [CrossRef]
- Usman, M.; Ashfaq, M.; Taj, S.; Abid, M. An economic analysis of cut-rose flower in Punjab, Pakistan. JAPS 2014, 24, 651–655. [Google Scholar]
- Sharma, R.; Kumari, P.; Sahare, H.; Paul, S. Insight to the biotechnological interventions in flower crops for abiotic stress tolerance. Sci. Hortic. 2023, 318, 112102. [Google Scholar] [CrossRef]
- Debener, T.; Byrne, D.H. Disease resistance breeding in rose: Current status and potential of biotechnological tools. Plant Sci. 2014, 228, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Vukosavljev, M.; Zhang, J.; Esselink, G.D.; Westende, W.V.; Cox, P.; Visser, R.; Arens, P.; Smulders, M.J. Genetic diversity and differentiation in roses: A garden rose perspective. Sci. Hortic. 2013, 162, 320–332. [Google Scholar] [CrossRef]
- Sazzad, S.; Rajbongshi, A.; Shakil, R.; Akter, B.; Kaiser, M.S. RoseNet: Rose leave dataset for the development of an automation system to recognize the diseases of rose. Data Brief 2022, 44, 108497. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhang, C.; Wang, W.; Cao, Z.; Li, S.; Li, H.; Zhu, W.; Huang, Y.; Bao, M.; He, Y.; et al. Comparative transcriptome analysis of different heat stress responses between self-root grafting line and heterogeneous grafting line in rose. Hortic. Plant J. 2021, 7, 243–255. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, S.; Yu, S.; Zhang, Y.; Su, L.; Geng, L.Z.; Cheng, C.; Jiang, X. Exogenous calcium enhances the physiological status and photosynthetic capacity of rose (Rosa hybrida L.) under drought stress. Hortic. Plant J. 2023; in press. [Google Scholar] [CrossRef]
- Carvalho, D.R.; Vasconcelos, M.W.; Lee, S.; Vreugdenhil, D.; Heuvelink, E.; Carvalho, S.M. Moderate salinity improves stomatal functioning in rose plants grown at high relative air humidity. Environ. Exp. Bot. 2017, 143, 1–9. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, F.; Chen, H.; Zhang, T.; Yan, J.; Hu, Y. Unraveling arbuscular mycorrhizal fungi-induced resistance of purple branch rose (Rosa rugosa ‘Zizhi’) to Lymantria dispar based on metabolomics. Biol. Control 2022, 172, 104971. [Google Scholar] [CrossRef]
- Khan, S.; Siraj, S.; Shahid, M.; Haque, M.M.; Islam, A. Osmolytes: Wonder molecules to combat protein misfolding against stress conditions. Int. J. Biol. Macromol. 2023, 234, 123662. [Google Scholar] [CrossRef]
- Fedotova, M.V. Compatible osmolytes-bioprotectants: Is there a common link between their hydration and their protective action under abiotic stresses? J. Mol. Liq. 2019, 292, 111339. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Park, E.-J.; Jeknic, Z.; Chen, T.H.H. Exogenous application of glycine betaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Murata, N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynth. Res. 1995, 44, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Biouki, R.Y.; Beyrami, H. Investigating the effects of glycine betaine on growth and flower yield of Damask Rose under salinity stress. J. Crop Improv. 2020, 22, 119–134. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Čapelja, E.; Vuksanović, V.; Stojnić, S.; Gavranović Markić, A.; Zlatković, M.; Milović, M.; Galović, V.; Orlović, S. Ectomycorrhizal fungi modulate pedunculate Oak’s heat stress responses through the alternation of polyamines, phenolics, and osmotica Content. Plants 2022, 11, 3360. [Google Scholar] [CrossRef]
