Effects of Protein Hydrolysate Derived from Anchovy By-Product on Plant Growth of Primrose and Root System Architecture Analysis with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Production Protein Hydrolysate from Fish By-Product
Amino Acid Composition of FPH
2.2. Plant Material and Growing Conditions
2.3. Application of Protein Hydrolysate
2.4. Plant Growth Characteristics and Assessment of Root System Architecture
2.5. Modeling Procedure
2.5.1. Multilayer Perceptron
2.5.2. Gaussian Process
2.5.3. Random Forest
2.5.4. Extreme Gradient Boosting
3. Results
3.1. Effects of FPH on Plant Growth and Root System
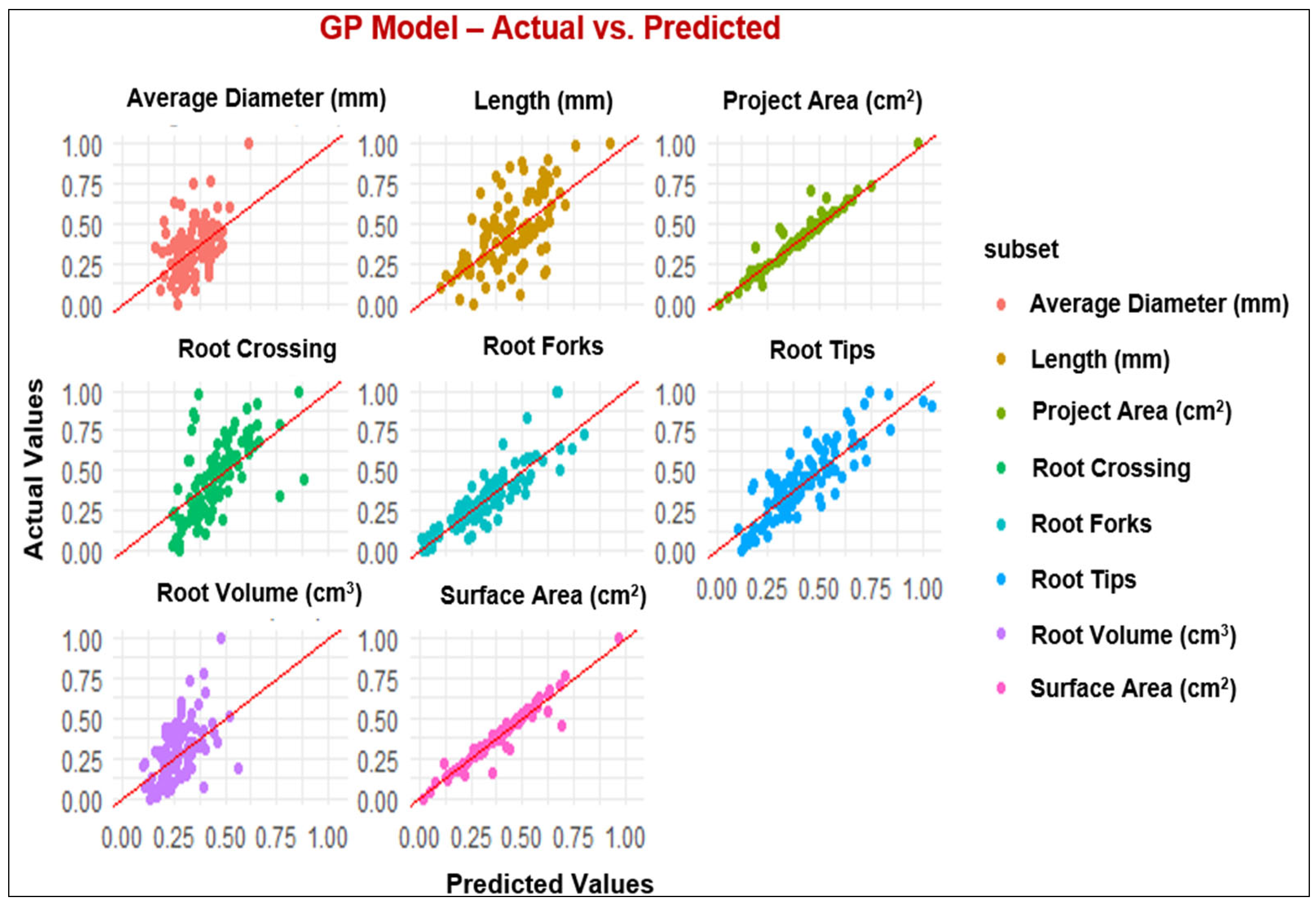
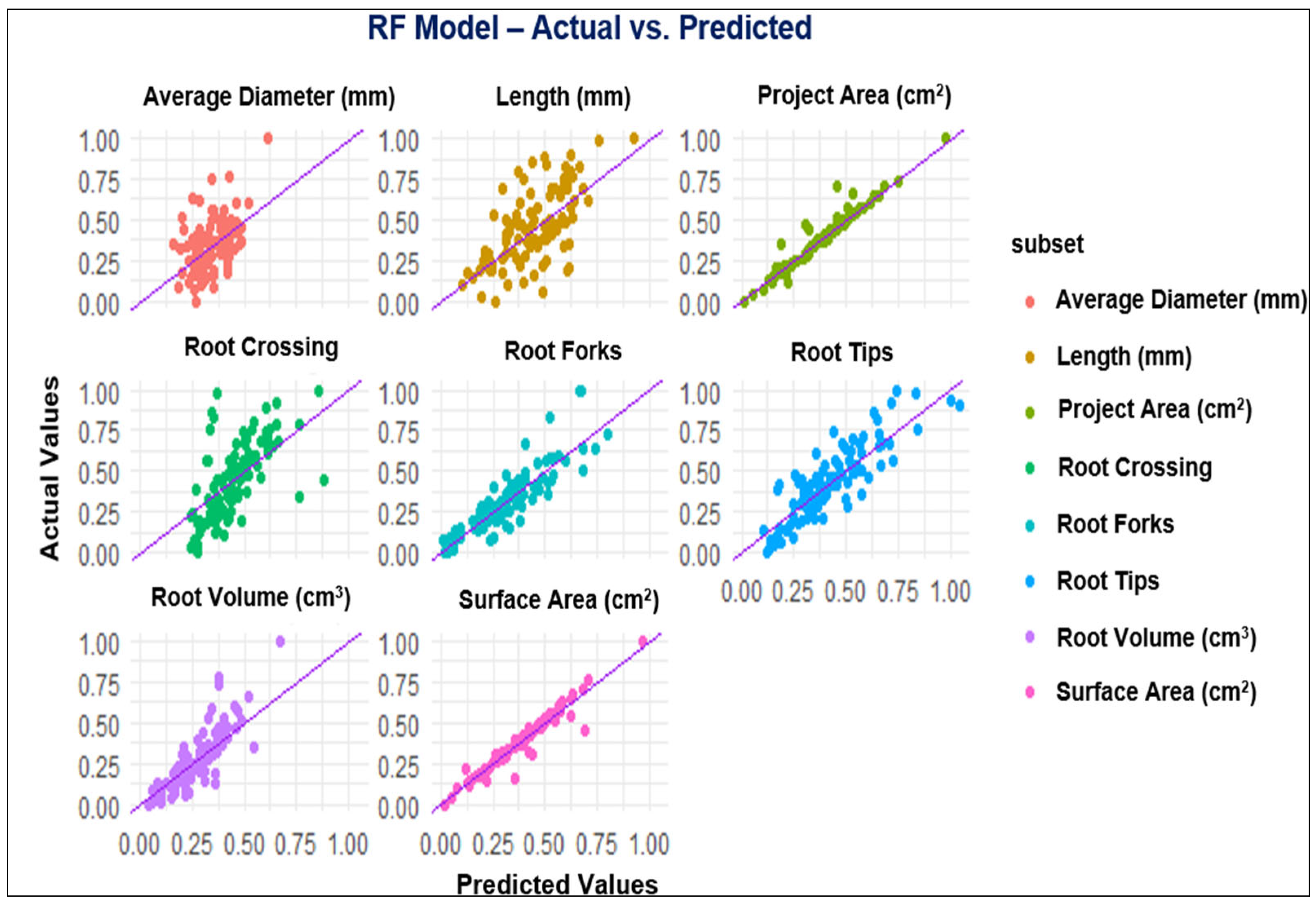
3.2. ML Modeling Analysis
4. Discussion
4.1. Performances of Plant Growth and Root System Architecture
4.2. Performance of Modeling of Root System Architecture
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karlsson, M. Primula culture and production. HortTechnology 2001, 11, 627–635. [Google Scholar] [CrossRef]
- Tütüncü, M.; Dalda-Sekerci, A.; Bulut, F.; Şimşek, Ö. Comprehensive assessment of genetic variation in native heterostylous primrose genotypes of Türkiye. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13276. [Google Scholar] [CrossRef]
- Davitkovska, M.; Kabranova, R.; Bogevska, Z.; Popsimonova, G.; Agic, R.; Zeljković, S.; Dorbić, B. Quality examination of Primula acaulis Hill. treated with two different fertilizers. J. Agric. Food Environ. Sci. 2021, 75, 45–51. [Google Scholar]
- Salehpour, T.; Khanali, M.; Rajabipour, A. Environmental impact assessment for ornamental plant greenhouse: Life cycle assessment approach for primrose production. Environ. Pollut. 2020, 266, 115258. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 511937. [Google Scholar] [CrossRef] [PubMed]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Choudhary, A.K.; Sahoo, M.R.; Dasgupta, M. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef]
- Ugolini, L.; Malaguti, L.; Matteo, R.; Pagnotta, E.; Beleggia, R.; Righetti, L. Protein Hydrolysates from Crambe abyssinica Seed Cake as Potential Biostimulants for Root Development. Agronomy 2023, 13, 2755. [Google Scholar] [CrossRef]
