Effect of Soaking, Germination, and Roasting on Phenolic Composition, Antioxidant Activities, and Fatty Acid Profile of Sunflower (Helianthus annuus L.) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sunflower Samples
- W1 = initial weight of the sample (mg);
- W2 = weight of the sample after drying (mg).
| Cultivars | Maturity (Days) | Characteristics | ||
|---|---|---|---|---|
| Average Size and Shape of Seed | Color of Shell | Use | ||
| Artuel | 90–100 | 9.3 × 4.8 mm, oval shape | Stripe black and white | Directly consumed as seed |
| Peredovik | 95–120 | 9.0 × 4.9 mm, oval shape | Black | Edible oil |
2.3. Processing
2.3.1. Soaking
2.3.2. Germination
2.3.3. Roasting
2.4. Extraction
2.5. Determination of TPC
2.6. Determination of TFC
2.7. Determination of Phenolic Compounds and Flavonoids by HPLC
2.8. Determination of Antioxidant Activity
2.8.1. DPPH Free Radical Scavenging Activity
- A0 = initial absorbance of DPPH solution (without sample);
- A1 = final absorbance of the DPPH solution after reaction with the sample.
2.8.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.8.3. ABTS Free Radical Scavenging Activity
- A0 = initial absorbance of ABTS solution (without sample);
- A1 = final absorbance of the ABTS solution after reaction with the sample.
2.9. Extraction and Determination of the Fatty Acid Composition
2.10. Statistical Analysis
3. Results and Discussion
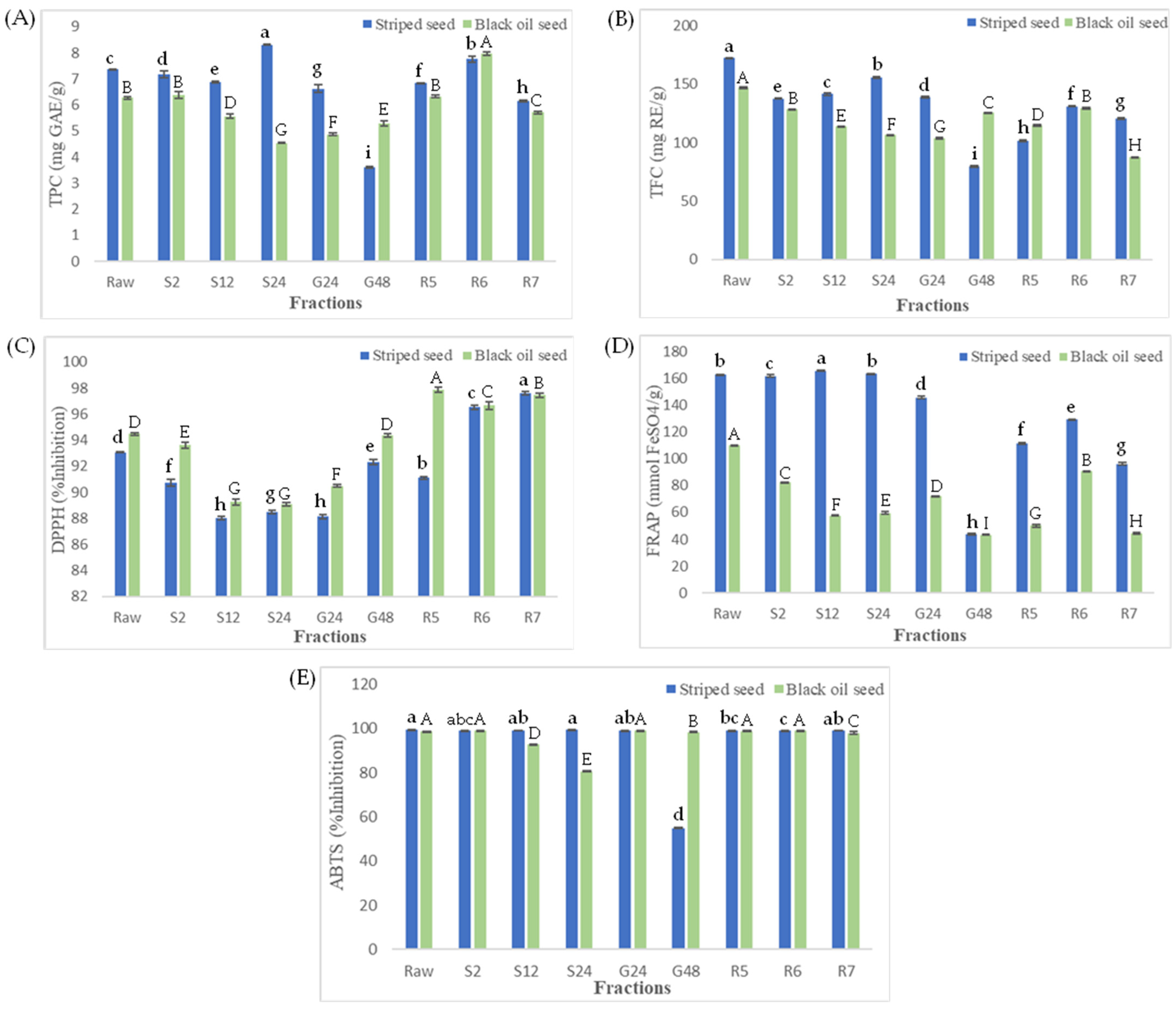
3.1. Total Phenolic and Total Flavonoid Contents
3.2. Change in Antioxidant Activity
3.3. Changes in Content and Composition of Phenolic Acids
3.4. Fatty Acid Composition and Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira Filho, J.G.; Egea, M.B. Sunflower seed byproduct and its fractions for food application: An attempt to improve the sustainability of the oil process. J. Food Sci. 2021, 86, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of Seed Oil Cakes as Industrial Co-Streams for Production of Innovative Bioplastics. A Review. Trends Food Sci. Tech. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Oilseed Crop Sunflower (Helianthus annuus) as a Source of Food: Nutritional and Health Benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Bester, D.; Esterhuyse, A.J.; Truter, E.J.; van Rooyen, J. Cardiovascular effects of edible oils: A comparison between four popular edible oils. Nutr. Res. Rev. 2010, 23, 334–348. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Nutrition Board of the Institute of Medicine Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- So, V.; Poul, P.; Oeung, S.; Srey, P.; Mao, K.; Ung, H.; Eng, P.; Heim, M.; Srun, M.; Chheng, C.; et al. Bioactive Compounds, Antioxidant Activities, and HPLC Analysis of Nine Edible Sprouts in Cambodia. Molecules 2023, 28, 2874. [Google Scholar] [CrossRef] [PubMed]