- Pottosin, I.; Shabala, S. Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.S.; Scherer, G.F.E. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef]
- Gerlin, L.; Baroukh, C.; Genin, S. Polyamines: Double agents in disease and plant immunity. Trends Plant. Sci. 2021, 26, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Rashan, A.I.; Altaee, R.T.; Salh, F.S.; Al-Abbasy, O.Y.; Al-Lehebe, N. The role of polyamines in plants: A review. Plant Sci. Today 2023, 10, 164–171. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 429326. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, X.; Yan, J.; Fan, L.; Rong, C.; Mo, C.; Zhang, M. Effect of exogenous spermidine on floral induction, endogenous polyamine and hormone production, and expression of related genes in ‘Fuji’ apple (Malus domestica Borkh.). Sci. Rep. 2019, 9, 12777. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Jabbarzadeh, Z.; Amiri, J.; Rasouli-Sadaghiani, M.H. Response of roses (Rosa hybrida L. ‘Herbert Stevens’) to foliar application of polyamines on root development, flowering, photosynthetic pigments, antioxidant enzymes activity and NPK. Sci. Rep. 2019, 9, 16025. [Google Scholar] [CrossRef]
- Ochir, S.; Park, B.; Nishizawa, M.; Kanazawa, T.; Funaki, M.; Yamagishi, T. Simultaneous determination of hydrolysable tannins in the petals of Rosa rugosa and allied plants. J. Nat. Med. 2010, 64, 383–387. [Google Scholar] [CrossRef]
- Koopman, W.J.M.; Wissemann, V.; De Cock, K.; Van Huylen-Broeck, J.; De Riek, J.; Sabatino, G.J.H.; Visser, D.; Vosman, B.; Ritz, C.M.; Maes, B.; et al. AFLP markers as a tool to reconstruct complex relationships: A case study in Rosa (Rosaceae). Am. J. Bot. 2008, 95, 353–366. [Google Scholar] [CrossRef]
- Bendahmane, M.; Dubois, A.; Raymond, O.; Bris, M.L. Genetics and genomics of flower initiation and development in roses. J. Exp. Bot. 2013, 64, 847–857. [Google Scholar] [CrossRef]
- Smulders, M.J.; Arens, P.; Bourke, P.M.; Debener, T.; Linde, M.; De Riek, J.; Leus, L.; Ruttink, T.; Baudino, S.; Hibrant Saint-Oyant, L.; et al. In the name of the rose: A roadmap for rose research in the genome era. Hortic. Res. 2019, 6, 65. [Google Scholar] [CrossRef]
- Scaramagli, S.; Franceschetti, M.; Michael, A.J.; Torrigiani, P.; Bagni, N. Polyamines and flowering: Spermidine biosynthesis in the different whorls of developing flowers of Nicotiana tabacum L. Plant Biosyst. 1999, 133, 229–237. [Google Scholar] [CrossRef]
- Biondi, S.; Antognoni, F.; Marincich, L.; Lianza, M.; Tejos, R.; Ruiz, K.B. The polyamine “multiverse” and stress mitigation in crops: A case study with seed priming in quinoa. Sci. Hortic. 2022, 304, 111292. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kumaravel, S. Study on phenolic content, antioxidant activity and CHNS elemental analysis of Amorphophallus sylvaticus. Int. J. Agric. Life Sci. 2016, 2, 12–17. Available online: https://skyfox.co/ijals/index.php/als/article/view/9 (accessed on 8 March 2024).
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Rašeta, M.; Kebert, M.; Mišković, J.; Rakić, M.; Kostić, S.; Čapelja, E.; Karaman, M. Polyamines in edible and medicinal fungi from Serbia: A novel perspective on neuroprotective properties. J. Fungi 2024, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Green, L.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 15 March 2024).