- Madende, M.; Hayes, M. Fish by-product use as biostimulants: An overview of the current state of the art, including relevant legislation and regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Himaya, S.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Siddik, M.A.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic fish protein hydrolysates in finfish aquaculture: A review. Rev. Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, bioactive properties, and potential applications of fish protein hydrolysates: Developments and challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.-J. Effects of plant-derived protein hydrolysates on yield, quality, and nitrogen use efficiency of greenhouse grown lettuce and tomato. Agronomy 2022, 12, 1018. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Drench application of fish-derived protein hydrolysates affects lettuce growth, chlorophyll content, and gas exchange. Horttechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Liatile, P.C.; Potgieter, G.; Moloi, M.J. A Natural Bio-Stimulant Consisting of a Mixture of Fish Protein Hydrolysates and Kelp Extract Enhances the Physiological, Biochemical and Growth Responses of Spinach under Different Water Levels. Plants 2022, 11, 3374. [Google Scholar] [CrossRef] [PubMed]
- Dewang, S.P.; Devi, C.U. Influence of soil-application of fish-protein hydrolysate liquid on growth and yield of spinach (Spinacia oleracea L.). Asian J. Dairy Food Res. 2021, 40, 69–75. [Google Scholar] [CrossRef]
- Mironenko, G.A.; Zagorskii, I.A.; Bystrova, N.A.; Kochetkov, K.A. The effect of a biostimulant based on a protein hydrolysate of rainbow trout (Oncorhynchus mykiss) on the growth and yield of wheat (Triticum aestivum L.). Molecules 2022, 27, 6663. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, J.; Jia, C.; Feng, J.; Liang, L.; Cheng, Q.; Li, T.; Hao, J. Foliar application of fish protein peptide improved the quality of deep-netted melon. J. Plant Nutr. 2023, 46, 3683–3696. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Palta, J.A.; Turner, N.C. Crop root system traits cannot be seen as a silver bullet delivering drought resistance. Plant Soil 2019, 439, 31–43. [Google Scholar] [CrossRef]
- Sanada, A.; Agehara, S. Characterizing root morphological responses to exogenous tryptophan in soybean (Glycine max) seedlings using a scanner-based rhizotron system. Plants 2023, 12, 186. [Google Scholar] [CrossRef]
- Chung, Y.S.; Lee, U.; Heo, S.; Na, C.-I.; Kim, Y. Image-based machine learning characterizes root nodule in soybean exposed to silicon. Front. Plant Sci. 2020, 11, 520161. [Google Scholar] [CrossRef]
- Zhao, J.; Bodner, G.; Rewald, B. Phenotyping: Using machine learning for improved pairwise genotype classification based on root traits. Front. Plant Sci. 2016, 7, 221684. [Google Scholar] [CrossRef]