- Gai, F.; Karamać, M.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Sunflower (Helianthus annuus L.) Plants at Various Growth Stages Subjected to Extraction—Comparison of the Antioxidant Activity and Phenolic Profile. Antioxidants 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ge, Y.; Na Jom, K. A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, T.D.; da Silva, M.G.; Ferrari, R.A.; Ruiz, A.L.T.G.; Duarte, R.M.T.; Simabuco, F.M.; Bezerra, R.M.N.; Pacheco, M.T.B. Evaluation of some in vitro bioactivities of sunflower phenolic compounds. Curr. Res. Food Sci. 2021, 4, 662–669. [Google Scholar] [CrossRef]
- Igual, M.; Martínez-Monzó, J. Physicochemical Properties and Structure Changes of Food Products during Processing. Foods 2022, 11, 2365. [Google Scholar] [CrossRef] [PubMed]
- Tenyang, N.; Ponka, R.; Tiencheu, B.; Tonfack Djikeng, F.; Womeni, H.M. Effect of boiling and oven roasting on some physicochemical properties of sunflower seeds produced in Far North, Cameroon. Food Sci. Nutr. 2022, 10, 402–411. [Google Scholar] [CrossRef]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of Extrusion and Traditional Processing Methods on Antinutrients and In Vitro Digestibility of Protein and Starch in Faba and Kidney Beans. Food Chem. 2000, 68, 159–165. [Google Scholar] [CrossRef]
- Abdel-Aleem, W.M.; Abdel-Hameed, S.; Latif, S. Effect of Soaking and Cooking on Nutritional and Quality Properties of Faba Bean. J. Food Dairy Sci. 2019, 10, 389–395. [Google Scholar] [CrossRef]
- Mecha, E.; Leitão, S.T.; Carbas, B.; Serra, A.T.; Moreira, P.M.; Veloso, M.M.; Gomes, R.; Figueira, M.E.; Brites, C.; Vaz Patto, M.C.; et al. Characterization of Soaking Process’ Impact in Common Beans Phenolic Composition: Contribute from the Unexplored Portuguese Germplasm. Foods 2019, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.C.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Thongchuang, M.; Kunsombat, C.; Taothong, R.; Naknawa, W.; Kraboun, K.; Ajavakom, V.; Wutipraditkul, N. Antioxidant capacity in different cultivars of sunflower sprouts and their harvesting indices. J. Appl. Sci. 2019, 18, 79–96. [Google Scholar] [CrossRef]
- Jan, K.; Ahmad, M.; Rehman, S.; Gani, A.; Khaqan, K. Effect of Roasting on Physicochemical and Antioxidant Properties of Kalonji (Nigella sativa) Seed Flour. J. Food Meas. Charact. 2019, 13, 1364–1372. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Lu, X.; Sun, H.; Wang, F. Effect of Oilseed Roasting on the Quality, Flavor and Safety of Oil: A Comprehensive Review. Int. Food Res. 2021, 150, 110791. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Lu, D.; Liu, J.; Jiang, B.; Chen, J. Effect of Roasting on the Antioxidant Activity, Phenolic Composition, and Nutritional Quality of Pumpkin (Cucurbita pepo L.) Seeds. Front. Nutr. 2021, 8, 647354. [Google Scholar] [CrossRef]