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Galili, T. Dendextend: An R Package for Visualizing, Adjusting and Comparing Trees of Hierarchical Clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Simin, N.; Lesjak, M.; Živanović, N.; Božanić Tanjga, B.; Orčić, D.; Ljubojević, M. Morphological characters, phytochemical profile and biological activities of novel garden roses edible cultivars. Horticulturae 2023, 9, 1082. [Google Scholar] [CrossRef]
- Azizi, S.; Seyed Hajizadeh, H.; Aghaee, A.; Kaya, O. In vitro assessment of physiological traits and ROS detoxification pathways involved in tolerance of Damask rose genotypes under salt stress. Sci. Rep. 2023, 13, 17795. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol. Biochem. 2020, 155, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kebert, M.; Kostić, S.; Stojnić, S.; Čapelja, E.; Gavranović Markić, A.; Zorić, M.; Kesić, M.; Flors, V. A fine-tuning of the plant hormones, polyamines and osmolytes by ectomycorrhizal fungi enhances drought tolerance in pedunculate oak. Int. J. Mol. Sci. 2023, 24, 7510. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.; Tomar, R.S.; Sharma, R.A.; Singh, S.K. Proline: A key player to regulate biotic and abiotic stress in plants. In Towards Sustainable Natural Resources: Monitoring and Managing Ecosystem Biodiversity; Springer International Publishing: Cham, Switzerland, 2022; Volume 18, pp. 333–346. [Google Scholar] [CrossRef]
- Kumar, N.; Pal, M.; Singh, A.; SaiRam, R.K.; Srivastava, G.C. Exogenous proline alleviates oxidative stress and increase vase life in rose (Rosa hybrida L. ‘Grand Gala’). Sci. Hortic. 2010, 127, 79–85. [Google Scholar] [CrossRef]
- Zhang, L.; Becker, D. Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 2015, 6, 151855. [Google Scholar] [CrossRef]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Wang, J. Increasing the activities of protective enzymes is an important strategy to improve resistance in cucumber to powdery mildew disease and melon aphid under different infection/infestation patterns. Front. Plant Sci. 2022, 13, 950538. [Google Scholar] [CrossRef]
- Kebert, M.; Kostić, S.; Zlatković, M.; Stojnic, S.; Čapelja, E.; Zorić, M.; Kiprovski, B.; Budakov, D.; Orlović, S. Ectomycorrhizal fungi modulate biochemical response against powdery mildew disease in Quercus robur L. Forests 2022, 13, 1491. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Montilla-Bascón, G.; Rubiales, D.; Prats, E. Changes in polyamine profile in host and non-host oat–powdery mildew interactions. Phytochem. Lett. 2014, 8, 207–212. [Google Scholar] [CrossRef]
- Walters, D.R. Polyamines and plant disease. Phytochemistry 2003, 64, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik, W.; Kulma, A.; Namysł, K.; Preisner, M.; Szopa, J. Polyamine metabolism in flax in response to treatment with pathogenic and non–pathogenic Fusarium strains. Front. Plant Sci. 2015, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gai, C.; Shao, M.; Fang, L.; Li, X.; Song, Y.; Zeng, R.; Chen, D. Herbivory by striped stem borer triggers polyamine accumulation in host rice plants to promote its larval growth. Plants 2023, 12, 3249. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Gholami, M.; Baninasab, B. Changes in polyamines during bud dormancy in almond cultivars differing in their flowering date. Sci. Hortic. 2019, 258, 108788. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chaudhury, R.; Dutta, S.; Basak, M.; Dey, S.; Schäffner, A.R.; Das, M. Role of metabolites in flower development and discovery of compounds controlling flowering time. Plant Physiol. Biochem. 2022, 190, 109–118. [Google Scholar] [CrossRef]
- Tavallali, V.; Alhavi, N.; Gholami, H.; Abarghuei, F.M. Developmental and phytochemical changes in pot marigold (Calendula officinalis L.) using exogenous application of polyamines. Plant Physiol. Biochem. 2022, 183, 128–137. [Google Scholar] [CrossRef]
- Constabel, P.C.; Yoshida, K.; Walker, V. Diverse ecological roles of plant tannins: Plant defense and beyond. In Recent Advances in Polyphenol Research; Romani, A., Lattanzio, V., Quideau, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 115–142. [Google Scholar]
- Harding, S.A. Condensed tannins: Arbiters of abiotic stress tolerance? Tree Physiol. 2019, 39, 341–344. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2021, 40, 432–444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






