- Şimşek, Ö. Machine Learning Offers Insights into the Impact of In Vitro Drought Stress on Strawberry Cultivars. Agriculture 2024, 14, 294. [Google Scholar] [CrossRef]
- Aasim, M.; Katırcı, R.; Akgur, O.; Yildirim, B.; Mustafa, Z.; Nadeem, M.A.; Baloch, F.S.; Karakoy, T.; Yılmaz, G. Machine learning (ML) algorithms and artificial neural network for optimizing in vitro germination and growth indices of industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 181, 114801. [Google Scholar] [CrossRef]
- van Dijk, A.D.J.; Kootstra, G.; Kruijer, W.; de Ridder, D. Machine learning in plant science and plant breeding. Iscience 2021, 24, 101890. [Google Scholar] [CrossRef]
- Sommer, C.; Gerlich, D.W. Machine learning in cell biology–teaching computers to recognize phenotypes. J. Cell Sci. 2013, 126, 5529–5539. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A. The application of artificial neural networks in modeling and predicting the effects of melatonin on morphological responses of citrus to drought stress. PLoS ONE 2020, 15, e0240427. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Shariatpanahi, M.E.; Abdipour, M.; Oroojloo, M. Modeling callus induction and regeneration in an anther culture of tomato (Lycopersicon esculentum L.) using image processing and artificial neural network method. Protoplasma 2019, 256, 1317–1332. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Jones, A.M.P. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef]
- Kirtis, A.; Aasim, M.; Katırcı, R. Application of artificial neural network and machine learning algorithms for modeling the in vitro regeneration of chickpea (Cicer arietinum L.). Plant Cell Tissue Organ Cult. (PCTOC) 2022, 150, 141–152. [Google Scholar] [CrossRef]
- Korkmaz, K.; Tokur, B.; Yılmaz, U. Enzimatik hidroliz yöntemi kullanılarak balık işleme atıklarından balık protein hidrolizatı üretimi. Yuz. Yıl Univ. J. Agric. Sci. 2021, 31, 502–513. [Google Scholar]
- Korkmaz, K.; Tokur, B. Investigation of the quality parameters of hydrolysates obtained from fish by-products using response surface methodology. J. Food Process. Preserv. 2022, 46, e16296. [Google Scholar] [CrossRef]
- Korkmaz, K.; Tokur, B. Optimization of hydrolysis conditions for the production of protein hydrolysates from fish wastes using response surface methodology. Food Biosci. 2022, 45, 101312. [Google Scholar] [CrossRef]