- Valdés García, A.; Beltrán Sanahuja, A.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Effect of Frying and Roasting Processes on the Oxidative Stability of Sunflower Seeds (Helianthus annuus) under Normal and Accelerated Storage Conditions. Foods 2021, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- El-Safy, F.; Salem, R.; Ensaf Mukhtar, Y.Y. The Impact of Soaking and Germination on Chemical Composition, Carbohydrate Fractions, Digestibility, Antinutritional Factors and Minerals Content of Some Legumes and Cereals Grain Seeds. Alex. Sci. Exch. J. 2013, 34, 499–513. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; Villanueva-Suárez, M.J.; Mateos-Aparicio, I. Soybean seeds and its by-product okara as sources of dietary fibre. Measurement by AOAC and Englyst methods. Food Chem. 2008, 108, 1099–1105. [Google Scholar] [CrossRef]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). Acta Sci. Pol. Technol. Aliment. 2012, 11, 293–302. [Google Scholar] [PubMed]
- Tarasevičienė, Ž.; Viršilė, A.; Danilčenko, H.; Duchovskis, P.; Paulauskienė, A.; Gajewski, M. Effects of Germination Time on the Antioxidant Properties of Edible Seeds. CyTA J. Food 2019, 17, 447–454. [Google Scholar] [CrossRef]
- Abu Bakar, M.F.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and Antioxidant Activity of Different Parts of Bambangan (Mangifera Pajang) and Tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic Contents and Antioxidant Activities of Bitter Gourd (Momordica Charantia L.) Leaf, Stem and Fruit Fraction Extracts In Vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Yuan, S.H.; Chang, S.K.C. Comparative Analyses of Phenolic Composition, Antioxidant Capacity, and Color of Cool Season Legumes and Other Selected Food Legumes. J. Food Sci. 2007, 72, S167–S177. [Google Scholar] [CrossRef]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical Distribution in Hull and Cotyledon of Adzuki Bean (Vigna angularis L.) and Mung Bean (Vigna radiate L.), and Their Contribution to Antioxidant, Anti-Inflammatory and Anti-Diabetic Activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. Improving Public Health?: The Role of Antioxidant-Rich Fruit and Vegetable Beverages. Food Res. Int. 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Yang, L.-F.; Siriamornpun, S.; Li, D. Polyunsaturated fatty acid content of edible insects in thailand. J. Food Lipids. 2006, 13, 277–285. [Google Scholar] [CrossRef]
- Kataria, A.; Chauhan, B.M.; Punia, D. Antinutrients and Protein Digestibility (In Vitro) of Mungbean as Affected by Domestic Processing and Cooking. Food Chem. 1989, 32, 9–17. [Google Scholar] [CrossRef]
- Khandelwal, S.; Udipi, S.A.; Ghugre, P. Polyphenols and Tannins in Indian Pulses: Effect of Soaking, Germination and Pressure Cooking. Food Res. Int. 2010, 43, 526–530. [Google Scholar] [CrossRef]
- Jood, S.; Chauhan, B.M.; Kapoor, A.C. Saponin Content of Chickpea and Black Gram: Varietal Differences and Effects of Processing and Cooking Methods. J. Sci. Food Agric. 1986, 37, 1121–1124. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef]
- Saharan, K.; Khetarpaul, N.; Bishnoi, S. Variability in Physico-Chemical Properties and Nutrient Composition of Newly Released Ricebean and Fababean Cultivars. J. Food Compos. Anal. 2002, 15, 159–167. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Li, J.; Pei, Y.; Liang, Y. Antioxidant Properties of Tartary Buckwheat Extracts as Affected by Different Thermal Processing Methods. LWT Food Sci. 2010, 43, 181–185. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.-T.; Shetty, K. Stimulation of Phenolics, Antioxidant and Antimicrobial Activities in Dark Germinated Mung Bean Sprouts in Response to Peptide and Phytochemical Elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Huang, J.; Qian, J.; Wang, S.; Li, Y.; Zhai, X.; Olajide, T.M.; Shen, G.X.; Liao, X. Effect of selenium biofortification on bioactive compounds and antioxidant activity in germinated black soybean. J. Food Sci. 2022, 87, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Rizki, H.; Kzaiber, F.; Elharfi, M.; Ennahli, S.; Hanine, H. Effects of Roasting Temperature and Time on the Physicochemical Properties of Sesame (Sesamum Indicum .L) Seeds. Int. J. Innov. Appl. Stud. 2015, 11, 148–155. [Google Scholar]
- Chandrasekara, N.; Shahidi, F. Antioxidative Potential of Cashew Phenolics in Food and Biological Model Systems as Affected by Roasting. Food Chem. 2011, 129, 1388–1396. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. The Antioxidant and Free Radical Scavenging Activities of Processed Cowpea (Vigna unguiculata (L.) Walp.) Seed Extracts. Food Chem. 2007, 101, 10–19. [Google Scholar] [CrossRef]
- Boateng, J.; Verghese, M.; Walker, L.T.; Ogutu, S. Effect of Processing on Antioxidant Contents in Selected Dry Beans (Phaseolus spp. L.). LWT Food Sci. 2008, 41, 1541–1547. [Google Scholar] [CrossRef]
- Franke, A.A.; Custer, L.J.; Cerna, C.M.; Narala, K.K. Quantitation of Phytoestrogens in Legumes by HPLC. J. Agric. Food Chem. 1994, 42, 1905–1913. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, S.-C.; Hu, C.-C.; Shyu, Y.-S.; Hsu, C.-Y.; Yang, D.-J. Effects of Roasting Temperature and Duration on Fatty Acid Composition, Phenolic Composition, Maillard Reaction Degree and Antioxidant Attribute of Almond (Prunus dulcis) Kernel. Food Chem. 2016, 190, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Effect of Soaking, Boiling, and Steaming on Total Phenolic Contentand Antioxidant Activities of Cool Season Food Legumes. Food Chem. 2008, 110, 1–13. [Google Scholar] [CrossRef]