- Lee, Y.; Hwang, K.T. Changes in physicochemical properties of mulberry fruits (Morus alba L.) during ripening. Sci. Hortic. 2017, 217, 189–196. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P. Chemical composition and physicochemical properties of tropical red seaweed. Gracilaria changii. Food Chem. 2017, 221, 302–310. [Google Scholar] [CrossRef]
- Kıvrak, İ. Chemical constituents: Water-soluble vitamins, free amino acids and sugar profile from Ganoderma adspersum. Nat. Prod. Res. 2015, 29, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Joint, F. Energy and protein requirements: Report of a joint FAO/WHO/UNU Expert Consultation. In Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 1985; p. 206. [Google Scholar]
- Saribaş, S.; Balkaya, A.; Kandemir, D.; Karaağaç, O. Yerli patlican anaçlarinin (Solanum melongena × Solanum aethiopicum) köklenme potansiyeli ve fenotipik kök mimarisi. Black Sea J. Agric. 2019, 2, 137–145. [Google Scholar]
- Zehra, Ş.; Tütüncü, A.Ç.; Demirkaya, S.; Harun, Ö. Organik ve konvansiyonel fide yetiştiriciliğinin domates fidelerinin kalitesi üzerine etkileri. Anadolu Tarım Bilim. Derg. 2023, 38, 555–564. [Google Scholar]
- Hu, J.; Sun, Y.; Li, G.; Jiang, G.; Tao, B. Probability analysis for grasp planning facing the field of medical robotics. Measurement 2019, 141, 227–234. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Wangkheirakpam, M.; Mahanand, S.; Majumdar, R.; Sharma, S.; Hidangmayum, D.; Netam, S. Fish waste utilization with reference to fish protein hydrolisate—A review. Fish. Technol 2019, 56, 169–178. [Google Scholar]
- Colantoni, A.; Recchia, L.; Bernabei, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the environmental impact of chemically-produced protein hydrolysate from leather waste vs. enzymatically-produced protein hydrolysate from legume grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Ishak, N.; Sarbon, N. A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing. Food Bioprocess Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Zeljković, S.; Parađiković, N.; Vinković, T.; Tkalec, M. Biostimulant application in the production of seedlings of seasonal flowers. Agroznanje 2011, 12, 175–181. [Google Scholar]
- Carillo, P.; Pannico, A.; Cirillo, C.; Ciriello, M.; Colla, G.; Cardarelli, M.; De Pascale, S.; Rouphael, Y. Protein hydrolysates from animal or vegetal sources affect morpho-physiological traits, ornamental quality, mineral composition, and shelf-life of Chrysanthemum in a distinctive manner. Plants 2022, 11, 2321. [Google Scholar] [CrossRef]