- Win, M.M.; Abdul-Hamid, A.; Baharin, B.S.; Anwar, F.; Saari, N. Effects of Roasting on Phenolics Composition and Antioxidant Activity of Peanut (Arachis hypogaea L.) Kernel Flour. Eur. Food Res. Technol. 2011, 233, 599–608. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Kemsawasd, V.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Suttisansanee, U. The Effects of Different Roasting Methods on the Phenolic Contents, Antioxidant Potential, and In Vitro Inhibitory Activities of Sacha Inchi Seeds. Foods 2023, 12, 4178. [Google Scholar] [CrossRef]
- Shimoji, Y.; Tamura, Y.; Nakamura, Y.; Nanda, K.; Nishidai, S.; Nishikawa, Y.; Ishihara, N.; Uenakai, K.; Ohigashi, H. Isolation and Identification of DPPH Radical Scavenging Compounds in Kurosu (Japanese Unpolished Rice Vinegar). J. Agric. Food Chem. 2002, 50, 6501–6503. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef]
- Schmitzer, V.; Slatnar, A.; Veberic, R.; Stampar, F.; Solar, A. Roasting Affects Phenolic Composition and Antioxidative Activity of Hazelnuts (Corylus avellana L.). J. Food Sci. 2011, 76, S14–S19. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) Skins as a Potential Source of Bioactive Polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under Moderate Salinity Increases Phenolic Content and Antioxidant Activity in Rapeseed (Brassica napus var. oleifera Del.) Sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef] [PubMed]
- Criste, A.D.; Urcan, A.C.; Coroian, C.O.; Copolovici, L.; Copolovici, D.M.; Burtescu, R.F.; Oláh, N.K. Plant-Based Beverages from Germinated and Ungerminated Seeds, as a Source of Probiotics, and Bioactive Compounds with Health Benefits—Part 1: Legumes. Agriculture 2023, 13, 1185. [Google Scholar] [CrossRef]
- Hahm, T.-S.; Park, S.-J.; Martin Lo, Y. Effects of Germination on Chemical Composition and Functional Properties of Sesame (Sesamum indicum L.) Seeds. Bioresour. Technol. 2009, 100, 1643–1647. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, J.-H.; Xu, Y.; Chen, Y.; Qian, K.; Zhang, Y. Effect of germination on nutritional quality of soybean. Food Sci. Technol. 2023, 43, e008323. [Google Scholar] [CrossRef]
- Kita, A.; Figiel, A. Effect of Roasting on Properties of Walnuts. Pol. J. Food Nutr. Sci. 2007, 57, 89–94. [Google Scholar]
- Arab, R.; Casal, S.; Pinho, T.; Cruz, R.; Freidja, M.L.; Lorenzo, J.M.; Hano, C.; Madani, K.; Boulekbache-Makhlouf, L. Effects of Seed Roasting Temperature on Sesame Oil Fatty Acid Composition, Lignan, Sterol and Tocopherol Contents, Oxidative Stability and Antioxidant Potential for Food Applications. Molecules 2022, 27, 4508. [Google Scholar] [CrossRef]
- Durmaz, G.; Gökmen, V. Impacts of Roasting Oily Seeds and Nuts on Their Extracted Oils. Lipid Technol. 2010, 22, 179–182. [Google Scholar] [CrossRef]