- Cristiano, G.; Pallozzi, E.; Tufarelli, V.; De Lucia, B. Effects of an animal-derived biostimulant on the growth and physiological parameters of potted snapdragon (Antirrhinum majus L.). Front. Plant Sci. 2018, 9, 362419. [Google Scholar] [CrossRef] [PubMed]
- Al-Malieky, H.M.; Jerry, A.N. Preparation protein hydrolysates from fish by-product and study effected on lettuce (Lactuca sativa L.) growth, yield, quality and enhanced salt tolerance. Basrah J. Agric. Sci. 2019, 32, 246–255. [Google Scholar] [CrossRef]
- Raguraj, S.; Kasim, S.; Md Jaafar, N.; Nazli, M.H. Growth of tea nursery plants as influenced by different rates of protein hydrolysate derived from chicken feathers. Agronomy 2022, 12, 299. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Horii, A.; McCue, P.; Shetty, K. Seed vigour studies in corn, soybean and tomato in response to fish protein hydrolysates and consequences on phenolic-linked responses. Bioresour. Technol. 2007, 98, 2170–2177. [Google Scholar] [CrossRef]
- Cristiano, G.; De Lucia, B. Petunia performance under application of animal-based protein hydrolysates: Effects on visual quality, biomass, nutrient content, root morphology, and gas exchange. Front. Plant Sci. 2021, 12, 640608. [Google Scholar] [CrossRef]
- Gough, S.P.; Westergren, T.; Hansson, M. Chlorophyll biosynthesis in higher plants. Regulatory aspects of 5-aminolevulinate formation. J. Plant Biol. 2003, 46, 135–160. [Google Scholar] [CrossRef]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for production, characterization and application of protein-based biostimulants in agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Ryser, P. The mysterious root length. Plant Soil 2006, 286, 1–6. [Google Scholar] [CrossRef]
- Clothier, B.E.; Green, S.R. Roots: The big movers of water and chemical in soil. Soil Sci. 1997, 162, 534–543. [Google Scholar] [CrossRef]
- Zhao, J.; Sykacek, P.; Bodner, G.; Rewald, B. Root traits of European Vicia faba cultivars—Using machine learning to explore adaptations to agroclimatic conditions. Plant Cell Environ. 2018, 41, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; York, L.M.; Seethepalli, A.; Bucciarelli, B.; Cheng, H.; Samac, D.A. Objective phenotyping of root system architecture using image augmentation and machine learning in alfalfa (Medicago sativa L.). Plant Phenomics 2022, 2022, 9879610. [Google Scholar] [CrossRef]
- Duarte, A.B.; de Oliveira Ferreira, D.; Ferreria, L.B.; da Silva, F.L. Machine learning applied to the prediction of root architecture of soybean cultivars under two water availability conditions. Semin. Ciências Agrárias 2022, 43, 1017–1036. [Google Scholar] [CrossRef]


| Amino Acids | Amount of Total Amino Acids (g/100 g Dried Samples) |
|---|---|
| Arginine | 0.67 ± 0.13 |
| Aspartic acid | 6.98 ± 0.42 |
| Cystine | 0.56 ± 0.04 |
| Glutamic acid | 8.72 ± 0.06 |
| Histidine | 0.67 ± 0.08 |
| Isoleucine leucine | 1.35 ± 0.05 |
| Lysine | 1.42 ± 0.07 |
| Methionine | 0.97 ± 0.02 |
| Phenylalanine | 0.65 ± 0.03 |
| Proline | 1.31 ± 0.02 |
| Serine | 4.03 ± 0.03 |
| Threonine | 3.45 ± 0.05 |
| Tyrosine | 0.61 ± 0.02 |
| Valine | 1.33 ± 0.012 |
| Parameter for Greenhouse | Average | Highest | Lowest |
|---|---|---|---|
| Temperature (°C) | 12.63 | 25.01 | 3.64 |
| Humidity (%) | 61.98 | 89.10 | 29.07 |
| Variety | Treatments (g/L) | Leaf Area (cm2) | Leaf Number | Flower Number | Whole Plant Dry Weight (g) | SPAD Index | DAFT |
|---|---|---|---|---|---|---|---|
| Light violet | 0.0 | 29.39 ± 1.4 | 12.08 ± 0.6 | 13.58 ± 1.4 | 3.53 ± 0.08 | 28.01 ± 0.81 | 90.66 ± 0.37 |
| 0.5 | 32.37 ± 1.2 | 13.75 ± 1.1 | 17.25 ± 1.0 | 4.70 ± 0.18 | 33.22 ± 0.88 | 86.33 ± 0.84 | |
| 1.0 | 34.94 ± 3.0 | 12.58 ± 0.5 | 16.58 ± 1.2 | 4.20 ± 0.16 | 34.66 ± 0.71 | 84.83 ± 0.93 | |
| 1.5 | 39.99 ± 2.2 | 14.25 ± 1.2 | 16.83 ± 1.8 | 5.05 ± 0.13 | 33.69 ± 0.24 | 87.08 ± 0.58 | |
| Pink | 0.0 | 28.75 ± 2.9 | 11.58 ± 1.0 | 12.58 ± 1.0 | 3.39 ± 0.21 | 26.88 ± 1.68 | 92.66 ± 1.14 |
| 0.5 | 38.97 ± 3.8 | 13.83 ± 1.2 | 14.58 ± 0.7 | 4.83 ± 0.36 | 33.89 ± 2.39 | 88.25 ± 1.86 | |
| 1.0 | 31.73 ± 1.8 | 12.75 ± 1.4 | 15.25 ± 1.3 | 4.15 ± 0.43 | 34.04 ± 3.30 | 90.33 ± 1.73 | |
| 1.5 | 39.75 ± 3.0 | 14.00 ± 1.0 | 16.15 ± 1.3 | 4.64 ± 0.38 | 32.52 ± 1.73 | 88.08 ± 1.18 | |
| Main Effects | |||||||
| Variety | Light violet | 34.17 ± 1.19 | 13.16 ± 0.48 | 16.06 ± 0.70 | 4.37 ± 0.11 | 32.40 ± 0.51 | 87.22 ± 0.47 a |
| Pink | 34.80 ± 1.64 | 13.04 ± 0.65 | 14.39 ± 0.60 | 4.25 ± 0.19 | 31.83 ± 1.25 | 89.83 ± 0.80 b | |
| Treatments (g/L) | 0.0 | 29.07 ± 1.63 c | 11.83 ± 0.60 | 13.08 ± 0.78 | 3.46 ± 0.11 c | 27.45 ± 0.94 b | 91.66 ± 0.63 b |
| 0.5 | 35.67 ± 2.1 ab | 13.79 ± 0.87 | 15.91 ± 0.69 | 4.77 ± 0.20 a | 33.55 ± 1.27 a | 87.29 ± 1.03 a | |
| 1.0 | 33.32 ± bc | 12.66 ± 0.66 | 15.91 ± 0.91 | 4.18 ± 0.22 b | 34.35 ± 1.69 a | 87.58 ± 1.13 a | |
| 1.5 | 39.87 ± 1.91 a | 14.12 ± 0.95 | 16.00 ± 1.16 | 4.85 ± 0.20 a | 33.10 ± 0.88 a | 87.580.66 a | |
| LSD | Variety | ns | ns | ns | ns | ns | 1.740 * |
| Treatments | 5.388 * | ns | ns | 0.568 * | 3.635 * | 2.461 * | |
| Variety × Treatments | ns | ns | ns | ns | ns | ns | |