- Lagiso, T.M.; Singh, B.C.S.; Weyessa, B. Evaluation of sunflower (Helianthus annuus L.) genotypes for quantitative traits and character association of seed yield and yield components at Oromia region, Ethiopia. Euphytica 2021, 217, 27. [Google Scholar] [CrossRef]
| Hydroxybenzoic Acids (mg/g DW) | Fractions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | S2 | S12 | S24 | G24 | G48 | R5 | R6 | R7 | ||
| Striped Seeds | GA | 3.72 ± 0.01 d | ND | 4.31 ± 0.01 c | ND | ND | 5.38 ± 0.03 b | 6.39 ± 0.21 a | 6.54 ± 0.60 a | 6.23 ± 0.22 a |
| PCCA | 2.80 ± 0.01 e | 3.54 ± 0.01 d | 3.97 ± 0.01 c | 4.34 ± 0.03 b | ND | ND | 2.77 ± 0.02 e | 2.87 ± 0.23 e | 4.58 ± 0.14 a | |
| p-OH | 3.49 ± 0.02 c | 3.98 ± 0.01 b | ND | ND | 4.20 ± 0.02 a | ND | ND | ND | ND | |
| VA | 26.98 ± 0.23 c | 32.95 ± 0.41 a | 33.17 ± 0.33 a | 26.30 ± 0.40 cd | 31.69 ± 0.54 b | 6.65 ± 0.18 f | 18.90 ± 0.49 e | 25.94 ± 0.79 d | ND | |
| Total | 37.00 ± 0.22 c | 40.46 ± 0.43 b | 41.46 ± 0.33 a | 30.64 ± 0.38 e | 35.89 ± 0.53 d | 12.03 ± 0.21 g | 28.06 ± 0.71 f | 35.35 ± 0.77 d | 10.81 ± 0.20 h | |
| Black Oil Seeds | GA | 5.25 ± 0.05 b | 5.47 ± 0.03 a | 5.42 ± 0.02 a | ND | ND | ND | 5.06 ± 0.07 c | 4.67 ± 0.08 d | 5.22 ± 0.01 b |
| PCCA | ND | ND | 4.92 ± 0.04 c | 7.18 ± 0.05 b | 7.30 ± 0.20 a | ND | 3.22 ± 0.06 d | 2.76 ± 0.02 e | 2.77 ± 0.01 e | |
| p-OH | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| VA | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Total | 5.25 ± 0.05 g | 5.47 ± 0.03 f | 10.34 ± 0.04 a | 7.18 ± 0.05 e | 7.30 ± 0.20 de | ND | 8.28 ± 0.13 b | 7.43 ± 0.10 d | 7.99 ± 0.02 c | |
| Hydroxycinnamic Acids (mg/g DW) | Fractions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | S2 | S12 | S24 | G24 | G48 | R5 | R6 | R7 | ||
| Striped Seeds | ChA | 37.78 ± 0.39 b | 36.00 ± 0.35 d | 36.72 ± 0.06 c | 29.07 ± 0.29 e | 38.39 ± 0.46 a | 5.88 ± 0.14 h | 20.63 ± 0.20 f | 19.47 ± 0.51 g | ND |
| CFA | 150.98 ± 0.53 d | 192.01 ± 0.84 ab | 191.76 ± 0.77 b | 158.16 ± 0.26 c | 192.97 ± 0.89 a | 63.95 ± 0.63 h | 115.912 ± 0.56 e | 95.97 ± 0.34 f | 92.41 ± 0.16 g | |
| SyA | 3.09 ± 0.01 f | 8.35 ± 0.21 a | 8.07 ± 0.04 a | 6.23 ± 0.10 d | 6.62 ± 0.06 b | 4.82 ± 0.04 e | 3.10 ± 0.06 f | 3.02 ± 0.23 f | 3.07 ± 0.01 f | |
| p-CA | 3.10 ± 0.01 d | 3.84 ± 0.02 c | 4.19 ± 0.13 b | 4.10 ± 0.02 b | 4.11 ± 0.01 b | 4.71 ± 0.04 a | 2.95 ± 0.05 e | 2.99 ± 0.23 de | 3.06 ± 0.02 de | |
| FA | 711.10 ± 0.61 i | 901.84 ± 1.02 e | 1123.72 ± 1.06 a | 894.83 ± 0.66 f | 755.28 ± 0.85 h | 88.11 ± 0.89 j | 1092.85 ± 0.92 c | 1113.26 ± 0.89 b | 1069.97 ± 0.67 d | |
| SNA | 6.33 ± 0.09 f | 6.51 ± 0.11 ef | 8.04 ± 0.09 b | 6.71 ± 0.08 e | 6.02 ± 0.03 g | 7.46 ± 0.11 cd | 7.69 ± 0.15 c | 8.59 ± 0.30 a | 7.25 ± 0.10 d | |