| Variety | Treatments (g/L) | Surface Area (cm2) | Projected Area (cm2) | Root Volume (cm3) |
|---|---|---|---|---|
| Light violet | 0.0 | 208.45 ± 17.2 | 67.77 ± 6.69 | 20.09 ± 1.87 |
| 0.5 | 244.77 ± 20.8 | 83.37 ± 6.93 | 31.37 ± 2.54 | |
| 1.0 | 309.81 ± 16.5 | 102.03 ± 6.01 | 31.49 ± 2.02 | |
| 1.5 | 268.84 ± 21.6 | 87.36 ± 6.78 | 20.46 ± 1.47 | |
| Pink | 0.0 | 207.41 ± 24.2 | 68.79 ± 7.94 | 21.04 ± 2.71 |
| 0.5 | 245.62 ± 21.1 | 81.47 ± 7.00 | 31.16 ± 3.08 | |
| 1.0 | 327.19 ± 21.8 | 108.52 ± 7.23 | 33.94 ± 3.14 | |
| 1.5 | 252.10 ± 15.6 | 83.62 ± 5.19 | 20.18 ± 1.94 | |
| Main Effects | ||||
| Variety | Light violet | 257.97 ± 10.9 | 85.13 ± 3.74 | 25.85 ± 1.26 |
| Pink | 258.08 ± 11.5 | 85.60 ± 3.84 | 26.58 ± 1.47 | |
| Treatments | 0.0 | 207.93 ± 11.7 c | 68.28 ± 4.29 c | 20.57 ± 1.36 b |
| 0.5 | 245.20 ± 15.0 bc | 82.42 ± 4.96 b | 31.27 ± 2.03 a | |
| 1.0 | 318.50 ± 14.0 a | 105.27 ± 4.84 a | 32.74 ± 1.92 a | |
| 1.5 | 260.47 ± 13.2 b | 85.49 ± 4.26 b | 20.32 ± 1.24 b | |
| LSD | Variety | ns | ns | ns |
| Treatments | 39.738 * | 13.463 * | 4.749 * | |
| Variety × Treatments | ns | ns | ns | |
| Variety | Treatments (g/L) | Total Root Length (cm) | Average Root Diameter (mm) | The Number of Root Tips | The Number of Root Forks | The Number of Root Crossings |
|---|---|---|---|---|---|---|
| Light violet | 0.0 | 174.36 ± 10.2 | 3.48 ± 0.16 | 445.54 ± 26.1 | 715.27 ± 59.2 | 68.36 ± 6.01 |
| 0.5 | 217.94 ± 14.4 | 3.62 ± 0.24 | 493.61 ± 34.4 | 982.15 ± 86.6 | 123.61 ± 9.72 | |
| 1.0 | 253.59 ± 16.1 | 4.12 ± 0.17 | 596.41 ± 44.5 | 1262.08 ± 122.8 | 133.33 ± 7.37 | |
| 1.5 | 230.46 ± 23.0 | 3.98 ± 0.17 | 512.33 ± 56.64 | 969.16 ± 148.0 | 142.83 ± 12.1 | |
| Pink | 0.0 | 178.66 ± 9.9 | 3.53 ± 0.29 | 460.00 ± 38.2 | 785.00 ± 68.7 | 75.00 ± 5.10 |
| 0.5 | 221.60 ± 18.1 | 3.78 ± 0.35 | 498.50 ± 43.9 | 953.91 ± 91.1 | 120.41 ± 9.9 | |
| 1.0 | 265.58 ± 16.1 | 4.50 ± 0.41 | 628.91 ± 43.5 | 1311.83 ± 95.7 | 140.91 ± 7.0 | |
| 1.5 | 218.12 ± 20.1 | 3.91 ± 0.33 | 483.16 ± 39.3 | 898.41 ± 97.0 | 136.67 ± 10.8 | |
| Main Effects | ||||||
| Variety | Light violet | 219.09 ± 9.1 | 3.80 ± 0.10 | 511.97 ± 22.46 | 982.16 ± 60.6 | 117.03 ± 5.9 |
| Pink | 220.99 ± 9.4 | 3.93 ± 0.18 | 517.65 ± 22.71 | 987.29 ± 53.5 | 118.31 ± 5.8 | |
| Treatments | 0.0 | 176.51 ± 7.00 c | 3.51 ± 0.16 | 452.77 ± 23.25 b | 750.13 ± 45.3 c | 71.68 ± 3.9 b |
| 0.5 | 219.77 ± 11.5 b | 3.70 ± 0.21 | 496.05 ± 27.92 b | 968.03 ± 63.0 b | 122.01 ± 6.9 a | |
| 1.0 | 259.59 ± 11.4 a | 4.31 ± 0.23 | 612.66 ± 31.3 a | 1286.9 ± 78.0 a | 137.12 ± 5.1 a | |