| Total | 912.41 ± 1.28 g | 1148.54 ± 0.64 d | 1372.50 ± 1.04 a | 1099.10 ± 0.64 e | 1003.38 ± 1.34 f | 174.94 ± 1.77 h | 1243.14 ± 0.24 b | 1243.31 ± 1.17 b | 1175.76 ± 0.60 c | |
| Black Oil Seeds | ChA | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| CFA | 91.91 ± 0.96 f | 151.90 ± 0.45 a | 121.89 ± 0.35 d | 123.00 ± 0.59 c | 128.56 ± 0.52 b | 108.90 ± 0.73 e | 78.78 ± 0.43 g | 76.84 ± 0.09 h | 71.25 ± 0.42 i | |
| SyA | 3.02 ± 0.01 e | 6.63 ± 0.11 a | 6.70 ± 0.05 a | 5.78 ± 0.02 b | 5.37 ± 0.10 c | 5.02 ± 0.04 d | 2.87 ± 0.02 h | 2.84 ± 0.01 h | 2.89 ± 0.01 h | |
| p-CA | 2.94 ± 0.01 d | 3.68 ± 0.02 c | 3.91 ± 0.01 b | 3.90 ± 0.01 b | 3.90 ± 0.05 b | 5.30 ± 0.06 a | 2.76 ± 0.01 i | 2.83 ± 0.01 f | 2.89 ± 0.01 e | |
| FA | 795.28 ± 0.57 f | 929.18 ± 0.91 d | 941.11 ± 0.61 c | 593.71 ± 0.87 g | 583.62 ± 0.84 h | 578.78 ± 0.84 i | 1048.79 ± 0.95 a | 987.55 ± 0.83 b | 844.08 ± 0.68 e | |
| SNA | 5.23 ± 0.04 h | 5.90 ± 0.02 e | 6.66 ± 0.06 c | 5.58 ± 0.09 g | 5.51 ± 0.03 g | 6.07 ± 0.04 d | 7.47 ± 0.29 a | 7.03 ± 0.31 b | 5.84 ± 0.03 f | |
| Total | 898.38 ± 1.54 f | 1097.29 ± 1.27 c | 1080.27 ± 0.27 b | 732.01 ± 0.99 g | 726.98 ± 1.20 h | 674.07 ± 1.57 i | 1140.47 ± 1.14 a | 1077.10 ± 1.20 d | 926.95 ± 0.34 e | |
| Fractions | Fatty Acid Composition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | ΣSFA | ΣMUFA | ΣPUFA | Total | |
| Raw | 4.42 ± 0.08 c | 2.59 ± 0.07 c | 48.25 ± 0.19 a | 42.74 ± 0.38 f | 1.72 ± 0.07 a | 7.01 ± 0.15 cde | 48.25 ± 0.19 a | 44.46 ± 0.44 e | 100 |
| S2 | 4.37 ± 0.06 c | 2.46 ± 0.02 d | 46.50 ± 0.60 b | 45.58 ± 0.58 c | 1.09 ± 0.02 f | 6.83 ± 0.08 e | 46.50 ± 0.60 b | 46.83 ± 0.56 c | 100 |
| S12 | 4.42 ± 0.05 c | 2.76 ± 0.01 c | 47.09 ± 0.30 b | 44.44 ± 0.24 de | 1.30 ± 0.04 d | 7.18 ± 0.06 cd | 47.09 ± 0.30 b | 45.73 ± 0.26 d | 100 |
| S24 | 4.72 ± 0.17 b | 3.03 ± 0.01 a | 46.53 ± 0.32 b | 44.14 ± 0.36 e | 1.58 ± 0.01 b | 7.75 ± 0.18 a | 46.53 ± 0.32 b | 45.72 ± 0.35 d | 100 |
| G24 | 5.13 ± 0.08 a | 2.59 ± 0.03 c | 38.67 ± 0.57 d | 52.95 ± 0.54 a | 0.66 ± 0.01 g | 7.72 ± 0.11 a | 38.67 ± 0.57 d | 53.61 ± 0.54 a | 100 |
| G48 | 4.37 ± 0.04 c | 2.63 ± 0.04 c | 46.55 ± 0.38 b | 45.11 ± 0.38 cd | 1.35 ± 0.02 c | 6.99 ± 0.08 de | 46.55 ± 0.38 b | 46.46 ± 0.40 c | 100 |
| R5 | 4.71 ± 0.01 b | 2.73 ± 0.06 b | 44.96 ± 0.42 c | 46.31 ± 0.40 b | 1.29 ± 0.01 d | 7.44 ± 0.05 b | 44.96 ± 0.42 c | 47.60 ± 0.39 b | 100 |
| R6 | 4.45 ± 0.11 c | 2.60 ± 0.07 c | 45.59 ± 0.27 c | 46.31 ± 0.07 b | 1.05 ± 0.03 f | 7.05 ± 0.18 cd | 45.59 ± 0.27 c | 47.36 ± 0.10 b | 100 |
| R7 | 4.59 ± 0.03 b | 2.62 ± 0.01 b | 45.10 ± 0.30 c | 46.50 ± 0.31 b | 1.18 ± 0.02 e | 7.22 ± 0.02 c | 45.10 ± 0.30 c | 47.68 ± 0.29 b | 100 |
| Fractions | Fatty Acids Concentrations (mg/100 g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | ΣSFA | ΣMUFA | ΣPUFA | Total | |
| Raw | 76.64 ± 0.30 e | 44.85 ± 0.58 e | 836.52 ± 12.56 d | 740.96 ± 7.95 e | 29.74 ± 0.74 a | 121.49 ± 0.88 d | 836.52 ± 12.56 d | 770.70 ± 7.55 d | 1728.71 ± 16.95 d |
| S2 | 84.33 ± 0.97 c | 47.48 ± 0.32 d | 897.70 ± 11.04 c | 880.07 ± 13.82 c | 21.09 ± 0.31 d | 131.81 ± 1.30 c | 897.70 ± 11.04 c | 901.16 ± 13.51 c | 1930.68 ± 9.99 c |
| S12 | 67.42 ± 0.97 f | 42.03 ± 0.29 f | 717.82 ± 12.70 e | 677.36 ± 4.14 f | 19.75 ± 0.50 e | 109.45 ± 1.22 e | 717.82 ± 12.70 e | 697.10 ± 3.95 e | 1524.37 ± 17.47 e |
| S24 | 19.99 ± 0.69 i | 12.84 ± 0.05 i | 197.11 ± 1.11 h | 186.97 ± 1.84 g | 6.71 ± 0.05 i | 32.84 ± 0.73 h | 197.11 ± 1.11 h | 193.68 ± 1.79 h | 423.63 ± 0.68 h |
| G24 | 153.57 ± 1.84 a | 77.63 ± 0.53 a | 1158.22 ± 21.94 a | 1586.01 ± 35.47 a | 19.63 ± 0.21 f | 231.21 ± 2.30 a | 1158.22 ± 21.94 a | 1605.64 ± 35.62 a | 2995.07 ± 47.80 a |
| G48 | 82.65 ± 0.16 d | 49.69 ± 0.30 c | 881.10 ± 11.64 c | 853.78 ± 11.56 d | 25.61 ± 0.57 b | 132.34 ± 0.33 c | 881.10 ± 11.64 c | 879.39 ± 12.11 c | 1892.83 ± 18.66 c |
| R5 | 37.63 ± 0.15 h | 21.80 ± 0.45 h | 359.15 ± 3.83 g | 369.97 ± 2.70 h | 10.34 ± 0.09 h | 59.43 ± 0.31 g | 359.15 ± 3.83 g | 380.31 ± 2.65 g | 798.89 ± 1.28 g |
| R6 | 104.81 ± 0.66 b | 61.30 ± 0.58 b | 1074.45 ± 26.93 b | 1091.42 ± 19.18 b | 24.85 ± 0.26 c | 166.11 ± 1.23 b | 1074.45 ± 26.93 b | 1116.27 ± 19.02 b | 2356.83 ± 44.88 b |
| R7 | 64.65 ± 0.35 g | 36.93 ± 0.20 g | 634.63 ± 4.92 f | 654.25 ± 3.70 f | 16.63 ± 0.30 g | 101.57 ± 0.24 f | 634.63 ± 4.92 f | 670.88 ± 3.44 f | 1407.08 ± 1.48 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thepthanee, C.; Li, H.; Wei, H.; Prakitchaiwattana, C.; Siriamornpun, S. Effect of Soaking, Germination, and Roasting on Phenolic Composition, Antioxidant Activities, and Fatty Acid Profile of Sunflower (Helianthus annuus L.) Seeds. Horticulturae 2024, 10, 387. https://doi.org/10.3390/horticulturae10040387
Thepthanee C, Li H, Wei H, Prakitchaiwattana C, Siriamornpun S. Effect of Soaking, Germination, and Roasting on Phenolic Composition, Antioxidant Activities, and Fatty Acid Profile of Sunflower (Helianthus annuus L.) Seeds. Horticulturae. 2024; 10(4):387. https://doi.org/10.3390/horticulturae10040387
Chicago/Turabian StyleThepthanee, Chorpaka, Hua Li, Hu Wei, Cheunjit Prakitchaiwattana, and Sirithon Siriamornpun. 2024. "Effect of Soaking, Germination, and Roasting on Phenolic Composition, Antioxidant Activities, and Fatty Acid Profile of Sunflower (Helianthus annuus L.) Seeds" Horticulturae 10, no. 4: 387. https://doi.org/10.3390/horticulturae10040387
APA StyleThepthanee, C., Li, H., Wei, H., Prakitchaiwattana, C., & Siriamornpun, S. (2024). Effect of Soaking, Germination, and Roasting on Phenolic Composition, Antioxidant Activities, and Fatty Acid Profile of Sunflower (Helianthus annuus L.) Seeds. Horticulturae, 10(4), 387. https://doi.org/10.3390/horticulturae10040387