| 1.5 | 224.29 ± 15.3 b | 3.95 ± 0.18 | 497.75 ± 34.6 b | 933.79 ± 88.7 bc | 139.87 ± 8.17 a | |
| LSD | Variety | ns | ns | ns | ns | ns |
| Treatments | 34.503 * | ns | 86.789 * | 207.87 * | 18.405 * | |
| Variety × Treatments | ns | ns | ns | ns | ns | |
| Parameters | MLP and ML Models | R2 | RMSE | MAE |
|---|---|---|---|---|
| Projected Area (cm2) | MLP | 0.95 | 0.05 | 0.03 |
| GP | 0.94 | 0.03 | 0.02 | |
| RF | 0.91 | 0.06 | 0.04 | |
| XGBoost | 0.89 | 0.06 | 0.04 | |
| Surface Area (cm2) | MLP | 0.94 | 0.05 | 0.03 |
| GP | 0.95 | 0.04 | 0.03 | |
| RF | 0.92 | 0.06 | 0.04 | |
| XGBoost | 0.91 | 0.06 | 0.04 | |
| Length (cm) | MLP | 0.42 | 0.20 | 0.16 |
| GP | 0.43 | 0.19 | 0.15 | |
| RF | 0.42 | 0.19 | 0.15 | |
| XGBoost | 0.35 | 0.20 | 0.16 | |
| Average Diameter (mm) | MLP | 0.35 | 0.16 | 0.13 |
| GP | 0.35 | 0.16 | 0.12 | |
| RF | 0.22 | 0.17 | 0.14 | |
| XGBoost | 0.20 | 0.17 | 0.12 | |
| Root Volume (cm3) | MLP | 0.54 | 0.15 | 0.12 |
| GP | 0.52 | 0.15 | 0.12 | |
| RF | 0.47 | 0.15 | 0.12 | |
| XGBoost | 0.47 | 0.15 | 0.12 | |
| Root Tips | MLP | 0.78 | 0.11 | 0.09 |
| GP | 0.79 | 0.11 | 0.09 | |
| RF | 0.75 | 0.12 | 0.10 | |
| XGBoost | 0.75 | 0.12 | 0.09 | |
| Root Forks | MLP | 0.85 | 0.09 | 0.07 |
| GP | 0.81 | 0.09 | 0.07 | |
| RF | 0.81 | 0.09 | 0.07 | |
| XGBoost | 0.80 | 0.10 | 0.07 | |
| Root Crossing | MLP | 0.46 | 0.19 | 0.15 |
| GP | 0.46 | 0.18 | 0.15 | |
| RF | 0.55 | 0.17 | 0.13 | |
| XGBoost | 0.45 | 0.18 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tütüncü, M. Effects of Protein Hydrolysate Derived from Anchovy By-Product on Plant Growth of Primrose and Root System Architecture Analysis with Machine Learning. Horticulturae 2024, 10, 400. https://doi.org/10.3390/horticulturae10040400
Tütüncü M. Effects of Protein Hydrolysate Derived from Anchovy By-Product on Plant Growth of Primrose and Root System Architecture Analysis with Machine Learning. Horticulturae. 2024; 10(4):400. https://doi.org/10.3390/horticulturae10040400
Chicago/Turabian StyleTütüncü, Mehmet. 2024. "Effects of Protein Hydrolysate Derived from Anchovy By-Product on Plant Growth of Primrose and Root System Architecture Analysis with Machine Learning" Horticulturae 10, no. 4: 400. https://doi.org/10.3390/horticulturae10040400
APA StyleTütüncü, M. (2024). Effects of Protein Hydrolysate Derived from Anchovy By-Product on Plant Growth of Primrose and Root System Architecture Analysis with Machine Learning. Horticulturae, 10(4), 400. https://doi.org/10.3390/horticulturae10040400






